Insights into the Formation of Ternary Complexes Among Wheat Starch, Lauric Acid and Protein: Effects of Plasma Pretreatment Times and Protein Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ACP Treatment of WS
2.3. Fabrication of WS-LA-Protein Ternary Complexes
2.4. Amylose Content Analysis
2.5. Complexing Index (CI) Analysis
2.6. X-Ray Diffraction (XRD)
2.7. Fourier Transform Infrared (FTIR) Spectroscopy
2.8. Differential Scanning Calorimetry (DSC)
2.9. In Vitro Digestibility
2.10. Statistical Analysis
3. Results and Discussion
3.1. Amylose Content
3.2. Complexing Index (CI)
3.3. Crystalline Structure
3.4. Short-Range Ordered Structure
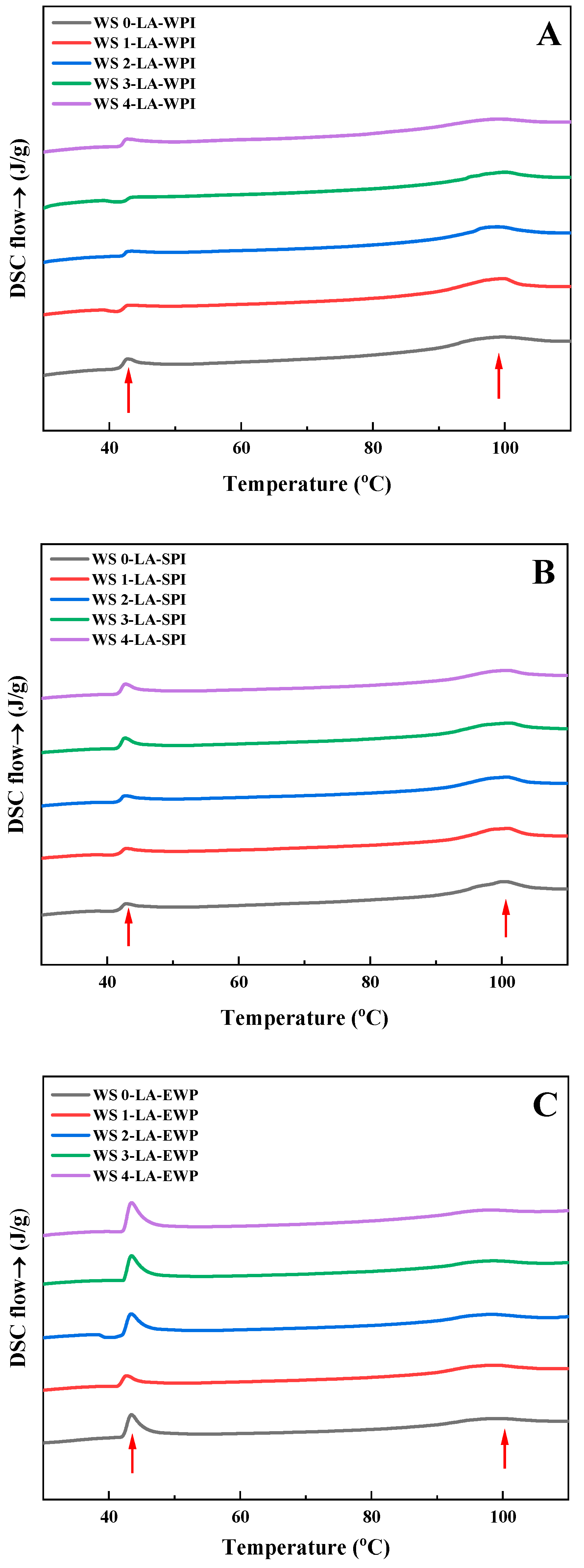
3.5. Thermal Properties
3.6. In Vitro Digestibility
3.7. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parada, J.; Santos, J.L. Interactions between starch, lipids, and proteins in foods: Microstructure control for glycemic response modulation. Crit. Rev. Food Sci. Nutr. 2016, 56, 2362–2369. [Google Scholar] [CrossRef]
- Wang, S.; Chao, C.; Cai, J.; Niu, B.; Copeland, L.; Wang, S. Starch-lipid and starch-lipid-protein complexes: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1056–1079. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chao, C.; Yu, J.; Copeland, L.; Huang, Y.; Wang, S. Mechanisms underlying the formation of amylose-lauric acid-β-lactoglobulin complexes: Experimental and molecular dynamics studies. J. Agric. Food Chem. 2022, 70, 10635–10643. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Chao, C.; Cai, J.; Yu, J.; Wang, S.; Wang, S. Effects of cooling rate and complexing temperature on the formation of starch-lauric acid-β-lactoglobulin complexes. Carbohydr. Polym. 2021, 253, 117301. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, M.; Yu, J.; Wang, S.; Copeland, L. Insights into the formation and structures of starch-protein-lipid complexes. J. Agric. Food Chem. 2017, 65, 1960–1966. [Google Scholar] [CrossRef]
- Zheng, M.; Chao, C.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. Effects of chain length and degree of unsaturation of fatty acids on structure and in vitro digestibility of starch-protein-fatty acid complexes. J. Agric. Food Chem. 2018, 66, 1872–1880. [Google Scholar] [CrossRef]
- Chao, C.; Huang, S.; Wang, C.; Sun, R.; Yu, J.; Copeland, L.; Wang, S. Interaction between amylose, fatty acid, and β-lactoglobulin to study multiple biomacromolecules self-assembly and application. Aggregate 2024, 5, 536. [Google Scholar] [CrossRef]
- Cai, J.; Chao, C.; Niu, B.; Copeland, L.; Yu, J.; Wang, S.; Wang, S. New insight into the interactions among starch, lipid and protein in model systems with different starches. Food Hydrocoll. 2021, 112, 106323. [Google Scholar] [CrossRef]
- Cai, J.; Chao, C.; Niu, B.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. Effects of debranching on the formation of maize starch-lauric acid-β-lactoglobulin complexes. J. Agric. Food Chem. 2021, 69, 9086–9093. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J.; Copeland, L.; Wang, S. Revisiting the formation of starch-monoglyceride-protein complexes: Effects of octenyl succinic anhydride modification. J. Agric. Food Chem. 2023, 71, 19033–19044. [Google Scholar] [CrossRef]
- Chao, C.; Yu, J.; Wang, S.; Copeland, L.; Wang, S. Mechanisms underlying the formation of complexes between maize starch and lipids. J. Agric. Food Chem. 2018, 66, 272–278. [Google Scholar] [CrossRef]
- Lin, L.; Yang, H.; Chi, C.; Ma, X. Effect of protein types on structure and digestibility of starch-protein-lipids complexes. LWT-Food Sci. Technol. 2020, 134, 110175. [Google Scholar] [CrossRef]
- Duan, Y.; Chao, C.; Yu, J.; Liu, Y.; Wang, S. Effects of different sources of proteins on the formation of starch-lipid-protein complexes. Int. J. Biol. Macromol. 2023, 253, 126853. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Lv, M.Y.; Zhang, H.L.; Xiao, S.S.; Zheng, S.Y.; Wang, X.D. Synergistic effect of endogenous gluten and oleic acid on wheat starch digestion by forming ordered starch-fatty acid-protein complexes during thermal processing. Curr. Res. Food Sci. 2023, 6, 100422. [Google Scholar] [CrossRef]
- Niu, B.; Qin, Y.; Xie, X.; Zhang, B.; Cheng, L.; Yan, Y. Effect of ultrasound-pretreated starch on the formation, structure and digestibility of starch ternary complexes from lauric acid and β-lactoglobulin. Ultrason. Sonochemistry 2024, 109, 106990. [Google Scholar] [CrossRef]
- Khumsupan, D.; Lin, S.P.; Hsieh, C.W.; Santoso, S.P.; Chou, Y.J.; Hsieh, K.C.; Lin, H.W.; Ting, Y.W.; Cheng, K.C. Current and potential applications of atmospheric cold plasma in the food industry. Molecules 2023, 28, 4903. [Google Scholar] [CrossRef]
- Chen, C.S.; Tong, F.; Sun, R.H.; Yang, J.F.; Pang, Z.H.; Liu, X.Q. Plasma effects on properties and structure of corn starch: Characterization and analysis. Foods 2023, 12, 4042. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Guha, P.; Srivastav, P.P. Physical action of nonthermal cold plasma technology for starch modification. Food Phys. 2024, 1, 100011. [Google Scholar] [CrossRef]
- Xu, H.B.; Zhou, J.P.; Liu, X.; Yu, J.L.; Copeland, L.; Wang, S.J. Methods for characterizing the structure of starch in relation to its applications: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2023, 63, 4799–4816. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Z.; Liu, S.; Zhang, X.; Ji, X.; Shi, M.; Niu, B. Effect of atmospheric cold plasma pretreatment on the formation of ternary complexes among wheat starch, β-lactoglobulin and fatty acids with different chain lengths. Food Chem. 2025, 471, 142798. [Google Scholar] [CrossRef]
- Devi, E.; Kalaivendan, R.G.T.; Eazhumalai, G.; Annapure, U.S. Impact of atmospheric pressure pin-to-plate cold plasma on the functionality of arrowroot starch. J. Agr. Food Res. 2023, 12, 100567. [Google Scholar] [CrossRef]
- Kaur, P.; Annapure, U.S. Rheological and gelling properties of atmospheric pressure cold plasma treated finger millet (Eleusine coracana) starch. Food Res. Int. 2024, 187, 114418. [Google Scholar] [CrossRef]
- Kaur, P.; Annapure, U.S. Effects of pin-to-plate atmospheric cold plasma for modification of pearl millet (Pennisetum glaucum) starch. Food Res. Int. 2023, 169, 112930. [Google Scholar] [CrossRef]
- Liang, Y.L.; Zheng, L.L.; Yang, Y.; Zheng, X.Y.; Xiao, D.; Ai, B.L.; Sheng, Z.W. Dielectric barrier discharge cold plasma modifies the multiscale structure and functional properties of banana starch. Int. J. Biol. Macromol. 2024, 264, 130462. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Liu, S.; Zhang, R.; Zhang, S.; Pei, J.F.; Liu, H. The multi-scale structures and in vitro digestibility of starches with different crystalline types induced by dielectric barrier discharge plasma. Int. J. Biol. Macromol. 2024, 263, 130281. [Google Scholar] [CrossRef]
- Gupta, R.K.; Guha, P.; Srivastav, P.P. Effect of high voltage dielectric barrier discharge (DBD) atmospheric cold plasma treatment on physicochemical and functional properties of taro (Colocasia esculenta) starch. Int. J. Biol. Macromol. 2023, 253, 126772. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ren, F.; Yu, J.; Copeland, L.; Wang, S. Octenyl succinate modification of starch enhances the formation of starch-lipid complexes. J. Agric. Food Chem. 2021, 69, 14938–14950. [Google Scholar] [CrossRef]
- van Soest, J.J.G.; Tournois, H.; de Wit, D.; Vliegenthart, J.F.G. Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohyd. Res. 1995, 279, 201–214. [Google Scholar] [CrossRef]
- Rafiq, S.I.; Singh, S.; Saxena, D.C. Effect of heat-moisture and acid treatment on physicochemical, pasting, thermal and morphological properties of horse chestnut (Aesculus indica) starch. Food Hydrocolloid. 2016, 57, 103–113. [Google Scholar] [CrossRef]
- Flores-morales, A.; Jiménez-estrada, M.; Mora-escobedo, R. Determination of the structural changes by FT-IR, Raman, and CP/MAS 13C NMR spectroscopy on retrograded starch of maize tortillas. Carbohydr. Polym. 2012, 87, 61–68. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Wang, S.; Wang, S. Annealing improves paste viscosity and stability of starch. Food Hydrocolloid. 2017, 62, 203–211. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Lal, M.K.; Singh, B.; Sharma, S.; Singh, M.P.; Kumar, A. Glycemic index of starchy crops and factors affecting its digestibility: A review. Trends Food Sci. Technol. 2021, 111, 741–755. [Google Scholar] [CrossRef]
- Chi, C.D.; Li, X.X.; Huang, S.X.; Chen, L.; Zhang, Y.P.; Li, L.; Miao, S. Basic principles in starch multi-scale structuration to mitigate digestibility: A review. Trends Food Sci. Technol. 2021, 109, 154–168. [Google Scholar] [CrossRef]
- Brennan, C.S. Dietary fibre, glycaemic response, and diabetes. Mol. Nutr. Food Res. 2005, 49, 716. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. 2), S33–S50. [Google Scholar]
- Miao, M.; Jiang, B.; Cui, S.W.; Zhang, T.; Jin, Z.Y. Slowly digestible starch-a review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1642–1657. [Google Scholar] [CrossRef]
- Warren, F.J.; Fukuma, N.M.; Mikkelsen, D.; Flanagan, B.M.; Williams, B.A.; Lisle, A.T.; Cuív, P.O.; Morrison, M.; Gidley, M.J. Food starch structure impacts gut microbiome composition. mSphere 2018, 3, e00086-18. [Google Scholar] [CrossRef]
- Duan, Y.F.; Wang, Y.; Liu, Q.S.; Dong, H.B.; Li, H.; Xiong, D.L.; Zhang, J.S. Changes in the intestine microbial, digestion and immunity of Litopenaeus vannamei in response to dietary resistant starch. Sci. Rep. 2019, 9, 6464. [Google Scholar] [CrossRef]
- Panyoo, A.E.; Emmambux, M.N. Amylose-lipid complex production and potential health benefits: A mini-review. Starch-Stärke 2017, 69, 1600203. [Google Scholar] [CrossRef]
- Chen, X.; He, X.W.; Fu, X.; Zhang, B.; Huang, Q. Complexation of rice starch/flour and maize oil through heat moisture treatment: Structural, in vitro digestion and physicochemical properties. Int. J. Biol. Macromol. 2017, 98, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.S.; Bezerra, M.A.; Cerqueira, U.; Rodrigues, C.J.O.; Santos, B.C.; Novaes, C.G.; Almeida, E.R.V. An introductory review on the application of principal component analysis in the data exploration of the chemical analysis of food samples. Food Sci. Biotechnol. 2024, 33, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
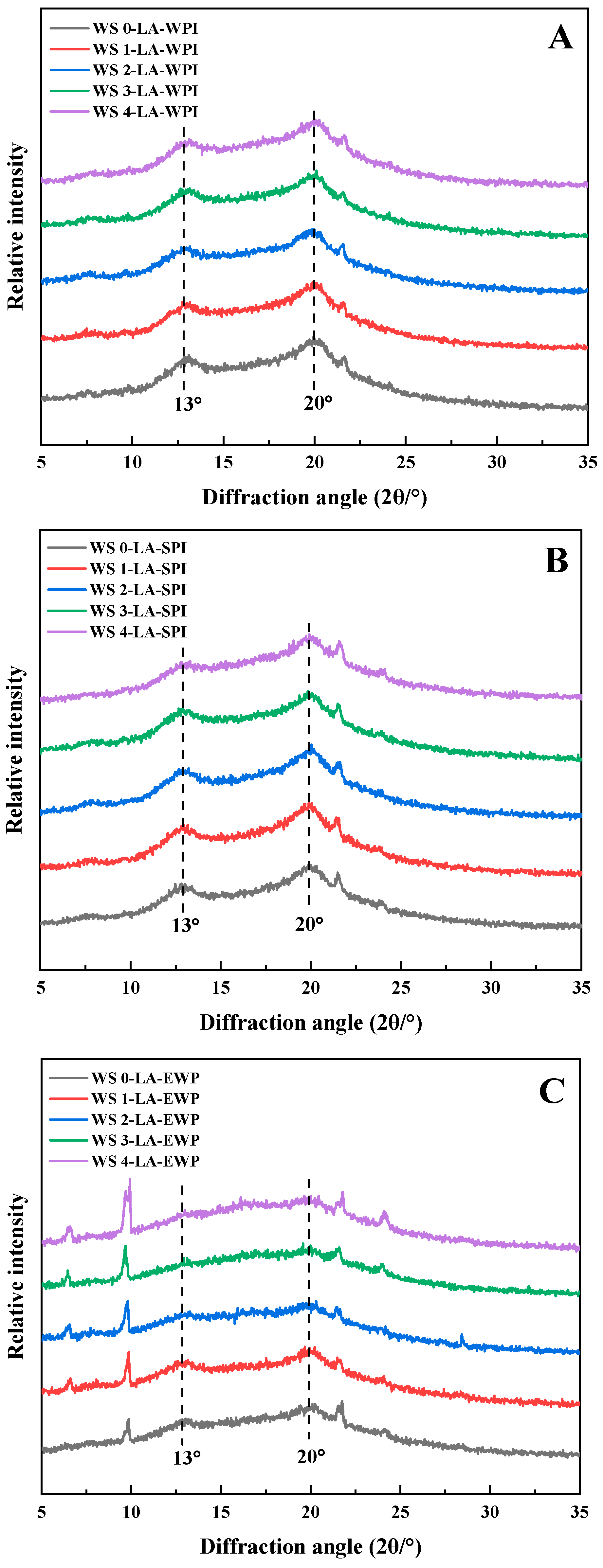



| Samples | AM (%) |
|---|---|
| WS0 | 28.71 ± 0.22 c |
| WS1 | 30.02 ± 0.19 a |
| WS2 | 29.29 ± 0.08 b |
| WS3 | 27.71 ± 0.10 d |
| WS4 | 27.23 ± 0.21 e |
| Samples | CI (%) | Xv (%) | R1047/1022 | To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) |
|---|---|---|---|---|---|---|---|
| WS0-LA-WPI | 68.33 ± 0.11 b | 10.18 ± 0.07 c | 0.798 ± 0.001 c | 91.26 ± 0.10 a | 99.58 ± 0.15 a | 106.67 ± 0.08 ab | 3.57 ± 0.11 c |
| WS1-LA-WPI | 69.21 ± 0.19 a | 10.56 ± 0.06 a | 0.811 ± 0.002 a | 91.49 ± 0.09 a | 99.82 ± 0.11 a | 107.02 ± 0.14 a | 4.26 ± 0.19 a |
| WS2-LA-WPI | 68.62 ± 0.16 b | 10.39 ± 0.07 b | 0.804 ± 0.001 b | 91.60 ± 0.20 a | 99.74 ± 0.06 a | 106.75 ± 0.16 ab | 3.93 ± 0.13 b |
| WS3-LA-WPI | 67.91 ± 0.10 c | 9.87 ± 0.04 d | 0.792 ± 0.002 d | 91.19 ± 0.19 a | 99.56 ± 0.21 a | 106.45 ± 0.11 b | 3.42 ± 0.08 cd |
| WS4-LA-WPI | 67.34 ± 0.12 d | 9.72 ± 0.06 e | 0.787 ± 0.003 e | 91.01 ± 0.18 a | 99.38 ± 0.06 a | 106.37 ± 0.10 b | 3.14 ± 0.14 d |
| WS0-LA-SPI | 65.94 ± 0.14 c | 9.93 ± 0.05 c | 0.784 ± 0.002 b | 92.53 ± 0.29 b | 98.6 ± 0.24 a | 106.72 ± 0.15 a | 3.36 ± 0.18 ab |
| WS1-LA-SPI | 67.41 ± 0.19 a | 10.13 ± 0.06 a | 0.792 ± 0.001 a | 92.98 ± 0.21 ab | 99.07 ± 0.21 a | 106.82 ± 0.14 a | 3.73 ± 0.23 a |
| WS2-LA-SPI | 66.73 ± 0.23 b | 10.02 ± 0.07 b | 0.786 ± 0.002 b | 93.20 ± 0.09 a | 98.80 ± 0.13 a | 106.71 ± 0.25 a | 3.53 ± 0.15 ab |
| WS3-LA-SPI | 65.36 ± 0.16 d | 9.82 ± 0.04 d | 0.781 ± 0.003 c | 92.51 ± 0.24 b | 98.64 ± 0.14 a | 106.65 ± 0.11 a | 3.28 ± 0.13 b |
| WS4-LA-SPI | 64.78 ± 0.22 e | 9.76 ± 0.07 e | 0.779 ± 0.002 d | 92.41 ± 0.19 b | 98.53 ± 0.16 a | 106.56 ± 0.08 a | 3.19 ± 0.05 b |
| WS0-LA-EWP | 61.31 ± 0.10 c | 9.14 ± 0.04 b | 0.757 ± 0.001 c | 90.23 ± 0.11 a | 97.78 ± 0.13 a | 105.57 ± 0.22 a | 2.69 ± 0.15 bc |
| WS1-LA-EWP | 62.81 ± 0.23 a | 9.43 ± 0.07 a | 0.770 ± 0.002 a | 90.29 ± 0.16 a | 97.77 ± 0.12 a | 105.65 ± 0.09 a | 3.13 ± 0.14 a |
| WS2-LA-EWP | 62.19 ± 0.19 b | 9.32 ± 0.05 ab | 0.762 ± 0.001 b | 90.61 ± 0.30 a | 97.69 ± 0.19 a | 105.77 ± 0.08 a | 2.95 ± 0.13 ab |
| WS3-LA-EWP | 60.38 ± 0.15 d | 8.97 ± 0.04 c | 0.751 ± 0.002 d | 90.32 ± 0.16 a | 97.25 ± 0.13 b | 105.45 ± 0.11 a | 2.43 ± 0.09 cd |
| WS4-LA-EWP | 59.51 ± 0.17 e | 8.86 ± 0.07 d | 0.748 ± 0.001 e | 90.16 ± 0.13 a | 97.37 ± 0.21 ab | 105.36 ± 0.21 a | 2.29 ± 0.12 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, B.; Wang, Z.; Yan, Y. Insights into the Formation of Ternary Complexes Among Wheat Starch, Lauric Acid and Protein: Effects of Plasma Pretreatment Times and Protein Types. Foods 2025, 14, 1922. https://doi.org/10.3390/foods14111922
Niu B, Wang Z, Yan Y. Insights into the Formation of Ternary Complexes Among Wheat Starch, Lauric Acid and Protein: Effects of Plasma Pretreatment Times and Protein Types. Foods. 2025; 14(11):1922. https://doi.org/10.3390/foods14111922
Chicago/Turabian StyleNiu, Bin, Ziyu Wang, and Yizhe Yan. 2025. "Insights into the Formation of Ternary Complexes Among Wheat Starch, Lauric Acid and Protein: Effects of Plasma Pretreatment Times and Protein Types" Foods 14, no. 11: 1922. https://doi.org/10.3390/foods14111922
APA StyleNiu, B., Wang, Z., & Yan, Y. (2025). Insights into the Formation of Ternary Complexes Among Wheat Starch, Lauric Acid and Protein: Effects of Plasma Pretreatment Times and Protein Types. Foods, 14(11), 1922. https://doi.org/10.3390/foods14111922






