Impact of Barley Malt with Different Row-Types on the Volatile Compounds in Beer
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Wort Preparation
2.3. Beer Fermentation
2.4. Determination of Physicochemical Parameters
2.5. Wort of FSs and FAAs Analysis
2.6. GC-MS Analysis
2.7. GC-E-Nose Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Analysis of FSs and Amino FAAs in Barley Malt
3.1.1. Physicochemical Analysis
3.1.2. Analysis of FSs in Wort
3.1.3. FAAs Analysis in Wort
3.2. Flavor Analysis of Beer
3.2.1. Physicochemical Parameters of the Beer
3.2.2. Analysis of VCs by GC-E-Nose
3.2.3. Analysis of VCs by GC-MS
3.2.4. Effect of Different Row-Type Malt on the Ratio of Higher Alcohols to Esters
3.3. Multivariate Analysis
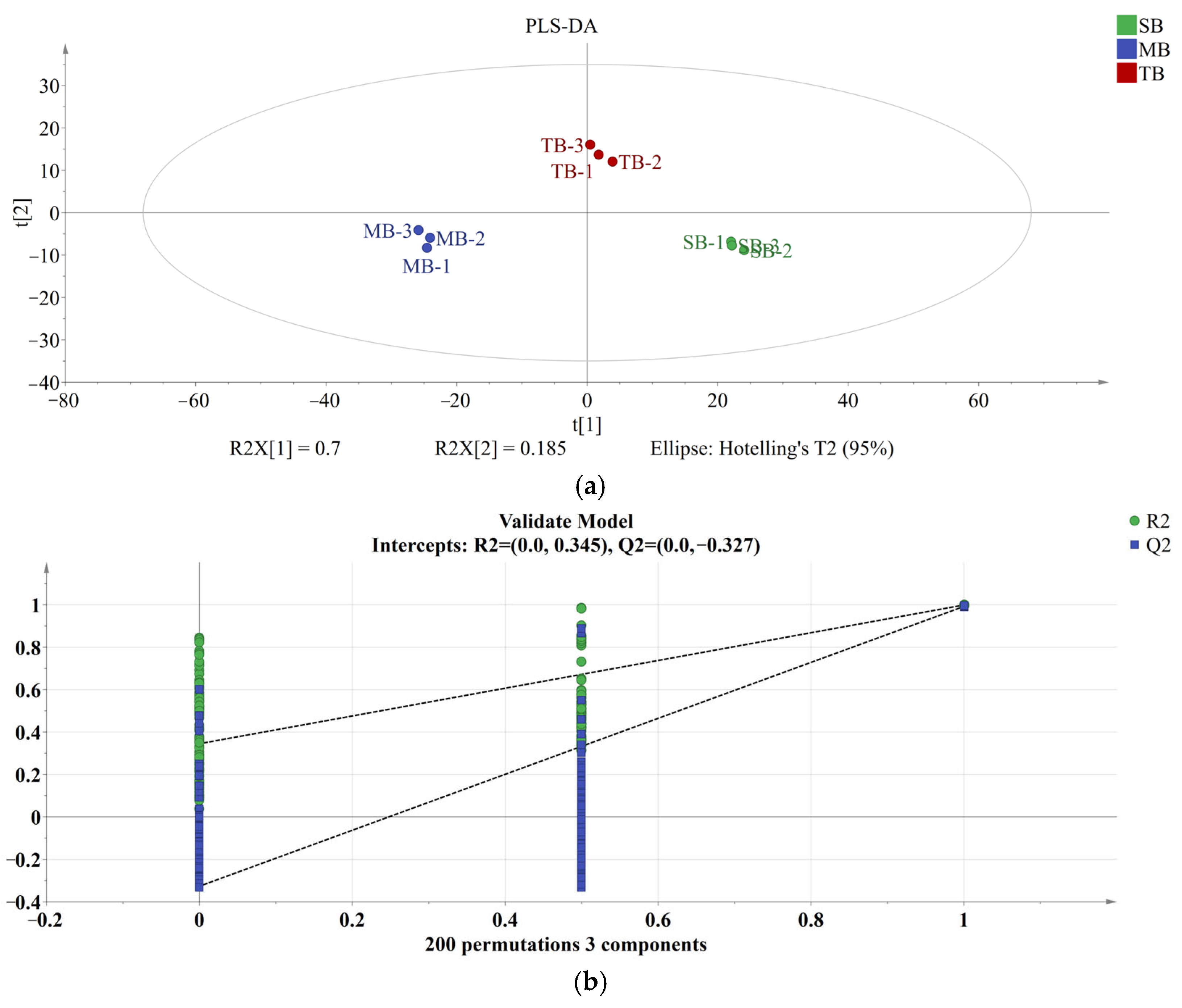
3.3.1. PLS-DA Analysis
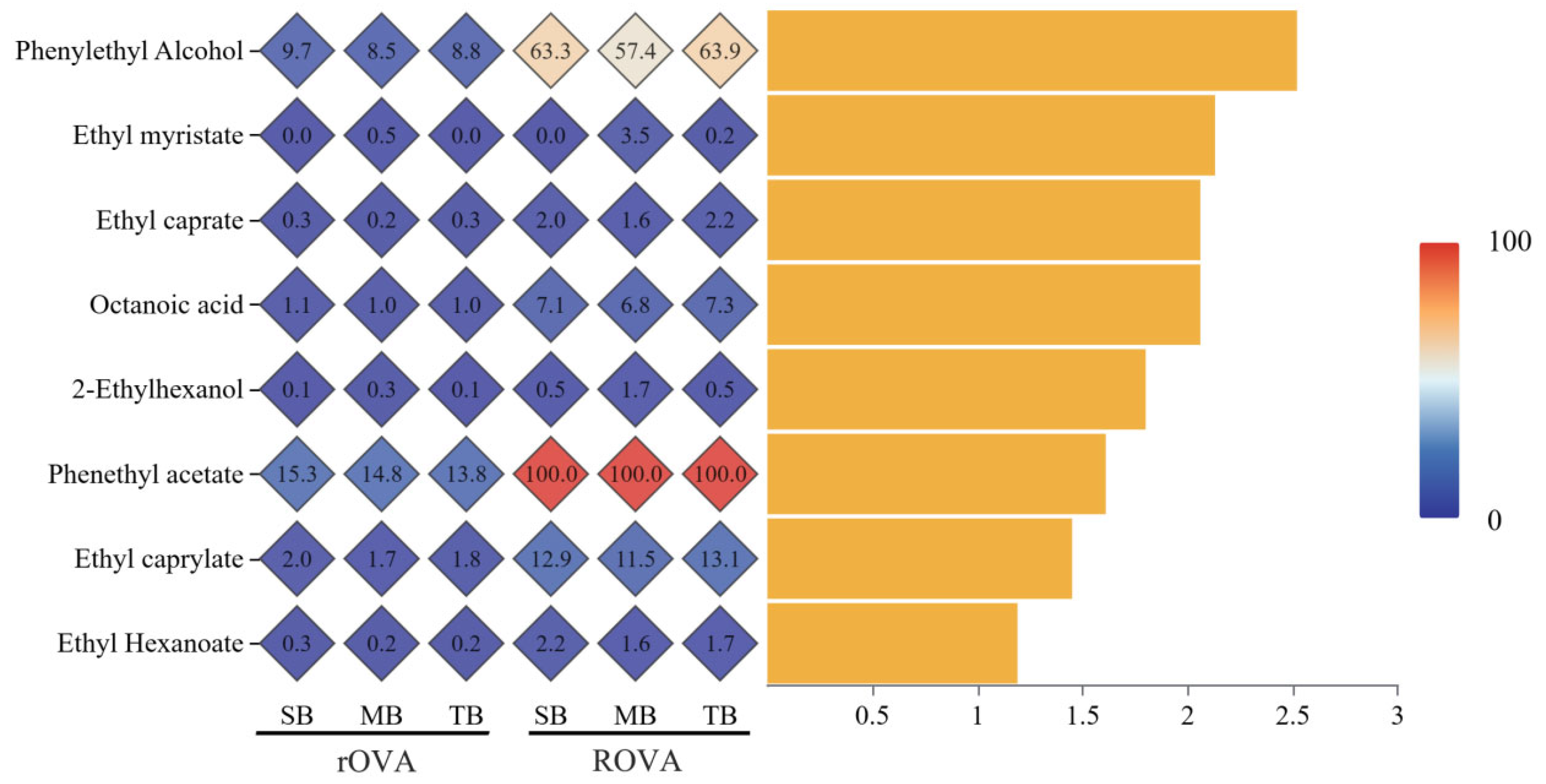
3.3.2. rOAV and ROAV Analysis
3.3.3. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, R.; Chen, X.G.; Peng, Z.C.; Li, W.Z.; Chu, J.Y.; Lu, J.; Xie, G.F.; Wu, D.H. Evaluation of polyphenols content and antioxidant ability of commercial herbal beer. Syst. Microbiol. Biomanufacturing 2024, 4, 77–85. [Google Scholar] [CrossRef]
- Postigo, V.; Mauro, L.; Diaz, T.; Saiz, R.; Arroyo, T.; Garcia, M. Autochthonous Ingredients for Craft Beer Production. Fermentation 2024, 10, 225. [Google Scholar] [CrossRef]
- Ji Hyun, K.; Ji Hyo, K.; Eun Ji, C.; Seung Ju, L.; Young An, K.; Kwang Won, H.; Wang June, K. Multivariate analysis for feasibility of Korean six-row barleys for beer brewing. J. Inst. Brew. 2014, 120, 371–378. [Google Scholar]
- Han, H.; Kim, J.; Choi, E.; Ahn, H.; Kim, W.J. Characteristics of beer produced from Korean six-row barley with the addition of adjuncts. J. Inst. Brew. 2016, 122, 500–507. [Google Scholar] [CrossRef]
- De Flaviis, R.; Santarelli, V.; Grilli, S.; Sacchetti, G. An integrative multi-omics approach aimed to gain insight on the effect of composition, style, yeast, and wheat species on wheat craft beer flavour. Food Chem. 2024, 441, 138387. [Google Scholar] [CrossRef]
- Wang, X.; Lu, J.; Cai, G.; Wu, D. Advances in Maillard reaction products on color, flavor and biological activity of specialty malt. Syst. Microbiol. Biomanufacturing 2024, 4, 500–510. [Google Scholar] [CrossRef]
- De Flaviis, R.; Santarelli, V.; Mutarutwa, D.; Giuliani, M.; Sacchetti, G. Volatiles profile of ‘Blanche’ wheat craft beer as affected by wheat origin: A chemometric study. Food Chem. 2022, 385, 132696. [Google Scholar] [CrossRef]
- Rungue Alvim, R.P.; Oliveira Gomes, F.d.C.; Garcia, C.F.; Vieira, M.d.L.A. de Resende Machado AM. Identification of volatile organic compounds extracted by headspace solid-phase microextraction in specialty beers produced in Brazil. J. Inst. Brew. 2017, 123, 219–225. [Google Scholar] [CrossRef]
- Bettenhausen, H.M.; Barr, L.; Broeckling, C.D.; Chaparro, J.M.; Holbrook, C.; Sedin, D. Heuberger AL. Influence of malt source on beer chemistry, flavor, and flavor stability. Food Res. Int. 2018, 113, 487–504. [Google Scholar] [CrossRef]
- Herb, D.; Filichkin, T.; Fisk, S.; Helgerson, L.; Hayes, P.; Meints, B.; Jennings, R.; Monsour, R.; Tynan, S.; Vinkemeier, K.; et al. Effects of Barley (Hordeum vulgare L.) Variety Growing Environment on Beer Flavor. J. Am. Soc. Brew. Chem. 2017, 75, 345–353. [Google Scholar] [CrossRef]
- Yu, D.-x.; Zhang, X.; Guo, S.; Yan, H.; Wang, J.-m.; Zhou, J.-q.; Yang, J.; Duan, J.-A. Headspace GC/MS and fast GC e-nose combined with chemometric analysis to identify the varieties and geographical origins of ginger (Zingiber officinale Roscoe). Food Chem. 2022, 396, 133672. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Cui, Y.; Yu, J.; Yan, S.; Bai, J.; Xu, H.; Li, M. Volatile flavor behavior characterization of Hericium erinaceus during postharvest storage using E-nose, HS-GC-IMS, and HS-SPME-GC-MS after treated with electron-beam generated X-ray irradiation. Food Chem. 2024, 454, 139771. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, L.; Liu, H.; Liu, H.; Zhou, Y.; Li, M.; Gao, R. Analysis of the changes of volatile flavor compounds in a traditional Chinese shrimp paste during fermentation based on electronic nose, SPME-GC-MS and HS-GC-IMS. Food Sci. Hum. Wellness 2023, 12, 173–182. [Google Scholar] [CrossRef]
- Li, D.; Chu, B.; Li, B.; Wang, X.; Chen, X.; Gu, Q. The difference analysis of physicochemical indexes and volatile flavor compounds of chili oil prepared from different varieties of chili pepper. Food Res. Int. 2024, 190, 114657. [Google Scholar] [CrossRef]
- Wang, L.; Xie, J.; Wang, Q.; Hu, J.; Jiang, Y.; Wang, J.; Tong, H.; Yuan, H.; Yang, Y. Evaluation of the quality grade of Congou black tea by the fusion of GC-E-Nose, E-tongue, and E-eye. Food Chem.-X 2024, 23, 101519. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Meng, Q.; Song, H.; Wang, X. Characterization of Key Odor-Active Compounds in Draft Beers for the Chinese Market Using Molecular Sensory Science Approaches. Molecules 2024, 29, 2537. [Google Scholar] [CrossRef]
- Charmet, G.; Pin, P.A.; Schmitt, M.; Leroy, N.; Claustres, B.; Burt, C.; Genty, A. Genomic prediction of agronomic and malting quality traits in six-rowed winter barley. Euphytica 2023, 219, 63. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Liu, H.; Peng, Z.; Lu, J.; Wu, D. Applying huaimi (flos sophorae immaturus) as a brewing adjunct and its impact on sensory properties of beer. Int. J. Gastron Food Sci. 2024, 36, 100933. [Google Scholar] [CrossRef]
- QB/T 1686-2008; Barley Malt. National Development and Reform Commission: Beijing, China, 2008.
- GB/T 4928-2008; Method for Analysis of Beer. General Administration of Quality Supervision; National Standardization Administration: Beijing, China, 2008.
- Qi, M.; Jiang, L.; Song, J.; Li, L.; Xu, M.; Li, Y.; Ma, C.; Chen, S.; Li, H. Enhancing cassava beer quality: Extrusion-induced modification of cassava starch structure boosts fermentable sugar content in wort. Int. J. Biol. Macromol. 2024, 278, 134895. [Google Scholar] [CrossRef]
- Fang, X.; Xu, W.; Jiang, G.; Sui, M.; Xiao, J.; Ning, Y.; Niaz, R.; Wu, D.; Feng, X.; Chen, J.; et al. Monitoring the dynamic changes in aroma during the whole processing of Qingzhuan tea at an industrial scale: From fresh leaves to finished tea. Food Chem. 2024, 439, 137810. [Google Scholar] [CrossRef]
- Ma, M.; Chen, Z.; Huang, B.; Chen, X.; Liu, H.; Peng, Z.; Dong, P.; Lu, J.; Wu, D. Characterizing the key aroma compounds of barley malt from different origins using GC-E-Nose, HS-SPME-GC-MS, and HS-GC-IMS. Food Biosci. 2024, 58, 103707. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Guido, L.F. Impact of Wort Amino Acids on Beer Flavour: A Review. Fermentation 2018, 4, 23. [Google Scholar] [CrossRef]
- Grassi, S.; Amigo, J.M.; Lyndgaard, C.B.; Foschino, R.; Casiraghi, E. Assessment of the sugars and ethanol development in beer fermentation with FT-IR and multivariate curve resolution models. Food Res. Int. 2014, 62, 602–608. [Google Scholar] [CrossRef]
- Gu, Z.; Jin, Z.; Schwarz, P.; Rao, J.; Chen, B. Unraveling the role of germination days on the aroma variations of roasted barley malts via gas chromatography-mass spectrometry based untargeted and targeted flavoromics. Food Chem. 2023, 426, 136563. [Google Scholar] [CrossRef]
- Ledley, A.J.; Elias, R.J.; Cockburn, D.W. Evaluating the Role of Mashing in the Amino Acid Profiles of Worts Produced from Gluten-Free Malts. Beverages 2023, 9, 10. [Google Scholar] [CrossRef]
- Hill, A.E.; Stewart, G.G. Free Amino Nitrogen in Brewing. Fermentation 2019, 5, 22. [Google Scholar] [CrossRef]
- Bogdan, P.; Kordialik-Bogacka, E.; Czyzowska, A.; Oracz, J.; Zyzelewicz, D. The Profiles of Low Molecular Nitrogen Compounds and Fatty Acids in Wort and Beer Obtained with the Addition of Quinoa (Chenopodium quinoa Willd.), Amaranth (Amaranthus cruentus L.) or Maltose Syrup. Foods 2020, 9, 1626. [Google Scholar] [CrossRef]
- He, Y.; Dong, J.J.; Yin, H.; Zhao, Y.X.; Chen, R.; Wan, X.J.; Chen, P.; Hou, X.P.; Liu, J.; Chen, L. Wort composition and its impact on the flavour-active higher alcohol and ester formation of beer—A review. J. Inst. Brew. 2014, 120, 157–163. [Google Scholar] [CrossRef]
- Lei, H.J.; Zheng, L.Y.; Wang, C.X.; Zhao, H.F.; Zhao, M.M. Effects of worts treated with proteases on the assimilation of free amino acids and fermentation performance of lager yeast. Int. J. Food Microbiol. 2013, 161, 76–83. [Google Scholar] [CrossRef]
- Willaert, R.; Nedovic, V.A. Primary beer fermentation by immobilised yeast—A review on flavour formation and control strategies. J. Chem. Technol. Biotechnol. 2006, 81, 1353–1367. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Wang, Y.; Bai, C.; Jiang, Y.; Yuan, M.; Zhao, L.; Chen, L. Characterization of the flavor profile of four major Chinese carps using HS-SPME-GC-MS combined with ultra-fasted gas chromatography-electronic nose. Food Chem. 2025, 463, 141264. [Google Scholar] [CrossRef]
- Shevchuk, A.; Jayasinghe, L.; Kuhnert, N. Differentiation of black tea infusions according to origin, processing and botanical varieties using multivariate statistical analysis of LC-MS data. Food Res. Int. 2018, 109, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Huang, G.; Ren, L.; Li, Y.; Yu, J.; Lu, Q.; Yang, Y.; Deng, X.; Li, Y.; Zhou, H. Effects of Monascus purpureus on ripe Pu-erh tea in different fermentation methods and identification of characteristic volatile compounds. Food Chem. 2024, 440, 138249. [Google Scholar] [CrossRef] [PubMed]
- Pires, E.J.; Teixeira, J.A.; Branyik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma-a review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Viejo, C.G.; Fuentes, S.; Torrico, D.D.; Godbole, A.; Dunshea, F.R. Chemical characterization of aromas in beer and their effect on consumers liking. Food Chem. 2019, 293, 479–485. [Google Scholar] [CrossRef]
- Liu, S.Q.; Pilone, G.J. An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbiological implications. Int. J. Food Sci. Technol. 2000, 35, 49–61. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The chemistry of beer aging—A critical review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]
- Song, L.; Han, D.-Y.; Luo, L.-J.; Wei, Y.-H.; Yu, Y.-J.; Wen, Z.; Zhu, H.-Y.; Bai, M.; Wang, J.-W.; Bai, F.-Y.; et al. Exploring Non- Saccharomyces yeasts from Daqu for beer production. LWT-Food Sci. Technol. 2024, 209, 116803. [Google Scholar] [CrossRef]
- Yan, Y.; Zou, M.; Tang, C.; Ao, H.; He, L.; Qiu, S.; Li, C. The insights into sour flavor and organic acids in alcoholic beverages. Food Chem. 2024, 460, 140676. [Google Scholar] [CrossRef]
- Saison, D.; De Schutter, D.P.; Uyttenhove, B.; Delvaux, F.; Delvaux, F.R. Contribution of staling compounds to the aged flavour of lager beer by studying their flavour thresholds. Food Chem. 2009, 114, 1206–1215. [Google Scholar] [CrossRef]
- Goncalves Moreira, M.T.; Pereira, P.R.; Aquino, A.; Conte-Junior, C.A. Flosi Paschoalin VM. Aldehyde Accumulation in Aged Alcoholic Beer: Addressing Acetaldehyde Impacts on Upper Aerodigestive Tract Cancer Risks. Int. J. Mol. Sci. 2022, 23, 14147. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Yu, P.; Wu, D.; Cai, G. Screening of Several Strains of Brewer’s Yeast Strains and Comparison of Their Fermentability. Chin. J. Biotechnol. 2022, 42, 45–53. [Google Scholar]
- Cui, D.-y.; Ge, J.-l.; Song, Y.-m.; Feng, P.-p.; Lin, L.-c.; Guo L-y Zhang, C.-y. Regulating the ratio of higher alcohols to esters by simultaneously overexpressing ATF1 and deleting BAT2 in brewer’s yeast Saccharomyces pastorianus. Food Biosci. 2021, 43, 101231. [Google Scholar] [CrossRef]
- Brendel, S.; Hofmann, T.; Granvogl, M. Dry-Hopping to Modify the Aroma of Alcohol-Free Beer on a Molecular Level-Loss and Transfer of Odor-Active Compounds. J. Agric. Food Chem. 2020, 68, 8602–8612. [Google Scholar] [CrossRef]
- Mutz, Y.S.; do Rosario, D.; Silva, L.R.G.; Santos, F.D.; Santos, L.P.; Janegitz, B.C.; Filgueiras, P.R.; Romao, W.; Ferreira, R.d.Q.; Conte-Junior, C.A. Portable electronic tongue based on screen-printed electrodes coupled with chemometrics for rapid differentiation of Brazilian lager beer. Food Control 2021, 127, 108163. [Google Scholar] [CrossRef]
- Cordente, A.G.; Solomon, M.; Schulkin, A.; Francis, I.L.; Barker, A.; Borneman, A.R.; Curtin, C.D. Novel wine yeast with ARO4 and TYR1 mutations that overproduce ‘floral’ aroma compounds 2-phenylethanol and 2-phenylethyl acetate. Appl. Microbiol. Biotechnol. 2018, 102, 5977–5988. [Google Scholar] [CrossRef]
- Yang, M.; Zhai, X.; Huang, X.; Li, Z.; Shi, J.; Li, Q.; Zou, X.; Battino, M. Rapid discrimination of beer based on quantitative aroma determination using colorimetric sensor array. Food Chem. 2021, 363, 130297. [Google Scholar] [CrossRef]
- Gao, Y.-F.; Li, X.-Y.; Wang, Q.-L.; Li, Z.-H.; Chi, S.-X.; Dong, Y.; Guo, L.; Zhang, Y.-H. Discrimination and quantification of volatile compounds in beer by FTIR combined with machine learning approaches. Food Chem.-X 2024, 22, 101300. [Google Scholar] [CrossRef]
- Jablonski, S.J.; Mielko-Nizialek, K.A.; Leszczynski, P.; Gasinski, A.; Kawa-Rygielska, J.; Mlynarz, P.; Lukaszewicz, M. Examination of internal metabolome and VOCs profile of brewery yeast and their mutants producing beer with improved aroma. Sci. Rep. 2024, 14, 16531. [Google Scholar]
- Nisbet, M.A.; Tobias, H.J.; Brenna, J.T.; Sacks, G.L.; Mansfield, A.K. Quantifying the Contribution of Grape Hexoses to Wine Volatiles by High-Precision [U13C]-Glucose Tracer Studies. J. Agric. Food Chem. 2014, 62, 6820–6827. [Google Scholar] [CrossRef]





| Class | Flavor Compounds | CAS | TB | MB | SB |
|---|---|---|---|---|---|
| Alcohols | 1-Propanol | 71-23-8 | 26.09 ± 4.80 a | 21.29 ± 1.53 a | 25.57 ± 0.94 a |
| 2-Methyl-1-propanol | 78-83-1 | 107.39 ± 2.29 b | 97.33 ± 3.87 c | 115.15 ± 1.52 a | |
| 1-Butanol | 71-36-3 | 2.16 ± 0.05 b | 1.78 ± 0.10 c | 2.43 ± 0.11 a | |
| 3-Methyl-1-butanol | 123-51-3 | 1032.20 ± 22.15 b | 936.55 ± 14.56 c | 1146.50 ± 4.30 a | |
| 1-Hexanol | 111-27-3 | 3.49 ± 0.21 a | 2.57 ± 0.01 b | 3.63 ± 0.31 a | |
| 1-Octen-3-ol | 3391-86-4 | 1.25 ± 0.02 b | 0.00 ± 0.00 c | 1.52 ± 0.02 a | |
| 2-Ethylhexanol | 104-76-7 | 20.21 ± 0.48 c | 77.63 ± 1.17 a | 23.13 ± 0.04 b | |
| 2-Nonanol | 628-99-9 | 1.37 ± 0.13 b | 1.01 ± 0.03 c | 1.73 ± 0.25 a | |
| Linalool | 78-70-6 | 37.25 ± 0.85 b | 26.93 ± 0.64 c | 42.79 ± 0.16 a | |
| 1-Octanol | 111-87-5 | 21.19 ± 0.58 b | 18.76 ± 0.47 c | 24.76 ± 0.02 a | |
| 1-Nonanol | 143-08-8 | 7.60 ± 0.22 b | 6.60 ± 0.25 c | 8.96 ± 0.17 a | |
| 3(Z)-Nonenol | 10340-23-5 | 3.07 ± 0.11 b | 0.00 ± 0.00 c | 3.25 ± 0.04 a | |
| 3-Methylthiopropanol | 505-10-2 | 2.62 ± 0.13 b | 3.03 ± 0.27 a | 2.91 ± 0.07 ab | |
| 2-Undecanol | 1653-30-1 | 4.26 ± 0.21 b | 2.48 ± 0.05 c | 4.70 ± 0.04 a | |
| 1-Decanol | 112-30-1 | 21.45 ± 0.56 b | 18.34 ± 1.21 c | 25.58 ± 0.50 a | |
| Citronellol | 106-22-9 | 38.47 ± 1.30 b | 30.96 ± 2.07 c | 42.73 ± 0.88 a | |
| Neraniol | 106-25-2 | 4.89 ± 0.20 a | 0.00 ± 0.00 b | 5.21 ± 0.28 a | |
| Geraniol | 106-24-1 | 10.99 ± 0.34 b | 9.37 ± 0.26 c | 11.57 ± 0.20 a | |
| Phenylethyl Alcohol | 60-12-8 | 1236.93 ± 27.65 b | 1192.19 ± 17.44 b | 1353.47 ± 22.07 a | |
| 1-Hexadecanol | 36653-82-4 | 0.00 ± 0.00 b | 12.37 ± 1.04 a | 11.84 ± 1.06 a | |
| T-cadinol | 5937-11-1 | 10.24 ± 0.43 b | 10.57 ± 0.48 b | 12.06 ± 0.85 a | |
| Total | 2469.77 ± 11.27 c | 2593.11 ± 7.52 b | 2869.49 ± 28.52 a | ||
| Esters | Ethyl Acetate | 141-78-6 | 75.37 ± 0.90 b | 75.05 ± 1.43 b | 94.07 ± 2.27 a |
| Ethyl propionate | 105-37-3 | 2.31 ± 0.10 c | 2.75 ± 0.05 b | 3.16 ± 0.17 a | |
| Isobutyl acetate | 110-19-0 | 2.56 ± 0.10 c | 3.05 ± 0.04 b | 3.65 ± 0.17 a | |
| Ethyl butyrate | 105-54-4 | 7.76 ± 0.13 b | 7.62 ± 0.16 b | 10.16 ± 0.29 a | |
| Isoamyl acetate | 123-92-2 | 100.63 ± 4.23 c | 120.14 ± 1.76 b | 150.88 ± 6.17 a | |
| Ethyl Hexanoate | 123-66-0 | 49.91 ± 0.89 b | 49.84 ± 1.59 b | 71.27 ± 1.58 a | |
| Ethyl heptanoate | 106-30-9 | 1.91 ± 0.04 b | 1.44 ± 0.11 c | 2.28 ± 0.03 a | |
| 2-Ethylhexyl acetate | 103-09-3 | 0.93 ± 0.93 c | 4.59 ± 0.23 a | 2.42 ± 0.07 b | |
| Ethyl caprylate | 106-32-1 | 524.22 ± 39.03 ab | 494.24 ± 45.46 b | 573.89 ± 9.39 a | |
| Heptylformiat | 112-23-2 | 5.49 ± 0.23 a | 3.94 ± 0.03 b | 5.68 ± 0.06 a | |
| Ethyl nonanoate | 123-29-5 | 11.52 ± 0.93 a | 8.34 ± 1.11 b | 11.33 ± 0.38 a | |
| Ethyl caprate | 110-38-3 | 456.32 ± 31.98 a | 366.73 ± 39.52 b | 455.15 ± 5.99 a | |
| Octanoic acid isoamyl | 2035-99-6 | 19.74 ± 1.79 a | 16.63 ± 2.00 a | 17.72 ± 0.43 a | |
| Phenethyl acetate | 103-45-7 | 262.86 ± 12.28 b | 281.81 ± 10.17 ab | 290.31 ± 4.43 a | |
| Ethyl laurate | 106-33-2 | 298.20 ± 10.11 b | 354.15 ± 22.01 a | 319.75 ± 1.63 b | |
| 3-methylbutyl decanoate | 2306-91-4 | 10.34 ± 0.61 ab | 9.38 ± 0.89 b | 10.84 ± 0.13 a | |
| Pentadecanoic acid, ethyl ester | 41114-00-5 | 6.96 ± 0.19 c | 15.75 ± 1.47 a | 9.64 ± 0.80 b | |
| Palmitic acid ethyl ester | 628-97-7 | 57.69 ± 0.93 b | 76.74 ± 7.60 a | 60.41 ± 3.69 b | |
| Ethyl 9-hexadecenoate | 54546-22-4 | 29.34 ± 0.39 b | 81.71 ± 6.16 a | 33.65 ± 1.19 b | |
| Ethyl myristate | 124-06-1 | 5.08 ± 0.04 b | 93.05 ± 9.11 a | 0.00 ± 0.00 b | |
| Total | 2066.96 ± 47.34 b | 1929.16 ± 32.02 b | 2126.27 ± 18.17 a | ||
| Acids | Acetic acid | 64-19-7 | 14.83 ± 0.14 b | 16.79 ± 1.44 b | 22.41 ± 2.42 a |
| 6-Methylheptanoic acid | 929-10-2 | 3.88 ± 0.29 b | 5.18 ± 0.11 a | 3.57 ± 0.10 b | |
| Octanoic acid | 124-07-2 | 917.10 ± 37.44 b | 912.15 ± 24.26 b | 992.82 ± 6.79 a | |
| Decanoic acid | 334-48-5 | 144.56 ± 1.22 b | 112.62 ± 8.36 c | 171.16 ± 2.53 a | |
| 9-Decenoic acid | 14436-32-9 | 29.61 ± 0.85 a | 22.80 ± 3.01 b | 29.24 ± 0.67 a | |
| Total | 1069.53 ± 32.54 b | 1109.98 ± 38.16 b | 1219.20 ± 5.72 a | ||
| Ketones | Acetone | 67-64-1 | 6.84 ± 0.13 c | 7.04 ± 1.01 b | 8.59 ± 1.11 a |
| Methyl Isobutyl Ketone | 108-10-1 | 0.77 ± 0.03 b | 0.63 ± 0.06 c | 0.94 ± 0.05 a | |
| 6-Methyl-5-hepten-2-one | 110-93-0 | 1.49 ± 0.05 b | 1.07 ± 0.05 c | 1.75 ± 0.02 a | |
| 2-Nonanone | 821-55-6 | 2.84 ± 0.07 b | 2.11 ± 0.03 c | 3.53 ± 0.08 a | |
| Dihydropseudoionone | 689-67-8 | 0.66 ± 0.05 b | 0.51 ± 0.04 c | 0.79 ± 0.05 a | |
| Total | 15.48 ± 0.52 b | 17.81 ± 0.04 c | 20.44 ± 0.34 a | ||
| Aldehydes | Acetaldehyde | 75-07-0 | 12.04 ± 0.02 b | 11.17 ± 0.55 c | 13.43 ± 0.42 a |
| Isobutyraldehyde | 78-84-2 | 68.19 ± 0.59 a | 62.83 ± 0.79 c | 65.89 ± 0.53 b | |
| Isovaleraldehyde | 590-86-3 | 0.33 ± 0.02 a | 0.30 ± 0.01 a | 0.32 ± 0.01 a | |
| Nonanal | 124-19-6 | 0.56 ± 0.05 b | 0.53 ± 0.02 b | 0.68 ± 0.04 a | |
| 2,5-Dimethylbenzaldehyde | 5779-94-2 | 1.16 ± 0.29 b | 1.63 ± 0.08 ab | 1.95 ± 0.32 a | |
| Total | 70.72 ± 0.44 c | 75.64 ± 0.50 b | 78.13 ± 1.70 a | ||
| Others | Dimethyl sulfide | 75-18-3 | 5.40 ± 0.19 a | 5.43 ± 1.16 a | 9.29 ± 1.04 a |
| Caryophyllene oxide | 1139-30-6 | 1.25 ± 0.09 b | 1.33 ± 0.18 b | 1.51 ± 0.27 a | |
| 2,4-Di-tert-butylphenol | 96-76-4 | 20.20 ± 0.51 b | 11.59 ± 0.44 c | 22.09 ± 0.34 a | |
| 1,6-Dimethyl-4-isopropylnaphthaIene | 483-78-3 | 5.77 ± 0.15 c | 7.05 ± 0.70 b | 9.08 ± 0.49 a | |
| Total | 27.01 ± 1.81 c | 34.06 ± 0.45 b | 41.27 ± 1.57 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Chen, Z.; Zhang, Y.; Shi, Z.; Lu, J.; Wu, D. Impact of Barley Malt with Different Row-Types on the Volatile Compounds in Beer. Foods 2025, 14, 2010. https://doi.org/10.3390/foods14122010
Zhang J, Chen Z, Zhang Y, Shi Z, Lu J, Wu D. Impact of Barley Malt with Different Row-Types on the Volatile Compounds in Beer. Foods. 2025; 14(12):2010. https://doi.org/10.3390/foods14122010
Chicago/Turabian StyleZhang, Jinglong, Ziqiang Chen, Yongxin Zhang, Zhenbao Shi, Jian Lu, and Dianhui Wu. 2025. "Impact of Barley Malt with Different Row-Types on the Volatile Compounds in Beer" Foods 14, no. 12: 2010. https://doi.org/10.3390/foods14122010
APA StyleZhang, J., Chen, Z., Zhang, Y., Shi, Z., Lu, J., & Wu, D. (2025). Impact of Barley Malt with Different Row-Types on the Volatile Compounds in Beer. Foods, 14(12), 2010. https://doi.org/10.3390/foods14122010




