A Comparative Study Based on HS-SPME-GC-MS of Volatile Compounds in Large Yellow Croaker (Pseudosciaena crocea) During Varied Cold Storage Conditions
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Design and Sampling
2.2. Extraction of Volatile Compounds
2.3. Volatile Compound Profiling
2.4. Statistical Analysis
3. Results and Discussion
3.1. Volatile Compound Analysis: Component Identification
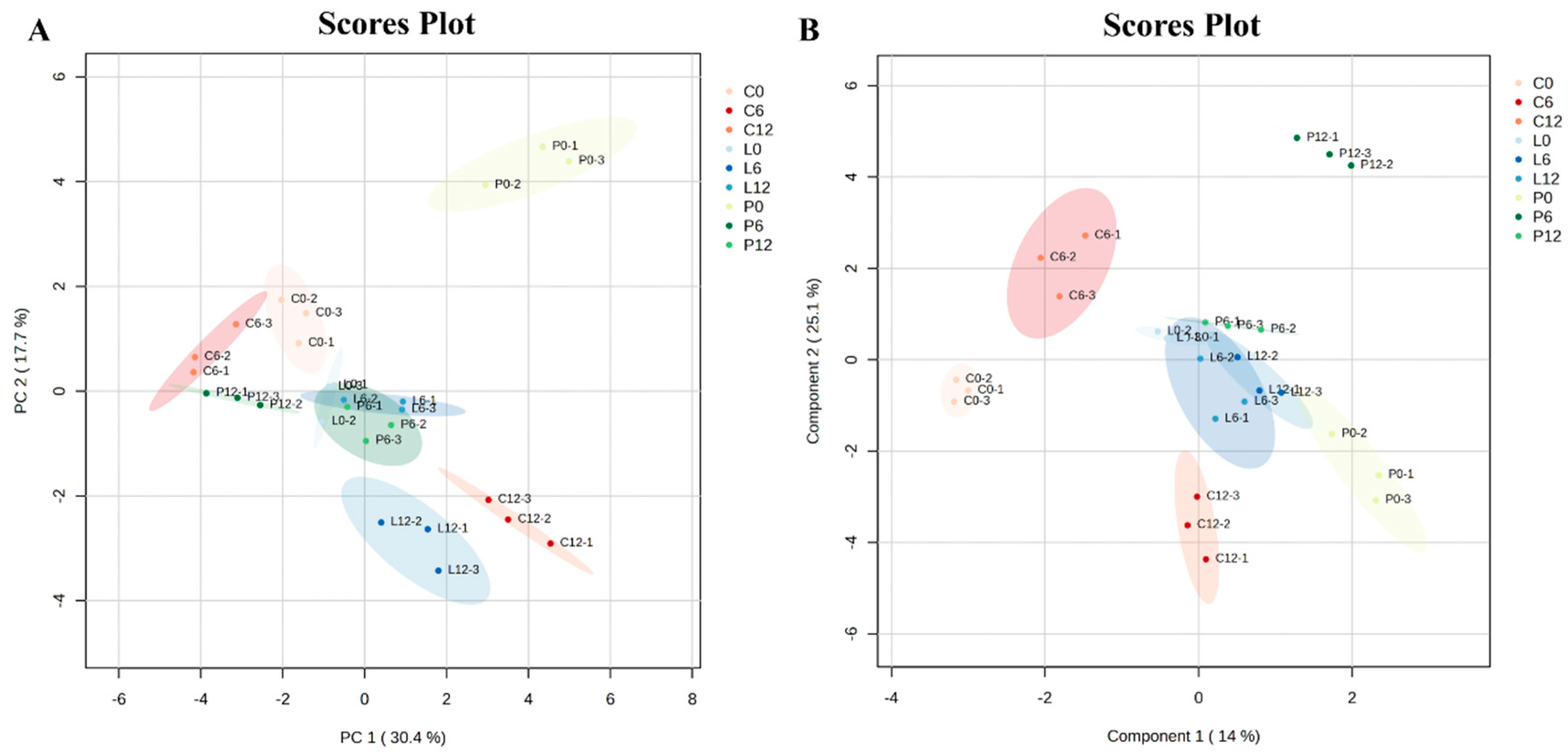
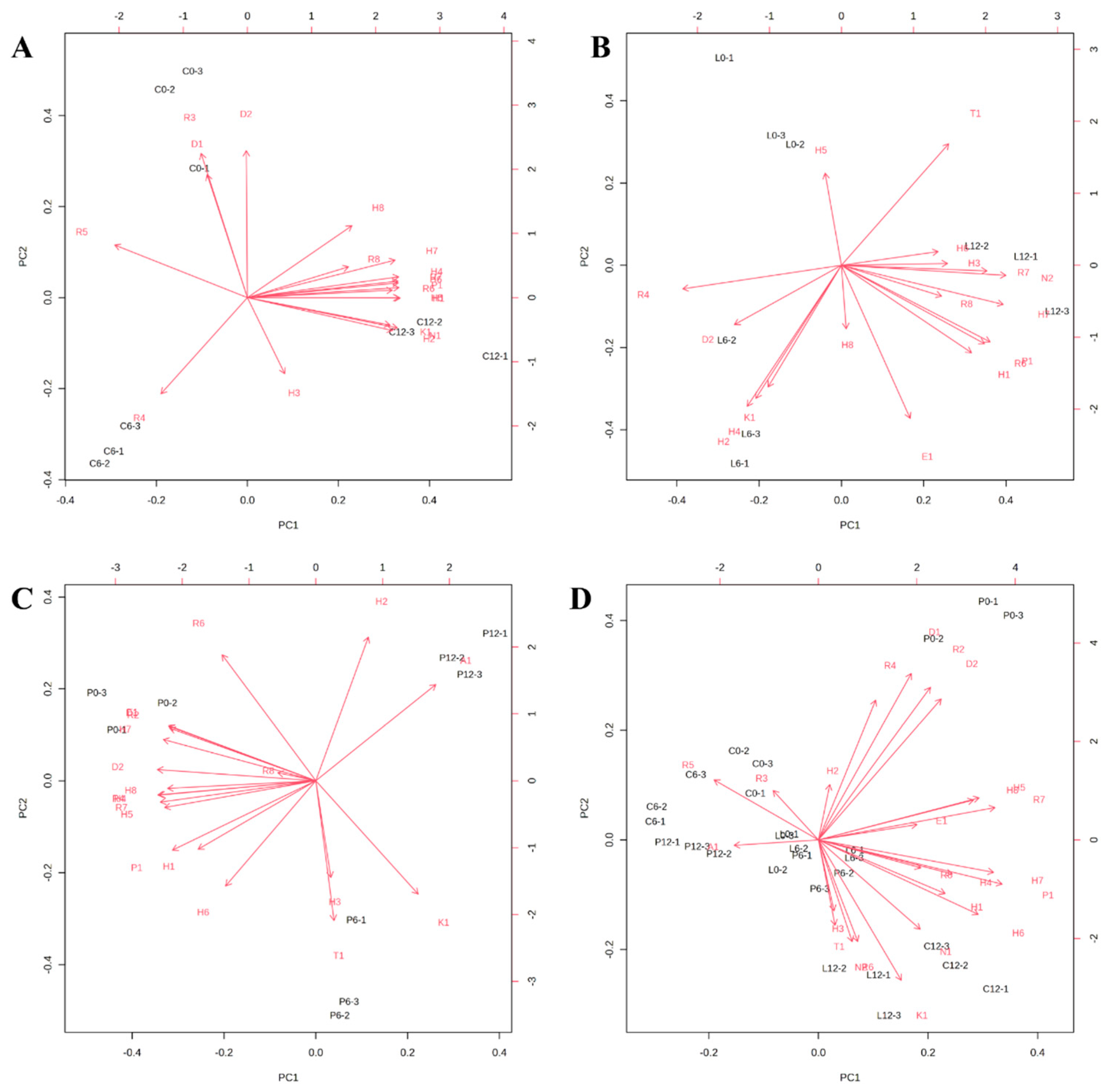
3.2. Multivariate Data Analysis
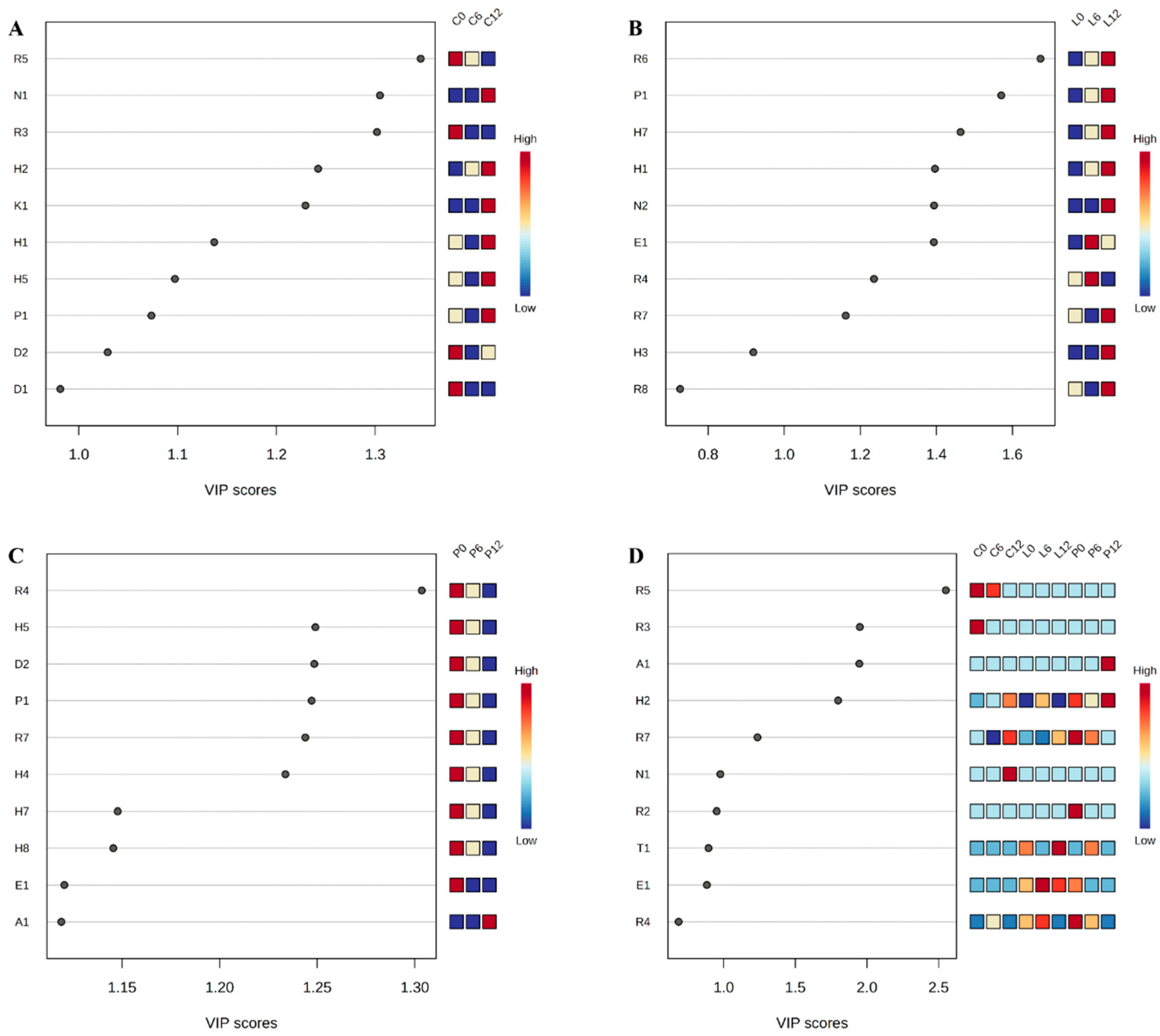
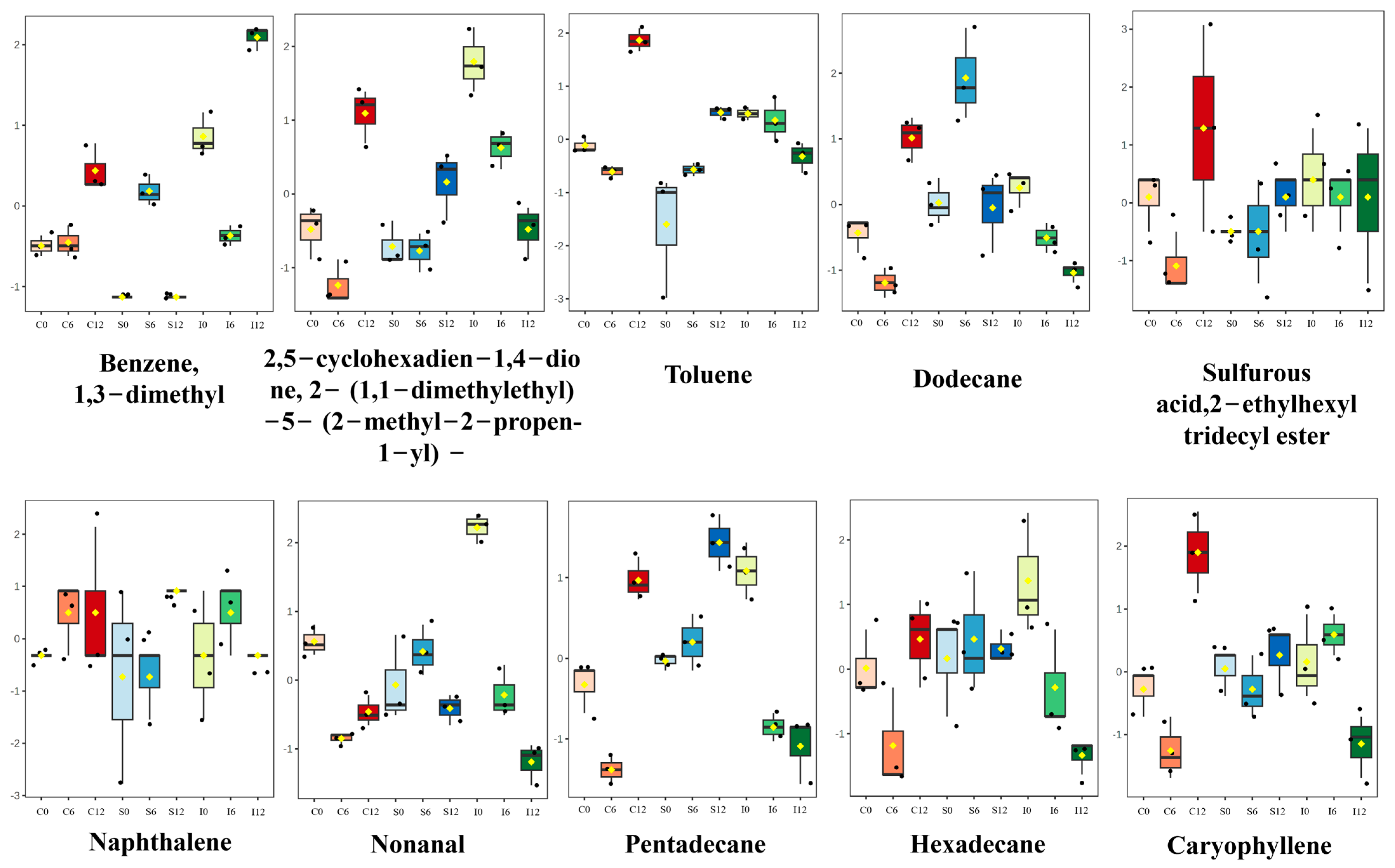
3.3. Identification of Characteristic Volatile Compounds
3.4. Identification of Potential Characteristic Volatile Compounds
3.5. Differences in Volatile Compounds of Large Yellow Croaker Under Different Storage Conditions
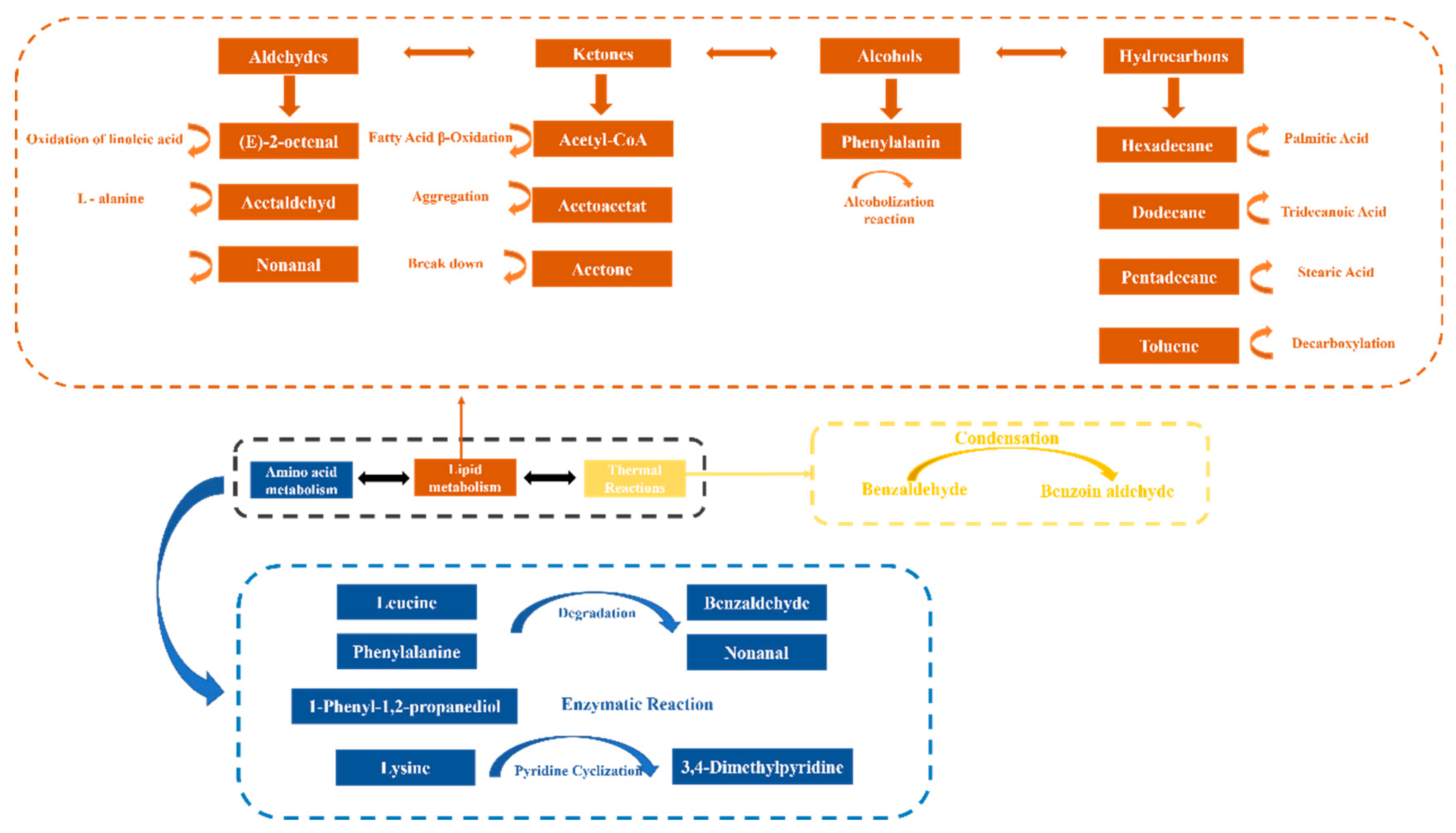
3.5.1. Thermal Reaction Volatile Compounds Pathway
3.5.2. Lipid Oxidation Pathway
3.5.3. Amino Acid Degradation Pathway
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Fisheries Administration Bureau, M.O.A. China Fifishery Statics Yearbooks; China Agriculture Press: Beijing, China, 2020.
- Chen, Y.; Ning, Q.; Wang, S.; Chen, Y.; Mo, X.; Liang, P.; Pang, J.; Dong, X. Effects of Cold Treatments on Lipidomics Profiles of Large Yellow Croaker (Larimichthys crocea) Fillets by UPLC-Q-Exactive Orbitrap MS Analysis. J. Food Compos. Anal. 2022, 109, 104481. [Google Scholar] [CrossRef]
- Fisheries Bureau of Agriculture Ministry of China. Fisheries Production Statistics for 2022; China Agriculture Press: Beijing, China, 2022.
- Prabhakar, P.K.; Vatsa, S.; Srivastav, P.P.; Pathak, S.S. A Comprehensive Review on Freshness of Fish and Assessment: Analytical Methods and Recent Innovations. Food Res. Int. 2020, 133, 109157. [Google Scholar] [CrossRef] [PubMed]
- Ba, H.V.; Oliveros, C.M.; Park, K.; Dashdorj, D.; Hwang, I. Effect of Marbling and Chilled Ageing on Meat-Quality Traits, Volatile Compounds and Sensory Characteristics of Beef Longissimus Dorsi Muscle. Anim. Prod. Sci. 2016, 57, 981–992. [Google Scholar] [CrossRef]
- Cardona, E.; Milhade, L.; Pourtau, A.; Panserat, S.; Terrier, F.; Lanuque, A.; Roy, J.; Marandel, L.; Bobe, J.; Skiba-Cassy, S. Tissue Origin of Circulating microRNAs and Their Response to Nutritional and Environmental Stress in Rainbow Trout (Oncorhynchus Mykiss). Sci. Total Environ. 2022, 853, 158584. [Google Scholar] [CrossRef]
- López-Cánovas, A.E.; Cabas, I.; Ros-Chumillas, M.; Navarro-Segura, L.; López-Gómez, A.; García-Ayala, A. Nanoencapsulated Clove Essential Oil Applied in Low Dose Decreases Stress in Farmed Gilthead Seabream (Sparus aurata L.) during Slaughter by Hypothermia in Ice Slurry. Aquaculture 2019, 504, 437–445. [Google Scholar] [CrossRef]
- Liu, L.; Lan, W.; Pu, T.; Zhou, Y.; Xie, J. Combining slightly acidic electrolyzed water and slurry ice to prolong the shelf-life of mackerel (Pneumatophorus japonicus). J. Food Process. Preserv. 2021, 45, 15762. [Google Scholar] [CrossRef]
- Melinder, A.; Ignatowicz, M. Properties of Seawater with Ice Slurry Use in Focus. Int. J. Refrig. 2015, 52, 51–58. [Google Scholar] [CrossRef]
- Kauffeld, M.; Wang, M.J.; Goldstein, V.; Kasza, K.E. Ice Slurry Applications. Int. J. Refrig. 2010, 33, 1491–1505. [Google Scholar] [CrossRef]
- Huidobro, A.; Mendes, R.; Nunes, M. Slaughtering of Gilthead Seabream (Sparus aurata) in Liquid Ice: Influence on Fish Quality. Eur. Food Res. Technol. 2001, 213, 267–272. [Google Scholar] [CrossRef]
- Wang, J.; Goldstein, V. Ice slurry: Advanced fish chilling and preservation technology. Am. Fish. Soc. Symp. 2003, 38, 379–386. [Google Scholar]
- Lan, W.-Q.; Zhang, W.-J.; Liu, J.-L.; Xie, J. Effects of Precooling with Slurry Ice on the Freshness of Farmed Perch (Lateolabrax Japonicus) during Logistics Process. J. Aquat. Food Prod. Technol. 2021, 30, 162–175. [Google Scholar] [CrossRef]
- Guan, W.; Liu, T.; Yan, W.; Cai, L. The Impact of Ice Slurry as a Medium on Oxidation Status and Flesh Quality of Shrimp (Litopenaeus vannamei) during Refrigeration Storage. J. Food Sci. 2023, 88, 4918–4927. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Li, K.; Guo, J.; Lu, M.; Hong, Y.; Cai, Z. Mass Spectrometry for Analysis of Changes during Food Storage and Processing. J. Agric. Food Chem. 2020, 68, 6956–6966. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-C.; Park, J.-H.; Kim, J.-K.; Park, H.-Y.; Yoon, Y.-S.; Eun, J.-B.; Song, B.-S. Rapid Identification Method for Gamma-Irradiated Soybeans Using Gas Chromatography–Mass Spectrometry Coupled with a Headspace Solid-Phase Microextraction Technique. J. Agric. Food Chem. 2020, 68, 2803–2815. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Shi, W. Odor Characteristics of White Croaker and Small Yellow Croaker Fish during Refrigerated Storage. J. Food Biochem. 2019, 43, e12852. [Google Scholar] [CrossRef]
- Wei, H.; Wei, Y.; Qiu, X.; Yang, S.; Chen, F.; Ni, H.; Li, Q. Comparison of Potent Odorants in Raw and Cooked Mildly Salted Large Yellow Croaker Using Odor-Active Value Calculation and Omission Test: Understanding the Role of Cooking Method. Food Chem. 2023, 402, 134015. [Google Scholar] [CrossRef]
- Utpott, M.; Rodrigues, E.; de Oliveira Rios, A.; Mercali, G.D.; Flôres, S.H. Metabolomics: An Analytical Technique for Food Processing Evaluation. Food Chem. 2022, 366, 130685. [Google Scholar] [CrossRef]
- Zhao, T.; Cao, Z.; Yu, J.; Weng, X.; Benjakul, S.; Guidi, A.; Ying, X.; Ma, L.; Xiao, G.; Deng, S. Gas-Phase Ion Migration Spectrum Analysis of the Volatile Flavors of Large Yellow Croaker Oil after Different Storage Periods. Curr. Res. Food Sci. 2022, 5, 813–822. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, Y.; Lin, N.; Yang, X.; Wu, J.; Guo, Q. Comparison of Volatile and Non-Volatile Compounds of Ice-Stored Large Yellow Croaker (Larimichthys crocea) Affected by Different Post-Harvest Handling Methods. Foods 2025, 14, 431. [Google Scholar] [CrossRef]
- Syropoulou, F.; Parlapani, F.F.; Anagnostopoulos, D.A.; Stamatiou, A.; Mallouchos, A.; Boziaris, I.S. Spoilage Investigation of Chill Stored Meagre (Argyrosomus regius) Using Modern Microbiological and Analytical Techniques. Foods 2021, 10, 3109. [Google Scholar] [CrossRef]
- Liu, T.; Liu, J.; Wang, P.; Li, X.; Zhong, Y.; Yan, W.; Guan, W.; Cai, L. Effect of Slurry Ice on Quality Characteristics and Microbiota Composition of Pacific White Shrimp during Refrigerated Storage. J. Agric. Food Res. 2023, 14, 100792. [Google Scholar] [CrossRef]
- Xu, Y.; Meng, L.; Chen, X.; Chen, X.; Su, L.; Yuan, L.; Shi, W.; Huang, G. A Strategy to Significantly Improve the Classification Accuracy of LIBS Data: Application for the Determination of Heavy Metals in Tegillarca Granosa. Plasma Sci. Technol. 2021, 23, 85503. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Yang, X.; Cheng, W.; Huang, Y.; Liang, P. Oxidative Stability of Marine Phospholipids Derived from Large Yellow Croaker Roe. Food Res. Int. 2022, 160, 111743. [Google Scholar] [CrossRef]
- Varlet, V.; Prost, C.; Serot, T. Volatile Aldehydes in Smoked Fish: Analysis Methods, Occurence and Mechanisms of Formation. Food Chem. 2007, 105, 1536–1556. [Google Scholar] [CrossRef]
- Dai, L.; Cao, X.; Miao, X.; Yang, X.; Zhang, J.; Shang, X. The Chemical Composition, Protective Effect of Rheum Officinale Leaf Juice and Its Mechanism against Dextran Sulfate Sodium-Induced Ulcerative Colitis. Phytomedicine 2024, 129, 155653. [Google Scholar] [CrossRef] [PubMed]
- Mozzon, M.; Foligni, R.; Mannozzi, C.; Galdenzi, F.; Laurita, R.; Tappi, S.; Dalla Rosa, M. Effect of plasma-activated water (PAW) soaking on the lipid oxidation of sardine (Sardina pilchardus) fillets. Food Res. Int. 2024, 176, 113823. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Mei, J.; Xie, J. Revealing the Evolution of Lipid Extracts from Tilapia (Oreochromis mossambicus) during Cold Storage: Based on Lipid Metabolism Analysis via GC-Q-MS and Characterization of Lipid Oxidation. LWT 2024, 201, 116268. [Google Scholar] [CrossRef]
- Hematyar, N.; Rustad, T.; Sampels, S.; Kastrup Dalsgaard, T. Relationship between Lipid and Protein Oxidation in Fish. Aquacult. Res. 2019, 50, 1393–1403. [Google Scholar] [CrossRef]
- Li, H.; Geng, W.; Haruna, S.A.; Zhou, C.; Wang, Y.; Ouyang, Q.; Chen, Q. Identification of Characteristic Volatiles and Metabolomic Pathway during Pork Storage Using HS-SPME-GC/MS Coupled with Multivariate Analysis. Food Chem. 2022, 373, 131431. [Google Scholar] [CrossRef]
| Super Class | R.T. (min) | Volatile Compounds | Abbreviation | CAS | Formula |
|---|---|---|---|---|---|
| Nitrogen compound | 8.971 | Nicotinic acid, 2-phenylethyl ester | N1 | 1000308-36-9 | C14H13NO2 |
| 9.794 | Pyridine, 3,4-dimethyl | N2 | 000583-58-4 | C7H9N | |
| Aldehydes | 10.549 | Benzaldehyde | D1 | 000100-52-7 | C7H6O |
| 13.521 | Nonanal | D2 | 000124-19-6 | C9H18O | |
| Hydrocarbons | 6.02 | Toluene | H1 | 000108-88-3 | C7H8 |
| 8.447 | Benzene,1,3-dimethyl | H2 | 000108-38-3 | C8H10 | |
| 15.083 | Naphthalene | H3 | 000091-20-3 | C10H8 | |
| 15.251 | Dodecane | H4 | 000112-40-3 | C12H26 | |
| 16.111 | Benzene,1,3-bis(1,1-dimethylethyl)- | H5 | 001014-60-4 | C14H22 | |
| 18.176 | Caryophyllene | H6 | 000087-44-5 | C15H24 | |
| 18.789 | Pentadecane | H7 | 000629-62-9 | C15H32 | |
| 20.467 | Hexadecane | H8 | 000544-76-3 | C16H34 | |
| Ketones | 10.539 | Ethanone, 2-(formyloxy)-1-phenyl | K1 | 055153-12-3 | C9H8O3 |
| Ethers | 11.991 | Heptane,1,1′-oxybis- | E1 | 000629-64-1 | C14H30O |
| Alcohols | 12.127 | 1,2-Propanediol,1-phenyl | A1 | 001855-09-0 | C9H12O2 |
| Phenols | 19.000 | Butylated Hydroxytoluene | P1 | 000128-37-0 | C15H24O |
| Esters | 13.181 | Benzeneacetic acid, | T1 | 056143-21-6 | C10H12O3 |
| Others | 13.175 | Benzene,(1-methoxypropyl) | R2 | 059588-12-4 | C10H14O |
| 14.009 | Triethyl phosphate | R3 | 000078-40-0 | C6H15O4P | |
| 14.014 | Phosphoric acid, diethyl pentyl ester | R4 | 020195-08-8 | C9H21O4P | |
| 17.070 | Benzoic acid, 2-methylpropyl ester | R5 | 000120-50-3 | C11H14O2 | |
| 18.438 | Dimethyl phthalate | R6 | 000131-11-3 | C10H10O4 | |
| 18.695 | 2,5-cyclohexadiene-1,4-dione, | R7 | 1000396-22-4 | C14H18O2 | |
| 20.520 | Sulfurous acid, 2-ethylhexyl tridecyl ester | R8 | 1000309-19-6 | C21H44O3S |
| Volatile Compounds | C0 | C6 | C12 | L0 | L6 | L12 | P0 | P6 | P12 |
|---|---|---|---|---|---|---|---|---|---|
| Toluene | 0.54 ab ± 0.023 | 0.46 a ± 0.02 | 0.86 b ± 0.035 | 0.28 a ± 0.24 | 0.47 a ± 0.02 | 0.64 a ± 0.020 | 0.64 b ± 0.020 | 0.62 b ± 0.06 | 0.51 a ± 0.04 |
| Benzene,1,3-dimethyl | 0.06 b ± 0.010 | 0.06 a ± 0.015 | 0.13 a ± 0.023 | 0.01 a ± 0.001 | 0.11 b ± 0.015 | n.d. | 0.17 c ± 0.020 | 0.07 a ± 0.01 | 0.26 b ± 0.01 |
| Nicotinic acid, 2-phenylethyl ester | n.d. | n.d. | 0.16 b ± 0.015 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ethanone, 2-(formyloxy)-1-phenyl | n.d. | n.d. | 0.04 a ± 0.015 | 0.02 b ± 0.005 | 0.04 b ± 0.005 | 0.02 a ± 0.005 | n.d. | 0.03 b ± 0.005 | 0.02 a ± 0.005 |
| Benzaldehyde | 0.03 a ± 0.026 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.09 b ± 0.011 | n.d. | n.d. |
| Heptane,1,1-oxybis- | n.d. | n.d. | n.d. | 0.02 a ± 0.015 | 0.16 b ± 0.015 | 0.15 b ± 0.015 | 0.13 b ± 0.015 | n.d. | n.d. |
| 1,2-Propanediol,1-phenyl | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.05 b ± 0.01 |
| Benzene,(1-methoxypropyl) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.08 b ± 0.002 | n.d. | n.d. |
| Benzeneacetic acid, alpha.-methoxy-,methyl ester | n.d. | n.d. | n.d. | 0.02 ± 0.005 | n.d. | 0.03 ± 0.005 | n.d. | 0.02 ± 0.01 | n.d. |
| Pyridine, 3,4-dimethyl | n.d. | n.d. | n.d. | n.d. | n.d. | 0.04 ± 0.010 | n.d. | n.d. | n.d. |
| Nonanal | 0.17 a ± 0.015 | 0.08 a ± 0.005 | 0.10 a ± 0.015 | 0.13 a ± 0.043 | 0.16 c ± 0.025 | 0.11 b ± 0.015 | 0.29 b ± 0.02 | 0.12 b ± 0.03 | 0.05 a ± 0.02 |
| Triethyl phosphate | 0.05 a ± 0.005 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.00 | 0.00 |
| Phosphoric acid, diethyl pentyl ester | n.d. | 0.02 a ± 0.001 | n.d. | 0.02 a | 0.02 a ± 0.005 | n.d. | 0.04 a ± 0.002 | 0.02 a ± 0.005 | n.d. |
| Naphthalene | 0.03 a ± 0.002 | 0.04 a ± 0.005 | 0.03 a ± 0.011 | 0.03 a ± 0.005 | 0.03 a ± 0.005 | 0.04 a ± 0.002 | 0.03 a ± 0.01 | 0.04 a ± 0.01 | 0.03 a ± 0.002 |
| Dodecane | 0.11 a ± 0.011 | 0.08 a ± 0.01 | 0.18 b ± 0.015 | 0.13 a ± 0.015 | 0.22 b ± 0.030 | 0.13 a ± 0.026 | 0.14 a ± 0.01 | 0.11 a ± 0.01 | 0.09 a ± 0.01 |
| Benzene,1,3-bis(1,1-dimethylethyl) | 0.09 a ± 0.01 | 0.06 a ± 0.152 | 0.18 b ± 0.081 | 0.12 ab ± 0.081 | 0.08 a ± 0.020 | 0.1 a ± 0.020 | 0.20 b ± 0.01 | 0.13 ab ± 0.03 | 0.06 a ± 0.01 |
| Benzoic acid,2-methylpropyl ester | 0.02 ± 0.005 | 0.02 ± 0.005 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Caryophyllene | 0.07 ab ± 0.011 | 0.04 a ± 0.152 | 0.14 b ± 0.011 | 0.08 ab ± 0.011 | 0.07 ab ± 0.015 | 0.09 ab ± 0.017 | 0.09 ab ± 0.02 | 0.10 ab ± 0.01 | 0.05 a ± 0.01 |
| Dimethyl phthalate | 0.02 a ± 0.005 | n.d. | 0.06 a ± 0.026 | 0.24 b ± 0.160 | 2.24 c ± 0.056 | 4.63 d ± 0.070 | 0.06 a ± 0.01 | n.d. | 0.03 a ± 0.01 |
| 2,5-cyclohexadiene-1,4dione, 2-(1,1-dimethylethyl)-5-(2-methyl-2-propen-1-yl) | 0.14 a ± 0.020 | 0.10 a ± 0.173 | 0.23 b ± 0.021 | 0.13 a ± 0.017 | 0.13 a ± 0.015 | 0.18 a ± 0.026 | 0.27 b ± 0.03 | 0.21 ab ± 0.01 | 0.14 a ± 0.02 |
| Pentadecane | 0.17 a ± 0.017 | 0.11 a ± 0.01 | 0.24 b ± 0.015 | 0.19 ab ± 0.005 | 0.20 ab ± 0.020 | 0.27 b ± 0.02 | 0.25 b ± 0.020 | 0.14 a ± 0.01 | 0.13 a ± 0.02 |
| Butylated Hydroxytoluene | 14.19 a ± 0.352 | 11.63 a ± 0.854 | 21.27 c ± 0.113 | 14.03 a ± 0.643 | 14.88 b ± 0.135 | 16.19 b ± 0.110 | 17.93 b ± 0.14 | 16.75 c ± 0.21 | 13.08 a ± 0.20 |
| Hexadecane | 0.05 ab ± 0.011 | 0.03 a ± 0.001 | 0.07 b ± 0.015 | 0.06 ab ± 0.001 | 0.07 b ± 0.020 | 0.06 ab ± 0.005 | 0.09 c ± 0.02 | 0.05 ab ± 0.02 | 0.03 a ± 0.01 |
| Sulfurous acid, 2-ethylhexyl tridecyl ester | 0.05 a ± 0.005 | 0.04 a ± 0.005 | 0.07 a ± 0.02 | 0.05 a ± 0.001 | 0.05 a ± 0.010 | 0.06 a ± 0.005 | 0.06 a ± 0.01 | 0.06 a ± 0.01 | 0.06 a ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Tian, F.; Cao, A.; Guan, W.; Liu, T.; Liu, Y.; Cai, L. A Comparative Study Based on HS-SPME-GC-MS of Volatile Compounds in Large Yellow Croaker (Pseudosciaena crocea) During Varied Cold Storage Conditions. Foods 2025, 14, 2063. https://doi.org/10.3390/foods14122063
Chen W, Tian F, Cao A, Guan W, Liu T, Liu Y, Cai L. A Comparative Study Based on HS-SPME-GC-MS of Volatile Compounds in Large Yellow Croaker (Pseudosciaena crocea) During Varied Cold Storage Conditions. Foods. 2025; 14(12):2063. https://doi.org/10.3390/foods14122063
Chicago/Turabian StyleChen, Wenyuchu, Fang Tian, Ailing Cao, Weiliang Guan, Tianyu Liu, Ying Liu, and Luyun Cai. 2025. "A Comparative Study Based on HS-SPME-GC-MS of Volatile Compounds in Large Yellow Croaker (Pseudosciaena crocea) During Varied Cold Storage Conditions" Foods 14, no. 12: 2063. https://doi.org/10.3390/foods14122063
APA StyleChen, W., Tian, F., Cao, A., Guan, W., Liu, T., Liu, Y., & Cai, L. (2025). A Comparative Study Based on HS-SPME-GC-MS of Volatile Compounds in Large Yellow Croaker (Pseudosciaena crocea) During Varied Cold Storage Conditions. Foods, 14(12), 2063. https://doi.org/10.3390/foods14122063







