Non-Targeted Lipidomics Analysis of Characteristic Milk Using High-Resolution Mass Spectrometry (UHPLC-HRMS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Sample Collection
2.1.2. Reagents and Consumables
2.1.3. Instruments
2.2. Methods
2.2.1. Total Lipid Extraction
2.2.2. Sample Preparation
2.2.3. UHPLC-HRMS Analysis
2.2.4. Data Processing
3. Results
3.1. Reliability of Methods and Results
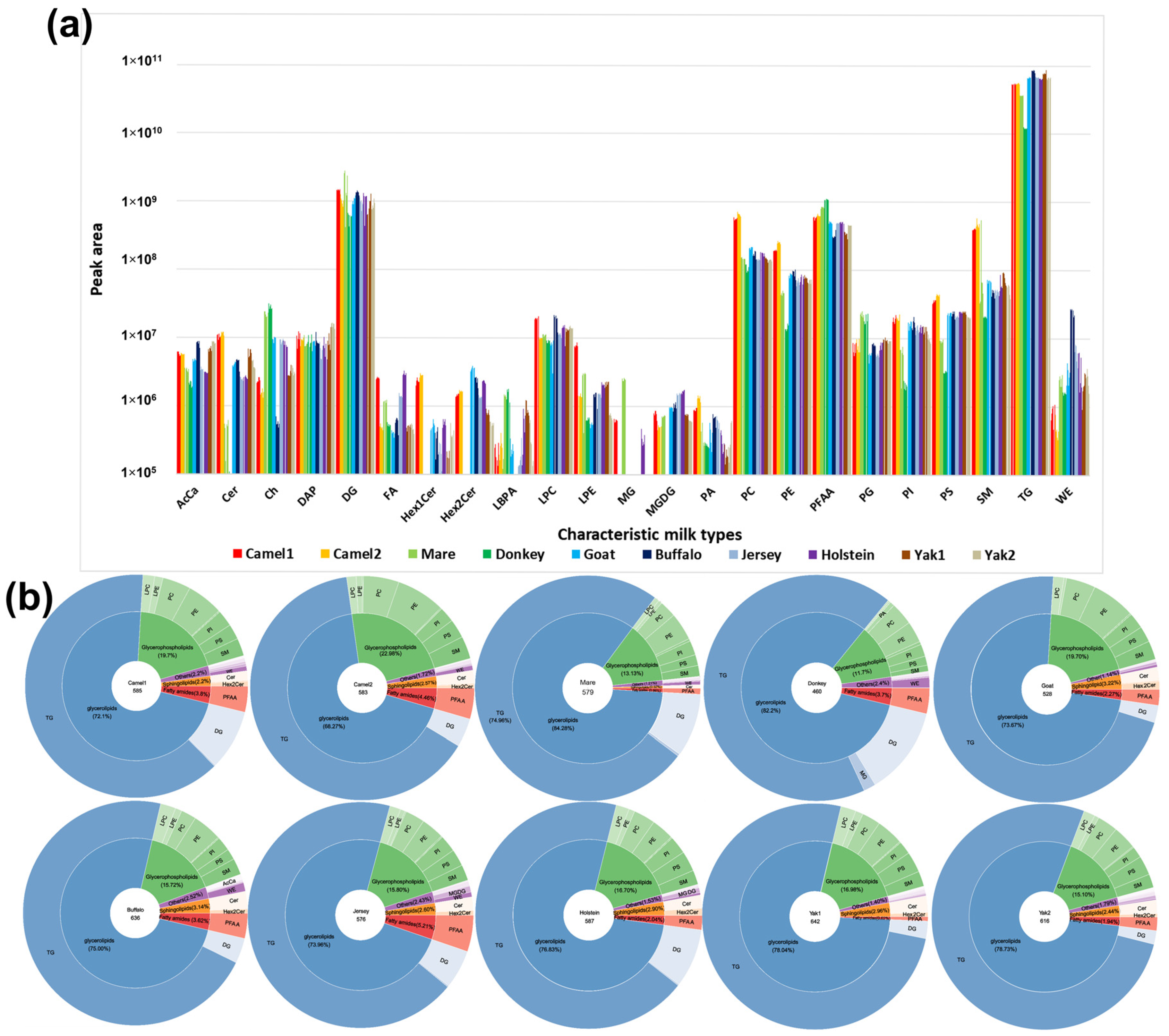
3.2. Differences in Total Lipid Content of Raw Milk Samples
3.3. Differences in Total Lipid Composition of Raw Milk Samples
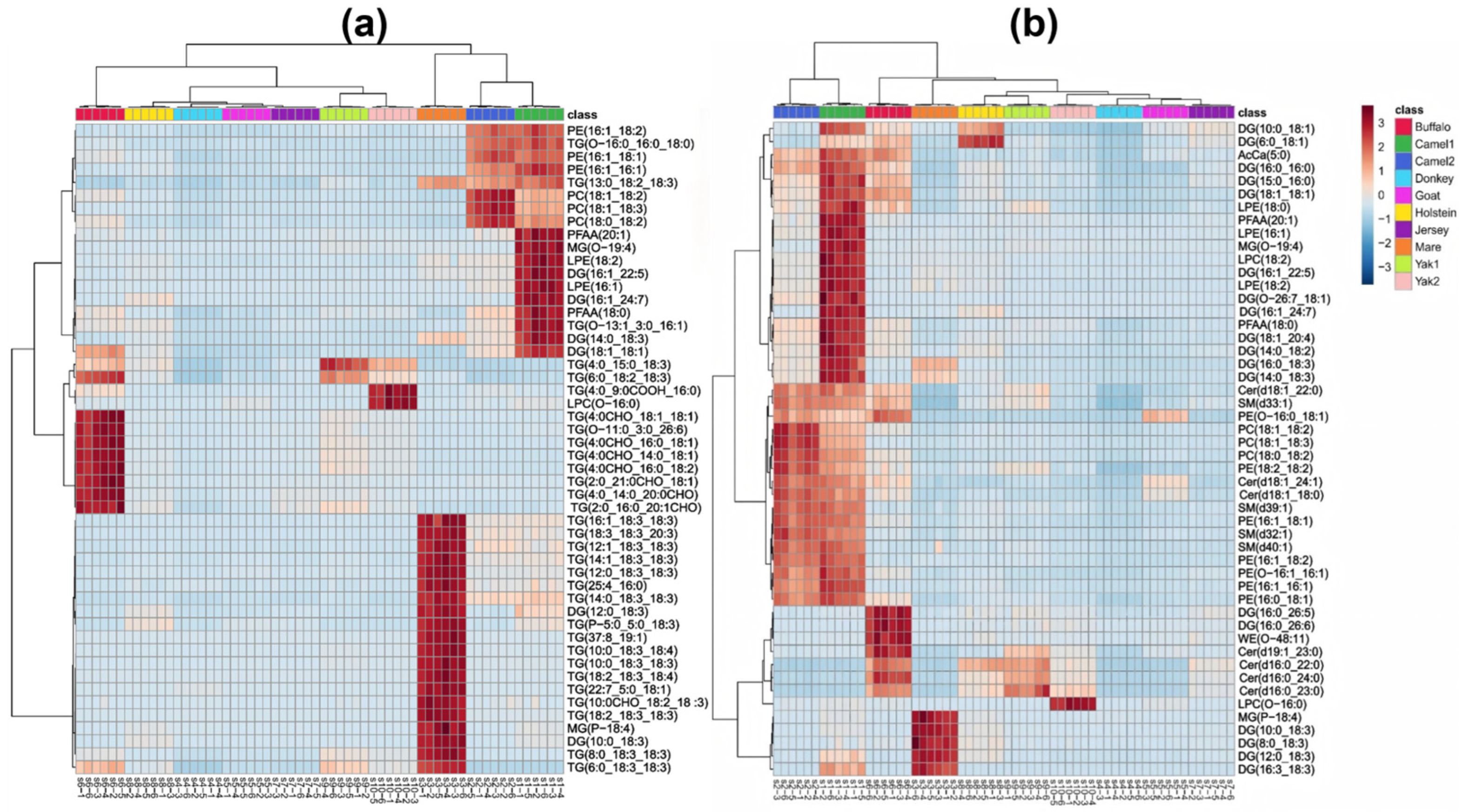
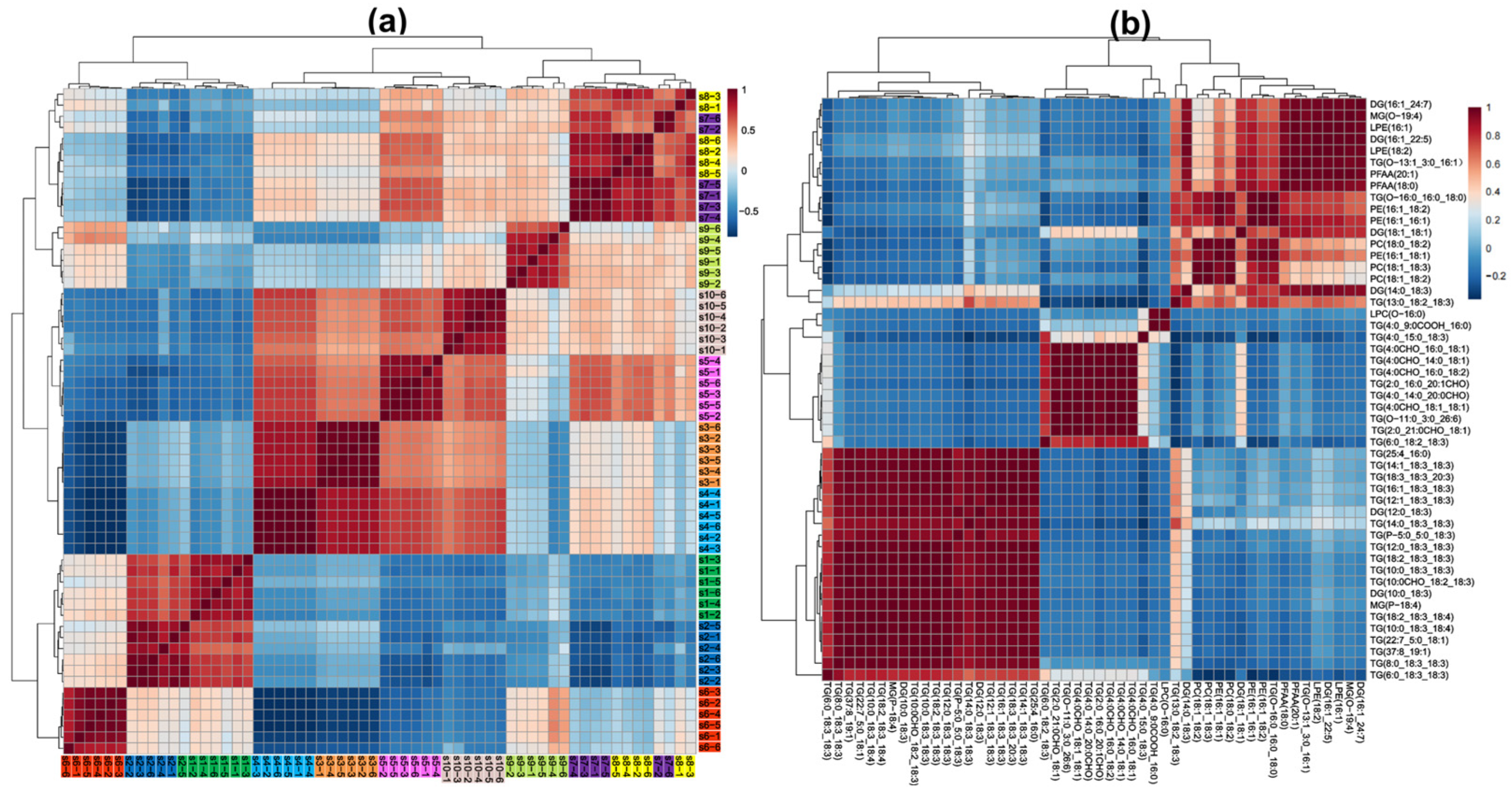
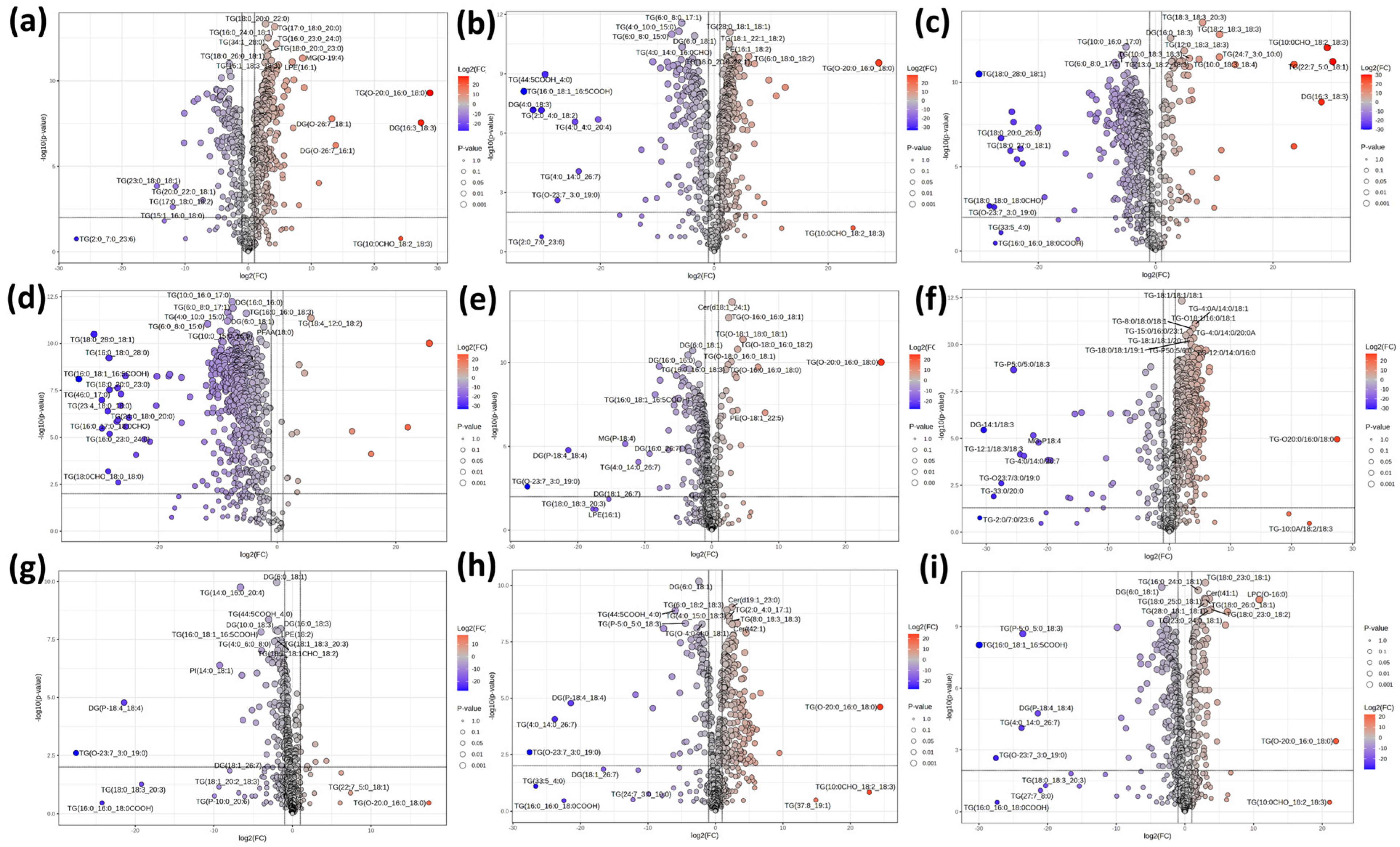
3.4. Multidimensional Statistical Analysis of Different Characteristic Milks
4. Discussion
4.1. Comparative Lipid Content Across Species
4.2. Ruminant Milk: Lipid Diversity, Regionality, and Conservation
4.3. Non-Ruminant Milks: PUFA-Rich but Lipid-Light
4.4. Methodological and Scientific Advancements
4.5. Implications for Dairy Product Development and Challenge
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FA | fatty acids |
| GL | glycerolipids |
| GP | glycerophospholipids |
| SP | sphingolipids |
| ST | sterol lipids |
| SL | saccharolipids |
| PR | prenol lipids |
| PK | polyketides |
| MCT | medium-chain triglycerides |
| MFGM | milk fat globule membranes |
| SFA | saturated fatty acids |
| HRMS | high-resolution mass spectrometry |
| HPLC-MS | High-performance liquid chromatography—mass spectrometry |
| MALDI-TOF MS | Matrix-assisted laser desorption ionization—time of flight mass spectrometry |
| GC-MS | gas chromatography—mass spectrometry |
| UHPLC-HRMS | ultra-high-performance liquid chromatography—high-resolution mass spectrometry |
| QC | quality control |
| PCA | principal component analysis |
| LOD | limit of detection |
| ACCa | Acyl carnitine |
| Cer | Ceramide |
| Ch | Cholesterol |
| DAP | Dialkyl phthalates |
| DG | Diglyceride |
| Hex1Cer | Hexosyl ceramide |
| Hex2Cer | Dihexosyl ceramide |
| LBPA | Monoacylglycerophosphomonoradylglycerols |
| LPC | Lyso phosphatidylcholine |
| LPE | Lyso phosphatidylethanolamine |
| MG | Monoglyceride |
| MGDG | Monogalactosyl diacylglycerol |
| PA | Phosphatidic acid |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| PFAA | Primary amides |
| PG | Phosphatidylglycerol |
| PI | Phosphatidylinositol |
| PS | Phosphatidylserine |
| SM | Sphingomyelin |
| TG | Triacylglycerol |
| WE | Wax ester |
| PLS-DA | partial least squares—discriminant analysis |
| PUFA | long-chain polyunsaturated fatty acids |
| DHA | docosahexaenoic acid |
| EPA | eicosapentaenoic acid |
| MUFA | monounsaturated fatty acids |
| CMPA | cow milk protein allergy |
| MCFA | medium-chain fatty acids |
References
- Ghide, M.K.; Yan, Y. 1,3-Dioleoyl-2-palmitoyl glycerol (OPO)-Enzymatic synthesis and use as an important supplement in infant formulas. J. Food Biochem. 2021, 45, e13799. [Google Scholar] [CrossRef]
- Ren, W.; Sun, M.; Shi, X.; Wang, T.; Wang, Y.; Wang, C.; Li, M. Progress of Mass Spectrometry-Based Lipidomics in the Dairy Field. Foods 2023, 12, 2098. [Google Scholar] [CrossRef]
- Ramos-Martín, F.; D’Amelio, N. Biomembrane lipids: When physics and chemistry join to shape biological activity. Biochimie 2022, 203, 118–138. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Zhu, D.; Pang, X.; Liu, Y.; Frew, R.; Chen, G. Lipidomics profiling of goat milk, soymilk and bovine milk by UPLC-Q-Exactive Orbitrap Mass Spectrometry. Food Chem. 2017, 224, 302–309. [Google Scholar] [CrossRef]
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Köfeler, H.; et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef] [PubMed]
- Ganeshalingam, M.; Enstad, S.; Sen, S.; Cheema, S.; Esposito, F.; Thomas, R. Role of lipidomics in assessing the functional lipid composition in breast milk. Front. Nutr. 2022, 9, 899401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yi, W.; Liu, B.; Dai, Y.; Jiang, T.; Chen, S.; Wang, J.; Feng, B.; Qiao, W.; Liu, Y.; et al. MFGM components promote gut Bifidobacterium growth in infant and in vitro. Eur. J. Nutr. 2022, 61, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Zhao, Y.; Wang, X.; Li, Y.; Fang, B.; Wang, R.; Wang, X.; Liao, H.; Li, G.; Wang, P.; et al. Structure, Biological Functions, Separation, Properties, and Potential Applications of Milk Fat Globule Membrane (MFGM): A Review. Nutrients 2024, 16, 587. [Google Scholar] [CrossRef]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef]
- Liu, Z.; Rochfort, S.; Cocks, B. Milk lipidomics: What we know and what we don’t. Prog. Lipid Res. 2018, 71, 70–85. [Google Scholar] [CrossRef]
- George, A.D.; Gay, M.C.L.; Trengove, R.D.; Geddes, D.T. Human Milk Lipidomics: Current Techniques and Methodologies. Nutrients 2018, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, S.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Optimized Identification of Triacylglycerols in Milk by HPLC-HRMS. Food Anal. Method 2022, 15, 2084–2094. [Google Scholar] [CrossRef]
- Miao, Q.Y.; Gao, W.; Li, J.; Chen, J. Progress on lipidomics analytical methods and their applications in studies of traditional Chinese medicines. China J. Chin. Mater. Medica 2019, 44, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.M.; Prabutzki, P.; Leopold, J.; Nimptsch, A.; Lemmnitzer, K.; Vos, D.R.N.; Hopf, C.; Schiller, J. A new update of MALDI-TOF mass spectrometry in lipid research. Prog. Lipid Res. 2022, 86, 101145. [Google Scholar] [CrossRef] [PubMed]
- Ghidotti, M.; Fabbri, D.; Torri, C.; Piccinini, S. Determination of volatile fatty acids in digestate by solvent extraction with dimethyl carbonate and gas chromatography-mass spectrometry. Anal. Chim. Acta 2018, 1034, 92–101. [Google Scholar] [CrossRef]
- Marable, C.A.; Frank, C.L.; Seim, R.F.; Hester, S.; Henderson, W.M.; Chorley, B.; Shafer, T.J. Integrated Omic Analyses Identify Pathways and Transcriptomic Regulators Associated With Chemical Alterations of In Vitro Neural Network Formation. Toxicol. Sci. 2022, 186, 118–133. [Google Scholar] [CrossRef]
- Reis, A.; Rudnitskaya, A.; Blackburn, G.J.; Mohd Fauzi, N.; Pitt, A.R.; Spickett, C.M. A comparison of five lipid extraction solvent systems for lipidomic studies of human LDL. J. Lipid Res. 2013, 54, 1812–1824. [Google Scholar] [CrossRef]
- Wang, W.; Wei, T.; Fan, J.; Yi, J.; Li, Y.; Wan, M.; Wang, J.; Bai, W. Repeated mutagenic effects of 60Co-γ irradiation coupled with high-throughput screening improves lipid accumulation in mutant strains of the microalgae Chlorella pyrenoidosa as a feedstock for bioenergy. Algal Res. 2018, 33, 71–77. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Song, Y.; Zhang, Y.; Zhao, L.; Zhang, L.; Lü, X.; Wang, H.; Zhang, X.; Zhang, J.; et al. Characterization and comparison of lipids from human and ewe colostrum based on lipidomics analysis. Food Chem. 2023, 400, 133998. [Google Scholar] [CrossRef]
- Li, M.; Li, Q.; Kang, S.; Cao, X.; Zheng, Y.; Wu, J.; Wu, R.; Shao, J.; Yang, M.; Yue, X. Characterization and comparison of lipids in bovine colostrum and mature milk based on UHPLC-QTOF-MS lipidomics. Food Res. Int. 2020, 136, 109490. [Google Scholar] [CrossRef]
- Ahn, J.K.; Kim, J.; Hwang, J.; Song, J.; Kim, K.H.; Cha, H.-S. Potential metabolomic biomarkers for reliable diagnosis of Behcet’s disease using gas chromatography/ time-of-flight-mass spectrometry. Jt. Bone Spine 2018, 85, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Swelum, A.A.; El-Saadony, M.T.; Abdo, M.; Ombarak, R.A.; Hussein, E.O.S.; Suliman, G.; Alhimaidi, A.R.; Ammari, A.A.; Ba-Awadh, H.; Taha, A.E.; et al. Nutritional, antimicrobial and medicinal properties of Camel’s milk: A review. Saudi J. Biol. Sci. 2021, 28, 3126–3136. [Google Scholar] [CrossRef] [PubMed]
- Seifu, E. Recent advances on camel milk: Nutritional and health benefits and processing implications—A review. AIMS Agric. Food 2022, 7, 777–804. [Google Scholar] [CrossRef]
- Lagutin, K.; MacKenzie, A.; Bloor, S.; Scott, D.; Vyssotski, M. HPLC-MS, GC and NMR Profiling of Bioactive Lipids of Human Milk and Milk of Dairy Animals (Cow, Sheep, Goat, Buffalo, Camel, Red Deer). Separations 2022, 9, 145. [Google Scholar] [CrossRef]
- Rodríguez-Alcalá, L.M.; Fontecha, J. Major lipid classes separation of buttermilk, and cows, goats and ewes milk by high performance liquid chromatography with an evaporative light scattering detector focused on the phospholipid fraction. J. Chromatogr. A 2010, 1217, 3063–3066. [Google Scholar] [CrossRef]
- Bitman, J.; Wood, D.L.; Miller, R.H.; Tyrrell, H.F.; Reynolds, C.K.; Baxter, H.D. Comparison of Milk and Blood Lipids in Jersey and Holstein Cows Fed Total Mixed Rations with or Without Whole Cottonseed. J. Dairy Sci. 1996, 79, 1596–1602. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, J.; Ge, W.; Wang, J. Comparative Lipidomics Analysis of Human and Ruminant Milk Reveals Variation in Composition and Structural Characteristics. J. Agric. Food Chem. 2022, 70, 8994–9006. [Google Scholar] [CrossRef]
- Živkov Baloš, M.; Ljubojević Pelić, D.; Jakšić, S.; Lazić, S. Donkey Milk: An Overview of its Chemical Composition and Main Nutritional Properties or Human Health Benefit Properties. J. Equine Vet. Sci. 2023, 121, 104225. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Chen, R.; Qiao, Y.; Zhang, Q.; Li, Q.; Wang, X.; Pan, Y.; Li, S.; Wang, Z. Analysis for lipid nutrient differences in the milk of 13 species from a quantitative non-targeted lipidomics perspective. Food Chem. X 2023, 20, 101024. [Google Scholar] [CrossRef]
- Emakpor, O.L.; Edo, G.I.; Jikah, A.N.; Ikpekoro, V.O.; Agbo, J.J.; Ainyanbhor, I.E.; Essaghah, A.E.A.; Ekokotu, H.A.; Oghroro, E.E.A.; Akpoghelie, P.O. Buffalo milk: An essential natural adjuvant. Discov. Food 2024, 4, 38. [Google Scholar] [CrossRef]
- Nie, P.; Pan, B.; Ahmad, M.J.; Zhang, X.; Chen, C.; Yao, Z.; Lv, H.; Wei, K.; Yang, L. Summer Buffalo Milk Produced in China: A Desirable Diet Enriched in Polyunsaturated Fatty Acids and Amino Acids. Foods 2022, 11, 3475. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Jin, Q.; Wang, X. Human milk fat substitutes: Past achievements and current trends. Prog. Lipid Res. 2019, 74, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Pei, J.; Wu, X.; Bao, P.; Guo, X.; Yan, P. Explaining Unsaturated Fatty Acids (UFAs), Especially Polyunsaturated Fatty Acid (PUFA) Content in Subcutaneous Fat of Yaks of Different Sex by Differential Proteome Analysis. Genes 2022, 13, 790. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Deshwal, G.; Borad, S.; Bam, J.; Paul, V. Characterization of lipid composition and physicochemical properties of clarified yak milk fat. Meas. Food 2024, 15, 100189. [Google Scholar] [CrossRef]
- Zhang, Z.; Qie, M.; Bai, L.; Zhao, S.; Li, Y.; Yang, X.; Liang, K.; Zhao, Y. Rapid authenticity assessment of PGI Hongyuan yak milk based on SICRIT-QTOF MS. Food Chem. 2024, 442, 138444. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Zeng, J.; Zhao, C.; Hou, Y.; Wu, T.; Deng, Z.; Zheng, L. Comparative Analysis of Protein Digestion Characteristics in Human, Cow, Goat, Sheep, Mare, and Camel Milk under Simulated Infant Condition. J. Agric. Food Chem. 2023, 71, 15035–15047. [Google Scholar] [CrossRef]
- Ho, T.M.; Zou, Z.; Bansal, N. Camel milk: A review of its nutritional value, heat stability, and potential food products. Food Res. Int. 2022, 153, 110870. [Google Scholar] [CrossRef]
- Bakry, I.A.; Yang, L.; Farag, M.A.; Korma, S.A.; Khalifa, I.; Cacciotti, I.; Ziedan, N.I.; Jin, J.; Jin, Q.; Wei, W.; et al. A Comprehensive Review of the Composition, Nutritional Value, and Functional Properties of Camel Milk Fat. Foods 2021, 10, 2158. [Google Scholar] [CrossRef]
- Sahoo, A.; Rahman, H. Donkey & Non-Bovine Milk Preface. 2024. Available online: https://www.researchgate.net/publication/378316795_DONKEY_NON-BOVINE_MILK_PREFACE (accessed on 8 May 2025).
- Chen, B.; Zhu, H.; Zhang, Y.; Wang, X.; Zhang, W.; Wang, Y.; Pang, X.; Zhang, S.; Lv, J. Comparison of species and lactation of different mammalian milk: The unique composition and stereospecificity of fatty acids of mare milk. Int. Dairy J. 2024, 150, 105822. [Google Scholar] [CrossRef]
- Gharaei, M.; Zadeh, M.; Ravari, S.S.; Negarestani, Z.; Samarin, M.; Mehdikhani, S.; Birjandi, R. Donkey’s Milk; A Novel Health-Promoting Dairy: A Scoping Review. 2024. Available online: https://www.researchgate.net/publication/378909417_Donkey’s_Milk_A_Novel_health-promoting_Dairy_A_Scoping_Review (accessed on 14 December 2024).
- Bari, M.; Bisogno, T.; Battista, N. Bioactive Lipids in Health and Disease. Biomolecules 2020, 10, 1698. [Google Scholar] [CrossRef]
- Salimei, E.; Fantuz, F. Equid milk for human consumption. Int. Dairy J. 2012, 24, 130–142. [Google Scholar] [CrossRef]
- Cimmino, F.; Catapano, A.; Villano, I.; Di Maio, G.; Petrella, L.; Traina, G.; Pizzella, A.; Raffaella, T.; Cavaliere, G. Invited review: Human, cow, and donkey milk comparison: Focus on metabolic effects. J. Dairy Sci. 2023, 106, 3072–3085. [Google Scholar] [CrossRef] [PubMed]
- Malacarne, M.; Martuzzi, F.; Summer, A.; Mariani, P. Protein and fat composition of mare’s milk: Some nutritional remarks with reference to human and cow’s milk. Int. Dairy J. 2002, 12, 869–877. [Google Scholar] [CrossRef]
- Nantapo, C.T.W.; Muchenje, V.; Hugo, A. Atherogenicity index and health-related fatty acids in different stages of lactation from Friesian, Jersey and Friesian × Jersey cross cow milk under a pasture-based dairy system. Food Chem. 2014, 146, 127–133. [Google Scholar] [CrossRef]
- Laboureur, L.; Ollero, M.; Touboul, D. Lipidomics by Supercritical Fluid Chromatography. Int. J. Mol. Sci. 2015, 16, 13868–13884. [Google Scholar] [CrossRef]
- Li, A.; Hines, K.M.; Xu, L. Lipidomics by HILIC-Ion Mobility-Mass Spectrometry. Methods Mol. Biol. 2020, 2084, 119–132. [Google Scholar] [CrossRef]
- Jackson, S.N.; Barbacci, D.; Egan, T.; Lewis, E.K.; Schultz, J.A.; Woods, A.S. MALDI-Ion Mobility Mass Spectrometry of Lipids in Negative Ion Mode. Anal. Methods 2014, 6, 5001–5007. [Google Scholar] [CrossRef]



| Reference | Milk Type(s) | Platform and Column | Lipid Identification | Lipid Species | Highlighted Features |
|---|---|---|---|---|---|
| Li et al., 2017 [4] | Goat, soy, and cow | UPLC-Q-Exactive Orbitrap, C18 | Lipidsearch 4.0 | 370 | Clear differences between plant-based and animal milk lipids. |
| Li et al., 2020 [20] | Bovine colostrum, mature milk | UHPLC-QTOF-MS, C18 | Lipid Analyzer | 335 | Covered different lactation stages; practical implications for nutritional quality evaluation. |
| Wang et al., 2023 [19] | Human, ewe colostrum | UHPLC-QTRAP-MS, C30 | Lipid Maps | 1004 | Detected a high number of lipid species using a wide-coverage platform. |
| Imperiale et al. [12] | Cow | UPLC-Q-Exactive Orbitrap, C18 | Lipid Maps | Optimized TG profiling | Developed TG isomer identification strategy. |
| Zhao et al., 2022 [27] | Human, cow, goat, sheep, camel | UHPLC-Q-Exactive Orbitrap, C18 | Lipidsearch 4.1 | 826~918 | Revealed significant differences between human and ruminant milk lipids; relevance to infant formula development. |
| Wu et al., 2023 [29] | Mare, donkey, camel, yak, pig, human | UHPLC-Q-Exactive Plus, C18 | Lipidsearch 4.2 | 2585 | Broad cross-species comparison with limited subgroup analysis. |
| Present Study | Camel, mare, donkey, goat, buffalo, yak, Jersey, Holstein | UHPLC-Q-Exactive Plus, C18 | Lipidsearch 5.0 | 640 | Featured the broadest species coverage and revealed a wide spectrum of lipid subclasses, including rarely reported ones. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, T.; Zhou, T.; Zhang, S.; Quan, Z.; Liu, Y. Non-Targeted Lipidomics Analysis of Characteristic Milk Using High-Resolution Mass Spectrometry (UHPLC-HRMS). Foods 2025, 14, 2068. https://doi.org/10.3390/foods14122068
Wei T, Zhou T, Zhang S, Quan Z, Liu Y. Non-Targeted Lipidomics Analysis of Characteristic Milk Using High-Resolution Mass Spectrometry (UHPLC-HRMS). Foods. 2025; 14(12):2068. https://doi.org/10.3390/foods14122068
Chicago/Turabian StyleWei, Tingting, Tianxiao Zhou, Shenping Zhang, Zhexue Quan, and Yang Liu. 2025. "Non-Targeted Lipidomics Analysis of Characteristic Milk Using High-Resolution Mass Spectrometry (UHPLC-HRMS)" Foods 14, no. 12: 2068. https://doi.org/10.3390/foods14122068
APA StyleWei, T., Zhou, T., Zhang, S., Quan, Z., & Liu, Y. (2025). Non-Targeted Lipidomics Analysis of Characteristic Milk Using High-Resolution Mass Spectrometry (UHPLC-HRMS). Foods, 14(12), 2068. https://doi.org/10.3390/foods14122068








