Strategic Approaches for Co-Encapsulation of Bioactive Compounds: Technological Advances and Mechanistic Insight
Abstract
1. Introduction
2. Types of Co-Delivered Active Substances
2.1. Polyphenols
2.2. Probiotics
2.3. Vitamins
2.4. Others
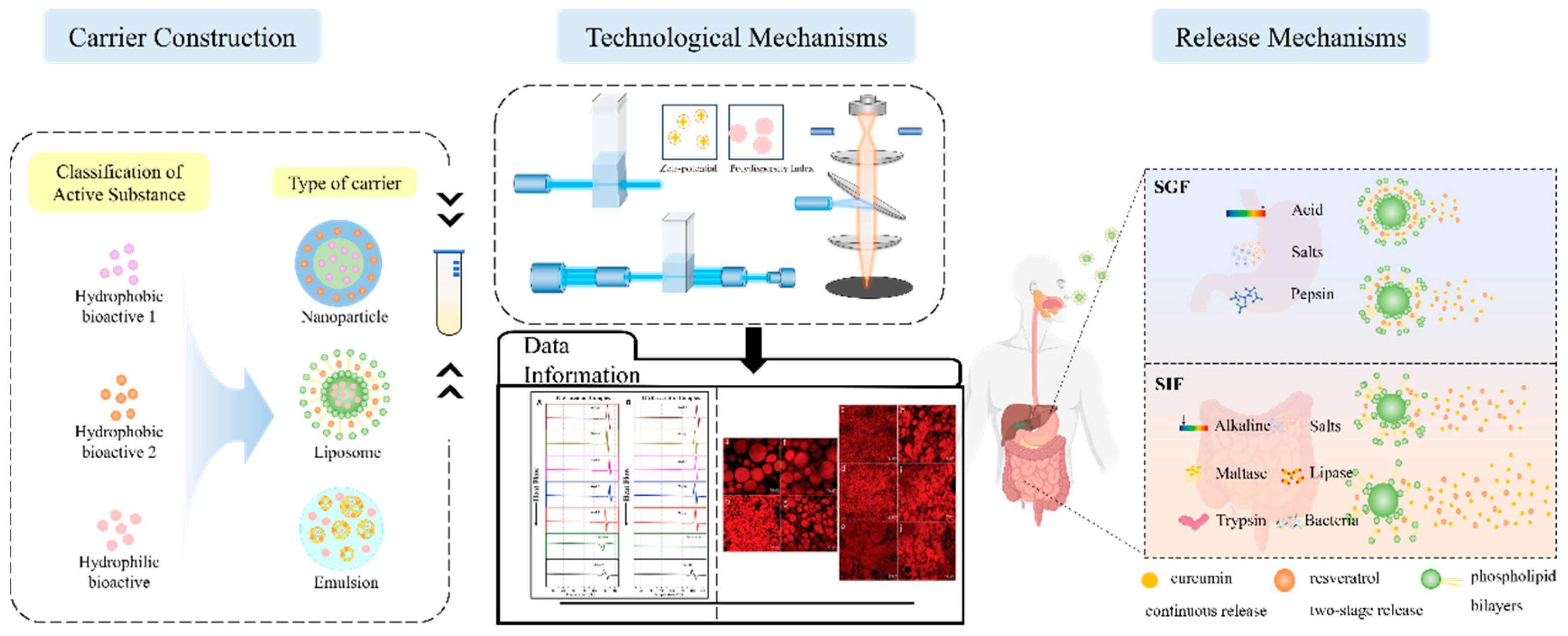
3. Delivery Carrier Classification and Characterization
3.1. Type of Carrier
3.1.1. Emulsions
3.1.2. Nanoparticles
3.1.3. Liposomes
3.1.4. Other Delivery Carriers
3.2. Methods for Characterization of Co-Delivery Structures
3.2.1. Conventional Generic Method
3.2.2. Specialized Methodology
3.3. Factors Affecting the Release Kinetics of Co-Delivery Carriers
3.3.1. Different Digestion Models
3.3.2. Release Dynamics Modeling
4. Application of Co-Delivery Carriers
5. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Coronel, J.; Yu, J.; Pilli, N.; Kane, M.A.; Amengual, J. The conversion of β-carotene to vitamin A in adipocytes drives the anti-obesogenic effects of β-carotene in mice. Mol. Metab. 2022, 66, 101640. [Google Scholar] [CrossRef]
- Medeiros, G.K.V.V.; Queiroga, R.C.R.E.; Costa, W.K.A.; Gadelha, C.A.A.; e Lacerda, R.R.; Lacerda, J.T.J.G.; Pinto, L.S.; Braganhol, E.; Teixeira, F.C.; de SBarbosa, P.P.; et al. Proteomic of goat milk whey and its bacteriostatic and antitumour potential. Int. J. Biol. Macromol. 2018, 113, 116–123. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Y.-Y.; Pan, L.-H.; Li, Q.-M.; Luo, J.-P.; Zha, X.-Q. Co-encapsulation systems for delivery of bioactive ingredients. Food Res. Int. 2022, 155, 111073. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Chi, Y.; Huang, Y.; He, Q.; Ren, Y. Synergistic antioxidant effect of glutathione and edible phenolic acids and improvement of the activity protection by coencapsulation into chitosan-coated liposomes. LWT 2020, 127, 109409. [Google Scholar] [CrossRef]
- Zhao, G.; Hu, C.; Xue, Y. In vitro evaluation of chitosan-coated liposome containing both coenzyme Q10 and alpha-lipoic acid: Cytotoxicity, antioxidant activity, and antimicrobial activity. J. Cosmet. Dermatol. 2018, 17, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Fan, Q.; Liu, T.; Wusigale; Liang, L. Co-encapsulation of α-tocopherol and resveratrol in oil-in-water emulsion stabilized by sodium caseinate: Impact of polysaccharide on the stability and bioaccessibility. J. Food Eng. 2020, 264, 109685. [Google Scholar] [CrossRef]
- Yeh, C.-T.; Shih, P.-H.; Yen, G.-C. Synergistic Effect of Antioxidant Phenolic Acids on Human Phenolsulfotransferase Activity. J. Agric. Food Chem. 2004, 52, 4139–4143. [Google Scholar] [CrossRef]
- Aditya, N.P.; Aditya, S.; Yang, H.; Kim, H.W.; Park, S.O.; Ko, S. Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chem. 2015, 173, 7–13. [Google Scholar] [CrossRef]
- Hussain, Z.; Pandey, M.; Choudhury, H.; Ying, P.C.; Xian, T.M.; Kaur, T.; Jia, G.W.; Gorain, B. Hyaluronic acid functionalized nanoparticles for simultaneous delivery of curcumin and resveratrol for management of chronic diabetic wounds: Fabrication, characterization, stability and in vitro release kinetics. J. Drug Deliv. Sci. Technol. 2020, 57, 101747. [Google Scholar] [CrossRef]
- Zhao, Y.; Cai, C.; Liu, M.; Zhao, Y.; Wu, Y.; Fan, Z.; Ding, Z.; Zhang, H.; Wang, Z.; Han, J. Drug-binding albumins forming stabilized nanoparticles for co-delivery of paclitaxel and resveratrol: In vitro/in vivo evaluation and binding properties investigation. Int. J. Biol. Macromol. 2020, 153, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, M.; Yuan, Y. The potential of curcumin-based co-delivery systems for applications in the food industry: Food preservation, freshness monitoring, and functional food. Food Res. Int. 2023, 171, 113070. [Google Scholar] [CrossRef]
- Choi, I.; Li, N.; Zhong, Q. Co-loading curcumin and quercetin in freeze-dried mushroom microparticles to inhibit lipid oxidation in beef patties. Food Chem. 2022, 374, 131625. [Google Scholar] [CrossRef]
- Yoha, K.S.; Nida, S.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Targeted Delivery of Probiotics: Perspectives on Research and Commercialization. Probiotics Antimicrob. Proteins 2022, 14, 15–48. [Google Scholar] [CrossRef]
- Köprüalan Aydın, Ö.; Baysan, U.; Altay, Ö.; İlter Baysan, I.; Kaymak Ertekin, F.; Jafari, S.M. Vitamin delivery systems by spray-drying encapsulation within plant protein-based carriers: A review. Food Biosci. 2023, 56, 103341. [Google Scholar] [CrossRef]
- Guan, C.; Zhou, X.; Li, H.; Ma, X.; Zhuang, J. NF-κB inhibitors gifted by nature: The anticancer promise of polyphenol compounds. Biomed. Pharmacother. 2022, 156, 113951. [Google Scholar] [CrossRef]
- Shen, X.; He, L.; Cui, Y.; Lin, Z.; Jafari, S.M.; Tan, C. Co-encapsulation of bioactive compounds in liposomal delivery systems for synergistic effects. Food Biosci. 2025, 68, 106306. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, J.; Lin, L.; Wang, X.; Zhang, L.; Tao, N. Co-delivery of astaxanthin using positive synergistic effect from biomaterials: From structural design to functional regulation. Food Chem. 2025, 470, 142731. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Liu, H.; Wang, C.; Guo, N.; Shen, Y.; Luo, S.; Jiang, S.; Zheng, Z. Remodeling the structure of soy protein fibrils to hydrogels for co-encapsulation of (−)-epigallocatechin gallate (EGCG) and curcumin: Role of EGCG. Food Hydrocoll. 2024, 147, 109439. [Google Scholar] [CrossRef]
- Zhao, G.; Hu, C.; Sun, R.; Ni, S.; Xia, Q. Effect of Emulsification Process on Multiple Lipid Particles Encapsulating Both Coenzyme Q10 and Tea Polyphenols. J. Food Process Eng. 2015, 38, 144–154. [Google Scholar] [CrossRef]
- Feng, F.; Gao, J.; Chen, L.; Xiao, S.; Huang, G. Scalable fabrication of uniform nanoparticles for the efficient co-encapsulation of curcumin and procyanidins driven by multiple interactions. Food Hydrocoll. 2023, 144, 108960. [Google Scholar] [CrossRef]
- Xiong, Y.; Feng, Y.-X.; Chang, M.; Wang, Q.; Yin, S.-N.; Jian, L.-Y.; Ren, D.-F. Formulated chitosan-sodium tripolyphosphate nanoparticles for co-encapsulation of ellagic acid and anti-inflammatory peptide: Characterization, stability and anti-inflammatory activity. J. Sci. Food Agric. 2023, 103, 3447–3456. [Google Scholar] [CrossRef]
- Yang, Z.; McClements, D.J.; Peng, X.; Xu, Z.; Meng, M.; Chen, L.; Jin, Z. Fabrication of zein–carboxymethyl cellulose nanoparticles for co-delivery of quercetin and resveratrol. J. Food Eng. 2023, 341, 111322. [Google Scholar] [CrossRef]
- Chen, W.; Zou, M.; Ma, X.; Lv, R.; Ding, T.; Liu, D. Co-Encapsulation of EGCG and Quercetin in Liposomes for Optimum Antioxidant Activity. J. Food Sci. 2019, 84, 111–120. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, X.; McClements, D.J.; Zou, L.; Liu, X.; Liu, F. Co-encapsulation of Epigallocatechin Gallate (EGCG) and Curcumin by Two Proteins-Based Nanoparticles: Role of EGCG. J. Agric. Food Chem. 2019, 67, 13228–13236. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Wu, X.; Zhao, Z.; Deng, Q.; Chen, Y.; Yin, L. Endothelial cell-targeting, ROS-ultrasensitive drug/siRNA co-delivery nanocomplexes mitigate early-stage neutrophil recruitment for the anti-inflammatory treatment of myocardial ischemia reperfusion injury. Acta Biomater. 2022, 143, 344–355. [Google Scholar] [CrossRef]
- Ghobadi-Oghaz, N.; Asoodeh, A.; Mohammadi, M. Fabrication, characterization and in vitro cell exposure study of zein-chitosan nanoparticles for co-delivery of curcumin and berberine. Int. J. Biol. Macromol. 2022, 204, 576–586. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Wang, R.; Wang, X.; Jiang, K.; Xie, C.; Zhan, C.; Wang, H.; Lu, W. Co-delivery of paclitaxel and melittin by glycopeptide-modified lipodisks for synergistic anti-glioma therapy. Nanoscale 2019, 11, 13069–13077. [Google Scholar] [CrossRef]
- Ma, L.; Su, C.; Li, X.; Wang, H.; Luo, M.; Chen, Z.; Zhang, B.; Zhu, J.; Yuan, Y. Preparation and characterization of bilayered microencapsulation for co-delivery Lactobacillus casei and polyphenols via Zein-chitosan complex coacervation. Food Hydrocoll. 2024, 148, 109410. [Google Scholar] [CrossRef]
- Su, J.; Cai, Y.; Zhi, Z.; Guo, Q.; Mao, L.; Gao, Y.; Yuan, F.; Van der Meeren, P. Assembly of propylene glycol alginate/β-lactoglobulin composite hydrogels induced by ethanol for co-delivery of probiotics and curcumin. Carbohydr. Polym. 2021, 254, 117446. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, X.; Li, R.; Cao, Y.; Zhou, W.; Li, J.; Zheng, B. Fabrication and Characterization of W/O/W Emulgels by Sipunculus nudus Salt-Soluble Proteins: Co-Encapsulation of Vitamin C and β-Carotene. Foods 2022, 11, 2720. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Rocha, F.; Estevinho, B.N. Co-encapsulation of retinoic acid, curcumin and resveratrol by spray-drying of alginic acid sodium-based emulsions and ethyl cellulose-based solutions: Impact on the co-delivery profiles. Int. J. Biol. Macromol. 2023, 224, 1217–1227. [Google Scholar] [CrossRef]
- Bajaj, S.R.; Marathe, S.J.; Singhal, R.S. Co-encapsulation of vitamins B12 and D3 using spray drying: Wall material optimization, product characterization, and release kinetics. Food Chem. 2021, 335, 127642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, Y.; Hou, Y.; Wang, H.; Tan, M. Fabrication of novel W/O/W emulsion gels using beeswax stabilized W/O: Preparation, characterization and co-delivery of phycocyanin and astaxanthin. Food Biosci. 2024, 57, 103536. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Abdullah; Tian, W.; Song, M.; Cao, Y.; Xiao, J. Co-delivery of EGCG and lycopene via a pickering double emulsion induced synergistic hypolipidemic effect. Food Funct. 2022, 13, 3419–3430. [Google Scholar] [CrossRef]
- Ferraz, M.C.; Procopio, F.R.; Furtado, G.d.F.; Hubinger, M.D. Co-encapsulation of paprika and cinnamon oleoresin by spray drying using whey protein isolate and maltodextrin as wall material: Development, characterization and storage stability. Food Res. Int. 2022, 162, 112164. [Google Scholar] [CrossRef]
- Niu, B.; Chen, H.; Wu, W.; Fang, X.; Mu, H.; Han, Y.; Gao, H. Co-encapsulation of chlorogenic acid and cinnamaldehyde essential oil in Pickering emulsion stablized by chitosan nanoparticles. Food Chem. X 2022, 14, 100312. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, Q.; Rehman, A.; Jafari, S.M.; Zuo, M.; Khan, M.A.; Ali, A.; Khan, S.; Karim, A.; Usman, M.; Hussain, A.; et al. Improving the oxidative stability of fish oil nanoemulsions by co-encapsulation with curcumin and resveratrol. Colloids Surf. B Biointerfaces 2021, 199, 111481. [Google Scholar] [CrossRef]
- Quichaba, M.B.; Moreira, T.F.M.; de Oliveira, A.; de Carvalho, A.S.; de Menezes, J.L.; Gonçalves, O.H.; de Abreu Filho, B.A.; Leimann, F.V. Biopreservatives against foodborne bacteria: Combined effect of nisin and nanoncapsulated curcumin and co-encapsulation of nisin and curcumin. J. Food Sci. Technol. 2023, 60, 581–589. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, H.; Wang, D.; Liu, T.; Zhang, R.; Wu, Y.; Zhang, Y.; Han, J.; Liu, M. Antioxidant activity, stability, in vitro digestion and cytotoxicity of two dietary polyphenols co-loaded by β-lactoglobulin. Food Chem. 2022, 371, 131385. [Google Scholar] [CrossRef]
- Zhang, F.; Khan, M.A.; Cheng, H.; Liang, L. Co-encapsulation of α-tocopherol and resveratrol within zein nanoparticles: Impact on antioxidant activity and stability. J. Food Eng. 2019, 247, 9–18. [Google Scholar] [CrossRef]
- Oliviero, F.; Scanu, A.; Zamudio-Cuevas, Y.; Punzi, L.; Spinella, P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018, 98, 1653–1659. [Google Scholar] [CrossRef]
- Leri, M.; Vasarri, M.; Carnemolla, F.; Oriente, F.; Cabaro, S.; Stio, M.; Degl’Innocenti, D.; Stefani, M.; Bucciantini, M. EVOO Polyphenols Exert Anti-Inflammatory Effects on the Microglia Cell through TREM2 Signaling Pathway. Pharmaceuticals 2023, 16, 933. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Kumar, A.; Girisa, S.; Aswani, B.S.; Vishwa, R.; Sethi, G.; Kunnumakkara, A.B. Nanoformulations of curcumin: An alliance for effective cancer therapeutics. Food Biosci. 2023, 56, 103095. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Abbasi, P.; Eshaghi, M.M.; Bakhshi, A.; Ezra Manicum, A.-L.; Rahdar, A.; Pandey, S.; Jadoun, S.; Díez-Pascual, A.M. Curcumin delivery and co-delivery based on nanomaterials as an effective approach for cancer therapy. J. Drug Deliv. Sci. Technol. 2022, 78, 103982. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Q.; Hu, Z.; Cai, J.; Qin, X. Maillard-Reacted Whey Protein Isolates and Epigallocatechin Gallate Complex Enhance the Thermal Stability of the Pickering Emulsion Delivery of Curcumin. J. Agric. Food Chem. 2019, 67, 5212–5220. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, H.; Chen, Y.; Chen, L.; Fang, Z.; Liang, L. Formation of a Multiligand Complex of Bovine Serum Albumin with Retinol, Resveratrol, and (−)-Epigallocatechin-3-gallate for the Protection of Bioactive Components. J. Agric. Food Chem. 2017, 65, 3019–3030. [Google Scholar] [CrossRef]
- Liu, T.; Liu, M.; Liu, H.; Ren, Y.; Zhao, Y.; Yan, H.; Wang, Q.; Zhang, N.; Ding, Z.; Wang, Z. Co-encapsulation of (−)-epigallocatechin-3-gallate and piceatannol/oxyresveratrol in β-lactoglobulin: Effect of ligand–protein binding on the antioxidant activity, stability, solubility and cytotoxicity. Food Funct. 2021, 12, 7126–7144. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Han, S.; Wang, J.; McClements, D.J.; Liu, X.; Liu, F. Co-delivery of curcumin and epigallocatechin gallate in W/O/W emulsions stabilized by protein fibril-cellulose complexes. Colloids Surf. B Biointerfaces 2023, 222, 113072. [Google Scholar] [CrossRef]
- Eom, D.W.; Lee, J.H.; Kim, Y.J.; Hwang, G.S.; Kim, S.N.; Kwak, J.H.; Cheon, G.J.; Kim, K.H.; Jang, H.J.; Ham, J.; et al. Synergistic effect of curcumin on epigallocatechin gallate-induced anticancer action in PC3 prostate cancer cells. BMB Rep. 2015, 48, 461–466. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, Q.; Liu, Q.; Wang, M.; Wei, W.; Gong, G.; He, Y.; Wang, Y.; Zheng, Y.; Yang, L.; et al. Oral polyphenol-based microbeads with synergistic demulsification and fat locking for obesity treatment. Cell Biomater. 2025, 1, 100019. [Google Scholar] [CrossRef]
- Chen, X.; McClements, D.J.; Wang, J.; Zou, L.; Deng, S.; Liu, W.; Yan, C.; Zhu, Y.; Cheng, C.; Liu, C. Coencapsulation of (−)-Epigallocatechin-3-gallate and Quercetin in Particle-Stabilized W/O/W Emulsion Gels: Controlled Release and Bioaccessibility. J. Agric. Food Chem. 2018, 66, 3691–3699. [Google Scholar] [CrossRef]
- Lu, Y.; Zhong, Y.; Guo, X.; Zhang, J.; Gao, Y.; Mao, L. Structural Modification of O/W Bigels by Glycerol Monostearate for Improved Co-Delivery of Curcumin and Epigallocatechin Gallate. ACS Food Sci. Technol. 2022, 2, 975–983. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Q.; Oliveira, H.; Mateus, N.; Ye, S.; Jiang, S.; He, J.; Wu, M. Preparation of nanoliposomes loaded with anthocyanins from grape skin extracts: Stability, gastric absorption and antiproliferative properties. Food Funct. 2022, 13, 10912–10922. [Google Scholar] [CrossRef]
- Nascimento, A.L.A.A.; Borges, L.L.R.; Fernandes, J.G.; Freitas, V.V.; Martins, E.; Campelo, P.H.; Stringheta, P.C. Exploring strategies to enhance anthocyanin bioavailability and bioaccessibility in food: A literature review. Food Biosci. 2023, 56, 103388. [Google Scholar] [CrossRef]
- do Carmo, E.L.; Teixeira, M.A.; e Souza, I.S.; Figueiredo, J.d.A.; Fernandes, R.V.d.B.; Botrel, D.A.; Borges, S.V. Co-encapsulation of anthocyanins extracted from grape skins (Vitis vinifera var. Syrah) and α-tocopherol via spray drying. J. Food Process. Preserv. 2021, 45, e16038. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, W.; Ye, Y.; Cui, W.; Dong, L.; Yao, Y.; Li, K.; Han, J.; Liu, W. Novel Nanoliposome Codelivered DHA and Anthocyanidin: Characterization, In Vitro Infant Digestibility, and Improved Cell Uptake. J. Agric. Food Chem. 2021, 69, 9395–9406. [Google Scholar] [CrossRef]
- Moshfegh, N.; Niakousary, M.; Hosseini, S.M.H.; Mazloomi, S.M.; Abbasi, A. Effect of maltodextrin and Persian gum as wall materials and tannic acid as copigment on some properties of encapsulated sour cherry anthocyanin microcapsules. Food Chem. 2025, 463, 141165. [Google Scholar] [CrossRef]
- Guo, C.; Yin, J.; Chen, D. Co-encapsulation of curcumin and resveratrol into novel nutraceutical hyalurosomes nano-food delivery system based on oligo-hyaluronic acid-curcumin polymer. Carbohydr. Polym. 2018, 181, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Coradini, K.; Lima, F.O.; Oliveira, C.M.; Chaves, P.S.; Athayde, M.L.; Carvalho, L.M.; Beck, R.C.R. Co-encapsulation of resveratrol and curcumin in lipid-core nanocapsules improves their in vitro antioxidant effects. Eur. J. Pharm. Biopharm. 2014, 88, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, Z.; Xue, C.; Yang, L. Co-delivery system based on multilayer structural nanoparticles for programmed sequential release of fucoxanthin and curcumin. Food Hydrocoll. 2023, 141, 108729. [Google Scholar] [CrossRef]
- Kavimughil, M.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. 3D printed MCT oleogel as a co-delivery carrier for curcumin and resveratrol. Biomaterials 2022, 287, 121616. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.d.; Santos, P.D.d.F.; Silva, J.T.d.P.; Leimann, F.V.; Bracht, L.; Gonçalves, O.H. Impact of curcumin nanoformulation on its antimicrobial activity. Trends Food Sci. Technol. 2018, 72, 74–82. [Google Scholar] [CrossRef]
- Rafiee, Z.; Nejatian, M.; Daeihamed, M.; Jafari, S.M. Application of curcumin-loaded nanocarriers for food, drug and cosmetic purposes. Trends Food Sci. Technol. 2019, 88, 445–458. [Google Scholar] [CrossRef]
- Yang, K.-Y.; Lin, L.-C.; Tseng, T.-Y.; Wang, S.-C.; Tsai, T.-H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC–MS/MS. J. Chromatogr. B 2007, 853, 183–189. [Google Scholar] [CrossRef]
- Wen, C.; Cao, L.; Yu, Z.; Liu, G.; Zhang, J.; Xu, X. Advances in lipo-solubility delivery vehicles for curcumin: Bioavailability, precise targeting, possibilities and challenges. Crit. Rev. Food Sci. Nutr. 2024, 64, 10835–10854. [Google Scholar] [CrossRef]
- Huang, M.; Liang, C.; Tan, C.; Huang, S.; Ying, R.; Wang, Y.; Wang, Z.; Zhang, Y. Liposome co-encapsulation as a strategy for the delivery of curcumin and resveratrol. Food Funct. 2019, 10, 6447–6458. [Google Scholar] [CrossRef]
- Guo, Q.; Shu, X.; Hu, Y.; Su, J.; Chen, S.; Decker, E.A.; Gao, Y. Formulated protein-polysaccharide-surfactant ternary complexes for co-encapsulation of curcumin and resveratrol: Characterization, stability and in vitro digestibility. Food Hydrocoll. 2021, 111, 106265. [Google Scholar] [CrossRef]
- Coradini, K.; Friedrich, R.B.; Fonseca, F.N.; Vencato, M.S.; Andrade, D.F.; Oliveira, C.M.; Battistel, A.P.; Guterres, S.S.; da Rocha, M.I.U.M.; Pohlmann, A.R.; et al. A novel approach to arthritis treatment based on resveratrol and curcumin co-encapsulated in lipid-core nanocapsules: In vivo studies. Eur. J. Pharm. Sci. 2015, 78, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Rashidinejad, A.; Bahrami, A.; Rehman, A.; Rezaei, A.; Babazadeh, A.; Singh, H.; Jafari, S.M. Co-encapsulation of probiotics with prebiotics and their application in functional/synbiotic dairy products. Crit. Rev. Food Sci. Nutr. 2022, 62, 2470–2494. [Google Scholar] [CrossRef] [PubMed]
- Menegazzi, G.d.S.; Ribeiro, E.S.; de Farias, B.S.; Luz, G.d.Q.d.; Oliveira, G.M.; Cadaval Junior, T.R.S.A.; Pinto, L.A.d.A.; Diaz, P.S. Microencapsulation of Lacticaseibacillus casei CSL3 using cheese whey, fructo-oligosaccharide and xanthan gum by spray drying. Food Biosci. 2023, 56, 103348. [Google Scholar] [CrossRef]
- Gu, Q.; Jiang, Z.; Li, K.; Li, Y.; Yan, X.; McClements, D.J.; Ma, C.; Liu, F. Effectiveness of probiotic- and fish oil-loaded water-in-oil-in-water (W1/O/W2) emulsions at alleviating ulcerative colitis. Food Funct. 2024, 15, 5797–5812. [Google Scholar] [CrossRef]
- Holkem, A.T.; Favaro-Trindade, C.S.; Lacroix, M. Study of anticancer properties of proanthocyanidin-rich cinnamon extract in combination with Bifidobacterium animalis subsp. lactis BLC1 and resistance of these free and co-encapsulated materials under in vitro simulated gastrointestinal conditions. Food Res. Int. 2020, 134, 109274. [Google Scholar] [CrossRef]
- Cai, Q.; Zhong, Y.; Huang, Q.; Huang, G.; Lu, X. Co-incorporation of probiotics into 3D printed custard cream with hydrophilic and hydrophobic bioactives. Food Hydrocoll. 2023, 142, 108809. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Liu, B.; Xiao, J.; Stuart, M.A.C.; Hou, G.; Zhang, H.; Liang, S.; Li, Z.; Wang, Q.; et al. Natural Phenolic-Metal Framework Strengthened Mesona Chinensis Polysaccharides Microgels for Improved Viability of Probiotics to Alleviate the Liver Injury and Gut Microbiota Dysbiosis. Adv. Funct. Mater. 2024, 34, 2401064. [Google Scholar] [CrossRef]
- Cheng, J.; Li, X.; Ke, T.; Gong, J.; Jiang, T.; Chen, L. Advances in polysaccharide-based probiotic delivery systems: A review. Carbohydr. Polym. Technol. Appl. 2025, 10, 100804. [Google Scholar] [CrossRef]
- Ahmad, M.; Hassan, I.; Shah, M.A.; Gani, A.; Muthukumarappan, K. Co-encapsulation of multivitamins in micro & nano-sized starch, target release, capsule characterization and interaction studies. Int. J. Biol. Macromol. 2023, 240, 124367. [Google Scholar] [CrossRef]
- Fang, Z.; Wusigale; Bao, H.; Ni, Y.; Choijilsuren, N.; Liang, L. Partition and digestive stability of α-tocopherol and resveratrol/naringenin in whey protein isolate emulsions. Int. Dairy J. 2019, 93, 116–123. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, W.; Pu, C.; Li, R.; Sun, Q.; Wang, H. Improved stability of liposome-stabilized emulsions as a co-encapsulation delivery system for vitamin B2, vitamin E and β-carotene. Food Funct. 2022, 13, 2966–2984. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, P.; Zou, Y.-X.; Luo, Z.-G.; Tamer, T.M. Co-encapsulation of Vitamin C and β-Carotene in liposomes: Storage stability, antioxidant activity, and in vitro gastrointestinal digestion. Food Res. Int. 2020, 136, 109587. [Google Scholar] [CrossRef] [PubMed]
- Hassane Hamadou, A.; Zhang, J.; Chen, C.; Xu, J.; Xu, B. Vitamin C and β-carotene co-loaded in marine and egg nanoliposomes. J. Food Eng. 2023, 340, 111315. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Han, Y.; McClements, D.J.; Liao, W.; Mao, L.; Yuan, F.; Gao, Y. Fabrication of multilayer structural microparticles for co-encapsulating coenzyme Q10 and piperine: Effect of the encapsulation location and interface thickness. Food Hydrocoll. 2020, 109, 106090. [Google Scholar] [CrossRef]
- Yu, L.; Chao, C.; Li, Q.; Ye, S.; Lin, J.; Zhong, S.; Xuan, Q.; Xu, K.; Zhao, S. A Co-Encapsulation of Coenzyme Q10 and Curcumin in Liposomes Coated with Chitosan (Q10-Cur-Lip-Chi) with Enhanced Solubility and Stability for Good Release Performance and Antioxidative Activity. Curr. Drug Deliv. 2023, 20, 1391–1403. [Google Scholar] [CrossRef]
- Ma, M.; Liu, Y.; Chen, Y.; Zhang, S.; Yuan, Y. Co-encapsulation: An effective strategy to enhance the synergistic effects of probiotics and polyphenols. Trends Food Sci. Technol. 2025, 158, 104927. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, D.; Zhang, Q.; Chen, Y.; Zheng, D.; Hao, L.; Duan, C.; Jia, L.; Liu, G.; Liu, Y. Synergistic effect of folate-mediated targeting and verapamil-mediated P-gp inhibition with paclitaxel -polymer micelles to overcome multi-drug resistance. Biomaterials 2011, 32, 9444–9456. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, H.; Zhang, H.; Shi, C.; Zhou, W.; Zhang, X. Nanostructured lipid carrier as a strategy for encapsulation of formononetin and perilla seed oil: In vitro characterization and stability studies. Food Biosci. 2023, 53, 102707. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Wang, Z.-M.; Meng, H.-C.; Lin, J.-W.; Guo, X.-M.; Zhang, T.; Chen, H.-L.; Lei, C.-Y.; Yu, S.-J. Robust W/O/W Emulsion Stabilized by Genipin-Cross-Linked Sugar Beet Pectin-Bovine Serum Albumin Nanoparticles: Co-encapsulation of Betanin and Curcumin. J. Agric. Food Chem. 2021, 69, 1318–1328. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, C.; Liu, X.; Mackie, A.; Zhang, M.; Dai, L.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Co-encapsulation of curcumin and β-carotene in Pickering emulsions stabilized by complex nanoparticles: Effects of microfluidization and thermal treatment. Food Hydrocoll. 2022, 122, 107064. [Google Scholar] [CrossRef]
- Han, L.; Lu, K.; Zhou, S.; Qi, B.; Li, Y. Co-delivery of insulin and quercetin in W/O/W double emulsions stabilized by different hydrophilic emulsifiers. Food Chem. 2022, 369, 130918. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wang, J.; Tan, C.; Ying, R.; Wu, X.; Chen, W.; Liu, J.; Ahmad, M. Liposomal co-delivery strategy to improve stability and antioxidant activity of trans-resveratrol and naringenin. Int. J. Food Sci. Technol. 2022, 57, 2701–2714. [Google Scholar] [CrossRef]
- Li, J.; Zhou, S.; Yu, J.; Cai, W.; Yang, Y.; Kuang, X.; Liu, H.; He, Z.; Wang, Y. Low dose shikonin and anthracyclines coloaded liposomes induce robust immunogenetic cell death for synergistic chemo-immunotherapy. J. Control. Release 2021, 335, 306–319. [Google Scholar] [CrossRef]
- Khatib, N.; Varidi, M.J.; Mohebbi, M.; Varidi, M.; Hosseini, S.M.H. Co-encapsulation of lupulon and xanthohumol in lecithin-based nanoliposomes developed by sonication method. J. Food Process. Preserv. 2019, 43, e14075. [Google Scholar] [CrossRef]
- Jaiswal, S.; Mishra, P. Co-delivery of curcumin and serratiopeptidase in HeLa and MCF-7 cells through nanoparticles show improved anti-cancer activity. Mater. Sci. Eng. C 2018, 92, 673–684. [Google Scholar] [CrossRef]
- Chu, P.-Y.; Tsai, S.-C.; Ko, H.-Y.; Wu, C.-C.; Lin, Y.-H. Co-Delivery of Natural Compounds with a Dual-Targeted Nanoparticle Delivery System for Improving Synergistic Therapy in an Orthotopic Tumor Model. ACS Appl. Mater. Interfaces 2019, 11, 23880–23892. [Google Scholar] [CrossRef]
- Dong, H.; Yin, X.; Wusigale; Cheng, H.; Choijilsuren, N.; Chen, X.; Liang, L. Antioxidant activity and stability of α-tocopherol, resveratrol and epigallocatechin-3-gallate in mixture and complexation with bovine serum albumin. Int. J. Food Sci. Technol. 2021, 56, 1788–1800. [Google Scholar] [CrossRef]
- Sun, H.; Sun, Y.; Tang, X.; Cui, Y.; Meng, D.; Zhang, Y.; Li, K.; Guo, H.; Chen, H.; Yang, R. The interaction mechanism and the functionality of yeast protein with hydrophilic and hydrophobic bioactive molecules. Food Biosci. 2023, 52, 102448. [Google Scholar] [CrossRef]
- Zhong, M.; Sun, Y.; Sun, Y.; Fang, L.; Wang, Q.; Qi, B.; Li, Y. Soy lipophilic protein self-assembled by pH-shift combined with heat treatment: Structure, hydrophobic resveratrol encapsulation, emulsification, and digestion. Food Chem. 2022, 394, 133514. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, J.; Li, Y.; Yang, Q.; Liu, X.; Liu, C.; Ma, S.; Liu, B.; Zhang, T.; Xiao, H.; et al. A self-assembled amphiphilic polysaccharide-based co-delivery system for egg white derived peptides and curcumin with oral bioavailability enhancement. Food Funct. 2021, 12, 10512–10523. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fu, D.; Zhang, J.; Li, T.; Wang, H.; Hou, W.; Niu, B.; Guo, R.; Liu, Y. Poly(aspartic acid)-based pH-responsive targeting co-delivery nanoparticles. J. Biomater. Appl. 2021, 36, 579–591. [Google Scholar] [CrossRef]
- Peng, Z.; He, Y.; Wang, D.; Fan, P.; Xie, M.; Xiong, T. Double emulsion (W/O/W) microcapsule preparation of novel bacteriocin lactococcin036019 with synergistic compound vitamin C prolongs the antibacterial activity in food matrix. Food Biosci. 2024, 57, 103597. [Google Scholar] [CrossRef]
- Santos, M.G.; Carpinteiro, D.A.; Thomazini, M.; Rocha-Selmi, G.A.; da Cruz, A.G.; Rodrigues, C.E.C.; Favaro-Trindade, C.S. Coencapsulation of xylitol and menthol by double emulsion followed by complex coacervation and microcapsule application in chewing gum. Food Res. Int. 2014, 66, 454–462. [Google Scholar] [CrossRef]
- Zahra, H.; Iran, A.; Manouchehr, V. Investigation of co-encapsulation of pancreatic beta cells and curcumin within alginate microcapsules. bioRxiv 2023. [Google Scholar] [CrossRef]
- Cheng, H.; Chang, X.; Luo, H.; Tang, H.; Chen, L.; Liang, L. Co-encapsulation of resveratrol in fish oil microcapsules optimally stabilized by enzyme-crosslinked whey protein with gum Arabic. Colloids Surf. B Biointerfaces 2023, 223, 113172. [Google Scholar] [CrossRef]
- Yang, X.; Shen, J.; Liu, J.; Yang, Y.; Hu, A.; Ren, N.; Cheng, Z.; Liu, W. Spray-Drying of Hydroxypropyl β-Cyclodextrin Microcapsules for Co-Encapsulation of Resveratrol and Piperine with Enhanced Solubility. Crystals 2022, 12, 596. [Google Scholar] [CrossRef]
- Leena, M.M.; Anukiruthika, T.; Moses, J.A.; Anandharamakrishnan, C. Co-delivery of curcumin and resveratrol through electrosprayed core-shell nanoparticles in 3D printed hydrogel. Food Hydrocoll. 2022, 124, 107200. [Google Scholar] [CrossRef]
- Yao, T.; Janaswamy, S. Ordered hydrocolloids networks as delivery vehicles of nutraceuticals: Optimal encapsulation of curcumin and resveratrol. Food Hydrocoll. 2022, 126, 107466. [Google Scholar] [CrossRef]
- Yang, J.; Fu, Y.; Zheng, H.; Jia, Y.; Gao, Y.; Yin, S.; Mao, L. Structural Design of Oleogel-Hydrogel Bigels for Co-Delivery of Curcumin and Epigallocatechin Gallate with Synergistic Stability and Bioactivity. Adv. Mater. Technol. 2023, 8, 2202185. [Google Scholar] [CrossRef]
- Abedi, F.; Davaran, S.; Hekmati, M.; Akbarzadeh, A.; Baradaran, B.; Moghaddam, S.V. An improved method in fabrication of smart dual-responsive nanogels for controlled release of doxorubicin and curcumin in HT-29 colon cancer cells. J. Nanobiotechnol. 2021, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Olga, G.; Styliani, C.; Ioannis, R.G. Coencapsulation of Ferulic and Gallic acid in hp-b-cyclodextrin. Food Chem. 2015, 185, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Zhu, F.; Li, B.; Zhao, L.; Liang, H.; Yan, Y.; Tan, H. Folate-conjugated and pH-triggered doxorubicin and paclitaxel co-delivery micellar system for targeted anticancer drug delivery. Mater. Chem. Front. 2018, 2, 1529–1538. [Google Scholar] [CrossRef]
- Song, P.; Lu, Z.; Jiang, T.; Han, W.; Chen, X.; Zhao, X. Chitosan coated pH/redox-responsive hyaluronic acid micelles for enhanced tumor targeted co-delivery of doxorubicin and siPD-L1. Int. J. Biol. Macromol. 2022, 222, 1078–1091. [Google Scholar] [CrossRef]
- Hu, M.; Liu, G.; Zhang, W.; Du, X.; Qi, B.; Li, Y. Co-encapsulation of (–)-epigallocatechin-3-gallate and quercetin in double emulsion hydrogel beads: Microstructures, functional properties, and digestion behaviors. Food Chem. 2022, 373, 131427. [Google Scholar] [CrossRef]
- Kuhn, F.; Santagapita, P.R.; Noreña, C.P.Z. Influence of egg albumin and whey protein in the co-encapsulation of betalains and phenolic compounds from Bougainvillea glabra bracts in Ca(II)-alginate beads. J. Food Process. Preserv. 2021, 45, e15918. [Google Scholar] [CrossRef]
- Karami Ghaleseiedi, Z.; Dadkhah Tehrani, A.; Parsamanesh, M. Starch-based dual amphiphilic graft copolymer as a new pH-sensitive maltidrug co-delivery system. Int. J. Biol. Macromol. 2018, 118, 913–920. [Google Scholar] [CrossRef]
- Salehi, S.; Nourbakhsh, M.S.; Yousefpour, M.; Rajabzadeh, G.; Sahab-Negah, S. Co-encapsulation of curcumin and boswellic acids in chitosan-coated niosome: An in-vitro digestion study. J. Microencapsul. 2022, 39, 226–238. [Google Scholar] [CrossRef]
- Ashkar, A.; Sosnik, A.; Davidovich-Pinhas, M. Structured edible lipid-based particle systems for oral drug-delivery. Biotechnol. Adv. 2022, 54, 107789. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, J.; Kim, Y.-J.; Wong, M.K.; Wang, P. Codelivery of Doxorubicin and Paclitaxel by Cross-Linked Multilamellar Liposome Enables Synergistic Antitumor Activity. Mol. Pharm. 2014, 11, 1651–1661. [Google Scholar] [CrossRef]
- Wang, T.; Mu, W.; Li, F.; Zhang, J.; Hou, T.; Pang, X.; Yin, X.; Zhang, N. “Layer peeling” co-delivery system for enhanced RNA interference-based tumor associated macrophages-specific chemoimmunotherapy. Nanoscale 2020, 12, 16851–16863. [Google Scholar] [CrossRef]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, S.; Hui, W.; He, J.; Liu, Z.; Cheng, J. Chitosan based pH-responsive polymeric prodrug vector for enhanced tumor targeted co-delivery of doxorubicin and siRNA. Carbohydr. Polym. 2020, 250, 116781. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, R.; Beulaja, M.; Arulvasu, C.; Sellamuthu, S.; Dinesh, D.; Prabhu, D.; Babu, G.; Vaseeharan, B.; Prabhu, N.M. Synergistic anticancer activity of curcumin and catechin: An in vitro study using human cancer cell lines. Microsc. Res. Tech. 2012, 75, 112–116. [Google Scholar] [CrossRef]
- Chen, H.; Dai, H.; Zhu, H.; Ma, L.; Fu, Y.; Feng, X.; Sun, Y.; Zhang, Y. Construction of dual-compartmental micro-droplet via shrimp ferritin nanocages stabilized Pickering emulsions for co-encapsulation of hydrophobic/hydrophilic bioactive compounds. Food Hydrocoll. 2022, 126, 107443. [Google Scholar] [CrossRef]
- Guan, X.; Ngai, T. pH-Sensitive W/O Pickering High Internal Phase Emulsions and W/O/W High Internal Water-Phase Double Emulsions with Tailored Microstructures Costabilized by Lecithin and Silica Inorganic Particles. Langmuir 2021, 37, 2843–2854. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.A.; Shah, A.G.; Wani, S.M.; Wani, I.A.; Masoodi, F.A.; Nissar, N.; Shagoo, M.A. Suitability of Different Food Grade Materials for the Encapsulation of Some Functional Foods Well Reported for Their Advantages and Susceptibility. Crit. Rev. Food Sci. Nutr. 2016, 56, 2431–2454. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, Y. Structured emulsion-based delivery systems: Controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 2010, 159, 213–228. [Google Scholar] [CrossRef]
- Chen, S.; Deng, J.; Zhang, L.-M. Cationic nanoparticles self-assembled from amphiphilic chitosan derivatives containing poly(amidoamine) dendrons and deoxycholic acid as a vector for co-delivery of doxorubicin and gene. Carbohydr. Polym. 2021, 258, 117706. [Google Scholar] [CrossRef] [PubMed]
- Uz, M.; Kalaga, M.; Pothuraju, R.; Ju, J.; Junker, W.M.; Batra, S.K.; Mallapragada, S.; Rachagani, S. Dual delivery nanoscale device for miR-345 and gemcitabine co-delivery to treat pancreatic cancer. J. Control. Release 2019, 294, 237–246. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, Z.; Lin, K.; Sun, C.; Dai, L.; Yang, S.; Mao, L.; Yuan, F.; Gao, Y. Fabrication and characterization of resveratrol loaded zein-propylene glycol alginate-rhamnolipid composite nanoparticles: Physicochemical stability, formation mechanism and in vitro digestion. Food Hydrocoll. 2019, 95, 336–348. [Google Scholar] [CrossRef]
- Yang, M.; Liu, J.; Li, Y.; Yang, Q.; Liu, C.; Liu, X.; Zhang, B.; Zhang, H.; Zhang, T.; Du, Z. Co-encapsulation of Egg-White-Derived Peptides (EWDP) and Curcumin within the Polysaccharide-Based Amphiphilic Nanoparticles for Promising Oral Bioavailability Enhancement: Role of EWDP. J. Agric. Food Chem. 2022, 70, 5126–5136. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Qing, J.; Han, Y.; McClements, D.J.; Gao, Y. Core-shell nanoparticles for co-encapsulation of coenzyme Q10 and piperine: Surface engineering of hydrogel shell around protein core. Food Hydrocoll. 2020, 103, 105651. [Google Scholar] [CrossRef]
- Bao, C.; Liu, B.; Li, B.; Chai, J.; Zhang, L.; Jiao, L.; Li, D.; Yu, Z.; Ren, F.; Shi, X.; et al. Enhanced Transport of Shape and Rigidity-Tuned α-Lactalbumin Nanotubes across Intestinal Mucus and Cellular Barriers. Nano Lett. 2020, 20, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Roblegg, E.; Fröhlich, E.; Meindl, C.; Teubl, B.; Zaversky, M.; Zimmer, A. Evaluation of a physiological in vitro system to study the transport of nanoparticles through the buccal mucosa. Nanotoxicology 2012, 6, 399–413. [Google Scholar] [CrossRef]
- Reza Baradaran, E.; Niloufar, M.; Shabnam, S.; Ali, Z.; Farid Abedin, D. Co-Delivery Nanosystems for Cancer Treatment: A Review. Pharm. Nanotechnol. 2019, 7, 90–112. [Google Scholar] [CrossRef]
- Bardania, H.; Tarvirdipour, S.; Dorkoosh, F. Liposome-targeted delivery for highly potent drugs. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1478–1489. [Google Scholar] [CrossRef]
- Thapa Magar, K.; Boafo, G.F.; Li, X.; Chen, Z.; He, W. Liposome-based delivery of biological drugs. Chin. Chem. Lett. 2022, 33, 587–596. [Google Scholar] [CrossRef]
- Tian, L.-W.; Luo, D.; Chen, D.; Zhou, H.; Zhang, X.-C.; Yang, X.-L.; Wang, Y.-L.; Liu, W. Co-delivery of bioactive peptides by nanoliposomes for promotion of hair growth. J. Drug Deliv. Sci. Technol. 2022, 72, 103381. [Google Scholar] [CrossRef]
- Liu, B.; Zan, L.; Li, X.; Cai, Y.; Hao, G. Modification of liposomes: Preparation, purpose, methods and the application in food. Int. J. Food Sci. Technol. 2024, 59, 3523–3536. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, Z.; Ju, X.; Deng, F.; Yang, F.; He, R. Co-encapsulation of rutinoside and β-carotene in liposomes modified by rhamnolipid: Antioxidant activity, antibacterial activity, storage stability, and in vitro gastrointestinal digestion. J. Food Sci. 2023, 88, 2064–2077. [Google Scholar] [CrossRef]
- Imani, S.; Alizadeh, A.; Tabibiazar, M.; Hamishehkar, H.; Roufegarinejad, L. Nanoliposomal co-encapsulation of cinnamon extract and zein hydrolysates with synergistic antioxidant activity for nutraceutical applications. Chem. Pap. 2022, 76, 2059–2069. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, J.; Jiang, J.; He, Y.; Zhang, W.; Mo, X.; Kang, X.; Xu, Q.; Wang, B.; Huang, Y. Remodeling tumor immune microenvironment (TIME) for glioma therapy using multi-targeting liposomal codelivery. J. Immunother. Cancer 2020, 8, e000207. [Google Scholar] [CrossRef]
- Joshi, S.; White, R.; Sahu, R.; Dennis, V.A.; Singh, S.R. Comprehensive Screening of Drug Encapsulation and Co-Encapsulation into Niosomes Produced Using a Microfluidic Device. Processes 2020, 8, 535. [Google Scholar] [CrossRef]
- Tavano, L.; Muzzalupo, R.; Picci, N.; de Cindio, B. Co-encapsulation of antioxidants into niosomal carriers: Gastrointestinal release studies for nutraceutical applications. Colloids Surf. B Biointerfaces 2014, 114, 82–88. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Y.; Yuan, F.; Gao, Y.; Mao, L. Effect of interfacial compositions on the physical properties of alginate-based emulsion gels and chemical stability of co-encapsulated bioactives. Food Hydrocoll. 2021, 111, 106389. [Google Scholar] [CrossRef]
- Yu, H.; Wang, H.; Su, W.; Song, Y.; Zaky, A.A.; Abd El-Aty, A.M.; Tan, M. Co-delivery of hydrophobic astaxanthin and hydrophilic phycocyanin by a pH-sensitive water-in-oil-in-water double emulsion-filled gellan gum hydrogel. Food Hydrocoll. 2022, 131, 107810. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, N.; Cheng, R.; Zhao, C.; Liu, Z.; Li, X.; Liu, J.; Tian, Z. pH multistage responsive micellar system with charge-switch and PEG layer detachment for co-delivery of paclitaxel and curcumin to synergistically eliminate breast cancer stem cells. Biomaterials 2017, 147, 53–67. [Google Scholar] [CrossRef]
- Meng, D.; Shi, L.; Zhu, L.; Wang, Q.; Liu, J.; Kong, Y.; Hou, M.; Yang, R.; Zhou, Z. Coencapsulation and Stability Evaluation of Hydrophilic and Hydrophobic Bioactive Compounds in a Cagelike Phytoferritin. J. Agric. Food Chem. 2020, 68, 3238–3249. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Tian, J.; Liu, Y.; Meng, D.; Blanchard, C.L.; Zhou, Z. One-step fabrication of phytoferritin-chitosan-epigallocatechin shell-core nanoparticles by thermal treatment. Food Hydrocoll. 2018, 80, 24–32. [Google Scholar] [CrossRef]
- Leena, M.M.; Silvia, M.G.; Vinitha, K.; Moses, J.A.; Anandharamakrishnan, C. Synergistic potential of nutraceuticals: Mechanisms and prospects for futuristic medicine. Food Funct. 2020, 11, 9317–9337. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, G.; Fan, S.; Ma, J.; Yan, Y.; Wang, M.; Tang, X.; Lv, P.; Zhang, Y. Targeted delivery of food functional ingredients in precise nutrition: Design strategy and application of nutritional intervention. Crit. Rev. Food Sci. Nutr. 2024, 64, 7854–7877. [Google Scholar] [CrossRef]
- Geng, M.; Wu, X.; Tan, X.; Li, L.; Teng, F.; Li, Y. Co-encapsulation of vitamins C and E in SPI-polysaccharide stabilized double emulsion prepared by ultrasound: Fabrication, stability, and in vitro digestion. Food Biosci. 2024, 59, 104113. [Google Scholar] [CrossRef]
- Praça, F.G.; Viegas, J.S.R.; Peh, H.Y.; Garbin, T.N.; Medina, W.S.G.; Bentley, M.V.L.B. Microemulsion co-delivering vitamin A and vitamin E as a new platform for topical treatment of acute skin inflammation. Mater. Sci. Eng. C 2020, 110, 110639. [Google Scholar] [CrossRef]
- Anzini, P.; Biganzoli, D.; Cherniukh, I.; Kovalenko, M.V.; Parola, A.; Ferri, F. Variance analysis of dynamic light scattering data. Rev. Sci. Instrum. 2023, 94, 095117. [Google Scholar] [CrossRef]
- Feng, X.; Huang, G.; Qiu, J.; Peng, L.; Luo, K.; Liu, D.; Han, P. Dynamic light scattering in flowing dispersion. Opt. Commun. 2023, 531, 129225. [Google Scholar] [CrossRef]
- Anzini, P.; Redoglio, D.; Rocco, M.; Masciocchi, N.; Ferri, F. Light Scattering and Turbidimetry Techniques for the Characterization of Nanoparticles and Nanostructured Networks. Nanomaterials 2022, 12, 2214. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov. Food Sci. Emerg. Technol. 2013, 19, 29–43. [Google Scholar] [CrossRef]
- Lu, Q.; Lu, P.-M.; Piao, J.-H.; Xu, X.-L.; Chen, J.; Zhu, L.; Jiang, J.-G. Preparation and physicochemical characteristics of an allicin nanoliposome and its release behavior. LWT-Food Sci. Technol. 2014, 57, 686–695. [Google Scholar] [CrossRef]
- Gui, C.; Zhang, Z.; Li, Z.; Luo, C.; Xia, J.; Wu, X.; Chu, J. Deep learning analysis on transmission electron microscope imaging of atomic defects in two-dimensional materials. iScience 2023, 26, 107982. [Google Scholar] [CrossRef] [PubMed]
- Falsafi, S.R.; Rostamabadi, H.; Assadpour, E.; Jafari, S.M. Morphology and microstructural analysis of bioactive-loaded micro/nanocarriers via microscopy techniques; CLSM/SEM/TEM/AFM. Adv. Colloid Interface Sci. 2020, 280, 102166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Su, D.S. Transmission Electron Microscopy and the Science of Carbon Nanomaterials. Small 2014, 10, 222–229. [Google Scholar] [CrossRef]
- Amjadi, S.; Almasi, H.; Hamishehkar, H.; Alizadeh Khaledabad, M.; Lim, L.-T. Coating of betanin and carvone Co-loaded nanoliposomes with synthesized cationic inulin: A strategy for enhancing the stability and bioavailability. Food Chem. 2022, 373, 131403. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, L.; Xu, M.; Luo, Y.; Wang, B.; Kuang, M.; Liu, X.; Sun, M.; Guo, Y.; Teng, L.; et al. Pulmonary delivery of liposomes co-loaded with SN38 prodrug and curcumin for the treatment of lung cancer. Eur. J. Pharm. Biopharm. 2022, 179, 156–165. [Google Scholar] [CrossRef]
- Rani, G.E.; Murugeswari, R.; Rajini, N. Detection of material strength of composite eggshell powders with an analysis of scanning electron microscopy. Bull. Mater. Sci. 2023, 46, 74. [Google Scholar] [CrossRef]
- Golinejad, S.; Mirjalili, M.H. Fast and cost-effective preparation of plant cells for scanning electron microscopy (SEM) analysis. Anal. Biochem. 2020, 609, 113920. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, C.-T.; Xu, Q.; Lou, Z.; Xu, Z.; Thierry, B.; Gu, N. Gold Nanoparticle Probe-Assisted Antigen-Counting Chip Using SEM. ACS Appl. Mater. Interfaces 2019, 11, 6769–6776. [Google Scholar] [CrossRef]
- Huang, Y.; Zhan, M.; Sun, H.; Zhang, C.; Shen, M.; Ma, J.; Zhang, G.; Shi, X. Electrosprayed core–shell microspheres co-deliver fibronectin and resveratrol for combined treatment of acute lung injury. J. Colloid Interface Sci. 2025, 686, 498–508. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Liu, Q.; Lu, S.; Chen, X.; Xu, W.; Shi, F. Co-delivery of bufalin and nintedanib via albumin sub-microspheres for synergistic cancer therapy. J. Control. Release 2021, 338, 705–718. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Menta, M.; Dacoba, T.G.; Crecente-Campo, J.; Alonso, M.J.; Dupin, D.; Loinaz, I.; Grassl, B.; Séby, F. Advanced nanomedicine characterization by DLS and AF4-UV-MALS: Application to a HIV nanovaccine. J. Pharm. Biomed. Anal. 2020, 179, 113017. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Jarand, C.W.; Brader, M.L.; Reed, W.F. Angle-dependent effects in DLS measurements of polydisperse particles. Meas. Sci. Technol. 2022, 33, 045202. [Google Scholar] [CrossRef]
- Ngo, D.-T.; Kuhn, L.T. In situ transmission electron microscopy for magnetic nanostructures. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 045001. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, M.; Tai, X.; Li, H.; Han, X.; Yu, J. Analytical transmission electron microscopy for emerging advanced materials. Matter 2021, 4, 2309–2339. [Google Scholar] [CrossRef]
- Sun, W.; Xu, Y.; Zhou, Y.; Zeng, Z.; Wang, L.; Ouyang, J. Topographic Scanning Electronic Microscopy Reveals the 3D Surface Structure of Materials. Adv. Funct. Mater. 2024, 35, 2420372. [Google Scholar] [CrossRef]
- Zhou, W.; Greer, H.F. What Can Electron Microscopy Tell Us Beyond Crystal Structures? Eur. J. Inorg. Chem. 2016, 2016, 941–950. [Google Scholar] [CrossRef]
- Lin, X.; Liu, J.; Wu, G.; Yang, X.; Yan, W.; Fan, N.; Li, H. Enhanced chemotherapy response in hepatocellular carcinoma: Synergistic effects of miR-122 and doxorubicin co-delivery system inducing apoptosis and DNA damage. Cancer Nanotechnol. 2024, 15, 48. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, S.; Zhang, L.; Dai, L.; Tai, K.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y.; Mackie, A. Fabrication, characterization and in vitro digestion of food grade complex nanoparticles for co-delivery of resveratrol and coenzyme Q10. Food Hydrocoll. 2020, 105, 105791. [Google Scholar] [CrossRef]
- Yang, Z.; McClements, D.J.; Peng, X.; Qiu, C.; Long, J.; Zhao, J.; Xu, Z.; Meng, M.; Chen, L.; Jin, Z. Co-encapsulation of quercetin and resveratrol in zein/carboxymethyl cellulose nanoparticles: Characterization, stability and in vitro digestion. Food Funct. 2022, 13, 11652–11663. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhu, J.; Pan, Y.; Huang, Q. Assessment of dynamic bioaccessibility of curcumin encapsulated in milled starch particle stabilized Pickering emulsions using TNO’s gastrointestinal model. Food Funct. 2019, 10, 2583–2594. [Google Scholar] [CrossRef] [PubMed]
- Effinger, A.; McAllister, M.; Tomaszewska, I.; O’Driscoll, C.M.; Taylor, M.; Gomersall, S.; Heaton, J.; Smith, K.L.; Sarcevica, I.; Young, S.L.; et al. Investigating the Impact of Crohn’s Disease on the Bioaccessibility of a Lipid-Based Formulation with an In Vitro Dynamic Gastrointestinal Model. Mol. Pharm. 2021, 18, 1530–1543. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, R.; Xiu, H.; Feng, J.; Jin Park, H.; Prabhakar, H.; Kong, F. Effect of cinnamon on starch hydrolysis of rice pudding: Comparing static and dynamic in vitro digestion models. Food Res. Int. 2022, 161, 111813. [Google Scholar] [CrossRef]
- Zheng, H.; Wijaya, W.; Zhang, H.; Feng, K.; Liu, Q.; Zheng, T.; Yin, Z.; Cao, Y.; Huang, Q. Improving the bioaccessibility and bioavailability of carnosic acid using a lecithin-based nanoemulsion: Complementary in vitro and in vivo studies. Food Funct. 2020, 11, 8141–8149. [Google Scholar] [CrossRef]
- Garvey, S.M.; Madden, E.N.; Qu, Y.; Best, C.H.; Tinker, K.M. The Effects of a Microbial Enzyme Mixture on Macronutrient Hydrolysis in a Static Simulation of Oro-Gastric Digestion That Models Human Digestive Senescence. Foods 2025, 14, 937. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y.; Wang, Z.; Zhang, Y.; Guo, W.; Li, S.; Wang, X.; Mao, Y.; Wang, S. A new intracellularly regulated release pattern controlled by coordinated carrier cracking and drug release. Chem. Eng. J. 2024, 497, 154514. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef]
- Askarizadeh, M.; Esfandiari, N.; Honarvar, B.; Sajadian, S.A.; Azdarpour, A. Kinetic Modeling to Explain the Release of Medicine from Drug Delivery Systems. ChemBioEng Rev. 2023, 10, 1006–1049. [Google Scholar] [CrossRef]
- Xu, X.; Shan, G.R.; Pan, P. Controlled co-delivery of hydrophilic and hydrophobic drugs from thermosensitive and crystallizable copolymer nanoparticles. J. Appl. Polym. Sci. 2016, 133, 44132. [Google Scholar] [CrossRef]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef] [PubMed]
- Sakellari, G.I.; Zafeiri, I.; Pawlik, A.; Kurukji, D.; Taylor, P.; Norton, I.T.; Spyropoulos, F. Independent co-delivery of model actives with different degrees of hydrophilicity from oil-in-water and water-in-oil emulsions stabilised by solid lipid particles via a Pickering mechanism: A-proof-of-principle study. J. Colloid Interface Sci. 2021, 587, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Polo, J.; Soto, A.; Zamorano, M.; Silva-Weiss, A.; Oyarzun-Ampuero, F.A.; Brossard, N.; Fuentes, J.; Osorio, F.A. Effect of the incorporation of liposomes loaded with rutin on the transport properties of edible film produced with hydroxypropyl methylcellulose: An in vitro release study. LWT 2024, 191, 115583. [Google Scholar]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Spray-drying of oil-in-water emulsions for encapsulation of retinoic acid: Polysaccharide- and protein-based microparticles characterization and controlled release studies. Food Hydrocoll. 2022, 124, 107193. [Google Scholar] [CrossRef]
- Jiang, Y.; Zang, K.; Xu, L.; Zeng, X.-A.; Li, H.; Brennan, C.; Zhao, D.; Sun, J. Co-delivery of riboflavin and rhein based on properties improved Jiuzao glutelin: Binding mechanism, stability, and antioxidant activities. J. Mol. Liq. 2022, 367, 120490. [Google Scholar] [CrossRef]
- Xinyi, W.; Shujun, F.U.; Xiangjun, M.; Wei, W.U.; Jingkai, G.U. Research Progress of New Methods and Technologies for in Vivo Fate Analysis of Nanocarrier Drug Delivery Systems. Prog. Pharm. Sci. 2024, 48, 747–760. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, X.; Chen, N.; Yang, X.; Tang, F. Recent Advance of Liposome Nanoparticles for Nucleic Acid Therapy. Pharmaceutics 2023, 15, 178. [Google Scholar] [CrossRef]
- Sun, C.; Lu, J.; Wang, J.; Hao, P.; Li, C.; Qi, L.; Yang, L.; He, B.; Zhong, Z.; Hao, N. Redox-sensitive polymeric micelles with aggregation-induced emission for bioimaging and delivery of anticancer drugs. J. Nanobiotechnol. 2021, 19, 14. [Google Scholar] [CrossRef]
- Laskar, P.; Dhasmana, A.; Kotnala, S.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Glutathione-Responsive Tannic Acid-Assisted FRET Nanomedicine for Cancer Therapy. Pharmaceutics 2023, 15, 1326. [Google Scholar] [CrossRef]
- Ilgin, P.; Ozay, H.; Ozay, O. A new dual stimuli responsive hydrogel: Modeling approaches for the prediction of drug loading and release profile. Eur. Polym. J. 2019, 113, 244–253. [Google Scholar] [CrossRef]
- Jie, Y.; Chen, F. Progress in the Application of Food-Grade Emulsions. Foods 2022, 11, 2883. [Google Scholar] [CrossRef]
- Ghorbanzade, T.; Jafari, S.M.; Akhavan, S.; Hadavi, R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. 2017, 216, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Oliver, M.; Ponce-Alquicira, E. The Role of Microencapsulation in Food Application. Molecules 2022, 27, 1499. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, B.; Sharma, A.; Akshit, F.N.U.; Mohan, M.S.; Salunke, P.; Anand, S. A review of oleogels applications in dairy foods. Crit. Rev. Food Sci. Nutr. 2024, 64, 9691–9709. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Vahabi, S.; Eatemadi, A. Nanoliposome encapsulated anesthetics for local anesthesia application. Biomed. Pharmacother. 2017, 86, 1–7. [Google Scholar] [CrossRef]
- Bah, M.G.; Bilal, H.M.; Wang, J. Fabrication and application of complex microcapsules: A review. Soft Matter 2020, 16, 570–590. [Google Scholar] [CrossRef]
- Alpaslan, D.; Olak, T.; Turan, A.; Ersen Dudu, T.; Aktas, N. Use of Coconut Oil-Based Organo-Hydrogels in Pharmaceutical Applications. J. Polym. Environ. 2022, 30, 666–680. [Google Scholar] [CrossRef]
- Tao, J.; Chow, S.F.; Zheng, Y. Application of flash nanoprecipitation to fabricate poorly water-soluble drug nanoparticles. Acta Pharm. Sin. B 2019, 9, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Dong, Y.; Liu, Z.; Li, Z.; Shao, J.; Li, M.; Yue, X. A Review of Plant-Based Drinks Addressing Nutrients, Flavor, and Processing Technologies. Foods 2023, 12, 3952. [Google Scholar] [CrossRef]
- Aditya, N.P.; Aditya, S.; Yang, H.-J.; Kim, H.W.; Park, S.O.; Lee, J.; Ko, S. Curcumin and catechin co-loaded water-in-oil-in-water emulsion and its beverage application. J. Funct. Foods 2015, 15, 35–43. [Google Scholar] [CrossRef]
- Huang, E.; Quek, S.Y.; Fu, N.; Wu, W.D.; Chen, X.D. Co-encapsulation of coenzyme Q10 and vitamin E: A study of microcapsule formation and its relation to structure and functionalities using single droplet drying and micro-fluidic-jet spray drying. J. Food Eng. 2019, 247, 45–55. [Google Scholar] [CrossRef]
- Colín-Cruz, M.A.; Pimentel-González, D.J.; Carrillo-Navas, H.; Alvarez-Ramírez, J.; Guadarrama-Lezama, A.Y. Co-encapsulation of bioactive compounds from blackberry juice and probiotic bacteria in biopolymeric matrices. LWT 2019, 110, 94–101. [Google Scholar] [CrossRef]
- Wang, J.; Shang, M.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Ji, H.; Jin, Z.; et al. Polysaccharide-based colloids as fat replacers in reduced-fat foods. Trends Food Sci. Technol. 2023, 141, 104195. [Google Scholar] [CrossRef]
- Zhou, D.-W.; Wang, K.; Zhang, Y.-A.; Ma, K.; Yang, X.-C.; Li, Z.-Y.; Yu, S.-S.; Chen, K.-Z.; Qiao, S.-L. mRNA therapeutics for disease therapy: Principles, delivery, and clinical translation. J. Mater. Chem. B 2023, 11, 3484–3510. [Google Scholar] [CrossRef]




| Active Substance | Structural Formula | Characteristic | Function | Bioavailability | Stability | References |
|---|---|---|---|---|---|---|
| EGCG |  | Hydrophilic | Anticancer | -- | Thermal stability and UV light stability | [19] |
| Tea polyphenols | 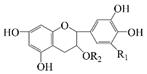 | Hydrophilic | Antioxidant and anticarcinogenic | -- | Storage stability | [20] |
| Procyanidins |  | Hydrophilic | Antibiotic, antioxidant, and anti-inflammatory | -- | -- | [21] |
| Ellagic acid | 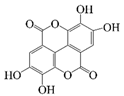 | Hydrophobic | Antidiabetic, antioxidant, and anti-inflammatory | -- | Storage stability | [22] |
| Resveratrol | 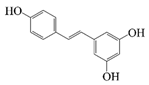 | Hydrophobic | Anti-inflammatory, antioxidant, anticancer, liver-protective | -- | pH stability, salt stability, and thermal stability | [23] |
| Quercetin | 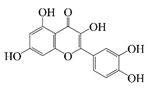 | Hydrophobic | Antioxidant, anti-inflammatory and antibacterial activities, improvement of cardiovascular health | -- | Storage stability | [24] |
| Curcumin | 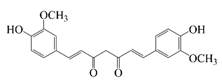 | Hydrophobic | Reduce blood lipid levels, antitumor activity, anti-inflammatory activity, antioxidant activity, and inhibition of Alzheimer’s disease | 87.3 ± 2.8% | pH stability | [25] |
| VCAM-1 siRNA | -- | Hydrophilic | anti-inflammatory | -- | -- | [26] |
| Berberine |  | Hydrophobic | Anticholinergic, antihypertensive, antibacterial, anti-inflammatory, and antioxidative | -- | Storage stability | [27] |
| Paclitaxel | 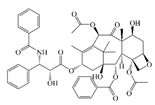 | Hydrophobic | Glioma | -- | -- | [28] |
| Lactobacillus casei | -- | Hydrophilic | Maintain gut balance by shaping gut microbiota and intestinal barrier | -- | Thermal stability, and storage stability | [29] |
| Probiotics | -- | -- | Regulate the intestinal microbiota | -- | Photochemical stability and storage stability | [30] |
| Vitamin C | 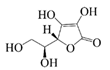 | Hydrophilic | Antioxidant | -- | Storage stability | [31] |
| Retinoic acid |  | Hydrophobic | Acute promyelocytic leukemia | -- | -- | [32] |
| Vitamin B12 |  | Hydrophobic | -- | 99% | Storage stability | [33] |
| Vitamin D3 |  | Hydrophobic | Bone development and bone health | 97% | Storage stability | [33] |
| Astaxanthin | 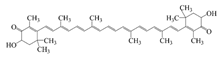 | Hydrophobic | Anti-oxidative, anti-inflammatory, and anti-apoptotic | 43.2 ± 0.3% | Freeze–thaw stability | [31,34] |
| Lycopene | 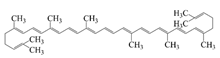 | Hydrophobic | Antioxidant and cholesterol metabolism | -- | Storage stability | [35] |
| β-Carotene |  | Hydrophobic | Antioxidant | -- | Storage stability | [31] |
| Cinnamon oleoresin | -- | Hydrophobic | Antimicrobial | -- | Storage stability | [36] |
| Cinnamaldehyde essential oil | -- | Hydrophobic | Antimicrobial | -- | Storage stability | [37] |
| Tuna fish oil | -- | Hydrophobic | Health-promoting benefits and disorder-prevention attributes | -- | Oxidative stability | [38] |
| Coenzyme Q10 |  | Hydrophobic | -- | -- | Storage stability | [20] |
| Phycocyanin | -- | Hydrophilic | Hepatoprotective, neuroprotective, and antitumor | 41.6 ± 0.5% | Freeze–thaw stability | [31,34] |
| Glutathione (γ-L-glutamyl-l-cysteinylglycine | -- | Hydrophilic | Antioxidant | -- | Storage stability | [5] |
| Melittin | -- | Hydrophilic | Glioma | -- | -- | [28] |
| Nisin | -- | -- | Antimicrobial | -- | -- | [39] |
| Carrier Type | Active Substances | Materials | Preparation Methods | Encapsulation Efficiency | Bioavailability | Precision Nutrition | References |
|---|---|---|---|---|---|---|---|
| O/W emulsion | α-tocopherol and resveratrol | Sodium caseinate and sunflower oil | High-speed blend, high-pressure homogenize | 92–96% | 40%, 90% | -- | [7] |
| Pickering emulsion | EGCG and Cur | MCT, WPI, and d-lactose | High-speed homogenize | -- | -- | -- | [49] |
| Pickering emulsion | EGCG and lycopene | Soybean oil, PGPR, and bacterial cellulose | Magnetic stir, high-speed homogenize | -- | -- | Hypolipidemic | [35] |
| Pickering emulsion | Betanin and Cur | Gelatin and PGPR | -- | 65.3%, 84.1% | 42.7%, 53.5% | Antitumor | [90] |
| Pickering emulsions | Cur and β-carotene | Zein, MCT, and tea saponin | Microfluidization | 97.58 ± 0.12%, 96.49 ± 0.46% | 75.33 ± 5.11%, 19.46 ± 0.59% | -- | [91] |
| W/O/W emulsion | Vitamin C and β-carotene | Soybean oil, PGPR, and sipunculus nudus | Magnetic stir, high-speed homogenize | 91.2%, 99.8% | -- | -- | [31] |
| W/O/W emulsion | Insulin and quercetin | Tween 80 and soybean oil | Stir, ultra-turrax, shear | 95.7%, 93.4% | 52.33%, 58.7% | Diabetes | [92] |
| W/O/W emulsion | Cur and catechin | Olive oil | Two-step emulsification method | 88–97% | 54% | -- | [9] |
| W/O/W emulsion | Cur and EGCG | Protein fibril-cellulose, cellulose nanocrystals, and PGPR | Two-step emulsification method | 98.0 ± 1.2%, 89.7 ± 0.3% | 67.8%, 68.9% | -- | [52] |
| Nanoliposome | Coenzyme Q10 and α-lipoic acid | CS, Soy phosphatidylcholine, agar, cholesterol, and Tween 80 | Magnetic stir, inject | -- | -- | -- | [6] |
| Nanoliposome | Reduced glutathione and caffeic acid | CS, soybean lecithin, and cholesterol | Two-step emulsification procedure | 61.32%, 68.92% | -- | -- | [5] |
| Nanoliposome | Vitamin C and β-carotene | Egg yolk phosphatidylcholine and cholesterol | Ethanol injection method | 77.90 ± 1.92%, 97.91 ± 0.20% | -- | -- | [83] |
| Nanoliposome | Cur and resveratrol | Egg yolk phosphatidylcholine | Thin-film evaporation method | 80.42 ± 2.12% | -- | -- | [70] |
| Nanoliposome | Coenzyme Q10 and Cur | CS | -- | 98.5% | -- | -- | [86] |
| Nanoliposome | Vitamin B2, vitamin E, and β-carotene | Lecithin from soybean and cholesterol | Thin-layer dispersion method | -- | -- | -- | [82] |
| Nanoliposome | Trans-resveratrol and naringenin | Egg yolk phosphatidylcholine and Tween 80 | Thin-film evaporation method | 77.72 ± 2.42%, 61.98 ± 1.68% | -- | Antioxidant | [93] |
| Nanoliposome | Shikonin and anthracyclines | Hydrogenated soybean phosphatidylcholine, cholesterol, and DSPE-PEG2000 | Thin-film hydration method | 95% | -- | Antitumor | [94] |
| Nanoliposome | Lupulon and xanthohumol | Egg yolk lecithin | Sonication method | 81.7%, 97.13% | -- | Antimicrobial | [95] |
| Nanoparticle | Cur and serrati peptidase | BSA | Desolvation method | 80%, 8.4% | -- | Anticancer | [96] |
| Nanoparticle | Cur and EGCG | HA and mPEG5000-NHS | Stir | 46.01 ± 1.96, 67.76 ± 6.67% | -- | Anticancer | [97] |
| Nanoparticle | Cur and PC | SPI | Flash nanoprecipitation | 95% | -- | -- | [21] |
| Nanoparticle | α-Tocopherol, resveratrol, and EGCG | BSA | Mix | -- | -- | Antioxidant | [98] |
| Nanoparticle | Cur and EGCG | Yeast protein | Stir | -- | -- | -- | [99] |
| Nanoparticle | FUC and Cur | Gliadin, CS hydrochloride, and CMK | Stir | 96.3%, 72.8% | -- | Colon-targeted | [64] |
| Nanoparticle | Ellagic acid and anti-inflammatory peptide | CS, TPP, Dex, and LPS | Ionic gelation method | -- | -- | Anti-inflammatory | [22] |
| Nanoparticle | Resveratrol and vitamin D3 | Olive oil | pH shift combined with heat treatment process | 86.74%, 53.24% | 81.2%, 93.2% | -- | [100] |
| Nanoparticle | Procyanidin B2, dihydromyricetin | β-lactoglobulin | -- | -- | -- | -- | [43] |
| Nanoparticle | Egg white-derived peptides and Cur | β-Cyclodextrin and HTCC | Spontaneous self-assembly | 94.7–98.9% | -- | -- | [101] |
| Nanoparticle | Cur and doxorubicin hydrochloride | Tyrosine, PASP, HA, and EDA | -- | Loading capacity: 50.9 ± 4.3%, 26.0 ± 1.9% | -- | Anticancer | [102] |
| Nanoparticle | Cur and berberine | Zein and CS | Stir | 75%, 60% | -- | -- | [27] |
| Microcapsule | Novel bacteriocin lactococcin036019, and vitamin C | S. Aureus cmcc 26003 | High-speed disperse, spray-dry | 56.18% | -- | Antibacterial | [103] |
| Microcapsule | Cur, quercetin, tea polyphenols, and lyophilized lactobacillus casei | Zein, CS, and rutin | Anti-solvent process, coacervate, | 68.44 ± 1.3%%, 57.35%, 58.13% | -- | -- | [29] |
| Microcapsule | RA, Cur, and resveratrol | Alginic acid sodium salt and coconut oil | Spray-dry | 96 ± 5%, 92 ± 4%, 93 ± 4% | -- | -- | [32] |
| Microcapsule | Xylitol and menthol | Pork gelatin type B, gum Arabic, corn oil, and PGPR | Spray-dry | -- | -- | -- | [104] |
| Microcapsule | Cur and pancreatic beta cells | Alginate | Jet-break regime of the syringe pump | -- | -- | Diabetes | [105] |
| Microcapsule | Resveratrol and fish oil | Whey protein, gum Arabic, and transglutaminase | Spray-dry | 84–88% | -- | -- | [106] |
| Microcapsule | Paprika and cinnamon oleoresin | WPI and maltodextrin | Spray-dry | 83% | -- | -- | [36] |
| Microcapsule | Resveratrol and piperine | Hydroxypropyl β-cyclodextrin | Spray-dry | -- | -- | -- | [107] |
| Microcapsule | Anthocyanins and α-tocopherol | Gum Arabic | Spray-dry | -- | -- | -- | [59] |
| Emulsion gels | Astaxanthin and phycocyanin | Beeswax, gelatin, sodium caseinate, sodium alginate, low acylated gellan gum, and corn oil | Fast digital high-speed shear, homogenize | 96.4 ± 0.9%; 94.9 ± 2.1% | 43.2 ± 0.3%; 41.6 ± 0.5% | -- | [34] |
| Emulsion gels | Quercetin and EGCG | PGPR, corn oil, wheat gluten, and gelatin | Antisolvent precipitation method, high shear mix | 65.5%, 97.2% | 48.4%, 49% | -- | [55] |
| Hydrogel | EGCG and Cur | Defatted soy flour | Heat followed by ice water bath | 97.71%, 91.02% | -- | Anticancer | [19] |
| Hydrogel | Cur and resveratrol | Zein, gelatin, gellan gum, and polyethylene glycol | Stir under heating followed by cooling at room temperature | 68.33%, 83.39% | 82%, 79% | -- | [108] |
| Hydrogel | Cur and resveratrol | Iota-carrageenan | -- | -- | -- | -- | [109] |
| Bigel | EGCG and Cur | Corn oil, gelatin, and Tween 20 | Stir | -- | -- | -- | [56] |
| Bigel | EGCG and Cur | Corn oil, κ-carrageenan, monoglycerides, Tween 20, and PGPR | Stir | -- | -- | Colon adenocarcinoma | [110] |
| Oleogel | Cur and resveratrol | Gelatin, gellan gum, and MCT | Stir | 80.06 ± 1.37%, 86.41 ± 2.28% | 50.08 ± 1.27%, 89.19 ± 0.89% | -- | [65] |
| Nanogel | Cur and doxorubicin | NIPAAm, TEGDMA, PPS, and PVA | Free radical polymerization methods, stir | 96%, 98% | -- | Colon cancer | [111] |
| Lipodisk | Paclitaxel and melittin | POPC, DSPE-PEG2000, and cholesterol | Film hydration adsorption method | -- | -- | Anti-glioma effect | [28] |
| β-cyclodextrin | Trans-ferulic acid and gallic acid | Hydroxypropyl-β-cyclodextrin | Spray-dry | 89.22%, 68.06% | -- | -- | [112] |
| β-cyclodextrin | Resveratrol and piperine | Hydroxypropyl-β-cyclodextrin | Spray-dry | -- | -- | -- | [107] |
| Micelle | Doxorubicin and paclitaxe | Folate, oxidized dextra, NHS | Schiff’s base reaction | -- | -- | Anticancer | [113] |
| Micelle | Doxorubicin and sipd-l1 | CS and HA | Nanoprecipitation technique | 91.3 ± 2.1% | -- | Antitumor | [114] |
| Hydrogel beads | EGCG and quercetin | Soybean protein isolate, PGPR, sodium alginate | Stir, inject | 98.75 ± 0.04%, 96.46 ± 0.76% | -- | IBD | [115] |
| Ca(ii)-alginate beads | Betalains, phenolic compounds | Egg albumin, whey protein | Ionic gelation | 70% | -- | -- | [116] |
| Copolymer | Polycaprolactone, poly (2-ethyl 2-oxazoline) | Starch | -- | -- | -- | Anticancer | [117] |
| Niosome | Cur and boswellic acids | CS | -- | -- | 79.02 ± 0.13%, 81 ± 0.10% | -- | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, C.; Tang, J.; Cao, L.; Fan, M.; Lin, X.; Liu, G.; Liang, L.; Liu, X.; Zhang, J.; Li, Y.; et al. Strategic Approaches for Co-Encapsulation of Bioactive Compounds: Technological Advances and Mechanistic Insight. Foods 2025, 14, 2024. https://doi.org/10.3390/foods14122024
Wen C, Tang J, Cao L, Fan M, Lin X, Liu G, Liang L, Liu X, Zhang J, Li Y, et al. Strategic Approaches for Co-Encapsulation of Bioactive Compounds: Technological Advances and Mechanistic Insight. Foods. 2025; 14(12):2024. https://doi.org/10.3390/foods14122024
Chicago/Turabian StyleWen, Chaoting, Jialuo Tang, Liyan Cao, Meidi Fan, Xinying Lin, Guoyan Liu, Li Liang, Xiaofang Liu, Jixian Zhang, Youdong Li, and et al. 2025. "Strategic Approaches for Co-Encapsulation of Bioactive Compounds: Technological Advances and Mechanistic Insight" Foods 14, no. 12: 2024. https://doi.org/10.3390/foods14122024
APA StyleWen, C., Tang, J., Cao, L., Fan, M., Lin, X., Liu, G., Liang, L., Liu, X., Zhang, J., Li, Y., & Xu, X. (2025). Strategic Approaches for Co-Encapsulation of Bioactive Compounds: Technological Advances and Mechanistic Insight. Foods, 14(12), 2024. https://doi.org/10.3390/foods14122024






