From Molecular to Macroscopic: Dual-Pathway Regulation of Carrot Whole Flour on the Gluten-Starch System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Chemical Compositions
2.3. Preparation of Steamed Cake
2.4. Steamed Cake Characteristics
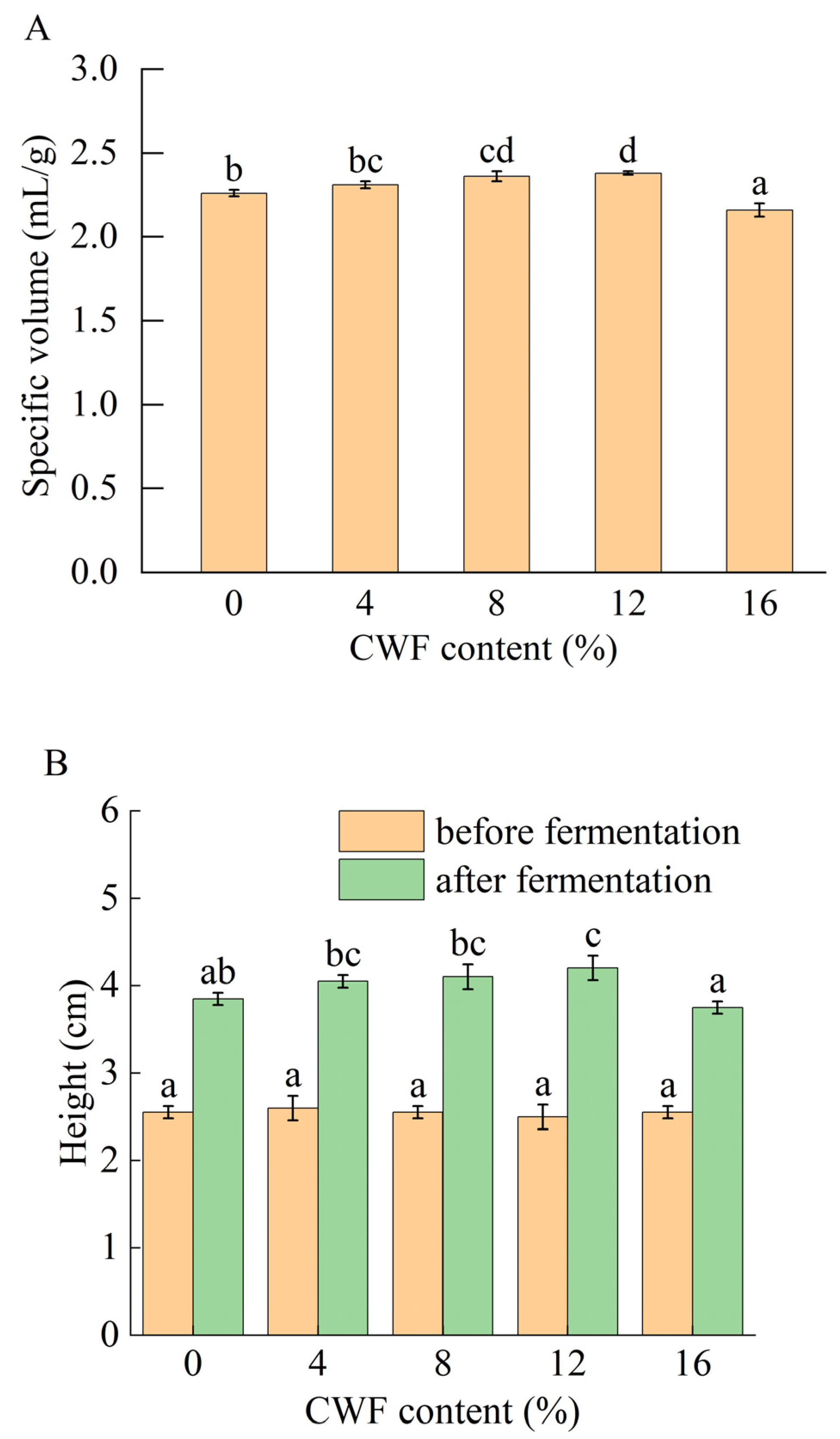
2.4.1. Specific Volume
2.4.2. Texture Analysis
2.4.3. Color
2.4.4. Sensory Evaluation
2.5. Preparation of Samples
2.5.1. Preparation of Dietary Fiber and Polyphenols
2.5.2. Preparation of Gluten and Starch
2.6. Gluten Properties
2.6.1. Free Sulfhydryl and Disulfide Bonds Analysis
2.6.2. Intermolecular Interactions Analysis
2.6.3. Secondary Structure Analysis
2.7. Starch Properties
2.7.1. Pasting Properties
2.7.2. Short-Range Order
2.7.3. Starch Crystallinity
2.8. Statistical Analysis
3. Results
3.1. Chemical Compositions
3.2. Steamed Cake Characteristics
3.2.1. Volumetric Properties
3.2.2. Texture Analysis
3.2.3. Color
3.2.4. Sensory Evaluation
3.3. Gluten Properties
3.3.1. Free Sulfhydryl and Disulfide Bond Content
3.3.2. Intermolecular Interaction Force
3.3.3. Secondary Structure
3.4. Starch Properties
3.4.1. Pasting Properties
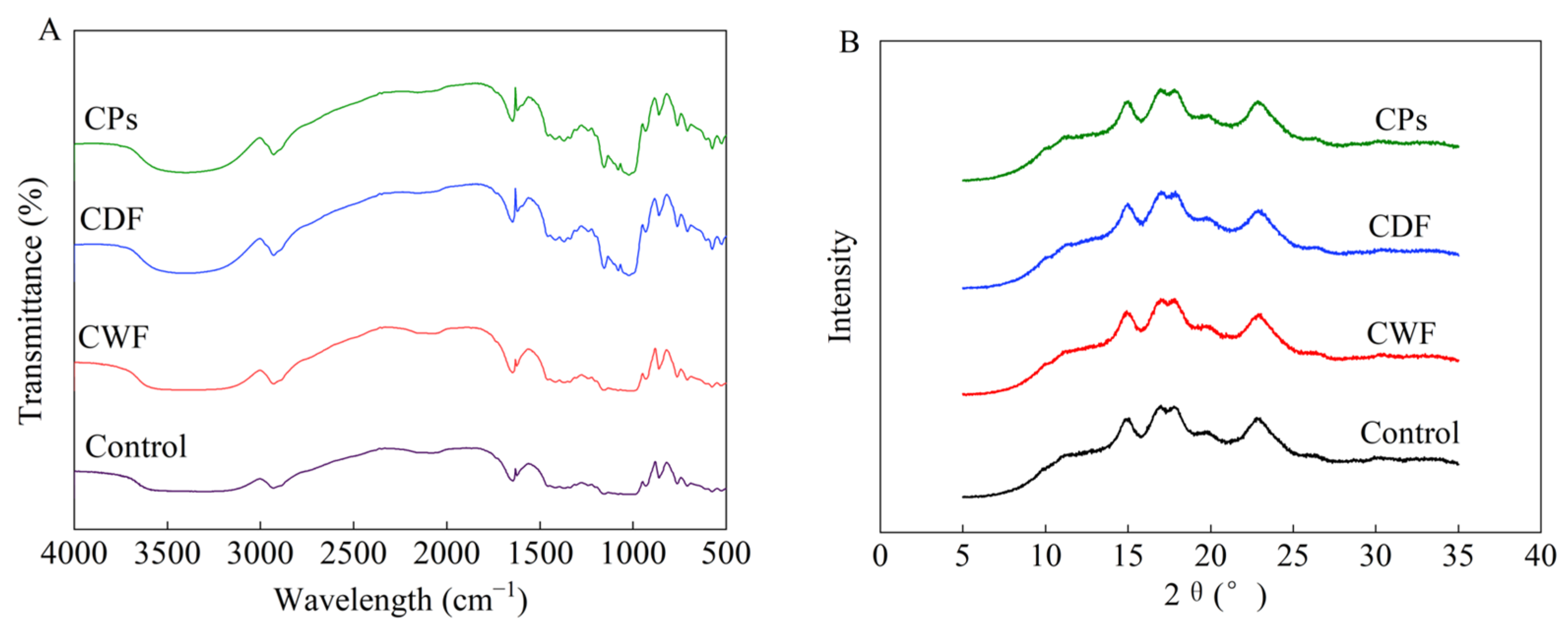
3.4.2. Short-Range Ordered Analysis
3.4.3. X-Ray Diffraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qian, X.; Sun, B.; Gu, Y.; Tian, X.; Ma, S.; Wang, X. Milling and roasting impact pasting and rheological properties of oat flours and quality of steamed oat cakes. LWT 2023, 175, 114477. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, F. Changes in structure and phenolic profiles during processing of steamed bread enriched with purple sweetpotato flour. Food Chem. 2022, 369, 130578. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Grover, K.; Dhillon, T.S.; Chawla, N.; Kaur, A. Development and quality evaluation of polyphenols enriched black carrot (Daucus carota L.) powder incorporated bread. Heliyon 2024, 10, e25109. [Google Scholar] [CrossRef]
- Ng, S.H.; Robert, S.D.; Wan Ahmad, W.A.; Wan Ishak, W.R. Incorporation of dietary fibre-rich oyster mushroom (Pleurotus sajor-caju) powder improves postprandial glycaemic response by interfering with starch granule structure and starch digestibility of biscuit. Food Chem. 2017, 227, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Tolve, R.; Pasini, G.; Vignale, F.; Favati, F.; Simonato, B. Effect of Grape Pomace Addition on the Technological, Sensory, and Nutritional Properties of Durum Wheat Pasta. Foods 2020, 9, 354. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A. Transformation of carrots into novel food ingredients and innovative healthy foods. Appl. Food Res. 2023, 3, 100303. [Google Scholar] [CrossRef]
- Sun, M.; Yang, T.; Qiao, X.-H.; Zhao, P.; Zhu, Z.-P.; Wang, G.-L.; Xu, L.-L.; Xiong, A.-S. Nitric oxide regulates the lignification and carotenoid biosynthesis of postharvest carrot (Daucus carota L.). Postharvest Biol. Technol. 2024, 207, 112593. [Google Scholar] [CrossRef]
- Bhatta, S.; Stevanovic Janezic, T.; Ratti, C. Freeze-Drying of Plant-Based Foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef]
- Li, X.; Yi, J.; He, J.; Dong, J.; Duan, X. Comparative evaluation of quality characteristics of fermented napa cabbage subjected to hot air drying, vacuum freeze drying, and microwave freeze drying. LWT 2024, 192, 115740. [Google Scholar] [CrossRef]
- Borah, M.S.; Bhagya Raj, G.V.S.; Tiwari, A.; Dash, K.K. Effect of intermittent microwave convective drying on quality characteristics of persimmon fruit. J. Agric. Food Res. 2023, 14, 100816. [Google Scholar] [CrossRef]
- Omidiran, A.T.; Odukoya, O.J.; Akinbule, O.O.; Sobukola, O.P. Effect of microwave-assisted pre-drying and deep-fat-frying conditions on some quality attributes of orange fleshed sweetpotato chips. Food Chem. Adv. 2023, 3, 100534. [Google Scholar] [CrossRef]
- Kriaa, K.; Nassar, A.F. Comparative study of pretreatment on microwave drying of Gala apples (Malus pumila): Effect of blanching, electric field and freezing. LWT 2022, 165, 113693. [Google Scholar] [CrossRef]
- Su, D.; Lv, W.; Wang, Y.; Wang, L.; Li, D. Influence of microwave hot-air flow rolling dry-blanching on microstructure, water migration and quality of pleurotus eryngii during hot-air drying. Food Control 2020, 114, 107228. [Google Scholar] [CrossRef]
- Keskin, M.; Guclu, G.; Sekerli, Y.E.; Soysal, Y.; Selli, S.; Kelebek, H. Comparative assessment of volatile and phenolic profiles of fresh black carrot (Daucus carota L.) and powders prepared by three drying methods. Sci. Hortic-Amst. 2021, 287, 110256. [Google Scholar] [CrossRef]
- Wang, X.; Appels, R.; Zhang, X.; Bekes, F.; Torok, K.; Tomoskozi, S.; Diepeveen, D.; Ma, W.; Islam, S. Protein-transitions in and out of the dough matrix in wheat flour mixing. Food Chem. 2017, 217, 542–551. [Google Scholar] [CrossRef]
- Chen, S.-X.; Ni, Z.-J.; Thakur, K.; Wang, S.; Zhang, J.-G.; Shang, Y.-F.; Wei, Z.-J. Effect of grape seed power on the structural and physicochemical properties of wheat gluten in noodle preparation system. Food Chem. 2021, 355, 129500. [Google Scholar] [CrossRef]
- Han, C.; Ma, M.; Li, M.; Sun, Q. Further interpretation of the underlying causes of the strengthening effect of alkali on gluten and noodle quality: Studies on gluten, gliadin, and glutenin. Food Hydrocolloid 2020, 103, 105661. [Google Scholar] [CrossRef]
- Ahmad, M.; Wani, T.A.; Wani, S.M.; Masoodi, F.A.; Gani, A. Incorporation of carrot pomace powder in wheat flour: Effect on flour, dough and cookie characteristics. J. Food Sci. Technol. 2016, 53, 3715–3724. [Google Scholar] [CrossRef] [PubMed]
- Kultys, E.; Moczkowska-Wyrwisz, M. Effect of using carrot pomace and beetroot-apple pomace on physicochemical and sensory properties of pasta. LWT 2022, 168, 113858. [Google Scholar] [CrossRef]
- Badjona, A.; Adubofuor, J.; Amoah, I.; Diako, C. Valorisation of carrot and pineapple pomaces for rock buns development. Sci. Afr. 2019, 6, e00160. [Google Scholar] [CrossRef]
- Landim Parente, G.D.; Nunes de Melo, B.D.; Albuquerque de Souza, J.; da Conceição, M.M.; Ubbink, J.; Mattos Braga, A.L. Fortification of traditional tapioca “pancakes” from the Brazilian northeast with microencapsulated carrot carotenoid. LWT 2021, 152, 112301. [Google Scholar] [CrossRef]
- Wunthunyarat, W.; Seo, H.S.; Wang, Y.J. Effects of germination conditions on enzyme activities and starch hydrolysis of long-grain brown rice in relation to flour properties and bread qualities. J. Food Sci. 2020, 85, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Influence of ingredients and chemical components on the quality of Chinese steamed bread. Food Chem. 2014, 163, 154–162. [Google Scholar] [CrossRef]
- He, L.; Chen, Y.; Zhang, H.; Wang, H.; Chen, S.; Liu, S.; Liu, A.; Li, Q.; Ao, X.; Liu, Y. Isolation and identification of Lactobacillus and yeast species and their effect on the quality of fermented rice cakes. Innov. Food Sci. Emerg. Technol. 2022, 77, 102984. [Google Scholar] [CrossRef]
- Dong, R.; Yu, Q.; Liao, W.; Liu, S.; He, Z.; Hu, X.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Composition of bound polyphenols from carrot dietary fiber and its in vivo and in vitro antioxidant activity. Food Chem. 2021, 339, 127879. [Google Scholar] [CrossRef]
- Dong, R.; Liu, S.; Xie, J.; Chen, Y.; Zheng, Y.; Zhang, X.; Zhao, E.; Wang, Z.; Xu, H.; Yu, Q. The recovery, catabolism and potential bioactivity of polyphenols from carrot subjected to in vitro simulated digestion and colonic fermentation. Food Res. Int. 2021, 143, 110263. [Google Scholar] [CrossRef]
- Gong, Y.; Meng, A.; Zhang, Y.; Zhang, B.; Ying, D.; Guo, B.; Wei, Y. Effect of water migration rate on the deformation characteristics of wheat starch/gluten extruded noodles. LWT 2022, 163, 113546. [Google Scholar] [CrossRef]
- Tian, B.; Zhou, C.; Li, D.; Pei, J.; Guo, A.; Liu, S.; Li, H. Monitoring the Effects of Hemicellulase on the Different Proofing Stages of Wheat Aleurone-Rich Bread Dough and Bread Quality. Foods 2021, 10, 2427. [Google Scholar] [CrossRef]
- Zhou, R.; Sun, J.; Qian, H.; Li, Y.; Zhang, H.; Qi, X.; Wang, L. Effect of the frying process on the properties of gluten protein of you-tiao. Food Chem. 2020, 310, 125973. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, X.; Xie, Q.; Chen, L.; Chang, C.; Wu, W.; Xiao, S.; Wang, X. Effects of Laminaria japonica polysaccharides on the texture, retrogradation, and structure performances in frozen dough bread. LWT 2021, 151, 112239. [Google Scholar] [CrossRef]
- Cai, X.; Hong, Y.; Gu, Z.; Zhang, Y. The effect of electrostatic interactions on pasting properties of potato starch/xanthan gum combinations. Food Res. Int. 2011, 44, 3079–3086. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Guo, Z.; Wang, H.; Wang, A.; Li, Z.; Chen, Y.; Qiu, J. Effect of buckwheat hull particle-size on bread staling quality. Food Chem. 2023, 405, 134851. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, Y.; Sun, F.; Li, X.; Wang, P.; Sun, J.; Zeng, J.; Wang, C.; Hu, W.; Chang, J.; et al. Tannins improve dough mixing properties through affecting physicochemical and structural properties of wheat gluten proteins. Food Res. Int. 2015, 69, 64–71. [Google Scholar] [CrossRef]
- Aubert, C.; Bruaut, M.; Chalot, G. Spatial distribution of sugars, organic acids, vitamin C, carotenoids, tocopherols, 6-methoxymellein, polyacetylenic compounds, polyphenols and terpenes in two orange Nantes type carrots (Daucus carota L.). J. Food Compos. Anal. 2022, 108, 104421. [Google Scholar] [CrossRef]
- Almoumen, A.; Mohamed, H.; Sobti, B.; Ayyash, M.; Kamleh, R.; Al-Marzouqi, A.H.; Kamal-Eldin, A. Quality of bread rolls fortified with date fruit pomace: Structure, proximate composition, staling, and sensory evaluation. NFS J. 2025, 38, 100214. [Google Scholar] [CrossRef]
- Wang, L.; Shi, D.; Chen, J.; Dong, H.; Chen, L. Effects of Chinese chestnut powder on starch digestion, texture properties, and staling characteristics of bread. Grain Oil Sci. Technol. 2023, 6, 82–90. [Google Scholar] [CrossRef]
- Hsieh, P.-H.; Weng, Y.-M.; Yu, Z.-R.; Wang, B.-J. Substitution of wheat flour with wholegrain flours affects physical properties, sensory acceptance, and starch digestion of Chinese steam bread (Mantou). LWT 2017, 86, 571–576. [Google Scholar] [CrossRef]
- Li, M.; Dhital, S.; Wei, Y. Multilevel Structure of Wheat Starch and Its Relationship to Noodle Eating Qualities. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1042–1055. [Google Scholar] [CrossRef]
- Cui, R.; Fei, Y.; Zhu, F. Physicochemical, structural and nutritional properties of steamed bread fortified with red beetroot powder and their changes during breadmaking process. Food Chem. 2022, 383, 132547. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, T.; Su, C.; Li, Q.; Yu, X. Fortification of Chinese steamed bread with flaxseed flour and evaluation of its physicochemical and sensory properties. Food Chem. X 2022, 13, 100267. [Google Scholar] [CrossRef]
- Chikpah, S.K.; Korese, J.K.; Hensel, O.; Sturm, B.; Pawelzik, E. Rheological properties of dough and bread quality characteristics as influenced by the proportion of wheat flour substitution with orange-fleshed sweet potato flour and baking conditions. LWT 2021, 147, 111515. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Xiao, Y.; Zhang, H.; Li, C. Effects of rose powder on the physicochemical properties of wheat flour and dough, and the quality of Chinese steamed bun. LWT 2025, 215, 117295. [Google Scholar] [CrossRef]
- Wu, C.; Liu, R.; Huang, W.; Rayas-Duarte, P.; Wang, F.; Yao, Y. Effect of sourdough fermentation on the quality of Chinese Northern-style steamed breads. J. Cereal Sci. 2012, 56, 127–133. [Google Scholar] [CrossRef]
- Johansson, E.; Malik, A.H.; Hussain, A.; Rasheed, F.; Newson, W.R.; Plivelic, T.; Hedenqvist, M.S.; Gällstedt, M.; Kuktaite, R. Wheat Gluten Polymer Structures: The Impact of Genotype, Environment, and Processing on Their Functionality in Various Applications. Cereal Chem. 2013, 90, 367–376. [Google Scholar] [CrossRef]
- Li, M.; Ma, S. Effects of interaction between wheat bran dietary fiber and gluten protein on gluten protein aggregation behavior. Int. J. Biol. Macromol. 2024, 283, 137692. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Hu, H.; Yang, J.; Wu, T.; Sun, X.; Fang, Y.; Huang, Q. Mechanistic study of the impact of germinated brown rice flour on gluten network formation, dough properties and bread quality. Innov. Food Sci. Emerg. Technol. 2023, 83, 103217. [Google Scholar] [CrossRef]
- Jia, F.; Wang, H.; Zhao, L.; Qiao, Z.; Wang, Y.; Wang, R.; Ma, J.; Zhang, L.; Liang, Y.; Wang, J. Effect of sweet potato flour on pasting, aggregation properties and dough quality of wheat flour. LWT 2023, 188, 115440. [Google Scholar] [CrossRef]
- Manu, B.T.; Prasada Rao, U.J.S. Influence of size distribution of proteins, thiol and disulfide content in whole wheat flour on rheological and chapati texture of Indian wheat varieties. Food Chem. 2008, 110, 88–95. [Google Scholar] [CrossRef]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, S.; Sun, B.; Wang, F.; Huang, J.; Wang, X.; Bao, Q. Effects of thermal properties and behavior of wheat starch and gluten on their interaction: A review. Int. J. Biol. Macromol. 2021, 177, 474–484. [Google Scholar] [CrossRef]
- Shewry, P.R.; Belton, P.S. What do we really understand about wheat gluten structure and functionality? J. Cereal Sci. 2024, 117, 103895. [Google Scholar] [CrossRef]
- Qin, W.; Pi, J.; Zhang, G. The interaction between tea polyphenols and wheat gluten in dough formation and bread making. Food Funct. 2022, 13, 12827–12835. [Google Scholar] [CrossRef] [PubMed]
- Nawrocka, A.; Miś, A.; Szymańska-Chargot, M. Characteristics of Relationships Between Structure of Gluten Proteins and Dough Rheology—Influence of Dietary Fibres Studied by FT-Raman Spectroscopy. Food Biophys. 2015, 11, 81–90. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, X.; Liu, T.; Cai, Y.; Deng, X.; Zhao, M.; Zhao, Q. Effects of apple fiber on the physicochemical properties and baking quality of frozen dough during frozen storage. Food Chem. 2024, 440, 138194. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Bai, J.; Zhang, J.; Liu, X.; Zhang, H. Effect of resting time on water distribution and gluten formation of dough. LWT 2024, 204, 116425. [Google Scholar] [CrossRef]
- Zhao, B.; Hou, L.; Liu, X.; Wu, C.; Liu, T.; Li, H. Effect of enzymatically interesterified rapeseed oil-based plastic fats on the dough properties and corresponding steamed bread. Food Biosci. 2024, 62, 105295. [Google Scholar] [CrossRef]
- Chen, G.; Hu, R.; Li, Y. Potassium chloride affects gluten microstructures and dough characteristics similarly as sodium chloride. J. Cereal Sci. 2018, 82, 155–163. [Google Scholar] [CrossRef]
- Fevzioglu, M.; Ozturk, O.K.; Hamaker, B.R.; Campanella, O.H. Quantitative approach to study secondary structure of proteins by FT-IR spectroscopy, using a model wheat gluten system. Int. J. Biol. Macromol. 2020, 164, 2753–2760. [Google Scholar] [CrossRef]
- Gao, X.; Liu, T.; Yu, J.; Li, L.; Feng, Y.; Li, X. Influence of high-molecular-weight glutenin subunit composition at Glu-B1 locus on secondary and micro structures of gluten in wheat (Triticum aestivum L.). Food Chem. 2016, 197, 1184–1190. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Zhao, J.; Mu, M.; Jia, F.; Wang, Q.; Liang, Y.; Wang, J. Aggregative and structural properties of wheat gluten induced by pectin. J. Cereal Sci. 2021, 100, 103247. [Google Scholar] [CrossRef]
- Xing, B.; Zhang, Z.; Zhu, M.; Teng, C.; Zou, L.; Liu, R.; Zhang, L.; Yang, X.; Ren, G.; Qin, P. The gluten structure, starch digestibility and quality properties of pasta supplemented with native or germinated quinoa flour. Food Chem. 2023, 399, 133976. [Google Scholar] [CrossRef] [PubMed]
- Bock, J.E.; Damodaran, S. Bran-induced changes in water structure and gluten conformation in model gluten dough studied by Fourier transform infrared spectroscopy. Food Hydrocolloid 2013, 31, 146–155. [Google Scholar] [CrossRef]
- Zhang, S.; Nie, Y.; Li, H.; Zhu, D.; Liu, H.; Yang, L. The gluten aggregation behavior and quality of whole wheat steamed buns during proofing. J. Cereal Sci. 2025, 121, 104081. [Google Scholar] [CrossRef]
- Nawrocka, A.; Szymańska-Chargot, M.; Miś, A.; Kowalski, R.; Gruszecki, W.I. Raman studies of gluten proteins aggregation induced by dietary fibres. Food Chem. 2016, 194, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, S.; Zheng, X.; Li, L. Studies on the formation mechanism and multiscale structure of wheat bran dietary fiber-gluten protein complex. Food Biosci. 2025, 66, 106219. [Google Scholar] [CrossRef]
- Zhang, K.; Wen, Q.; Li, T.; Liu, Q.; Wang, Y.; Huang, J. Comparison of interaction mechanism between chlorogenic acid/luteolin and glutenin/gliadin by multi-spectroscopic and thermodynamic methods. J. Mol. Struct. 2021, 1246, 131219. [Google Scholar] [CrossRef]
- Kotsiou, K.; Palassaros, G.; Matsakidou, A.; Mouzakitis, C.-K.; Biliaderis, C.G.; Lazaridou, A. Roasted-sprouted lentil flour as a novel ingredient for wheat flour substitution in breads: Impact on dough properties and quality attributes. Food Hydrocolloid 2023, 145, 109164. [Google Scholar] [CrossRef]
- Mei, Z.; Wang, W.; Feng, X.; Yu, C.; Chen, L.; Chen, H.; Lin, S. Mechanism underlying the effect of soluble oat β-glucan and tea polyphenols on wheat gluten aggregation characteristics. Int. J. Biol. Macromol. 2025, 288, 138669. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Williams, P.A.; Shu, J.; Luo, S.; Chen, J.; Liu, C. Pectin adsorption onto and penetration into starch granules and the effect on the gelatinization process and rheological properties. Food Hydrocolloid 2022, 129, 107618. [Google Scholar] [CrossRef]
- Bernaerts, T.; De Laet, E.; Hendrickx, M.; Van Loey, A. How particle size reduction improves pectin extraction efficiency in carrot pomace. LWT 2024, 201, 116242. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, X.; Wu, Y.; Zhou, T.; Zhang, S.; Leng, J.; Le, Y.; Zhao, W. Understanding the influence of β-glucan-based superabsorbent hydrogel on the digestibility of wheat starch: Gelatinization, rheological and structural properties. Food Biosci. 2024, 60, 104470. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.; Wu, N.-N.; Tan, B. Interaction of anthocyanins, soluble dietary fiber and waxy rice starch: Their effect on freeze-thaw stability, water migration, and pasting, rheological and microstructural properties of starch gels. Int. J. Biol. Macromol. 2024, 274, 133174. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liang, D.; Liu, Q.; Zheng, Y.; Shen, H.; Li, W. Investigation of the role of sodium chloride on wheat starch multi-structure, physicochemical and digestibility properties during X-ray irradiation. Food Chem. 2024, 447, 139012. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Ma, Z.; Hu, X. Controlling dough rheology and structural characteristics of chickpea-wheat composite flour-based noodles with different levels of Artemisia sphaerocephala Krasch. gum addition. Int. J. Biol. Macromol. 2020, 150, 605–616. [Google Scholar] [CrossRef]
- Yao, G.; Huang, Q. Theoretical and experimental study of the infrared and Raman spectra of L-lysine acetylation. Spectrochim. Acta Part. A: Mol. Biomol. Spectrosc. 2022, 278, 121371. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Yousefi, A.; Jekle, M.; Sharifianjazi, F. Ingenious wheat starch/Lepidium perfoliatum seed mucilage hybrid composite films: Synthesis, incorporating nanostructured Dy2Ce2O7 synthesized via an ultrasound-assisted approach and characterization. Carbohydr. Polym. Technol. Appl. 2025, 9, 100657. [Google Scholar] [CrossRef]
- Man, J.; Yang, Y.; Zhang, C.; Zhou, X.; Dong, Y.; Zhang, F.; Liu, Q.; Wei, C. Structural Changes of High-Amylose Rice Starch Residues following in Vitro and in Vivo Digestion. J. Agr. Food Chem. 2012, 60, 9332–9341. [Google Scholar] [CrossRef]
- González, M.; Vernon-Carter, E.J.; Alvarez-Ramirez, J.; Carrera-Tarela, Y. Effects of dry heat treatment temperature on the structure of wheat flour and starch in vitro digestibility of bread. Int. J. Biol. Macromol. 2021, 166, 1439–1447. [Google Scholar] [CrossRef]
- Li, J.; Shen, M.; Xiao, W.; Li, Y.; Pan, W.; Xie, J. Regulating the physicochemical and structural properties of different starches by complexation with tea polyphenols. Food Hydrocolloid 2023, 142, 108836. [Google Scholar] [CrossRef]
- Xiong, M.; Chen, B.; Chen, Y.; Li, S.; Fang, Z.; Wang, L.; Wang, C.; Chen, H. Effects of soluble dietary fiber from pomegranate peel on the physicochemical properties and in-vitro digestibility of sweet potato starch. Int. J. Biol. Macromol. 2024, 273, 133041. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, W.; Lin, Q.; Liu, X.; Zeng, J.; Gao, H.; Li, W. Deciphering the structure-function-quality improvement role of starch gels by wheat bran insoluble dietary fibers obtained from different fermentation patterns and its potential mechanisms. Food Chem. 2024, 460, 140641. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Zhu, F.; Jiang, S.; Sui, Z.; Kong, X. Differences in structure, physicochemical properties, and in vitro digestibility of three types of starch complexed with tannic acid. Food Hydrocolloid 2024, 157, 110419. [Google Scholar] [CrossRef]
- Li, D.; Yao, X.; Yang, Y.; Cao, G.; Yi, G. In vitro digestibility and fermentability profiles of wheat starch modified by chlorogenic acid. Int. J. Biol. Macromol. 2022, 215, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chi, C.; She, Z.; Liu, X.; Zhang, Y.; Wang, H.; Zhang, H. Understanding how starch constituent in frozen dough following freezing-thawing treatment affected quality of steamed bread. Food Chem. 2022, 366, 130614. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Z.; Liu, S.; Zhang, X.; Ji, X.; Shi, M.; Niu, B. Effect of atmospheric cold plasma pretreatment on the formation of ternary complexes among wheat starch, β-lactoglobulin and fatty acids with different chain lengths. Food Chem. 2025, 471, 142798. [Google Scholar] [CrossRef]
- Katyal, M.; Singh, N.; Chopra, N.; Kaur, A. Hard, medium-hard and extraordinarily soft wheat varieties: Comparison and relationship between various starch properties. Int. J. Biol. Macromol. 2019, 123, 1143–1149. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Li, D.; Jin, Z.; Xu, X. Roles of dextran, weak acidification and their combination in the quality of wheat bread. Food Chem. 2019, 286, 197–203. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Q.; Zhang, L.; Liu, W.; Richel, A.; Zhao, R.; Hu, H. Potato dietary fiber effectively inhibits structure damage and digestibility increase of potato starch gel due to freeze-thaw cycles. Int. J. Biol. Macromol. 2024, 279, 135034. [Google Scholar] [CrossRef]
- Liu, T.; Wang, K.; Xue, W.; Wang, L.; Zhang, C.; Zhang, X.; Chen, Z. In vitro starch digestibility, edible quality and microstructure of instant rice noodles enriched with rice bran insoluble dietary fiber. LWT 2021, 142, 111008. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Zhang, Y.; Chen, L.; Li, L.; Wang, Z. Digestibility and supramolecular structural changes of maize starch by non-covalent interactions with gallic acid. Food Funct. 2017, 8, 720–730. [Google Scholar] [CrossRef]
- Fan, J.; Qin, X.; Zeng, Z.; Li, Y.; Liu, X. Effects of deacetylated konjac glucomannan on the quality characteristics, staling and digestion of Chinese steamed bread. J. Cereal Sci. 2024, 117, 103910. [Google Scholar] [CrossRef]






| Wheat Flour | Carrot Whole Flour | |
|---|---|---|
| Moisture (g/100 g) | 12.51 ± 0.03 b | 7.45 ± 0.07 a |
| Protein (g/100 g) | 12.10 ± 0.23 b | 6.17 ± 0.21 a |
| Fat (g/100 g) | 0.86 ± 0.08 a | 2.27 ± 0.15 b |
| Ash (g/100 g) | 0.40 ± 0.01 a | 5.57 ± 0.06 b |
| Total dietary fiber (g/100 g) | 1.09 ± 0.10 a | 18.52 ± 0.14 b |
| Total polyphenolic (mg/100 g) | 75.93 ± 0.85 a | 190.58 ± 0.59 b |
| Carotenoid (mg/100 g) | 0.49 ± 0.03 a | 135.35 ± 1.93 b |
| Samples | Hardness (g) | Springiness | Cohesiveness | Chewiness (g) | Adhesiveness |
|---|---|---|---|---|---|
| Control | 7.50 ± 0.28 b | 9.34 ± 0.15 d | 0.72 ± 0.03 d | 43.78 ± 1.83 d | 5.23 ± 0.12 d |
| CWF4 | 7.38 ± 0.18 b | 8.86 ± 0.08 c | 0.65 ± 0.02 c | 42.64 ± 1.06 d | 4.81 ± 0.08 b |
| CWF8 | 7.25 ± 0.12 b | 8.59 ± 0.08 b | 0.61 ± 0.01 b | 37.91 ± 1.19 b | 3.97 ± 0.09 a |
| CWF12 | 6.50 ± 0.16 a | 8.45 ± 0.09 b | 0.61 ± 0.02 b | 32.14 ± 1.18 a | 3.91 ± 0.14 a |
| CWF16 | 8.75 ± 0.10 a | 8.01 ± 0.18 a | 0.57 ± 0.02 a | 40.23 ± 0.97 c | 5.02 ± 0.14 c |
| Samples | L* | a* | b* | ΔE |
|---|---|---|---|---|
| Control | 77.64 ± 0.21 e | 0.41 ± 0.04 a | 14.21 ± 0.04 a | |
| CWF4 | 65.59 ± 0.76 d | 11.40 ± 0.60 b | 40.36 ± 0.38 b | 31.20 ± 0.27 a |
| CWF8 | 63.96 ± 0.35 c | 17.10 ± 0.26 c | 48.64 ± 0.59 c | 41.03 ± 0.50 b |
| CWF12 | 60.85 ± 0.33 b | 18.14 ± 0.17 d | 53.34 ± 0.33 d | 46.49 ± 0.20 c |
| CWF16 | 58.76 ± 0.17 a | 20.39 ± 0.23 e | 55.80 ± 0.38 e | 50.24 ± 0.38 d |
| Samples | PV (mPa·s) | BD (mPa·s) | FV (mPa·s) | SB (mPa·s) | PT (°C) |
|---|---|---|---|---|---|
| Control | 4287.50 ± 28.99 c | 583.00 ± 50.91 a | 5532.50 ± 14.85 c | 1878.00 ± 24.04 c | 77.85 ± 0.57 b |
| CWF | 4593.50 ± 26.16 d | 892.00 ± 59.40 b | 5814.00 ± 5.66 d | 2062.50 ± 9.19 d | 77.50 ± 0.07 b |
| CDF | 2507.00 ± 8.49 a | 2206.00 ± 21.21 c | 448.00 ± 9.90 a | 147.00 ± 22.63 a | 69.43 ± 0.04 a |
| CPs | 4098.50 ± 33.23 b | 610.50 ± 10.61 a | 5140.00 ± 26.87 b | 1652.00 ± 16.97 b | 81.45 ± 0.00 c |
| Samples | R1047/1022 | R995/1022 | Relative Crystallinity |
|---|---|---|---|
| Control | 0.978 ± 0.017 c | 0.989 ± 0.010 c | 29.64 ± 0.38 a |
| CWF | 0.918 ± 0.008 b | 0.948 ± 0.006 b | 31.48 ± 0.06 b |
| CDF | 0.895 ± 0.009 ab | 0.892 ± 0.002 a | 32.29 ± 0.97 b |
| CPs | 0.880 ± 0.002 a | 0.898 ± 0.008 a | 32.72 ± 0.79 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Tian, X.; Zhang, R.; Li, H. From Molecular to Macroscopic: Dual-Pathway Regulation of Carrot Whole Flour on the Gluten-Starch System. Foods 2025, 14, 1964. https://doi.org/10.3390/foods14111964
Wang H, Tian X, Zhang R, Li H. From Molecular to Macroscopic: Dual-Pathway Regulation of Carrot Whole Flour on the Gluten-Starch System. Foods. 2025; 14(11):1964. https://doi.org/10.3390/foods14111964
Chicago/Turabian StyleWang, Han, Xiaoxuan Tian, Ruoyu Zhang, and Huijing Li. 2025. "From Molecular to Macroscopic: Dual-Pathway Regulation of Carrot Whole Flour on the Gluten-Starch System" Foods 14, no. 11: 1964. https://doi.org/10.3390/foods14111964
APA StyleWang, H., Tian, X., Zhang, R., & Li, H. (2025). From Molecular to Macroscopic: Dual-Pathway Regulation of Carrot Whole Flour on the Gluten-Starch System. Foods, 14(11), 1964. https://doi.org/10.3390/foods14111964






