An In Vitro Gut–Liver–Adipose Axis Model to Evaluate the Anti-Obesity Potential of a Novel Probiotic–Polycosanol Combination
Abstract
1. Introduction
2. Materials and Methods
2.1. Agents Preparation
2.2. Cells Culture
2.3. Experimental Protocol
2.4. In Vitro Intestinal Barrier Model
2.5. Cell Counting Kit-8 (Cytotoxicity Assay)
2.6. Analysis of ZO-1
2.7. Analysis of Claudina 4
2.8. Analysis of Occludin
2.9. Surface Hydrophobicity Assay
2.10. Reactive Oxygen Species (ROS) Production
2.11. TNFα Quantification Assay
2.12. Butyric Acid Quantification Assay
2.13. Lipid Accumulation Assay
2.14. Thiobarbituric Acid Reactive Substances Test (TBARS)
2.15. CD36 ELISA Kit
2.16. PPARγ ELISA Kit
2.17. Resistin ELISA Kit
2.18. GLP-1 ELISA Kit
2.19. SREBP-1 Detection Assay
2.20. AMPK ELISA Kit
2.21. Perilipin ELISA Kit
2.22. Mouse Mitochondrial Brown Fat Uncoupling Protein 1 (UCP1) ELISA Kit
2.23. Mouse PGC1 Alpha ELISA Kit
2.24. Western Blot
2.25. Statistical Analysis of Data
3. Results
3.1. Dose-Response Study on the Gut-Liver-Adipose Axis of Probiotics and Polycosanols Through Transwell® System
3.2. Assessment of the Effects of Probiotics and Polycosanols on an In Vitro Model of Intestinal Barrier
3.3. Assessment of the Influence of Probiotics and Polycosanols on the Hepatic System Compartment
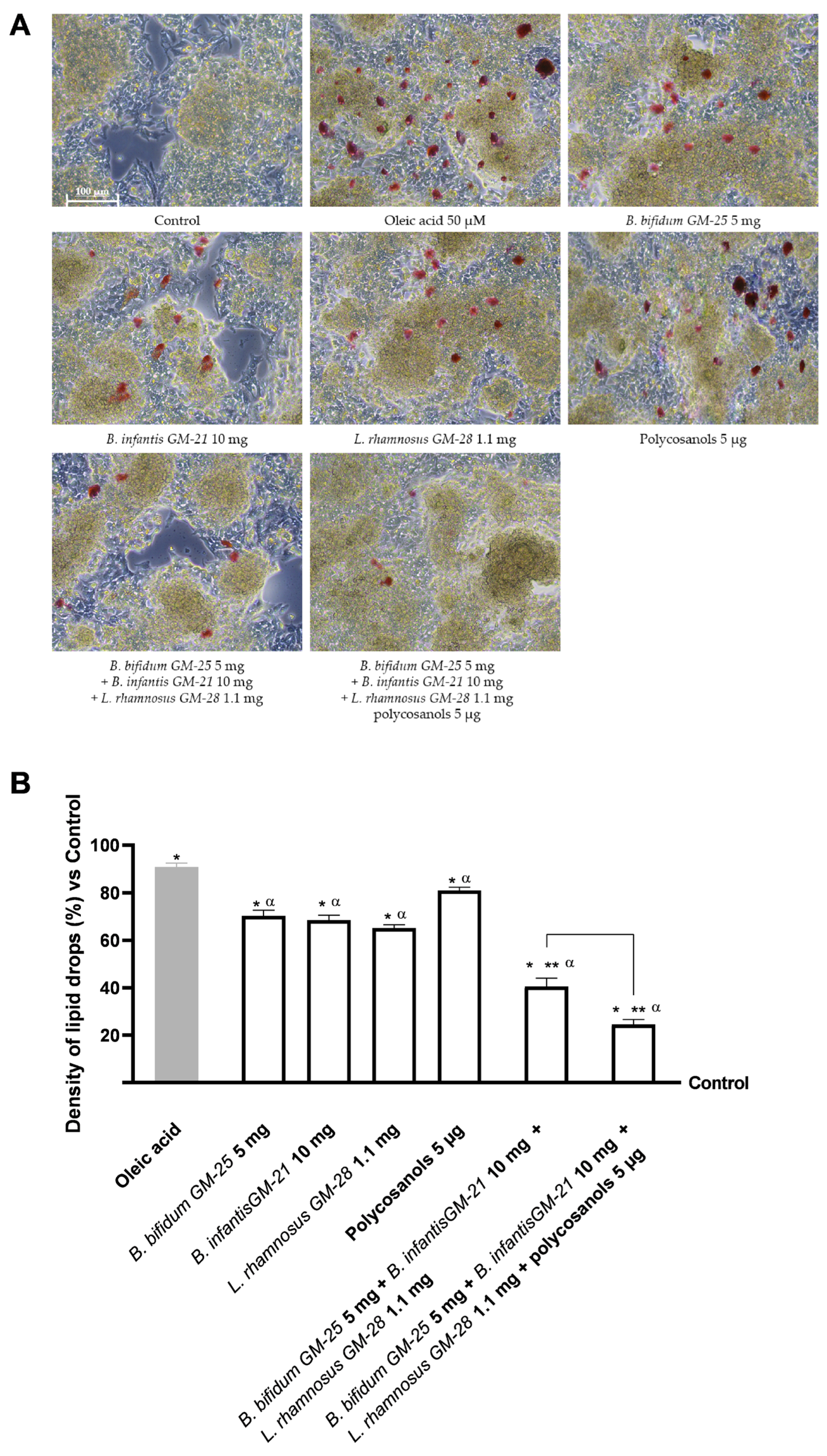
3.4. Evaluation of the Probiotics and Polycosanols’ Influence on 3T3-L1 Adipocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| aP2 | adipose-specific fatty acid-binding protein |
| ATCC | American Type Culture Collection |
| BAT | Brown Adipose Tissue |
| BMI | body mass index |
| C/EBP-α | CCAAT/enhancer-binding protein-α |
| C/EBPβ | CCAAT/enhancer-binding protein-β |
| C/EBPδ | CCAAT/enhancer-binding protein-δ |
| CCK8 | Cell Counting Kit 8 |
| CD36 | cluster of differentiation 36 |
| CFU | colony forming unit |
| CPT-1 | carnitine palmitoyltransferase-1 |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMEM-F12 | Dulbecco’s Modified Eagle’s Medium/Nutrient F-12 Ham |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EVOM3 | Epithelial Volt/Ohm Meter |
| FAS | fatty acid synthase |
| FBS | foetal bovine serum |
| FFA | free fatty acids |
| GLP-1 | glucagon-like peptide 1 |
| IL-6 | interleukin-6 |
| JNK | Jun N-terminal kinases |
| LC3β | microtubule- associated proteins 1A/1B light chain 3B |
| NF-kB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| PBS | Phosphate Buffer Saline |
| PCSK9 | Proprotein Convertase Subtilisin/Kexin type 9 |
| PGC-1α | Peroxisome Proliferator Activated Receptor 1 Alpha |
| PPAR-γ | Peroxisome activated receptor-γ |
| ROS | reactive oxygen species |
| SCFA | short-chain fatty acid |
| SREBP-1 | sterol regulatory element-binding protein-1 |
| SREBP-1c | sterol regulatory element-binding protein-1c |
| TEER | Trans-epithelial electric resistance |
| TJ | tight junctions |
| TNFα | tumor necrosis factor α |
| UCP1 | uncoupling protein 1 |
| WAT | White Adipose Tissue |
| YB | Bifidobacterium longum subsp. infantis YB0411 |
Appendix A

References
- Williams, E.P.; Mesidor, M.; Winters, K.; Dubbert, P.M.; Wyatt, S.B. Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Curr. Obes. Rep. 2015, 4, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, S.-I.; Jang, M.; Jeong, Y.; Kang, C.-H.; Kim, G.-H. Anti-Adipogenic Effect of Lactobacillus Fermentum MG4231 and MG4244 through AMPK Pathway in 3T3-L1 Preadipocytes. Food Sci. Biotechnol. 2020, 29, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-S.; Kim, W.-J.; Bae, W.-Y.; Lee, N.-K.; Paik, H.-D. Inula Britannica Inhibits Adipogenesis of 3T3-L1 Preadipocytes via Modulation of Mitotic Clonal Expansion Involving ERK 1/2 and Akt Signaling Pathways. Nutrients 2020, 12, 3037. [Google Scholar] [CrossRef] [PubMed]
- Schnabl, B.; Brenner, D.A. Interactions Between the Intestinal Microbiome and Liver Diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The Gut–Liver Axis and the Intersection with the Microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef]
- Casotti, V.; D’Antiga, L. Basic Principles of Liver Physiology. In Pediatric Hepatology and Liver Transplantation; Springer International Publishing: Cham, Switzerland, 2019; pp. 21–39. [Google Scholar]
- Item, F.; Konrad, D. Visceral Fat and Metabolic Inflammation: The Portal Theory Revisited. Obes. Rev. 2012, 13, 30–39. [Google Scholar] [CrossRef]
- Giralt, M.; Cereijo, R.; Villarroya, F. Adipokines and the Endocrine Role of Adipose Tissues. In Metabolic Control; Springer: Cham, Switzerland, 2015; pp. 265–282. [Google Scholar]
- Capurso, C.; Capurso, A. From Excess Adiposity to Insulin Resistance: The Role of Free Fatty Acids. Vasc. Pharmacol. 2012, 57, 91–97. [Google Scholar] [CrossRef]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.-E. Impact of the Gut Microbiota on Inflammation, Obesity, and Metabolic Disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- He, M.; Shi, B. Gut Microbiota as a Potential Target of Metabolic Syndrome: The Role of Probiotics and Prebiotics. Cell Biosci. 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Fabersani, E.; Marquez, A.; Gauffin-Cano, P. Adipose Tissue Inflammation and Metabolic Syndrome. The Proactive Role of Probiotics. Eur. J. Nutr. 2019, 58, 27–43. [Google Scholar] [CrossRef] [PubMed]
- George Kerry, R.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of Probiotics for Human Health: A Review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kang, I.; Lee, Y.; Habib, M.A.; Choi, B.J.; Kang, J.S.; Park, D.-S.; Kim, Y.-S. Bifidobacterium Longum Subsp. Infantis YB0411 Inhibits Adipogenesis in 3T3-L1 Pre-Adipocytes and Reduces High-Fat-Diet-Induced Obesity in Mice. J. Agric. Food Chem. 2021, 69, 6032–6042. [Google Scholar] [CrossRef]
- Caroline de Oliveira Melo, N.; Cuevas-Sierra, A.; Arellano-Garcia, L.; Portillo, M.P.; Milton-Laskibar, I.; Alfredo Martinez, J. Oral Administration of Viable or Heat-Inactivated Lacticaseibacillus Rhamnosus GG Influences on Metabolic Outcomes and Gut Microbiota in Rodents Fed a High-Fat High-Fructose Diet. J. Funct. Foods 2023, 109, 105808. [Google Scholar] [CrossRef]
- Vermaak, I.; Viljoen, A.M.; Hamman, J.H. Natural Products in Anti-Obesity Therapy. Nat. Prod. Rep. 2011, 28, 1493. [Google Scholar] [CrossRef]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef]
- Borah, A.K.; Sharma, P.; Singh, A.; Kalita, K.J.; Saha, S.; Chandra Borah, J. Adipose and Non-Adipose Perspectives of Plant Derived Natural Compounds for Mitigation of Obesity. J. Ethnopharmacol. 2021, 280, 114410. [Google Scholar] [CrossRef]
- Sharma, R.; Matsuzaka, T.; Kaushik, M.K.; Sugasawa, T.; Ohno, H.; Wang, Y.; Motomura, K.; Shimura, T.; Okajima, Y.; Mizunoe, Y.; et al. Octacosanol and Policosanol Prevent High-Fat Diet-Induced Obesity and Metabolic Disorders by Activating Brown Adipose Tissue and Improving Liver Metabolism. Sci. Rep. 2019, 9, 5169. [Google Scholar] [CrossRef]
- Siripattanakulkajorn, C.; Sombutsuwan, P.; Nakornsadet, A.; Chumsantea, S.; Lilitchan, S.; Krisnangkura, K.; Aryusuk, K. Policosanol and Other Bioactive Compounds in Different Thai Rice Varieties. J. Food Compos. Anal. 2024, 126, 105891. [Google Scholar] [CrossRef]
- Fernández-Arche, A.; Marquez-Martín, A.; de la Puerta Vazquez, R.; Perona, J.S.; Terencio, C.; Perez-Camino, C.; Ruiz-Gutierrez, V. Long-Chain Fatty Alcohols from Pomace Olive Oil Modulate the Release of Proinflammatory Mediators. J. Nutr. Biochem. 2009, 20, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-L.; Xu, R.-X.; Zhu, C.-G.; Wu, N.-Q.; Cui, Z.-P.; Li, J.-J. Policosanol Attenuates Statin-Induced Increases in Serum Proprotein Convertase Subtilisin/Kexin Type 9 When Combined with Atorvastatin. Evid. -Based Complement. Altern. Med. 2014, 2014, 926087. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Kim, S.-M.; Kim, S.-J.; Lee, E.-Y.; Kim, J.-R.; Cho, K.-H. Consumption of Policosanol Enhances HDL Functionality via CETP Inhibition and Reduces Blood Pressure and Visceral Fat in Young and Middle-Aged Subjects. Int. J. Mol. Med. 2017, 39, 889–899. [Google Scholar] [CrossRef]
- Olatunji, L.K.; Jimoh, A.O.; Tukur, U.M.; Imam, M.U. A Review of the Effects of Policosanol on Metabolic Syndrome. Clin. Complement. Med. Pharmacol. 2022, 2, 100058. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.Y.; Kim, B.; Seo, W.D.; Jia, Y.; Wu, C.; Jun, H.; Lee, S.-J. Barley Sprout Extract Containing Policosanols and Polyphenols Regulate AMPK, SREBP2 and ACAT2 Activity and Cholesterol and Glucose Metabolism in Vitro and in Vivo. Food Res. Int. 2015, 72, 174–183. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, S.-J.; Yadav, D.; Kim, J.-Y.; Kim, J.-R. Consumption of Cuban Policosanol Improves Blood Pressure and Lipid Profile via Enhancement of HDL Functionality in Healthy Women Subjects: Randomized, Double-Blinded, and Placebo-Controlled Study. Oxid. Med. Cell Longev. 2018, 2018, 4809525. [Google Scholar] [CrossRef]
- Kus, M.; Ibragimow, I.; Piotrowska-Kempisty, H. Caco-2 Cell Line Standardization with Pharmaceutical Requirements and In Vitro Model Suitability for Permeability Assays. Pharmaceutics 2023, 15, 2523. [Google Scholar] [CrossRef]
- Arzumanian, V.A.; Kiseleva, O.I.; Poverennaya, E.V. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int. J. Mol. Sci. 2021, 22, 13135. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Barjesteh, F.; Azedi, F.; Alipourfard, I.; Rezaei, Z.; Bahreini, E. Comparative analysis of β-Estradiol and testosterone on lipid droplet accumulation, and regulatory protein expression in palmitate/oleate-induced fatty HepG2 cells. BMC Gastroenterol. 2025, 25, 263. [Google Scholar] [CrossRef]
- Lee, S.Y.; Chung, K.S.; Son, S.R.; Lee, S.Y.; Jang, D.S.; Lee, J.K.; Lee, K.T. A Botanical Mixture Consisting of Inula japonica and Potentilla chinensis Relieves Obesity via the AMPK Signaling Pathway in 3T3-L1 Adipocytes and HFD-Fed Obese Mice. Nutrients 2022, 14, 3685. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liu, M.; Liu, H.; Xie, J.; Yan, J.; Hou, X.; Liu, J. Zeaxanthin promotes browning by enhancing mitochondrial biogenesis through the PKA pathway in 3T3-L1 adipocytes. Food Funct. 2021, 12, 6283–6293. [Google Scholar] [CrossRef] [PubMed]
- Menendez, R. Policosanol Modulates HMG-CoA Reductase Activity in Cultured Fibroblasts. Arch. Med. Res. 2001, 32, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Kodinova, S.; Dushkova, M.; Miteva-Petrova, M.; Yanakieva, V.; Petrov, S.; Denkova, Z. Production of probiotic Bulgarian yoghurts obtained from an ultrafiltered cow’s milk. IJAFR 2020, 59, 1–11. [Google Scholar] [CrossRef]
- International Organization for Standardization. Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 Degrees C by the Pour Plate Technique (ISO Standard No. 4833-1: 2013); International Organization for Standardization: Geneva, Switzerland, 2013. [Google Scholar]
- Kauffman, A.L.; Gyurdieva, A.V.; Mabus, J.R.; Ferguson, C.; Yan, Z.; Hornby, P.J. Alternative Functional in Vitro Models of Human Intestinal Epithelia. Front. Pharmacol. 2013, 4, 54371. [Google Scholar] [CrossRef]
- Jarc, T.; Novak, M.; Hevir, N.; Rižner, T.L.; Kreft, M.E.; Kristan, K. Demonstrating suitability of the Caco-2 cell model for BCS-based biowaiver according to the recent FDA and ICH harmonised guidelines. J. Pharm. Pharmacol. 2019, 71, 1231–1242. [Google Scholar] [CrossRef]
- Ozawa, M.; Tsume, Y.; Zur, M.; Dahan, A.; Amidon, G.L. Intestinal permeability study of minoxidil: Assessment of minoxidil as a high permeability reference drug for biopharmaceutics classification. Mol. Pharm. 2015, 12, 204–211. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Li, X.; Gong, W.; Xie, X.; Yang, Y.; Zheng, A. In vitro evaluation of absorption characteristics of peramivir for oral delivery. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 757–765. [Google Scholar] [CrossRef]
- Galla, R.; Ruga, S.; Aprile, S.; Ferrari, S.; Brovero, A.; Grosa, G.; Molinari, C.; Uberti, F. New Hyaluronic Acid from Plant Origin to Improve Joint Protection—An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 8114. [Google Scholar] [CrossRef]
- Uberti, F.; Morsanuto, V.; Ghirlanda, S.; Molinari, C. Iron Absorption from Three Commercially Available Supplements in Gastrointestinal Cell Lines. Nutrients 2017, 9, 1008. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of Drug Permeability and Prediction of Drug Absorption in Caco-2 Monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Shiu, S.-M.; Wu, M.-T.; Chen, W.-L.; Wang, S.-G.; Lee, H.-M. Monacolin K Affects Lipid Metabolism through SIRT1/AMPK Pathway in HepG2 Cells. Arch. Pharm. Res. 2013, 36, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Kim, H. Protective Effects of Lactic Acid Bacteria Against TLR4 Induced Inflammatory Response in Hepatoma HepG2 Cells Through Modulation of Toll-Like Receptor Negative Regulators of Mitogen-Activated Protein Kinase and NF-ΚB Signaling. Front. Immunol. 2018, 9, 1537. [Google Scholar] [CrossRef] [PubMed]
- Bort, A.; Sánchez, B.G.; Mateos-Gómez, P.A.; Díaz-Laviada, I.; Rodríguez-Henche, N. Capsaicin Targets Lipogenesis in HepG2 Cells Through AMPK Activation, AKT Inhibition and PPARs Regulation. Int. J. Mol. Sci. 2019, 20, 1660. [Google Scholar] [CrossRef]
- Sun, Z.; Cao, H.; Liu, Q.S.; Liang, Y.; Fiedler, H.; Zhang, J.; Zhou, Q.; Jiang, G. 4-Hexylphenol Influences Adipogenic Differentiation and Hepatic Lipid Accumulation in Vitro. Environ. Pollut. 2021, 268, 115635. [Google Scholar] [CrossRef]
- Rendine, M.; Venturi, S.; Marino, M.; Gardana, C.; Møller, P.; Martini, D.; Del Bo, C. Effects of Quercetin Metabolites on Glucose-Dependent Lipid Accumulation in 3T3-L1 Adipocytes. Mol. Nutr. Food Res. 2025, e70070. [Google Scholar] [CrossRef]
- Hwang, S.-H.; Lee, M. Autophagy Inhibition in 3T3-L1 Adipocytes Breaks the Crosstalk with Tumor Cells by Suppression of Adipokine Production. Anim. Cells Syst. 2020, 24, 17–25. [Google Scholar] [CrossRef]
- Park, S.S.; Lee, Y.J.; Kang, H.; Yang, G.; Hong, E.J.; Lim, J.Y.; Kim, E. Lactobacillus amylovorus KU4 ameliorates diet-induced obesity in mice by promoting adipose browning through PPARγ signaling. Sci. Rep. 2019, 9, 20152. [Google Scholar] [CrossRef]
- Hong, Y.; Lin, Y.; Si, Q.; Yang, L.; Dong, W.; Gu, X. Ginsenoside Rb2 alleviates obesity by activation of brown fat and induction of browning of white fat. Front. Endocrinol. 2019, 10, 153. [Google Scholar] [CrossRef]
- Christides, T.; Wray, D.; McBride, R.; Fairweather, R.; Sharp, P. Iron Bioavailability from Commercially Available Iron Supplements. Eur. J. Nutr. 2015, 54, 1345–1352. [Google Scholar] [CrossRef]
- Guha, S.; Alvarez, S.; Majumder, K. Transport of Dietary Anti-Inflammatory Peptide, γ-Glutamyl Valine (γ-EV), across the Intestinal Caco-2 Monolayer. Nutrients 2021, 13, 1448. [Google Scholar] [CrossRef] [PubMed]
- Challinor, V.L.; Stuthe, J.M.; Parsons, P.G.; Lambert, L.K.; Lehmann, R.P.; Kitching, W.; De Voss, J.J. Structure and bioactivity of steroidal saponins isolated from the roots of Chamaelirium luteum (false unicorn). J. Nat. Prod. 2012, 75, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Papazian, D.; Würtzen, P.A.; Hansen, S.W. Polarized airway epithelial models for immunological co-culture studies. Int. Arch. Allergy Immunol. 2016, 170, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, J.; Liu, Y.; Chen, L.; Yu, C. TNF-α Regulates the Proteolytic Degradation of ST6Gal-1 and Endothelial Cell-Cell Junctions through Upregulating Expression of BACE1. Sci. Rep. 2017, 7, 40256. [Google Scholar] [CrossRef]
- Galla, R.; Grisenti, P.; Farghali, M.; Saccuman, L.; Ferraboschi, P.; Uberti, F. Ovotransferrin Supplementation Improves the Iron Absorption: An In Vitro Gastro-Intestinal Model. Biomedicines 2021, 9, 1543. [Google Scholar] [CrossRef]
- Guan, C.; Chen, X.; Jiang, X.; Zhao, R.; Yuan, Y.; Chen, D.; Zhang, C.; Lu, M.; Lu, Z.; Gu, R. In Vitro Studies of Adhesion Properties of Six Lactic Acid Bacteria Isolated from the Longevous Population of China. RSC Adv. 2020, 10, 24234–24240. [Google Scholar] [CrossRef]
- Molinari, C.; Morsanuto, V.; Ghirlanda, S.; Ruga, S.; Notte, F.; Gaetano, L.; Uberti, F. Role of Combined Lipoic Acid and Vitamin D3 on Astrocytes as a Way to Prevent Brain Ageing by Induced Oxidative Stress and Iron Accumulation. Oxid. Med. Cell Longev. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Molinari, C.; Ruga, S.; Farghali, M.; Galla, R.; Bassiouny, A.; Uberti, F. Preventing C2c12 Muscular Cells Damage Combining Magnesium and Potassium with Vitamin D3 and Curcumin. J. Tradit. Complement. Med. 2021, 11, 532–544. [Google Scholar] [CrossRef]
- Allers, K.; Stahl-Hennig, C.; Fiedler, T.; Wibberg, D.; Hofmann, J.; Kunkel, D.; Moos, V.; Kreikemeyer, B.; Kalinowski, J.; Schneider, T. The Colonic Mucosa-Associated Microbiome in SIV Infection: Shift towards Bacteroidetes Coincides with Mucosal CD4+ T Cell Depletion and Enterocyte Damage. Sci. Rep. 2020, 10, 10887. [Google Scholar] [CrossRef]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, e61122. [Google Scholar] [CrossRef]
- Wang, P.; Liang, Y.; Chen, K.; Yau, S.-Y.; Sun, X.; Cheng, K.K.-Y.; Xu, A.; So, K.-F.; Li, A. Potential Involvement of Adiponectin Signaling in Regulating Physical Exercise-Elicited Hippocampal Neurogenesis and Dendritic Morphology in Stressed Mice. Front. Cell Neurosci. 2020, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.-N.; Lee, S.; Wang, X.; Wee, C.-D.; Yoon, H.-M.; Jung, E.-S.; Seo, M.-K.; Kwon, Y.; Jang, K.-A.; Kim, H.-R. What Is the Relationship between Antioxidant Efficacy, Functional Composition, and Genetic Characteristics in Comparing Soybean Resources by Year? Antioxidants 2022, 11, 2249. [Google Scholar] [CrossRef] [PubMed]
- Ruga, S.; Galla, R.; Ferrari, S.; Invernizzi, M.; Uberti, F. Novel Approach to the Treatment of Neuropathic Pain Using a Combination with Palmitoylethanolamide and Equisetum arvense L. in an In Vitro Study. Int. J. Mol. Sci. 2023, 24, 5503. [Google Scholar] [CrossRef] [PubMed]
- Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Ferrari, S.; Galla, R.; Mulè, S.; Uberti, F. Analysis of the Beneficial Effects of Probiotics on the Gut–Prostate Axis Using Prostatic Co-Culture Model. Foods 2024, 13, 3647. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity and Its Impact on Metabolic Syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Mese-Tayfur, S.; Demirel-Yalcıner, T.; Migni, A.; Bartolini, D.; Galli, F.; Ozer, N.K.; Sozen, E. Modulation of Inflammatory Signaling by Vitamin E Metabolites and Its Therapeutic Implications. Free Radic. Res. 2025, 59, 86–101. [Google Scholar] [CrossRef]
- Beyoğlu, D.; Popov, Y.V.; Idle, J.R. Metabolomic Hallmarks of Obesity and Metabolic Dysfunction-Associated Steatotic Liver Disease. Int. J. Mol. Sci. 2024, 25, 12809. [Google Scholar] [CrossRef]
- Hao, S. Liver Function. In Artificial Liver; Springer Nature: Singapore, 2020. [Google Scholar]
- Álvarez-Arraño, V.; Martín-Peláez, S. Effects of Probiotics and Synbiotics on Weight Loss in Subjects with Overweight or Obesity: A Systematic Review. Nutrients 2021, 13, 3627. [Google Scholar] [CrossRef]
- Angelakis, E.; Armougom, F.; Million, M.; Raoult, D. The Relationship Between Gut Microbiota and Weight Gain in Humans. Future Microbiol. 2012, 7, 91–109. [Google Scholar] [CrossRef]
- Baothman, O.A.; Zamzami, M.A.; Taher, I.; Abubaker, J.; Abu-Farha, M. The Role of Gut Microbiota in the Development of Obesity and Diabetes. Lipids Health Dis. 2016, 15, 108. [Google Scholar] [CrossRef]
- Yadav, H.; Lee, J.-H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial Metabolic Effects of a Probiotic via Butyrate-Induced GLP-1 Hormone Secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.-Y.; Zhang, H.; Tan, P.-C.; Zhou, S.-B.; Li, Q.-F. Adipose Tissue Aging: Mechanisms and Therapeutic Implications. Cell Death Dis. 2022, 13, 300. [Google Scholar] [CrossRef] [PubMed]
- Poulos, S.P.; Dodson, M.V.; Culver, M.F.; Hausman, G.J. The Increasingly Complex Regulation of Adipocyte Differentiation. Exp. Biol. Med. 2016, 241, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ojeda, F.; Rupérez, A.; Gomez-Llorente, C.; Gil, A.; Aguilera, C. Cell Models and Their Application for Studying Adipogenic Differentiation in Relation to Obesity: A Review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef]
- Urzì, O.; Gasparro, R.; Costanzo, E.; De Luca, A.; Giavaresi, G.; Fontana, S.; Alessandro, R. Three-Dimensional Cell Cultures: The Bridge between In Vitro and In Vivo Models. Int. J. Mol. Sci. 2023, 24, 12046. [Google Scholar] [CrossRef]
- Guru, A.; Issac, P.K.; Velayutham, M.; Saraswathi, N.T.; Arshad, A.; Arockiaraj, J. Molecular Mechanism of Down-Regulating Adipogenic Transcription Factors in 3T3-L1 Adipocyte Cells by Bioactive Anti-Adipogenic Compounds. Mol. Biol. Rep. 2021, 48, 743–761. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, Y.; Miao, K.; Zhang, S.; Deng, F.; Zhu, M.; Wang, C.; Gu, W.; Huang, Y.; Shao, Z.; et al. PPARγ As a Potential Target for Adipogenesis Induced by Fine Particulate Matter in 3T3-L1 Preadipocytes. Environ. Sci. Technol. 2023, 57, 7684–7697. [Google Scholar] [CrossRef]
- Malik, S.; Inamdar, S.; Acharya, J.; Goel, P.; Ghaskadbi, S. Characterization of Palmitic Acid Toxicity Induced Insulin Resistance in HepG2 Cells. Toxicol. In Vitro 2024, 97, 105802. [Google Scholar] [CrossRef]
- van Deuren, T.; Blaak, E.E.; Canfora, E.E. Butyrate to Combat Obesity and Obesity-associated Metabolic Disorders: Current Status and Future Implications for Therapeutic Use. Obes. Rev. 2022, 23, e13498. [Google Scholar] [CrossRef]
- Coppola, S.; Avagliano, C.; Calignano, A.; Berni Canani, R. The Protective Role of Butyrate against Obesity and Obesity-Related Diseases. Molecules 2021, 26, 682. [Google Scholar] [CrossRef]
- Coppola, S.; Nocerino, R.; Paparo, L.; Bedogni, G.; Calignano, A.; Di Scala, C.; de Giovanni di Santa Severina, A.F.; De Filippis, F.; Ercolini, D.; Berni Canani, R. Therapeutic Effects of Butyrate on Pediatric Obesity. JAMA Netw. Open 2022, 5, e2244912. [Google Scholar] [CrossRef] [PubMed]
- Daquinag, A.C.; Gao, Z.; Fussell, C.; Immaraj, L.; Pasqualini, R.; Arap, W.; Akimzhanov, A.M.; Febbraio, M.; Kolonin, M.G. Fatty Acid Mobilization from Adipose Tissue Is Mediated by CD36 Posttranslational Modifications and Intracellular Trafficking. JCI Insight 2021, 6, e147057. [Google Scholar] [CrossRef] [PubMed]
- Macchi, C.; Greco, M.F.; Botta, M.; Sperandeo, P.; Dongiovanni, P.; Valenti, L.; Cicero, A.F.G.; Borghi, C.; Lupo, M.G.; Romeo, S.; et al. Leptin, Resistin, and Proprotein Convertase Subtilisin/Kexin Type 9. Am. J. Pathol. 2020, 190, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Stienstra, R.; Duval, C.; Müller, M.; Kersten, S. PPARs, Obesity, and Inflammation. PPAR Res. 2007, 2007, 95974. [Google Scholar] [CrossRef]
- Zhou, D.; Samovski, D.; Okunade, A.L.; Stahl, P.D.; Abumrad, N.A.; Su, X. CD36 level and trafficking are determinants of lipolysis in adipocytes. FASEB J. 2012, 26, 4733. [Google Scholar] [CrossRef]
- Vroegrijk, I.O.; van Klinken, J.B.; van Diepen, J.A.; van den Berg, S.A.; Febbraio, M.; Steinbusch, L.K.; Glatz, J.F.; Havekes, L.M.; Voshol, P.J.; Rensen, P.C.; et al. CD36 is important for adipocyte recruitment and affects lipolysis. Obesity 2013, 21, 2037–2045. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, K.Y.; Jung, S.H.; Kim, H.S.; Shim, G.; Kim, M.G.; Lee, B.H. Activation of AMPK by berberine induces hepatic lipid accumulation by upregulation of fatty acid translocase CD36 in mice. Toxicol. Appl. Pharmacol. 2017, 316, 74–82. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Cui, W.; Silverstein, R.L. CD36, a signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate. J. Exp. Med. 2022, 219, e20211314. [Google Scholar] [CrossRef]
- Su, M.; Cao, J.; Huang, J.; Liu, S.; Im, D.; Yoo, J.-W.; Jung, J. The In Vitro and In Vivo Anti-Inflammatory Effects of a Phthalimide PPAR-γ Agonist. Mar. Drugs 2017, 15, 7. [Google Scholar] [CrossRef]
- Rowlands, J.; Heng, J.; Newsholme, P.; Carlessi, R. Pleiotropic Effects of GLP-1 and Analogs on Cell Signaling, Metabolism, and Function. Front. Endocrinol. 2018, 9, 95974. [Google Scholar] [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Measurement and Clinical Significance of Lipid Peroxidation as a Biomarker of Oxidative Stress: Oxidative Stress in Diabetes, Atherosclerosis, and Chronic Inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Crujeiras, A.B.; Parra, D.; Milagro, F.I.; Goyenechea, E.; Larrarte, E.; Margareto, J.; Martínez, J.A. Differential Expression of Oxidative Stress and Inflammation Related Genes in Peripheral Blood Mononuclear Cells in Response to a Low-Calorie Diet: A Nutrigenomics Study. OMICS 2008, 12, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Kuennen, M.R. Interaction Between Race and Weight Loss Intervention Strategy: Effect on Markers of Inflammation and Fat Distribution in Overweight Women. Obesity 2012, 20, 1335–1336. [Google Scholar] [CrossRef] [PubMed]
- WANG, S.-L.; LI, Y.; WEN, Y.; CHEN, Y.-F.; NA, L.-X.; LI, S.-T.; SUN, C.-H. Curcumin, a Potential Inhibitor of Up-Regulation of TNF-Alpha and IL-6 Induced by Palmitate in 3T3-L1 Adipocytes through NF-KappaB and JNK Pathway. Biomed. Environ. Sci. 2009, 22, 32–39. [Google Scholar] [CrossRef]
- Pratelli, G.; Di Liberto, D.; Carlisi, D.; Emanuele, S.; Giuliano, M.; Notaro, A.; De Blasio, A.; Calvaruso, G.; D’Anneo, A.; Lauricella, M. Hypertrophy and ER Stress Induced by Palmitate Are Counteracted by Mango Peel and Seed Extracts in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2023, 24, 5419. [Google Scholar] [CrossRef]
- Pelczyńska, M.; Moszak, M.; Wesołek, A.; Bogdański, P. The Preventive Mechanisms of Bioactive Food Compounds against Obesity-Induced Inflammation. Antioxidants 2023, 12, 1232. [Google Scholar] [CrossRef]
- Musso, G.; Bo, S.; Cassader, M.; De Michieli, F.; Gambino, R. Impact of Sterol Regulatory Element-Binding Factor-1c Polymorphism on Incidence of Nonalcoholic Fatty Liver Disease and on the Severity of Liver Disease and of Glucose and Lipid Dysmetabolism. Am. J. Clin. Nutr. 2013, 98, 895–906. [Google Scholar] [CrossRef]
- Barbhuiya, P.A.; Yoshitomi, R.; Pathak, M.P. Understanding the Link Between Sterol Regulatory Element Binding Protein (SREBPs) and Metabolic Dysfunction Associated Steatotic Liver Disease (MASLD). Curr. Obes. Rep. 2025, 14, 36. [Google Scholar] [CrossRef]
- Park, J.-E.; Oh, S.-H.; Cha, Y.-S. Lactobacillus Plantarum LG42 Isolated from Gajami Sik-Hae Inhibits Adipogenesis in 3T3-L1 Adipocyte. Biomed. Res. Int. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Dong, L.; Hu, X.; Feng, F.; Chen, F. 6-Gingerol, a Functional Polyphenol of Ginger, Promotes Browning through an AMPK-Dependent Pathway in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2019, 67, 14056–14065. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Mao, S.; Chen, S.; Zhang, W.; Liu, C. PPARs-orchestrated metabolic homeostasis in the adipose tissue. Int. J. Mol. Sci. 2021, 22, 8974. [Google Scholar] [CrossRef] [PubMed]
- Flori, L.; Galgani, G.; Bray, G.; Ippolito, C.; Segnani, C.; Pellegrini, C.; Calderone, V. Development of an adipocyte differentiation protocol using 3T3-L1 cells for the investigation of the browning process: Identification of the PPAR-γ agonist rosiglitazone as a browning reference drug. Front. Pharmacol. 2025, 16, 1546456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Fang, H.; Guo, F.; Li, F.; Chen, A.; Huang, S. Flavonoids as Inducers of White Adipose Tissue Browning and Thermogenesis: Signalling Pathways and Molecular Triggers. Nutr. Metab. 2019, 16, 47. [Google Scholar] [CrossRef]
- Sung, Y.-Y.; Son, E.; Im, G.; Kim, D.-S. Herbal Combination of Phyllostachys Pubescens and Scutellaria Baicalensis Inhibits Adipogenesis and Promotes Browning via AMPK Activation in 3T3-L1 Adipocytes. Plants 2020, 9, 1422. [Google Scholar] [CrossRef]
- Bu, S.; Yuan, C.; Cao, F.; Xu, Q.; Zhang, Y.; Ju, R.; Chen, L.; Li, Z. Concentrated Extract of Prunus Mume Fruit Exerts Dual Effects in 3T3-L1 Adipocytes by Inhibiting Adipogenesis and Inducing Beiging/Browning. Food Nutr. Res. 2021, 65, 5492. [Google Scholar] [CrossRef]
- Lo, K.A.; Sun, L. Turning WAT into BAT: A Review on Regulators Controlling the Browning of White Adipocytes. Biosci. Rep. 2013, 33, 711–719. [Google Scholar] [CrossRef]
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016, 26, 190–201. [Google Scholar] [CrossRef]









| Probiotic Strain | Accession Number | Concentration in CFU | Concentration in mg |
|---|---|---|---|
| Bifidobacterium bifidum GM-25 | DSM 34624 | 1 × 108 CFU–1 × 109 CFU | 1 mg–10 mg |
| Bifidobacterium infantis GM-21 | DSM 34621 | 1 × 108 CFU–1 × 109 CFU | 1 mg–10 mg |
| Lacticaseibacillus rhamnosus GM-28 | DSM 34619 | 1 × 108 CFU–1 × 109 CFU | 0.33 g–3.3 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulè, S.; Galla, R.; Parini, F.; Botta, M.; Ferrari, S.; Uberti, F. An In Vitro Gut–Liver–Adipose Axis Model to Evaluate the Anti-Obesity Potential of a Novel Probiotic–Polycosanol Combination. Foods 2025, 14, 2003. https://doi.org/10.3390/foods14112003
Mulè S, Galla R, Parini F, Botta M, Ferrari S, Uberti F. An In Vitro Gut–Liver–Adipose Axis Model to Evaluate the Anti-Obesity Potential of a Novel Probiotic–Polycosanol Combination. Foods. 2025; 14(11):2003. https://doi.org/10.3390/foods14112003
Chicago/Turabian StyleMulè, Simone, Rebecca Galla, Francesca Parini, Mattia Botta, Sara Ferrari, and Francesca Uberti. 2025. "An In Vitro Gut–Liver–Adipose Axis Model to Evaluate the Anti-Obesity Potential of a Novel Probiotic–Polycosanol Combination" Foods 14, no. 11: 2003. https://doi.org/10.3390/foods14112003
APA StyleMulè, S., Galla, R., Parini, F., Botta, M., Ferrari, S., & Uberti, F. (2025). An In Vitro Gut–Liver–Adipose Axis Model to Evaluate the Anti-Obesity Potential of a Novel Probiotic–Polycosanol Combination. Foods, 14(11), 2003. https://doi.org/10.3390/foods14112003







