Antioxidant Potential of Jostaberry Phytochemicals Encapsulated in Biopolymer Matrices During Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Vegetable Material and Carrier Encapsulation Agents
| Biopo2lymers | Characteristics |
|---|---|
| Maltodextrin | Maltodextrin with a food-grade DE of 18, certified under ISO 9001:2015 [34] and FSSC 22000, was supplied by Interstarch, Ukraine. It is a water-soluble polysaccharide produced by the partial enzymatic hydrolysis of starch. Recognized as a natural food additive, maltodextrin’s higher DE value corresponds to faster assimilation by the human body. As a carrier agent, it helps reduce the stickiness of plant extract powders during drying [35]. |
| Nutriose | Water-soluble dextrin, derived from highly branched corn starch, was marketed under the trade name Nutriose® by Gesundo, GM Gesundheits Manufactur GmbH, Germany. It is a highly branched polysaccharide composed of glucose units linked by 1–6, 1–2, and 1–3 glycosidic bonds. Due to this chemical structure, only approximately 10–15% of the resistant dextrin units are broken down in the stomach and small intestine, while the remainder is progressively fermented in the colon, providing a prebiotic effect [36]. Supplementation with Nutriose for 12 weeks has been shown to promote satiety, improve intestinal transit, and reduce body weight [37]. |
| Sodium alginate | Sodium alginate (100% food grade) was supplied by Shandong Jiejing Group Corporation, China. It is a natural anionic polysaccharide biopolymer derived from brown marine algae. Sodium alginate is widely used for its ability to eliminate toxins and heavy metals from the body [38], and as a carrier for drugs and bioactive substances due to its excellent biocompatibility [39]. |
| Pectin | Commercial pectin GRINDSTED® Pectin MRS 351 from DuPont Danisco (DE > 60%) is a heteropolysaccharide and a natural food additive (E440), serving as a source of soluble dietary fiber. It is obtained from fruits or vegetables. Pectin can protect encapsulated materials during digestion due to its resistance to proteases and amylase, which are active in the upper gastrointestinal tract. Partially degraded by colonic microflora, pectin exhibits a prebiotic effect, helps eliminate toxins, reduces cholesterol, and has anti-inflammatory properties [40]. Additionally, it serves as a carrier for medications and BACs [41]. |
2.3. Obtaining JE for Encapsulation
2.4. The Microencapsulation and Freeze-Drying Process
2.5. Physicochemical Analysis of Jostaberries and Microparticles
2.6. Evaluation of Color Parameters
2.7. Microparticles Production Efficiency (MPE)
2.8. Bulk Density (BD), TSS, Hygroscopicity, pH, Titrable Acidity (TA)
2.9. Solubility and Oil Holding Capacity (OHC)
2.10. Phytochemical Composition and AA
2.10.1. BAC Extraction for Spectrophotometric Analysis
2.10.2. Determination of TPC and Total Flavonoid Content (TFC)
2.10.3. Determination of TAC
2.10.4. Retention Efficiency (RE) of Polyphenols and Antocyanins
2.10.5. Assessment of DPPH Free Radical Scavenging Activity
2.10.6. Assessment of ABTS Free Cation-Radical Scavenging Activity
2.10.7. HPLC-PDA Detection
2.10.8. Quantitative Analysis of Organic Acids
2.11. Microstructure Analysis of Microparticles
2.12. Statistical Analysis
3. Results and Discussion
3.1. Jostaberry and Jostaberry Extracts Properties
| Indices | Samples | |
|---|---|---|
| FJ | FDJ | |
| DW, % | 19.59 ± 0.06 a | 93.42 ±0.02 b |
| FC, % | 0.58 ± 0.01 a | 1.99 ± 0.02 b |
| AC, % | 0.81 ± 0.02 a | 3.57 ± 0.03 b |
| TPC, mg GAE/g DW | 18.67 ± 0.32 a | 21.37 ± 0.16 b |
| TFC, mg RuE/g DW | 4.09 ± 0.04 b | 3.00 ± 0.08 a |
| TFC, mg QE/g DW | 2.09 ± 0.01 b | 1.59 ± 0.03 a |
| TAC, mg Cy3GE/g DW | 11.24 ± 0.07 b | 7.00 ± 0.05 a |
| AA by DPPH, mg TE/g DW | 29.47 ± 0.22 b | 7.80 ±0.16 a |
| AA by ABTS, mg TE/g DW | 82.96 ± 0.56 b | 36.76 ± 0.34 a |
3.2. Freeze-Dried Microparticles Preparation and Characterization
3.3. Color Analysis
3.4. Biological Value and Antioxidant Potential of Microparticles
- (a)
- Hydrogen bonds between the hydroxyl (–OH) and carboxyl (–COOH) groups of polyphenols (e.g., caffeic and chlorogenic acids), the hydroxyl groups of anthocyanins, and the hydroxyl groups of polysaccharides, such as maltodextrin and resistant dextrin, as well as the carboxyl groups of anionic polysaccharides, such as pectin and sodium alginate.
- (b)
- Electrostatic interactions, especially between the protonated forms of anthocyanins in acidic media, which may be stronger with sodium alginate (–COONa), thus enhancing retention and protection under acidic conditions.
- (c)
- Hydrophobic interactions, where hydrophobic regions of bioactive compounds associate with less polar regions of the wall encapsulating materials, such as resistant dextrin.
3.4.1. Total Phenolic, Total Anthocyanin Content and Antioxidant Activity
3.4.2. Retention Efficiency and Storage Stability of Microencapsulated Phytochemicals from Jostaberry
3.5. Microstructure of Microparticles
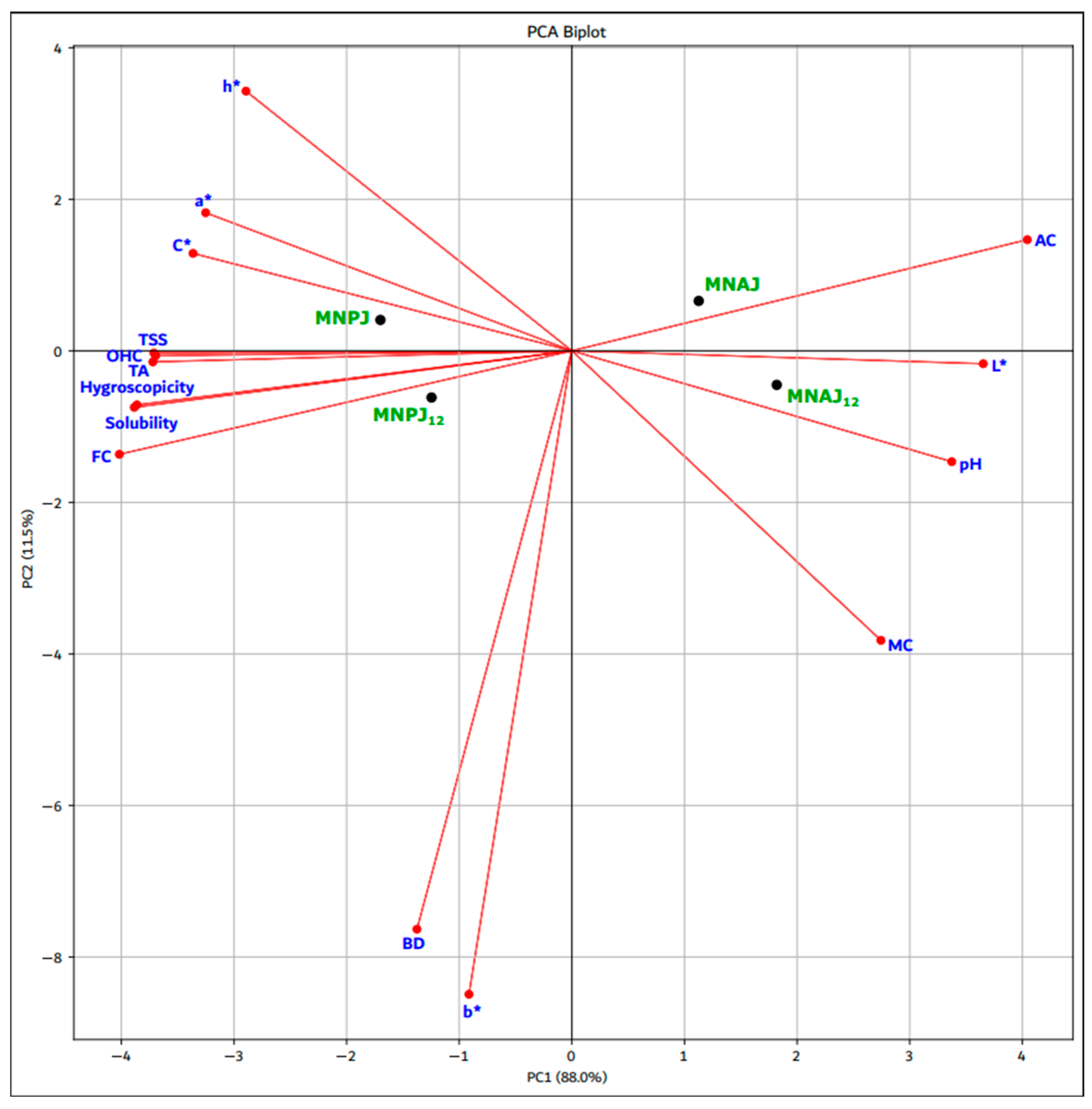
3.6. Relationship Between Physicochemical Characteristics, pH and Color Parameters
4. Limitations of the Study
5. Conclusions
6. Future Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives (Text with EEA Relevance). Current Version (16/12/2024); ELI. Available online: http://data.europa.eu/eli/reg/2008/1333/2023-10-05 (accessed on 2 March 2025).
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Cianciosi, D.; Simal-Gándara, J.; Forbes-Hernández, T.Y. The importance of berries in the human diet. Med. J. Nutr. Metab. 2019, 12, 335–340. [Google Scholar] [CrossRef]
- Bauer, A. New results of breeding Ribes nidigrolaria: Amphidiploid species hybrids between blackcurrant and gooseberry. ISHS Acta Hortic. 1986, 183, 107–110. [Google Scholar] [CrossRef]
- Bulgaru, V.; Gurev, A.; Baerle, A.; Dragancea, V.; Balan, G.; Cojocari, D.; Sturza, R.; Soran, M.-L.; Ghendov-Mosanu, A. Phytochemical, Antimicrobial, and Antioxidant Activity of Different Extracts from Frozen, Freeze-Dried, and Oven-Dried Jostaberries Grown in Moldova. Antioxidants 2024, 13, 890. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC—MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; You, L.; Zhao, Y.; Chang, X. Wild Lonicera caerulea berry polyphenol extract reduces cholesterol accumulation and enhances antioxidant capacity in vitro and in vivo. Food Res. Int. 2018, 107, 73–83. [Google Scholar] [CrossRef]
- Ribera-Fonseca, A.; Jiménez, D.; Leal, P.; Riquelme, I.; Roa, J.C.; Alberdi, M.; Peek, R.M.; Reyes-Díaz, M. The anti-proliferative and anti-invasive effect of leaf extracts of blueberry plants treated with methyl jasmonate on human gastric cancer in vitro is related to their antioxidant properties. Antioxidants 2020, 9, 45. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Fumagalli, M.; Dell’Agli, M. Berries: Gastrointestinal Protection Against Oxidative Stress and Inflammation; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128093009. [Google Scholar]
- Kranz, S.; Guellmar, A.; Olschowsky, P.; Tonndorf-Martini, S.; Heyder, M.; Pfister, W.; Reise, M.; Sigusch, B. Antimicrobial Effect of Natural Berry Juices on Common Oral Pathogenic Bacteria. Antibiotics 2020, 9, 533. [Google Scholar] [CrossRef]
- Jeon, Y.-D.; Kang, S.-H.; Moon, K.-H.; Lee, J.-H.; Kim, D.-G.; Kim, W.; Kim, J.-S.; Ahn, B.-Y.; Jin, J.-S. The Effect of Aronia Berry on Type 1 Diabetes In Vivo and In Vitro. J. Med. Food 2018, 21, 244–253. [Google Scholar] [CrossRef]
- Li, H.; Zheng, T.; Lian, F.; Xu, T.; Yin, W.; Jiang, Y. Anthocyanin-rich blueberry extracts and anthocyanin metabolite proto-catechuic acid promote autophagy-lysosomal pathway and alleviate neurons damage in in vivo and in vitro models of Alz-heimer’s disease. Nutrition 2022, 93, 111473. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacol. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Bakowska, A.M.; Kucharska, A.Z.; Oszmianski, J. The effects of heating, UV irradiation and storage on stability of anthocyanin-polyphenol copigment complex. Food Chem. 2003, 81, 349–355. [Google Scholar] [CrossRef]
- Wijesekara, T.; Xu, B. A critical review on the stability of natural food pigments and stabilization techniques. Food Res. Int. 2024, 179, 114011. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lin, B.; Hao, P.; Yi, K.; Li, X.; Hua, S. Multi-Omics Analysis Reveals That Anthocyanin Degradation and Phytohormone Changes Regulate Red Color Fading in Rapeseed (Brassica napus L.) Petals. Int. J. Mol. Sci. 2024, 25, 2577. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, K.; Andrzejewska, J.; Klóska, Ł. Influence of freezing, lyophilisation and air-drying on the total monomeric anthocyanins, vitamin C and antioxidant capacity of selected berries. Int. J. Food Sci. Technol. 2017, 52, 1246–1251. [Google Scholar] [CrossRef]
- Said, N.S.; Olawuyi, I.F.; Lee, W.Y. Pectin Hydrogels: Gel-Forming Behaviors, Mechanisms, and Food Applications. Gels 2023, 9, 732. [Google Scholar] [CrossRef]
- Basu, S.; Banerjee, D.; Chowdhury, R.; Bhattacharya, P. Controlled release of microencapsulated probiotics in food matrix. J. Food Eng. 2018, 238, 61–69. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. Off. J. Eur. Union 2012, 83, 1–295. [Google Scholar]
- Wanders, A.J.; Mars, M.; Borgonjen-van den Berg, K.J.; de Graaf, C.; Feskens, E.J. Satiety and energy intake after single and repeated exposure to gel-forming dietary fiber: Post-ingestive effects. Int. J. Obes. 2014, 38, 794–800. [Google Scholar] [CrossRef]
- Díaz-Bandera, D.; Villanueva-Carvajal, A.; Dublán-García, O.; Quintero-Salazar, B.; Dominguez-Lopez, A. Assessing release kinetics and dissolution of spray-dried Roselle (Hibiscus sabdariffa L.) extract encapsulated with dierent carrier agents. LWT-Food Sci. Technol. 2015, 64, 693–698. [Google Scholar] [CrossRef]
- Mehran, M.; Masoum, S.; Memarzadeh, M. Improvement of thermal stability and antioxidant activity of anthocyanins of Echium amoenum petal using maltodextrin/modified starch combination as wall material. Int. J. Biol. Macromol. 2020, 148, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, J.R.; Weis, G.C.C.; Moro, K.I.B.; Robalo, S.S.; Assmann, C.E.; da Silva, L.P.; Muller, E.I.; da Silva, C.d.B.; de Menezes, C.R.; da Rosa, C.S. Effect of wall materials and storage temperature on anthocyanin stability of microencapsulated blueberry extract. LWT 2021, 142, 111027. [Google Scholar] [CrossRef]
- Slavutsky, A.M.; Bertuzzi, M.A. Formulation and characterization of hydrogel based on pectin and brea gum. Int. J. Biol. Macromol. 2019, 123, 784–791. [Google Scholar] [CrossRef]
- Konovalova, M.V.; Kurek, D.V.; Litvinets, S.G.; Martinson, E.A.; Varlamov, V.P. Preparation and characterization of cryogels based on pectin and chitosan. Prog. Chem. Appl. Chitin Its Deriv. 2016, XXI, 114–121. [Google Scholar] [CrossRef]
- Méndez, D.A.; Schroeter, B.; Martínez-Abad, A.; Fabra, M.J.; Gurikov, P.; López-Rubio, A. Pectin-based aerogel particles for drug delivery: Effect of pectin composition on aerogel structure and release properties. Carbohydr. Polym. 2023, 306, 120604. [Google Scholar] [CrossRef]
- Bakowska-Barczak, A.M.; Kolodziejczyk, P.P. Black currant polyphenols: Their storage stability and microencapsulation. Ind. Crops Prod. 2011, 34, 1301–1309. [Google Scholar] [CrossRef]
- Murali, S.; Kar, A.; Mohapatra, D.; Kalia, P. Encapsulation of black carrot juice using spray and freeze drying. Food Sci. Technol. Int. 2015, 21, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Pashazadeh, H.; Redha, A.A.; Johnson, J.B.; Koca, I. Extraction optimization and microencapsulation of anthocyanins from okra flowers: Utilizing plant waste as a source of bioactive compounds. Food Biosci. 2025, 63, 105710. [Google Scholar] [CrossRef]
- Antonio-Gómez, M.V.; Salinas-Moreno, Y.; Hernández-Rosas, F.; Martínez-Bustos, F.; Andrade-González, I.; Herrera-Corredor, J.A. Optimized Extraction, Microencapsulation, and Stability of Anthocyanins from Ardisia compressa K. Fruit. Pol. J. Food Nutr. Sci. 2021, 71, 299–310. [Google Scholar] [CrossRef]
- Geng, Z.; Zhu, L.; Wang, J.; Yu, X.; Li, M.; Yang, W.; Hu, B.; Zhang, Q.; Yang, X. Drying sea buckthorn berries (Hippophae rhamnoides L.): Effects of different drying methods on drying kinetics, physicochemical properties, and microstructure. Front. Nutr. 2023, 10, 1106009. [Google Scholar] [CrossRef]
- Pateiro, M.; Vargas-Ramella, M.; Franco, D.; Gomes da Cruz, A.; Zengin, G.; Kumar, M.; Dhama, K.; Lorenzo, J.M. The role of emerging technologies in the dehydration of berries: Quality, bioactive compounds, and shelf life. Food Chem. 2022, 16, 100465. [Google Scholar] [CrossRef]
- ISO 9001:2015; Certification—Quality Management Systems. TÜV AUSTRIA, ROMANIA: Bucharest, Romania, 2015.
- Shofita, A.; Bindar, Y.; Samahi, T.; Jaelawijaya, A.; Fawwz, A. Reducing the stickiness of dragon fruit skin extract powder as food colorant by addition of maltodextrin during freeze drying. AIP Conf. Proc. 2020, 2219, 070011. [Google Scholar] [CrossRef]
- Castangia, I.; Fulgheri, F.; Leyva-Jimenez, F.J.; Alañón, M.E.; Cádiz-Gurrea, M.d.l.L.; Marongiu, F.; Meloni, M.C.; Aroffu, M.; Perra, M.; Allaw, M.; et al. From Grape By-Products to Enriched Yogurt Containing Pomace Extract Loaded in Nanotechnological Nutriosomes Tailored for Promoting Gastro-Intestinal Wellness. Antioxidants 2023, 12, 1285. [Google Scholar] [CrossRef]
- Guerin-Deremaux, L.; Li, S.; Pochat, M.; Wils, D.; Mubasher, M.; Reifer, C.; Miller, L.E. Effects of NUTRIOSE® dietary fiber supplementation on body weight, body composition, energy intake, and hunger in overweight men. Int. J. Food Sci. Nutr. 2011, 62, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.; Harrison, B.E.; Carr, T.E.; Barltrop, D. Reduction in the absorption of dietary strontium in children by an alginate derivative. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1971, 19, 79–85. [Google Scholar] [CrossRef]
- Farhad, A.; Sevil, M.; Effat, A.; Mohammad, F.; Mohammadali, T.; Abolfazl, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Engin. 2020, 14, 8. [Google Scholar]
- Blanco-Pérez, F.; Steigerwald, H.; Schülke, S.; Vieths, S.; Toda, M.; Scheurer, S. The Dietary Fiber Pectin: Health Benefits and Potential for the Treatment of Allergies by Modulation of Gut Microbiota. Curr. Allergy Asthma Rep. 2021, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fishman, M.L.; Kost, J.; Hicks, K.B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 2003, 24, 3333–3343. [Google Scholar] [CrossRef] [PubMed]
- Ataei, F.; Hojjatoleslamy, M. Physicochemical and sensory characteristics of sponge cake made with olive leaf. J. Food Meas. Charact. 2017, 11, 2259–2264. [Google Scholar] [CrossRef]
- Adetoro, A.O.; Opara, U.L.; Fawole, O.A. Effect of carrier agents on the physicochemical and technofunctional properties and antioxidant capacity of freeze-dried pomegranate juice (Punica granatum) powder. Foods 2020, 9, 1388. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M.; Mahoonak, A.S.; Mohammadi, A. Application of gum Arabic and maltodextrin for encapsulation of eggplant peel extract as a natural antioxidant and color source. Int. J. Biol. Macromol. 2019, 140, 59–68. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M.; Cal-Vidal, J. Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov. Food Sci. Emerg. Technol. 2005, 6, 420–428. [Google Scholar] [CrossRef]
- Bouyahya, A.; Dakka, N.; Talbaoui, A.; Moussaoui, N.E.; Abrini, J.; Bakri, Y. Phenolic contents and antiradical capacity of vegetable oil from Pistacia lentiscus (L.). J. Mater. Environ. Sci. 2018, 9, 1518–1524. [Google Scholar]
- Popescu, L.; Cesco, T.; Gurev, A.; Ghendov-Mosanu, A.; Sturza, R.; Tarna, R. Impact of Apple Pomace Powder on the Bioactivity, and the Sensory and Textural Characteristics of Yogurt. Foods 2022, 11, 3565. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolic compounds with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Paulpriya, K.; Packia Lincy, M.; Tresina Soris, P.; Veerabahu Ramasamy, M. In vitro antioxidant activity, total phenolic and total flavonoid contents of aerial part extracts of Daphniphyllum neilgherrense (wt.) Rosenth. Ethnopharm. J. Biol. Innov. 2015, 4, 257–268. [Google Scholar]
- Arnao, M.B.; Cano, A.; Alcolea, J.F.; Acosta, M. Estimation of free radical-quenching activity of leaf pigment extracts. Phytochem. Anal. 2001, 12, 138–143. [Google Scholar] [CrossRef]
- Peres, R.G.; Moraes, E.P.; Micke, G.A.; Tonin, F.G. Rapid method for the determination of organic acids in wine by capillary electrophoresis with indirect UV detection. Food Control 2009, 20, 548–552. [Google Scholar] [CrossRef]
- Monaico, E.I.; Monaico, E.V.; Ursaki, V.V.; Tiginyanu, I.M. Controlled electroplating of noble metals on III–V semiconductor nanotemplates fabricated by anodic etching of bulk substrates. Coatings 2022, 12, 1521. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Kalugina, I.; Kalugina, J. Structural and mechanical properties of the jostaberry jelly. Ukr. J. Food Sci. 2017, 5, 72–81. [Google Scholar] [CrossRef]
- Okatan, V. Antioxidant properties and phenolic profile of the most widely appreciated cultivated berry species: A comparative study. Folia Hort. 2020, 32, 79–85. [Google Scholar] [CrossRef]
- Chiang, C.J.; Kadouh, H.; Zhou, K. Phenolic compounds and antioxidant properties of gooseberry as affected by in vitro digestion. LWT-Food Sci. Technol. 2013, 51, 417–422. [Google Scholar] [CrossRef]
- Tsuda, H.; Kunitake, H.; Kawasaki-Takaki, R.; Nishiyama, K.; Yamasaki, M.; Komatsu, H.; Yukizaki, C. Antioxidant Activities and Anti-Cancer Cell Proliferation Properties of Natsuhaze (Vaccinium oldhamii Miq.), Shashanbo (V. bracteatum Thunb.) and Blueberry Cultivars. Plants 2013, 2, 57–71. [Google Scholar] [CrossRef]
- Kim, J.S. Antioxidant Activities of Selected Berries and Their Free, Esterified, and Insoluble-Bound Phenolic Acid Contents. Prev. Nutr. Food Sci. 2018, 23, 35–45. [Google Scholar] [CrossRef]
- Whang, L.; Wen, H.; Yang, N.; Li, H. Effect of vacuum freeze drying and hot air drying on dried mulberry fruit quality. PLoS ONE 2023, 18, e0283303. [Google Scholar] [CrossRef]
- Aziz, M.; Yusof, Y.; Blanchard, C.; Saifullah, M.; Farahnaky, A.; Scheiling, G. Material properties and tableting of fruit powders. Food Eng. Rev. 2018, 10, 66–80. [Google Scholar] [CrossRef]
- Dag, D.; Kilercioglu, M.; Oztop, M.H. Physical and chemical characteristics of encapsulated goldenberry (Physalis peruviana L.) juice powder. LWT-Food Sci. Technol. 2017, 83, 86–94. [Google Scholar] [CrossRef]
- Banu, K.; Sakin-Yılmazer, M.; Kaymak-Ertekin, F.; Balkır, P. Physical properties of yoghurt powder produced by spray drying. J. Food Sci. Technol. 2014, 51, 1377–1383. [Google Scholar]
- Franceschinis, L.; Salvatori, D.M.; Sosa, N.; Schebor, C. Physical and functional properties of blackberry freeze-and spray-dried powders. Dry. Technol. 2014, 32, 197–207. [Google Scholar] [CrossRef]
- Nthimole, C.T.; Kaseke, T.; Fawole, O.A. Micro-Encapsulation and Characterization of Anthocyanin-Rich Raspberry Juice Powder for Potential Applications in the Food Industry. Processes 2022, 10, 1038. [Google Scholar] [CrossRef]
- GEA Niro. Hygroscopicity—Method A14a: GEA Niro. In Analytical Methods of Dry Milk Products; GEA Niro: Soeborg, Denmark, 2005; pp. 1–3. Available online: https://www.gea.com/en/assets/170113/ (accessed on 12 March 2025).
- Nguyen, T.T.; Voilley, A.; Tran, T.T.T.; Waché, Y. Microencapsulation of Hibiscus sabdariffa L. Calyx Anthocyanins with Yeast Hulls. Plant Foods Hum. Nutr. 2022, 77, 83–89. [Google Scholar] [CrossRef]
- McClements, D.J. Designing biopolymer microgels to encapsulate, protect and deliver bioactive components: Physicochemical aspects. Adv. Colloid Interface Sci. 2017, 240, 31–59. [Google Scholar] [CrossRef]
- Wen, C.; Tang, J.; Cao, L.; Fan, M.; Lin, X.; Liu, G.; Liang, L.; Liu, X.; Zhang, J.; Li, Y.; et al. Strategic Approaches for Co-Encapsulation of Bioactive Compounds: Technological Advances and Mechanistic Insight. Foods 2025, 14, 2024. [Google Scholar] [CrossRef]
- Casati, C.B.; Baeza, R.; Sánchez, V. Physicochemical properties and bioactive compounds content in encapsulated freeze-dried powders obtained from blueberry, elderberry, blackcurrant and maqui berry. J. Berry Res. 2020, 9, 431–447. [Google Scholar] [CrossRef]
- Mazuco, R.A.; Cardoso, P.M.M.; Bindaco, É.S.; Scherer, R.; Castilho, R.O.; Faraco, A.A.G.; Ruas, F.G.; Oliveira, J.P.; Guimarães, M.C.C.; Andrade, T.U. Maltodextrin and gum Arabic-based microencapsulation methods for anthocyanin preservation in Juçara palm (Euterpe edulis Martius) fruit pulp. Plant Foods Hum. Nutr. 2018, 73, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lv, Y. Degradation kinetic of anthocyanins from rose (Rosa rugosa) as prepared by microencapsulation in freeze-drying and spray-drying. Int. J. Food Prop. 2019, 22, 2009–2021. [Google Scholar] [CrossRef]
- Laine, P.; Kylli, P.; Heinonen, M.; Jouppila, K. Storage stability of microencapsulated cloudberry (Rubus chamaemorus) phenolics. J. Agric. Food Chem. 2008, 56, 11251–11261. [Google Scholar] [CrossRef]
- Guan, Y.; Zhong, Q. The improved thermal stability of anthocyanins at pH 5.0 by gum arabic. LWT-Food Sci. Technol. 2015, 64, 706–712. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of grape polyphenols using maltodextrin and gum arabic as two alternative coating materials: Development and characterization. J. Biotech. 2016, 239, 23–33. [Google Scholar] [CrossRef] [PubMed]
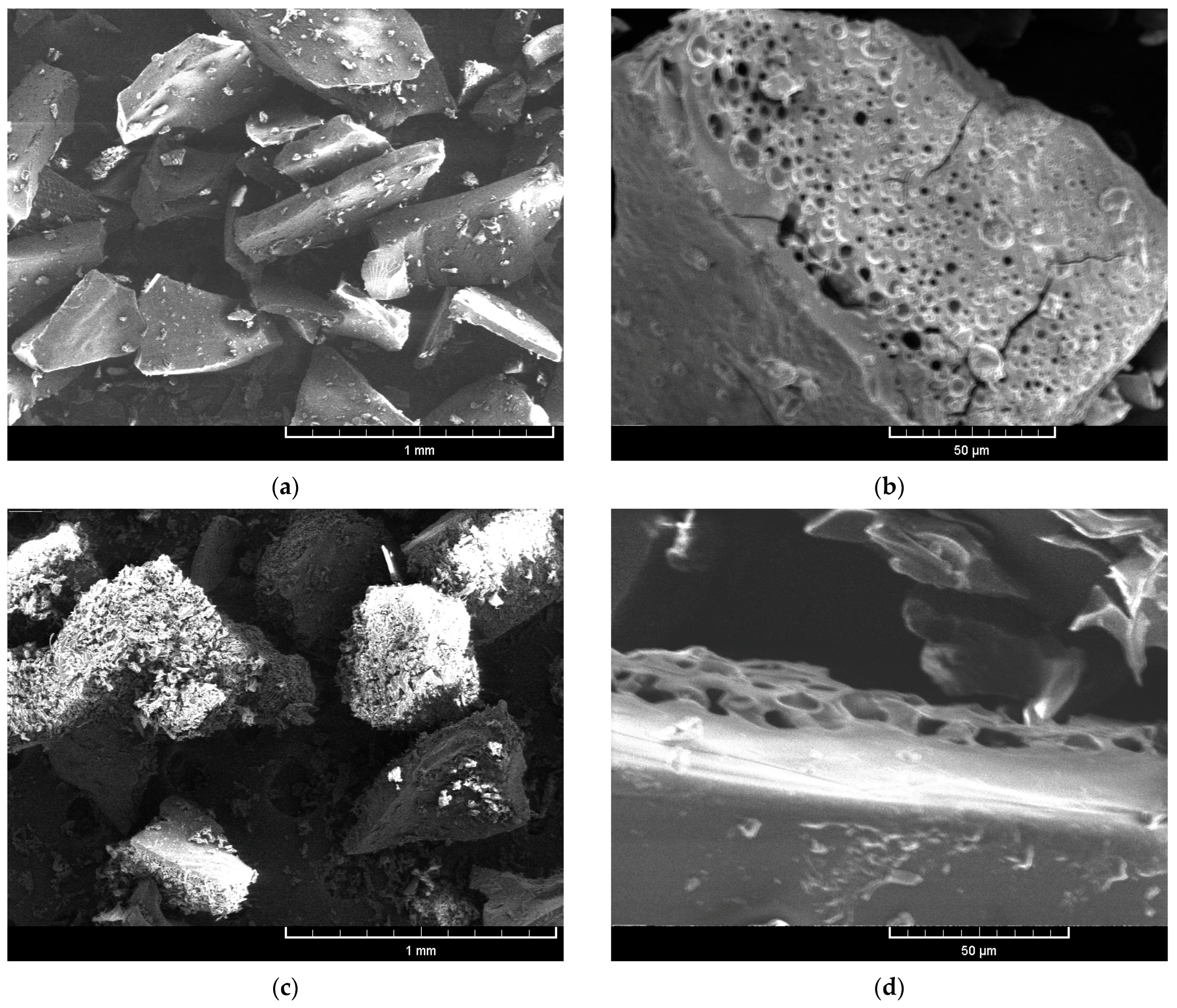
| Biological Active Compounds | Samples | |
|---|---|---|
| FJ | FDJ | |
| Anthocyanins, Cy3-O-Glc, mg/g DW | 14.00 ± 0.02 b | 5.95 ± 0.03 a |
| Ascorbic acid, mg/g DW | 3.60 ± 0.02 b | 1.99 ± 0.01 a |
| Chlorogenic acid, mg/g DW | 2.00 ± 0.02 b | 1.83 ±0.01 a |
| Caffeic acid, mg/g DW | 0.31 ± 0.01 b | 0.27 ± 0.01 a |
| Rutoside, mg/g DW | 1.36 ± 0.02 b | 0.95 ±0.02 a |
| Malic acid, mg/g DW | 3.43 ± 0.02 a | 4.11 ± 0.03 b |
| Citric acid, mg/g DW | 3.21 ± 0.01 a | 3.94 ± 0.02 b |
| Fumaric acid, mg/g DW | 5.02 ± 0.03 a | 7.15 ± 0.03 b |
| Indices | JE |
|---|---|
| TSS, °Bx | 18.12 ± 0.20 |
| TS, % | 19.95 ± 0.40 |
| TPC, mg GAE/g DW | 13.55 ± 1.23 |
| TAC, mg Cy3GE/g DW | 7.15 ± 0.13 |
| AA by DPPH, mg TE/g DW | 14.84 ± 0.33 |
| AA by ABTS, mg TE/g DW | 40.79 ± 2.37 |
| Physicochemical Indicators | Microparticles | |||
|---|---|---|---|---|
| MNPJ | MNPJ12 | MNAJ | MNAJ12 | |
| MPE, % | 87.74 ± 0.05 | nd | 88.90 ±0.01 | nd |
| MC, % | 2.61 ± 0.01 a | 2.76 ± 0.01 b | 2.80 ± 0.01 a | 2.93 ± 0.01 b |
| BD, g/cm3 | 0.781 ± 0.001 a | 0.820 ± 0.001 b | 0.752 ± 0.001 a | 0.790 ± 0.001 b |
| TSS, °Bx | 9.22 ± 0.01 b | 9.13 ± 0.03 a | 8.47 ± 0.02 b | 8.25 ± 0.03 a |
| Hygroscopicity, % | 7.62 ± 0.01 a | 7.61 ± 0.02 a | 7.43 ± 0.01 b | 7.39 ± 0.01 a |
| Solubility, % | 55.55 ± 0.06 b | 53.67 ± 0.09 a | 39.40 ± 0.02 b | 37.12 ± 0.05 a |
| OHC, % | 3.44 ± 0.06 b | 3.03 ± 0.03 a | 1.48 ± 0.05 b | 1.16 ± 0.03 a |
| TA, % | 16.02 ± 0.17 b | 15.45 ± 0.10 a | 12.88 ± 0.08 b | 12.31 ± 0.11 a |
| pH | 3.28 ± 0.01 a | 3.36 ± 0.01 b | 3.72 ± 0.01 a | 4.04 ± 0.01 b |
| FC, % | 0.07 ± 0.01 a | 0.07 ± 0.01 a | 0.05 ± 0.01 a | 0.05 ± 0.01 a |
| AC, % | 0.46 ± 0.02 b | 0.44 ± 0.04 a | 1.06 ± 0.03 b | 1.02 ± 0.02 a |
| Microparticles | CIELab Color Parameters | ||||
|---|---|---|---|---|---|
| L* | a* | b* | C* | h*,° | |
| MNPJ | 38.74 ± 0.24 a | 34.98 ± 0.21 b | −4.82 ± 0.08 b | 7.53 ± 0.04 b | 35.17 ± 0.09 b |
| MNPJ12 | 40.21 ± 0.56 b | 32.88 ± 0.11 a | −3.56 ± 0.02 a | 7.21 ± 0.03 a | 34.57 ± 0.08 a |
| MNAJ | 46.85 ± 0.29 a | 29.46 ± 0.09 b | −5.47 ± 0.06 b | 6.66 ± 0.05 b | 34.10 ± 0.01 b |
| MNAJ12 | 48.96 ± 0.14 b | 26.73 ±0.13 a | −4.22 ± 0.01 a | 6.40 ± 0.04 a | 32.74 ± 0.06 a |
| Parameters | MNPJ | MNAJ | ||
|---|---|---|---|---|
| Before Freeze-Drying | After Freeze-Drying | Before Freeze-Drying | After Freeze-Drying | |
| TPC, mg GAE/g DW | 5.02 ± 0.01 b | 4.67 ± 0.01 a | 4.57 ± 0.02 b | 4.07 ± 0.03 a |
| TAC, mg Cy3GE/g DW | 2.57 ± 0.02 b | 2.37 ± 0.01 a | 2.33 ± 0.01 b | 2.17 ± 0.0 a |
| AA by DPPH, mg TE/g DW | 6.32 ± 0.01 b | 5.69 ± 0.03 a | 6.49 ± 0.02 b | 6.06 ±0.02 a |
| AA by ABTS, mg TE/g DW | 17.89 ± 0.05 b | 11.35 ± 0.02 a | 18.92 ± 0.07 b | 13.76 ± 0.06 a |
| Indices | MNPJ | MNAJ | ||||||
|---|---|---|---|---|---|---|---|---|
| After Production | After 3 Months | After 6 Months | After 12 Months | After Production | After 3 Months | After 6 Months | After 12 Months | |
| TPC, mg GAE/g DW | 4.67 ± 0.02 b | 4.84 ± 0.03 c | 4.61 ± 0.01 b | 4.30 ± 0.0 a | 4.07 ± 0.02 a | 4.29 ± 0.01 b | 4.25 ± 0.02 b | 4.14 ± 0.01 a,b |
| TPC RE, % | 93.1 ± 0.1 b | 96.3 ± 0.1 c | 91.8 ± 0.2 b | 85.5 ± 0.0 a | 90.0 ± 0.2 a | 93.8 ± 0.4 c | 92.9 ± 0.3 b | 90.7 ± 0.2 a,b |
| TAC, mg Cy3GE/g DW | 2.46 ± 0.01 b | 2.39 ± 0.01 b | 2.39 ± 0.01 b | 2.16 ± 0.02 a | 2.17 ± 0.01 a | 2.16 ± 0.01 a | 2.30 ± 0.0 b | 2.11 ± 0.01 a |
| TAC RE, % | 95.7 ± 0.1 c | 92.9 ± 0.2 b | 91.4 ± 0.2 b | 84.0 ± 0.1 a | 93.4 ± 0.1 b | 92.9 ± 0.1 b | 89.3 ± 0.1 a | 89.8 ± 0.1 a |
| AA by DPPH, mg TE/g DW | 5.60 ± 0.02 c | 5.61 ± 0.01 c | 5.49 ± 0.02 b | 5.13 ± 0.01 a | 6.10 ± 0.03 b | 6.23 ± 0.02 c | 6.20 ± 0.02 c | 5.77 ± 0.01 a |
| AA by ABTS, mg TE/g DW | 11.02 ± 0.05 a,b | 11.20 ± 0.06 b | 11.11 ± 0.05 b | 10.72 ± 0.04 a | 13.37 ± 0.06 a,b | 13.42 ± 0.08 b | 13.25 ± 0.04 a,b | 12.87 ± 0.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurev, A.; Bulgaru, V.; Dragancea, V.; Smerea, O.; Baerle, A.; Codină, G.G.; Ghendov-Mosanu, A. Antioxidant Potential of Jostaberry Phytochemicals Encapsulated in Biopolymer Matrices During Storage. Foods 2025, 14, 3092. https://doi.org/10.3390/foods14173092
Gurev A, Bulgaru V, Dragancea V, Smerea O, Baerle A, Codină GG, Ghendov-Mosanu A. Antioxidant Potential of Jostaberry Phytochemicals Encapsulated in Biopolymer Matrices During Storage. Foods. 2025; 14(17):3092. https://doi.org/10.3390/foods14173092
Chicago/Turabian StyleGurev, Angela, Viorica Bulgaru, Veronica Dragancea, Olga Smerea, Alexei Baerle, Georgiana Gabriela Codină, and Aliona Ghendov-Mosanu. 2025. "Antioxidant Potential of Jostaberry Phytochemicals Encapsulated in Biopolymer Matrices During Storage" Foods 14, no. 17: 3092. https://doi.org/10.3390/foods14173092
APA StyleGurev, A., Bulgaru, V., Dragancea, V., Smerea, O., Baerle, A., Codină, G. G., & Ghendov-Mosanu, A. (2025). Antioxidant Potential of Jostaberry Phytochemicals Encapsulated in Biopolymer Matrices During Storage. Foods, 14(17), 3092. https://doi.org/10.3390/foods14173092






