The Application of Natural Phenolic Substances as Antimicrobial Agents in Agriculture and Food Industry
Abstract
1. Introduction
2. Classification of Phenolic Compounds
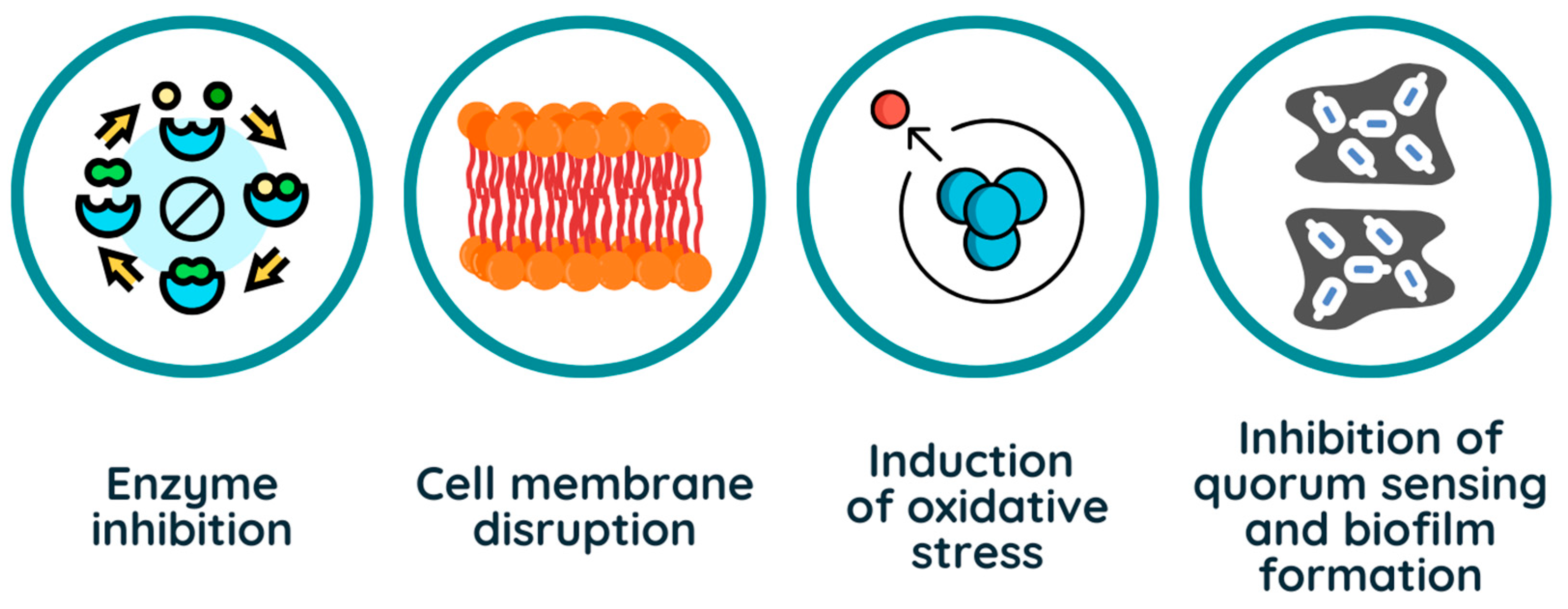
3. The Biocidal Mechanism of Action of Phenolic Compounds
4. Phenolic Compounds with the Highest Application Potential
4.1. In Agriculture
4.2. In Food Packaging
5. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CN | Cinnamaldehyde |
| CA | Cinnamic acid |
| CR | Carvacrol |
| RS | Resveratrol |
| CO | p-Coumaric acid |
| GA | Gallic acid |
| TA | Tannic acid |
| FA | Ferulic acid |
| SA | Salicylic acid |
| EU | Eugenol |
| TH | Thymol |
| ROS | Reactive oxygen species |
| MIC | Minimum inhibitory concentration |
| DON | Deoxynivalenol |
| PLA | Polylactide |
| PBAT | Poly(butylene adipate-co-terephthalate) |
| MFCAs | Medium-chain fatty acids |
| CH | Chitosan |
| PBS | Poly(butylene succinate) |
References
- Firmino, D.F.; Cavalcante, T.T.A.; Gomes, G.A.; Firmino, N.C.S.; Rosa, L.D.; De Carvalho, M.G.; Catunda, F.E.A., Jr. Antibacterial and Antibiofilm Activities of Cinnamomum sp. Essential Oil and Cinnamaldehyde: Antimicrobial Activities. Sci. World J. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2—Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 33–50. [Google Scholar] [CrossRef]
- Ginter, A. Plant Protection within the European Green Deal on the Example Starch Potato Cultivation. Prog. Plant Prot. 2022, 62, 208–215. [Google Scholar] [CrossRef]
- Kannan, M.; Bojan, N.; Swaminathan, J.; Zicarelli, G.; Hemalatha, D.; Zhang, Y.; Ramesh, M.; Faggio, C. Nanopesticides in Agricultural Pest Management and Their Environmental Risks: A Review. Int. J. Environ. Sci. Technol. 2023, 20, 10507–10532. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Xu, J.; Lynch, I.; Guo, Z.; Xie, C.; Zhang, P. Advanced Nanopesticides: Advantage and Action Mechanisms. Plant Physiol. Biochem. 2023, 203, 108051. [Google Scholar] [CrossRef]
- Santra, H.K.; Banerjee, D. Natural Products as Fungicide and Their Role in Crop Protection. In Natural Bioactive Products in Sustainable Agriculture; Singh, J., Yadav, A., Eds.; Springer: Singapore, 2020; pp. 131–219. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Thakur, N.; Kajla, P.; Kumar, M.; Trif, M. Natural Antimicrobials as Additives for Edible Food Packaging Applications: A Review. Foods 2021, 10, 2282. [Google Scholar] [CrossRef]
- Chalker-Scott, L.; Fuchigami, L.H. The Role of Phenolic Compounds in Plant Stress Responses. In Low Temperature Stress Physiology in Crops; CRC Press: Boca Raton, FL, USA, 2018; pp. 67–80. [Google Scholar]
- Bento, C.; Gonçalves, A.C.; Jesus, F.; Simões, M.; Silva, L.R. Phenolic compounds: Sources, properties and applications. In Bioactive Compounds: Sources, Properties and Applications; Porter, R., Parker, N., Eds.; Nova Science Publishers: New York, NY, USA, 2017; pp. 271–299. [Google Scholar]
- Zinn, S.; Betz, T.; Medcraft, C.; Schnell, M. Structure Determination of Trans-Cinnamaldehyde by Broadband Microwave Spectroscopy. Phys. Chem. Chem. Phys. 2015, 17, 16080–16085. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A Review of Cinnamaldehyde and Its Derivatives as Antibacterial Agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic Acid Derivatives and Their Biological Efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Nostro, N.A.; Papalia, N.T. Antimicrobial Activity of Carvacrol: Current Progress and Future Prospectives. Recent Pat. Anti-Infect. Drug Discov. 2012, 7, 28–35. [Google Scholar] [CrossRef]
- Mączka, W.; Twardawska, M.; Grabarczyk, M.; Wińska, K. Carvacrol—A Natural Phenolic Compound with Antimicrobial Properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Prasad, N.R.; Ganamani, A.; Balamurugan, E. Anticancer Activity of Resveratrol-Loaded Gelatin Nanoparticles on NCI-H460 Non-Small Cell Lung Cancer Cells. Biomed. Prev. Nutr. 2012, 3, 64–73. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric Acid and Its Conjugates: Dietary Sources, Pharmacokinetic Properties and Biological Activities. J. Sci. Food Agric. 2015, 96, 2952–2962. [Google Scholar] [CrossRef] [PubMed]
- Tsioptsias, C.; Tsivintzelis, I. Insights on Thermodynamic Thermal Properties and Infrared Spectroscopic Band Assignments of Gallic Acid. J. Pharm. Biomed. Anal. 2022, 221, 115065. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Zhen, L.; Lange, H.; Crestini, C. An Analytical Toolbox for Fast and Straightforward Structural Characterisation of Commercially Available Tannins. Molecules 2021, 26, 2532. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential Applications of Ferulic Acid from Natural Sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef]
- Meenu, M.; Khandare, K.; Singh, M.; Kenyanya, S.; Sharma, K.P.; Garg, M. Salicylic Acid: Food, Functions, and Future. In Plant Growth Regulators: Resilience for Sustainable Agriculture; Faizan, M., Hayat, S., Eds.; Springer: Singapore, 2024; pp. 21–39. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Liu, B.; Chen, B.; Zhang, J.; Wang, P.; Feng, G. The Environmental Fate of Thymol, a Novel Botanical Pesticide, in Tropical Agricultural Soil and Water. Toxicol. Environ. Chem. Rev. 2016, 99, 223–232. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Betts, G.; Hoskins, N.; Edwards, M.; Ercolini, D.; Mauriello, G. Membrane Toxicity of Antimicrobial Compounds from Essential Oils. J. Agric. Food Chem. 2007, 55, 4863–4870. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent Advances in Understanding the Antibacterial Properties of Flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Qu, S.; Yang, K.; Chen, L.; Liu, M.; Geng, Q.; He, X.; Li, Y.; Liu, Y.; Tian, J. Cinnamaldehyde, a Promising Natural Preservative Against Aspergillus flavus. Front. Microbiol. 2019, 10, 2895. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Lee, S.-J.; Park, N.-H.; Mechesso, A.F.; Birhanu, B.T.; Kang, J.; Reza, M.A.; Suh, J.-W.; Park, S.-C. Impact of Phenolic Compounds in the Acyl Homoserine Lactone-Mediated Quorum Sensing Regulatory Pathways. Sci. Rep. 2017, 7, 10618. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, L.; Rocchetti, G.; Lucini, L.; Rebecchi, A. Antimicrobial Potential of Polyphenols: Mechanisms of Action and Microbial Responses—A Narrative Review. Antioxidants 2025, 14, 200. [Google Scholar] [CrossRef]
- OuYang, Q.; Okwong, R.O.; Chen, Y.; Tao, N. Synergistic Activity of Cinnamaldehyde and Citronellal against Green Mold in Citrus Fruit. Postharvest Biol. Technol. 2019, 162, 111095. [Google Scholar] [CrossRef]
- Farhadi, K.; Rajabi, E.; Varpaei, H.A.; Iranzadasl, M.; Khodaparast, S.; Salehi, M. Thymol and carvacrol against Klebsiella: Anti-bacterial, anti-biofilm, and synergistic activities—A systematic review. Front. Pharmacol. 2024, 15, 1487083. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M.; Esfanjani, A.F. Protection of phenolic compounds within nanocarriers. CABI Rev. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Pasquet, P.L.; Julien-David, D.; Zhao, M.; Villain-Gambier, M.; Trébouet, D. Stability and preservation of phenolic compounds and related antioxidant capacity from agro-food matrix: Effect of pH and atmosphere. Food Biosci. 2024, 57, 103586. [Google Scholar] [CrossRef]
- Saarniit, K.; Lang, H.; Kuldjärv, R.; Laaksonen, O.; Rosenvald, S. The stability of phenolic compounds in fruit, berry, and vegetable purees based on accelerated shelf-life testing methodology. Foods 2023, 12, 1777. [Google Scholar] [CrossRef]
- Pinarli, B.; Simge Karliga, E.; Ozkan, G.; Capanoglu, E. Interaction of phenolics with food matrix: In vitro and in vivo approaches. Mediterr. J. Nutr. Metab. 2020, 13, 63–74. [Google Scholar] [CrossRef]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats Posed by the Fungal Kingdom to Humans, Wildlife, and Agriculture. mBio 2020, 11, 10–1128. [Google Scholar] [CrossRef]
- Bi, K.; Liang, Y.; Mengiste, T.; Sharon, A. Killing softly: A roadmap of Botrytis cinerea pathogenicity. Trends Plant Sci. 2023, 28, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, X.; Qu, Z.; Zhang, C.; Wang, F.; Gao, T.; Liang, J. Progress in Research on Prevention and Control of Crop Fungal Diseases in the Context of Climate Change. Agriculture 2024, 14, 1108. [Google Scholar] [CrossRef]
- Matan, N. Growth Inhibition of Aspergillus Niger by Cinnamaldehyde and Eugenol. Walailak J. Sci. Technol. (WJST) 2011, 4, 41–51. [Google Scholar]
- Sun, Q.; Shang, B.; Wang, L.; Lu, Z.; Liu, Y. Cinnamaldehyde Inhibits Fungal Growth and Aflatoxin B1 Biosynthesis by Modulating the Oxidative Stress Response of Aspergillus flavus. Appl. Microbiol. Biotechnol. 2015, 100, 1355–1364. [Google Scholar] [CrossRef]
- Niu, A.; Wu, H.; Ma, F.; Tan, S.; Wang, G.; Qiu, W. The Antifungal Activity of Cinnamaldehyde in Vapor Phase against Aspergillus niger Isolated from Spoiled Paddy. LWT 2022, 159, 113181. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Q.; Wang, Z.; Cao, H.; Zhang, D. Structure-Activity Relationships of Cinnamaldehyde and Eugenol Derivatives against Plant Pathogenic Fungi. Ind. Crop. Prod. 2017, 97, 388–394. [Google Scholar] [CrossRef]
- Zhou, L.-R.; Hu, H.-J.; Wang, J.; Zhu, Y.-X.; Zhu, X.-D.; Ma, J.-W.; Liu, Y.-Q. Cinnamaldehyde Acts as a Fungistat by Disrupting the Integrity of Fusarium oxysporum Fox-1 Cell Membranes. Horticulturae 2024, 10, 48. [Google Scholar] [CrossRef]
- Xing, F.; Hua, H.; Selvaraj, J.N.; Zhao, Y.; Zhou, L.; Liu, X.; Liu, Y. Growth Inhibition and Morphological Alterations of Fusarium verticillioides by Cinnamon Oil and Cinnamaldehyde. Food Control 2014, 46, 343–350. [Google Scholar] [CrossRef]
- Yang, R.; Miao, J.; Shen, Y.; Cai, N.; Wan, C.; Zou, L.; Chen, C.; Chen, J. Antifungal Effect of Cinnamaldehyde, Eugenol and Carvacrol Nanoemulsion against Penicillium digitatum and Application in Postharvest Preservation of Citrus Fruit. LWT 2021, 141, 110924. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Li, M.; Zhao, T.; Zhou, L. Cinnamaldehyde Inhibits the Growth of Phytophthora capsici through Disturbing Metabolic Homoeostasis. PeerJ 2021, 9, e11339. [Google Scholar] [CrossRef]
- Ibi, A.A.; Kyuka, C.K. Sources, Extraction and Biological Activities of Cinnamaldehyde. Trends Pharm. Sci. 2022, 8, 263–282. [Google Scholar] [CrossRef]
- Shen, Y.; Kahramanoğlu, İ.; Chen, C.; Chen, J.; Okatan, V.; Wan, C. Application of Cinnamaldehyde for the Postharvest Storage of Fresh Horticultural Products. Hortic. Int. J. 2021, 5, 103–105. [Google Scholar] [CrossRef]
- Yossa, N.; Patel, J.; Millner, P.; Lo, M. Inactivation ofSalmonellain Organic Soil by Cinnamaldehyde, Eugenol, Ecotrol, and Sporan. Foodborne Pathog. Dis. 2010, 8, 311–317. [Google Scholar] [CrossRef]
- Song, Y.-R.; Choi, M.-S.; Choi, G.-W.; Park, I.-K.; Oh, C.-S. Antibacterial Activity of Cinnamaldehyde and Estragole Extracted from Plant Essential Oils against Pseudomonas syringae pv. actinidiae Causing Bacterial Canker Disease in Kiwifruit. Plant Pathol. J. 2016, 32, 363–370. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jung, M.; Lee, S.-C.; Huh, M.-J.; Seo, S.-M.; Park, I.-K. Antibacterial Mode of Action of Trans-Cinnamaldehyde Derived from Cinnamon Bark (Cinnamomum Verum) Essential Oil against Agrobacterium tumefaciens. Pestic. Biochem. Physiol. 2020, 165, 104546. [Google Scholar] [CrossRef]
- Mohammed, T.G.M.; Rahman, A.F.A.E. Eco-Friendly Cinnamaldehyde Based Emulsion for Phytopathogenic Bacterial Growth Inhibitor. J. Adv. Microbiol. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Antimicrobial Mechanisms, and Antibiotic Activities of Cinnamaldehyde against Pathogenic Bacteria in Animal Feeds and Human Foods. J. Agric. Food Chem. 2017, 65, 10406–10423. [Google Scholar] [CrossRef]
- Wei, C.; Fan, C.; Xie, D.; Zhou, S.; Zhang, H.; Du, Q.; Jin, P. Fabrication of cinnamaldehyde-entrapped ethosome nanoparticles as antimicrobial agent. LWT 2023, 181, 114760. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Wang, J.; Zhou, M.; Wang, M.; Feng, J. Antifungal Activity and Action Mechanism of the Natural Product Cinnamic Acid Against Sclerotinia sclerotiorum. Plant Dis. 2019, 103, 944–950. [Google Scholar] [CrossRef]
- Liu, H.; Cai, C.; Zhang, X.; Li, W.; Ma, Z.; Feng, J.; Liu, X.; Lei, P. Discovery of Novel Cinnamic Acid Derivatives as Fungicide Candidates. J. Agric. Food Chem. 2024, 72, 2492–2500. [Google Scholar] [CrossRef]
- Yang, B.; Li, Z.; Liu, S.; Yang, J.; Wang, P.; Liu, H.; Zhou, X.; Liu, L.; Wu, Z.; Yang, S. Novel Cinnamic Acid Derivatives as a Versatile Tool for Developing Agrochemicals for Controlling Plant Virus and Bacterial Diseases by Enhancing Plant Defense Responses. Pest Manag. Sci. 2023, 79, 2556–2570. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhou, Y.; Zheng, Y.; Li, C.; Sheng, S.; Wang, J.; Wu, F. Enzymatic Modification of Chitosan by Cinnamic Acids: Antibacterial Activity against Ralstonia solanacearum. Int. J. Biol. Macromol. 2016, 87, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, S.; Sharifzadeh, A.; Shokri, H.; Khosravi, A.R.; Abbaszadeh, A. Antifungal Efficacy of Thymol, Carvacrol, Eugenol and Menthol as Alternative Agents to Control the Growth of Food-Relevant Fungi. J. Mycol. Médicale 2014, 24, e51–e56. [Google Scholar] [CrossRef]
- Saghrouchni, H.; Barnossi, A.E.; Salamatullah, A.M.; Bourhia, M.; Alzahrani, A.; Alkaltham, M.S.; Alyahya, H.K.; Tahiri, N.E.H.; Imtara, H.; Var, I. Carvacrol: A Promising Environmentally Friendly Agent to Fight Seeds Damping-Off Diseases Induced by Fungal Species. Agronomy 2021, 11, 985. [Google Scholar] [CrossRef]
- Babalık, Z.; Onursal, C.; Erbaş, D.; Koyuncu, M. Use of Carvacrol Helps Maintain Postharvest Quality of Red Globe Table Grape. J. Anim. Plant Sci. 2020, 30, 655–662. [Google Scholar] [CrossRef]
- Kotan, R.; Dadasoglu, F.; Kordali, S.; Cakir, A.; Dikbas, N.; Cakmakci, R. Antibacterial activity of essential oils extracted from some medicinal plants, carvacrol and thymol on Xanthomonas axonopodis pv. vesicatoria (Doidge) Dye causes bacterial spot disease on pepper and tomato. J. Agric. Technol. 2007, 3, 299–306. [Google Scholar]
- Qiao, K.; Liu, Q.; Huang, Y.; Xia, Y.; Zhang, S. Management of Bacterial Spot of Tomato Caused by Copper-Resistant Xanthomonas perforans Using a Small Molecule Compound Carvacrol. Crop Prot. 2020, 132, 105114. [Google Scholar] [CrossRef]
- Kmoch, M.; Loubová, V.; Veselská, M.; Jílková, B.; Víchová, J. Antifungal Activity of Essential Oils on Helminthosporium solani Causing Potato Silver Scurf under In Vitro and In Vivo Conditions. Agriculture 2023, 14, 66. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S.; Shikuku, V.; Dittrich, F.; Torjir, D.N.; Saini, M.; Getenga, Z. Soil sorption and effects on soil microorganisms of thymol and carvacrol monoterpenes from essential oils of aromatic plants. Front. Environ. Sci. 2024, 12, 1379018. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Y.; Li, S.; Ding, W. Resveratrol and Coumarin: Novel Agricultural Antibacterial Agent against Ralstonia solanacearum In Vitro and In Vivo. Molecules 2016, 21, 1501. [Google Scholar] [CrossRef]
- Luo, H.-Z.; Guan, Y.; Yang, R.; Qian, G.-L.; Yang, X.-H.; Wang, J.-S.; Jia, A.-Q. Growth Inhibition and Metabolomic Analysis of Xanthomonas oryzae pv. oryzae Treated with Resveratrol. BMC Microbiol. 2020, 20, 117. [Google Scholar] [CrossRef]
- El Khawand, T.; Gabaston, J.; Taillis, D.; Iglesias, M.-L.; Pedrot, E.; Pinto, A.P.; Fonayet, J.V.; Merillon, J.M.; Decendit, A.; Cluzet, S.; et al. A Dimeric Stilbene Extract Produced by Oxidative Coupling of Resveratrol Active against Plasmopara viticola and Botrytis cinerea for Vine Treatments. OENO One 2020, 54, 157–164. [Google Scholar] [CrossRef]
- Sohn, S.I.; Oh, Y.J.; Kim, B.Y.; Kweon, S.J.; Cho, H.S.; Ryu, T.H. Effect of genetically modified rice producing resveratrol on the soil microbial communities. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 795–805. [Google Scholar] [CrossRef]
- Liu, X.; Ji, D.; Cui, X.; Zhang, Z.; Li, B.; Xu, Y.; Chen, T.; Tian, S. P-Coumaric Acid Induces Antioxidant Capacity and Defense Responses of Sweet Cherry Fruit to Fungal Pathogens. Postharvest Biol. Technol. 2020, 169, 111297. [Google Scholar] [CrossRef]
- Tzintzun-Camacho, O.; Hernández-Jiménez, V.; González-Mendoza, D.; Pérez-Pérez, J.P.; Troncoso-Rojas, R.; Durán-Hernández, D.; Ceceña-Durán, C.; Moreno-Cruz, C.F. Characterization of Grape Marc Hydrolysates and Their Antifungal Effect against Phytopathogenic Fungi of Agricultural Importance. Chil. J. Agric. Res. 2021, 81, 151–160. [Google Scholar] [CrossRef]
- Kalwasińska, A.; Tarnawska, P.; Latos, M.; Pałubicka, K.; Janik, A.; Brzezinska, M.S. New P-Coumaric Acid Formulation in Sustainable Pest Management; Impact on Soil Bacterial Diversity and N-Cycle. Appl. Soil Ecol. 2022, 180, 104634. [Google Scholar] [CrossRef]
- Swiontek Brzezinska, M.; Pałubicka, K.; Latos, M.; Janik, A.; Tarnawska, P.; Krajnik, K.; Burkowska-But, A.; Świątczak, J.; Jedziniak, P.; Pietruszka, K.; et al. Natural compounds derived from Brassicaceae plants as an alternative to synthetic fungicides and their influence on soil fungus diversity. J. Sci. Food Agric. 2023, 103, 317–327. [Google Scholar] [CrossRef]
- Jia, M.; Wang, X.; Zhu, X.; Du, Y.; Zhou, P.; Wang, G.; Bai, Y. Accumulation of coumaric acid is a key factor in tobacco continuous cropping obstacles. Front. Plant Sci. 2024, 15, 1477324. [Google Scholar] [CrossRef]
- El-Nagar, A.; Elzaawely, A.A.; Taha, N.A.; Nehela, Y. The Antifungal Activity of Gallic Acid and Its Derivatives against Alternaria solani, the Causal Agent of Tomato Early Blight. Agronomy 2020, 10, 1402. [Google Scholar] [CrossRef]
- Karpova, N.; Shagdarova, B.; Lunkov, A.; Il’ina, A.; Varlamov, V. Antifungal Action of Chitosan in Combination with Fungicides in Vitro and Chitosan Conjugate with Gallic Acid on Tomatoes against Botrytis cinerea. Biotechnol. Lett. 2021, 43, 1565–1574. [Google Scholar] [CrossRef]
- Sobhy, S.; Al-Askar, A.A.; Bakhiet, E.K.; Elsharkawy, M.M.; Arishi, A.A.; Behiry, S.I.; Abdelkhalek, A. Phytochemical Characterization and Antifungal Efficacy of Camphor (Cinnamomum camphora L.) Extract against Phytopathogenic Fungi. Separations 2023, 10, 189. [Google Scholar] [CrossRef]
- Francesconi, S.; Tagliavento, V.; Ciarroni, S.; Sestili, F.; Balestra, G.M. Chitosan- and Gallic Acid-based (NPF) Displayed Antibacterial Activity against Three Pseudomonas spp. Plant Pathogens and Boosted Systemic Acquired Resistance in Kiwifruit and Olive Plants. Pest Manag. Sci. 2023, 80, 1300–1313. [Google Scholar] [CrossRef] [PubMed]
- Forrer, H.-R.; Musa, T.; Schwab, F.; Jenny, E.; Bucheli, T.; Wettstein, F.; Vogelgsang, S. Fusarium Head Blight Control and Prevention of Mycotoxin Contamination in Wheat with Botanicals and Tannic Acid. Toxins 2014, 6, 830–849. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Lei, M.; Andargie, M.; Zeng, J.; Li, J. Antifungal Activity and Mechanism of Action of Tannic Acid against Penicillium digitatum. Physiol. Mol. Plant Pathol. 2019, 107, 46–50. [Google Scholar] [CrossRef]
- Yao, J.; Zhi, H.; Shi, Q.; Zhang, Y.; Feng, J.; Liu, J.; Huang, H.; Xie, X. Tannic Acid Interfacial Modification of Prochloraz Ethyl Cellulose Nanoparticles for Enhancing the Antimicrobial Effect and Biosafety of Fungicides. ACS Appl. Mater. Interfaces 2023, 15, 41324–41336. [Google Scholar] [CrossRef]
- Han, X.; Gu, S.; Xu, R.; Kong, Y.; Lou, Y.; Wang, Q.; Gao, Y.; Shang, S.; Song, Z.; Song, J.; et al. Efficient Control of Rhizoctonia solani Using Environmentally Friendly pH-Responsive Tannic Acid–Rosin Nano-Microcapsules. ACS Appl. Mater. Interfaces 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Gusiatin, Z.M.; Kaal, J.; Wasilewska, A.; Kumpiene, J.; Radziemska, M. Short-term soil flushing with tannic acid and its effect on metal mobilization and selected properties of calcareous soil. Int. J. Environ. Res. Public Health 2021, 18, 5698. [Google Scholar] [CrossRef]
- Patzke, H.; Schieber, A. Growth-Inhibitory Activity of Phenolic Compounds Applied in an Emulsifiable Concentrate—Ferulic Acid as a Natural Pesticide against Botrytis cinerea. Food Res. Int. 2018, 113, 18–23. [Google Scholar] [CrossRef]
- Shu, P.; Li, Y.; Wang, X.; Yao, L.; Sheng, J.; Shen, L. Exogenous Ferulic Acid Treatment Increases Resistance against Botrytis cinerea in Tomato Fruit by Regulating Nitric Oxide Signaling Pathway. Postharvest Biol. Technol. 2021, 182, 111678. [Google Scholar] [CrossRef]
- Shirai, A.; Tanaka, A. Effects of Ferulic Acid Combined with Light Irradiation on Deoxynivalenol and Its Production in Fusarium graminearum. Fungal Biol. 2024, 128, 1684–1690. [Google Scholar] [CrossRef]
- El-Khateeb, A.Y.; Elsherbiny, E.A.; Tadros, L.K.; Ali, S.M.; Hamed, H.B. Phytochemical analysis and antifungal activity of fruit leaves extracts on the mycelial growth of fungal plant pathogens. J. Plant Pathol. Microbiol. 2013, 4, 1–6. [Google Scholar] [CrossRef]
- Dieryckx, C.; Gaudin, V.; Dupuy, J.-W.; Bonneu, M.; Girard, V.; Job, D. Beyond Plant Defense: Insights on the Potential of Salicylic and Methylsalicylic Acid to Contain Growth of the Phytopathogen Botrytis cinerea. Front. Plant Sci. 2015, 6, 859. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Bell, S.; Hernandez-Montiel, L.G.; Estrada, R.V.; Moreno-Hernández, C.; Gutierrez-Martinez, P. Chitosan and Salicylic Acid as Alternatives for the Control of Postharvest Fungal Diseases in Blueberries (Vaccinium Corymbosum). Int. Food Res. J. 2023, 30, 992–1000. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Hashem, A.H.; Okla, M.K.; Alwasel, Y.A.; Abdelgawad, H.; Attia, M.S. Protective Role of Endophytic Fungi and Salicylic Acid as Therapeutic Nutrients to Improve Immune Responses of Tomato Plants against Fusarial Wilt Disease. Not. Bot. Horti Agrobot. Cluj-Napoca 2024, 52, 13497. [Google Scholar] [CrossRef]
- Amiri, A.; Dugas, R.; Pichot, A.; Bompeix, G. In Vitro and in Vitro Activity of Eugenol Oil (Eugenia Caryophylata) against Four Important Postharvest Apple Pathogens. Int. J. Food Microbiol. 2008, 126, 13–19. [Google Scholar] [CrossRef]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M. Antifungal Activity of Eugenol against Penicillium, Aspergillus, and Fusarium Species. J. Food Prot. 2010, 73, 1124–1128. [Google Scholar] [CrossRef]
- Cui, W.; Du, K.-Y.; Ling, Y.-X.; Yang, C.-J. Activity of Eugenol Derivatives against Fusarium graminearum Q1 Strain and Screening of Isoeugenol Mixtures. J. Plant Pathol. 2021, 103, 915–921. [Google Scholar] [CrossRef]
- Jing, C.; Gou, J.; Han, X.; Wu, Q.; Zhang, C. In Vitro and in Vivo Activities of Eugenol against Tobacco Black Shank Caused by Phytophthora nicotianae. Pestic. Biochem. Physiol. 2017, 142, 148–154. [Google Scholar] [CrossRef]
- Wang, C.; Fan, Y. Eugenol Enhances the Resistance of Tomato against Tomato Yellow Leaf Curl Virus. J. Sci. Food Agric. 2013, 94, 677–682. [Google Scholar] [CrossRef]
- Yossa, N.; Patel, J.; Macarisin, D.; Millner, P.; Murphy, C.; Bauchan, G.; Lo, Y.M. Antibacterial Activity of Cinnamaldehyde and Sporan against Escherichia coli O157:H7 and Salmonella. J. Food Process. Preserv. 2012, 38, 749–757. [Google Scholar] [CrossRef][Green Version]
- Yang, L.; Ma, X.; Wang, L.; Yang, G.; Zhou, L.; Zhang, Z.; Li, X. In Vitro Antifungal Activity and Mechanism of Action of Carvacrol against Sclerotinia sclerotiorum (Lib.) de Bary. Plant Prot. Sci. 2024, 60, 172–180. [Google Scholar] [CrossRef]
- Oluoch, G.; Mamati, E.G.; Matiru, V.; Nyongesa, M. Efficacy of thymol and eugenol against bacterial wilt bacterium Ralstonia solanacearum. Afr. J. Biotechnol. 2021, 20, 256–265. [Google Scholar] [CrossRef]
- Ji, P.; Momol, M.T.; Olson, S.M.; Hong, J.; Pradhanang, P.; Anith, K.N.; Jones, J.B. New tactics for bacterial wilt management on tomatoes in the Southern US. Acta Hortic. 2005, 695, 153. [Google Scholar] [CrossRef]
- Kumari, S.; Kumaraswamy, R.V.; Choudhary, R.C.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Thymol Nanoemulsion Exhibits Potential Antibacterial Activity against Bacterial Pustule Disease and Growth Promotory Effect on Soybean. Sci. Rep. 2018, 8, 6650. [Google Scholar] [CrossRef]
- Sreelatha, S.; Kumar, N.; Yin, T.S.; Rajani, S. Evaluating the Antibacterial Activity and Mode of Action of Thymol-Loaded Chitosan Nanoparticles Against Plant Bacterial Pathogen Xanthomonas campestris pv. campestris. Front. Microbiol. 2022, 12, 792737. [Google Scholar] [CrossRef]
- Gill, T.A.; Li, J.; Saenger, M.; Scofield, S.R. Thymol-Based Submicron Emulsions Exhibit Antifungal Activity against Fusarium graminearum and Inhibit Fusarium Head Blight in Wheat. J. Appl. Microbiol. 2016, 121, 1103–1116. [Google Scholar] [CrossRef]
- Shcherbakova, L.; Mikityuk, O.; Arslanova, L.; Stakheev, A.; Erokhin, D.; Zavriev, S.; Dzhavakhiya, V. Studying the Ability of Thymol to Improve Fungicidal Effects of Tebuconazole and Difenoconazole against Some Plant Pathogenic Fungi in Seed or Foliar Treatments. Front. Microbiol. 2021, 12, 629429. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, Y.; Lu, H.; Li, P.; Chen, J.; Shi, Z.; Xie, Y.; Mo, H.; Hu, L. Nano-Thymol Emulsion Inhibits Botrytis cinerea to Control Postharvest Gray Mold on Tomato Fruit. Agronomy 2022, 12, 2973. [Google Scholar] [CrossRef]
- Song, C.; Guo, N.; Xue, A.; Jia, C.; Shi, W.; Liu, M.; Zhang, M.; Qin, J. Self-Assembled Thymol-Betaine Co-Crystals with Controlled Release and Hygroscopic Properties as Green Preservatives for Aflatoxin Prevention. Food Chem. 2024, 456, 140037. [Google Scholar] [CrossRef]
- Aladhadh, M. A review of modern methods for the detection of foodborne pathogens. Microorganisms 2023, 11, 1111. [Google Scholar] [CrossRef]
- Almasi, H.; Jahanbakhsh Oskouie, M.; Saleh, A. A review on techniques utilized for design of controlled release food active packaging. Crit. Rev. Food Sci. Nutr. 2021, 61, 2601–2621. [Google Scholar] [CrossRef] [PubMed]
- Honma, M.; Yamada, M.; Yasui, M.; Horibata, K.; Sugiyama, K.I.; Masumura, K. In vivo and in vitro mutagenicity of perillaldehyde and cinnamaldehyde. Genes Environ. 2021, 43, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Makwana, S.; Choudhary, R.; Dogra, N.; Kohli, P.; Haddock, J. Nanoencapsulation and Immobilization of Cinnamaldehyde for Developing Antimicrobial Food Packaging Material. LWT 2014, 57, 470–476. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Lopez-Carballo, G.; Catala, R.; Gavara, R.; Hernandez-Munoz, P. Antifungal Properties of Gliadin Films Incorporating Cinnamaldehyde and Application in Active Food Packaging of Bread and Cheese Spread Foodstuffs. Int. J. Food Microbiol. 2013, 166, 369–377. [Google Scholar] [CrossRef]
- Srisa, A.; Harnkarnsujarit, N. Antifungal Films from Trans-Cinnamaldehyde Incorporated Poly(Lactic Acid) and Poly(Butylene Adipate-Co-Terephthalate) for Bread Packaging. Food Chem. 2020, 333, 127537. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Z.; Chen, S.; Dong, H.; Zhang, X.; Qin, Y.; Yao, C.; Xu, F. High-Barrier, Strong, and Antibacterial Paper Fabricated by Coating Acetylated Cellulose and Cinnamaldehyde for Food Packaging. Cellulose 2021, 28, 4371–4384. [Google Scholar] [CrossRef]
- Wan, S.; Liu, Q.; Yang, D.; Guo, P.; Gao, Y.; Mo, R.; Zhang, Y. Characterization of High Amylose Corn Starch-Cinnamaldehyde Inclusion Films for Food Packaging. Food Chem. 2022, 403, 134219. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton Jr, G.; Cancellieri, M.; Tokura, Y. RIFM fragrance ingredient safety assessment, cinnamic acid, CAS Registry Number 621-82-9. Food Chem. Toxicol. 2022, 167, 113232. [Google Scholar] [CrossRef]
- Tong, W.Y.; Rafiee, A.R.A.; Leong, C.R.; Tan, W.-N.; Dailin, D.J.; Almarhoon, Z.M.; Shelkh, M.; Nawaz, A.; Chuah, L.F. Development of Sodium Alginate-Pectin Biodegradable Active Food Packaging Film Containing Cinnamic Acid. Chemosphere 2023, 336, 139212. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Multilayer Antimicrobial Films Based on Starch and PLA with Superficially Incorporated Ferulic or Cinnamic Acids for Active Food Packaging Purposes. Food Chem. Adv. 2023, 2, 100250. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Physicochemical and Antimicrobial Properties of Cassava Starch Films with Ferulic or Cinnamic Acid. LWT 2021, 144, 111242. [Google Scholar] [CrossRef]
- Letsididi, K.S.; Lou, Z.; Letsididi, R.; Mohammed, K.; Maguy, B.L. Antimicrobial and Antibiofilm Effects of Trans-Cinnamic Acid Nanoemulsion and Its Potential Application on Lettuce. LWT 2018, 94, 25–32. [Google Scholar] [CrossRef]
- Ghorani, V.; Alavinezhad, A.; Rajabi, O.; Mohammadpour, A.H.; Boskabady, M.H. Safety and tolerability of carvacrol in healthy subjects: A phase I clinical study. Drug Chem. Toxicol. 2021, 44, 177–189. [Google Scholar] [CrossRef]
- López-Mata, M.; Ruiz-Cruz, S.; Silva-Beltrán, N.; Ornelas-Paz, J.; Zamudio-Flores, P.; Burruel-Ibarra, S. Physicochemical, Antimicrobial and Antioxidant Properties of Chitosan Films Incorporated with Carvacrol. Molecules 2013, 18, 13735–13753. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Maté, J.I.; Gardrat, C.; Coma, V. Effect of Chitosan Molecular Weight on the Antimicrobial Activity and Release Rate of Carvacrol-Enriched Films. Food Hydrocoll. 2015, 51, 60–68. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, H.; Yang, B.; Chen, X.; Sun, H. Physical Properties, Antioxidant and Antimicrobial Activity of Chitosan Films Containing Carvacrol and Pomegranate Peel Extract. Molecules 2015, 20, 11034–11045. [Google Scholar] [CrossRef]
- Tastan, Ö.; Ferrari, G.; Baysal, T.; Donsì, F. Understanding the Effect of Formulation on Functionality of Modified Chitosan Films Containing Carvacrol Nanoemulsions. Food Hydrocoll. 2016, 61, 756–771. [Google Scholar] [CrossRef]
- Kamdem, D.P.; Shen, Z.; Nabinejad, O.; Shu, Z. Development of Biodegradable Composite Chitosan-Based Films Incorporated with Xylan and Carvacrol for Food Packaging Application. Food Packag. Shelf Life 2019, 21, 100344. [Google Scholar] [CrossRef]
- Higueras, L.; López-Carballo, G.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Antimicrobial Packaging of Chicken Fillets Based on the Release of Carvacrol from Chitosan/Cyclodextrin Films. Int. J. Food Microbiol. 2014, 188, 53–59. [Google Scholar] [CrossRef]
- Xiao, L.; Lapu, M.; Cui, L.; Li, J.; Wang, X.; Li, X.; Liu, M.; Liu, D. Impacts of Chitosan/Pullulan/Carvacrol Film on the Quality and Microbial Diversity of Refrigerated Goat Meat. Meat Sci. 2024, 220, 109704. [Google Scholar] [CrossRef]
- Kim, S.A. Rhee Highly Enhanced Bactericidal Effects of Medium Chain Fatty Acids (Caprylic, Capric, and Lauric Acid) Combined with Edible Plant Essential Oils (Carvacrol, Eugenol, β-Resorcylic Acid, Trans-Cinnamaldehyde, Thymol, and Vanillin) against Escherichia coli O157:H7. Food Control 2015, 60, 447–454. [Google Scholar] [CrossRef]
- Laroque, D.A.; Jong, N.R.D.; Müller, L.; Paganini, C.C.; De Araújo, P.H.H.; De Aragão, G.M.F.; Carciofi, B.A.M. Carvacrol Release Kinetics from Cellulose Acetate Films and Its Antibacterial Effect on the Shelf Life of Cooked Ham. J. Food Eng. 2023, 358, 111681. [Google Scholar] [CrossRef]
- Krepker, M.; Prinz-Setter, O.; Shemesh, R.; Vaxman, A.; Alperstein, D.; Segal, E. Antimicrobial Carvacrol-Containing Polypropylene Films: Composition, Structure and Function. Polymers 2018, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, F.; Botta, L.; La Carrubba, V.; Di Pasquale, L.; Settanni, L.; Gaglio, R. Combining Carvacrol and Nisin in Biodegradable Films for Antibacterial Packaging Applications. Int. J. Biol. Macromol. 2021, 193, 117–126. [Google Scholar] [CrossRef]
- Neira, L.M.; Martucci, J.F.; Stejskal, N.; Ruseckaite, R.A. Time-Dependent Evolution of Properties of Fish Gelatin Edible Films Enriched with Carvacrol during Storage. Food Hydrocoll. 2019, 94, 304–310. [Google Scholar] [CrossRef]
- Tao, R.; Sedman, J.; Ismail, A. Characterization and in Vitro Antimicrobial Study of Soy Protein Isolate Films Incorporating Carvacrol. Food Hydrocoll. 2021, 122, 107091. [Google Scholar] [CrossRef]
- Tavares, A.G.; Andrade, J.; Silva, R.R.A.; Marques, C.S.; Da Silva, J.O.R.; Vanetti, M.C.D.; De Melo, N.R.; De Fátima Ferreira Soares, N. Carvacrol-Loaded Liposome Suspension: Optimization, Characterization and Incorporation into Poly(Vinyl Alcohol) Films. Food Funct. 2021, 12, 6549–6557. [Google Scholar] [CrossRef]
- Altan, A.; Aytac, Z.; Uyar, T. Carvacrol Loaded Electrospun Fibrous Films from Zein and Poly(Lactic Acid) for Active Food Packaging. Food Hydrocoll. 2018, 81, 48–59. [Google Scholar] [CrossRef]
- Klinmalai, P.; Srisa, A.; Laorenza, Y.; Katekhong, W.; Harnkarnsujarit, N. Antifungal and Plasticization Effects of Carvacrol in Biodegradable Poly(Lactic Acid) and Poly(Butylene Adipate Terephthalate) Blend Films for Bakery Packaging. LWT 2021, 152, 112356. [Google Scholar] [CrossRef]
- Mao, S.; Li, F.; Zhou, X.; Lu, C.; Zhang, T. Characterization and Sustained Release Study of Starch-Based Films Loaded with Carvacrol: A Promising UV-Shielding and Bioactive Nanocomposite Film. LWT 2023, 180, 114719. [Google Scholar] [CrossRef]
- Requena, R.; Vargas, M.; Chiralt, A. Obtaining Antimicrobial Bilayer Starch and Polyester-Blend Films with Carvacrol. Food Hydrocoll. 2018, 83, 118–133. [Google Scholar] [CrossRef]
- Jahdkaran, E.; Hosseini, S.E.; Nafchi, A.M.; Nouri, L. The Effects of Methylcellulose Coating Containing Carvacrol or Menthol on the Physicochemical, Mechanical, and Antimicrobial Activity of Polyethylene Films. Food Sci. Nutr. 2021, 9, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Busolo, M.A.; Lagaron, J.M. Antioxidant Polyethylene Films Based on a Resveratrol Containing Clay of Interest in Food Packaging Applications. Food Packag. Shelf Life 2015, 6, 30–41. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Chen, M.; Jiang, S.; Cheng, J.; Li, X.; Zhang, M.; Jiang, S. Gelatin/Zein Fiber Mats Encapsulated with Resveratrol: Kinetics, Antibacterial Activity and Application for Pork Preservation. Food Hydrocoll. 2019, 101, 105577. [Google Scholar] [CrossRef]
- Silva, Â.; Duarte, A.; Sousa, S.; Ramos, A.; Domingues, F.C. Characterization and Antimicrobial Activity of Cellulose Derivatives Films Incorporated with a Resveratrol Inclusion Complex. LWT 2016, 73, 481–489. [Google Scholar] [CrossRef]
- Duarte, A.; Martinho, A.; Luís, Â.; Figueiras, A.; Oleastro, M.; Domingues, F.C.; Silva, F. Resveratrol encapsulation with methyl-β-cyclodextrin for antibacterial and antioxidant delivery applications. LWT-Food Sci. Technol. 2015, 63, 1254–1260. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Panda, S.K.; Navitha, M.; Asha, K.K.; Anandan, R.; Mathew, S. Vanillic Acid and Coumaric Acid Grafted Chitosan Derivatives: Improved Grafting Ratio and Potential Application in Functional Food. J. Food Sci. Technol. 2015, 52, 7153–7162. [Google Scholar] [CrossRef]
- Liu, X.; Sun, X.; Du, H.; Li, Y.; Wen, Y.; Zhu, Z. A Transparent P-Coumaric Acid-Grafted-Chitosan Coating with Antimicrobial, Antioxidant and Antifogging Properties for Fruit Packaging Applications. Carbohydr. Polym. 2024, 339, 122238. [Google Scholar] [CrossRef]
- Lee, S.; Zhang, M.; Wang, G.; Meng, W.; Zhang, X.; Wang, D.; Zhou, Y.; Wang, Z. Characterization of Polyvinyl Alcohol/Starch Composite Films Incorporated with p-Coumaric Acid Modified Chitosan and Chitosan Nanoparticles: A Comparative Study. Carbohydr. Polym. 2021, 262, 117930. [Google Scholar] [CrossRef]
- Noman, R.R.A.; Wong, C.S.; Law, K.P.; Neo, Y.P. Fabrication and characterisation of electrospun zein-based fibres functionalised by caffeic and p-coumaric acid for potential active packaging applications. Int. J. Food Sci. Technol. 2024, 59, 7942–7951. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, C.; Zhou, Y.; Lu, Z.; Zhao, H.; Bie, X.; Lu, F. Preparation of Gallic Acid-Grafted Chitosan Using Recombinant Bacterial Laccase and Its Application in Chilled Meat Preservation. Front. Microbiol. 2018, 9, 1729. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, C.; Sun, J.; Lv, S. Bioactive Edible Sodium Alginate Films Incorporated with Tannic Acid as Antimicrobial and Antioxidative Food Packaging. Foods 2022, 11, 3044. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, H.; Yang, X.; Ma, Q. Tannic Acid: A Crosslinker Leading to Versatile Functional Polymeric Networks: A Review. RSC Adv. 2022, 12, 7689–7711. [Google Scholar] [CrossRef]
- Zou, J.; Wong, J.; Lee, C.-R.; Nitin, N.; Wang, L.; Sun, G. Protein-Based Rechargeable and Replaceable Antimicrobial and Antifouling Coatings on Hydrophobic Food-Contact Surfaces. ACS Appl. Bio Mater. 2024, 7, 1842–1851. [Google Scholar] [CrossRef]
- Venkatesan, R.; Sivaprakash, P.; Kim, I.; Eldesoky, G.E.; Kim, S.-C. Tannic Acid as a Crosslinking Agent in Poly(Butylene Adipate-Co-Terephthalate) Composite Films Enhanced with Carbon Nanoparticles: Processing, Characterization, and Antimicrobial Activities for Food Packaging. J. Environ. Chem. Eng. 2023, 11, 110194. [Google Scholar] [CrossRef]
- Sharma, S.; Jaiswal, A.K.; Duffy, B.; Jaiswal, S. Ferulic Acid Incorporated Active Films Based on Poly(Lactide)/Poly(Butylene Adipate-Co-Terephthalate) Blend for Food Packaging. Food Packag. Shelf Life 2020, 24, 100491. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, H.; Ren, F.; Wang, J.; Yin, S. Preparation and Characterization of Ferulic Acid Wheat Gluten Nanofiber Films with Excellent Antimicrobial Properties. Foods 2023, 12, 2778. [Google Scholar] [CrossRef]
- Ou, S.; Wang, Y.; Tang, S.; Huang, C.; Jackson, M.G. Role of Ferulic Acid in Preparing Edible Films from Soy Protein Isolate. J. Food Eng. 2004, 70, 205–210. [Google Scholar] [CrossRef]
- Fang, Y.; Fu, J.; Tao, C.; Liu, P.; Cui, B. Mechanical Properties and Antibacterial Activities of Novel Starch-Based Composite Films Incorporated with Salicylic Acid. Int. J. Biol. Macromol. 2019, 155, 1350–1358. [Google Scholar] [CrossRef]
- Hu, F.; Sun, T.; Xie, J.; Xue, B.; Li, X.; Gan, J.; Li, L.; Bian, X.; Shao, Z. Functional Properties of Chitosan Films with Conjugated or Incorporated Salicylic Acid. J. Mol. Struct. 2020, 1223, 129237. [Google Scholar] [CrossRef]
- Kurczewska, J.; Ratajczak, M.; Gajecka, M. Alginate and pectin films covering halloysite with encapsulated salicylic acid as food packaging components. Appl. Clay Sci. 2021, 214, 106270. [Google Scholar] [CrossRef]
- Sanla-Ead, N.; Jangchud, A.; Chonhenchob, V.; Suppakul, P. Antimicrobial Activity of Cinnamaldehyde and Eugenol and Their Activity after Incorporation into Cellulose-based Packaging Films. Packag. Technol. Sci. 2011, 25, 7–17. [Google Scholar] [CrossRef]
- Narayanan, A.; Neera, N.; Mallesha, N.; Ramana, K.V. Synergized Antimicrobial Activity of Eugenol Incorporated Polyhydroxybutyrate Films Against Food Spoilage Microorganisms in Conjunction with Pediocin. Appl. Biochem. Biotechnol. 2013, 170, 1379–1388. [Google Scholar] [CrossRef]
- Huang, X.; Ge, X.; Zhou, L.; Wang, Y. Eugenol Embedded Zein and Poly(Lactic Acid) Film as Active Food Packaging: Formation, Characterization, and Antimicrobial Effects. Food Chem. 2022, 384, 132482. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, H.; Kang, S.; Xia, L.; Jiang, S.; Chen, M.; Jiang, S. An Active Packaging Film Based on Yam Starch with Eugenol and Its Application for Pork Preservation. Food Hydrocoll. 2019, 96, 546–554. [Google Scholar] [CrossRef]
- Sivaram, S.; Somanathan, H.; Kumaresan, S.M.; Muthuraman, M.S. The Beneficial Role of Plant Based Thymol in Food Packaging Application: A Comprehensive Review. Appl. Food Res. 2022, 2, 100214. [Google Scholar] [CrossRef]
- Michalska-Sionkowska, M.; Walczak, M.; Sionkowska, A. Antimicrobial Activity of Collagen Material with Thymol Addition for Potential Application as Wound Dressing. Polym. Test. 2017, 63, 360–366. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and Antimicrobial Activity Studies of Polypropylene Films with Carvacrol and Thymol for Active Packaging. J. Food Eng. 2011, 109, 513–519. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Redhwi, H.H.; Tsagkalias, I.; Vouvoudi, E.C.; Achilias, D.S. Development of Bio-Composites with Enhanced Antioxidant Activity Based on Poly(Lactic Acid) with Thymol, Carvacrol, Limonene, or Cinnamaldehyde for Active Food Packaging. Polymers 2021, 13, 3652. [Google Scholar] [CrossRef]
- Pleva, P.; Bartošová, L.; Máčalová, D.; Zálešáková, L.; Sedlaříková, J.; Janalíková, M. Biofilm Formation Reduction by Eugenol and Thymol on Biodegradable Food Packaging Material. Foods 2021, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Chen, D.; Li, B.; Zhang, B.; Miao, F.; Zhou, L. Bioactivity and Structure-Activity Relationship of Cinnamic Acid Esters and Their Derivatives as Potential Antifungal Agents for Plant Protection. PLoS ONE 2017, 12, e0176189. [Google Scholar] [CrossRef] [PubMed]
- Chavan, P.S.; Tupe, S.G. Antifungal Activity and Mechanism of Action of Carvacrol and Thymol against Vineyard and Wine Spoilage Yeasts. Food Control 2014, 46, 115–120. [Google Scholar] [CrossRef]
| Substance | Chemical Name | Group | Occurrence | References |
|---|---|---|---|---|
| Cinnamaldehyde (CN) | (E)-3-phenyl-2-propenal | Phenylpropanoid | Cinnamon oil (60–75%) from Cinnamomum cassia and Cinnamomum zeylanicum | [10,11] |
| Cinnamic acid (CA) | 3-phenylprop-2-enoic acid | Phenolic acid (hydroxycinnamic acid) | Cinnamomum spp., vegetables, whole grains | [12] |
| Carvacrol (CR) | 2-methyl-5-(1-methylenthyl)-phenol | Monoterpenoid phenol | Essential oils of the Labiatae family, including Origanum, Satureja, Thymbra, Thymus and Corydothymus | [13,14] |
| Resveratrol (RS) | 3,5,4′-trihydroxystilbene | Stilbene | Various food products, including grapes, red wine, and peanuts | [15] |
| p-Coumaric acid (CO) | 4-hydroxycinnamic acid | Phenolic acid (hydroxycinnamic acid) | Fruits, vegetables and grains | [16] |
| Gallic acid (GA) | 3,4,5-trihydroxybenzoic acid | Phenolic acid (hydroxybenzoic acid) | Oak bark, tea leaves, as well as fruits and walnuts | [17,18] |
| Tannic acid (TA) | 1,2,3,4,6-penta-O-{3,4-dihydroxy-5-[(3,4,5-trihydroxybenzoyl)oxy]benzoyl}-D-glucopyranose | Tannin | Common plants | [19] |
| Ferulic acid (FA) | 4-hydroxy-3-methoxycinnamic acid | Phenolic acid (hydroxycinnamic acid) | Ubiquitous in seeds, and leaves | [20] |
| Salicylic acid (SA) | 2-hydroxybenzoic acid | Phenolic acid (hydroxybenzoic acid) | Willow bark, fruits (berries, grapes), vegetables | [21] |
| Eugenol (EU) | 4-Allyl-2-methoxy phenol | Phenylpropanoid | Amiaceae, Lauraceae, Myrtaceae and Myristicaceae families, and in clove oil from Syzygium aromaticum | [22] |
| Thymol (TH) | 2-Isopropyl-5-methylphenol | Monoterpenoid phenol | Thyme (Thymus spp.) | [23] |
| Substance | Target Microorganisms | Mechanism of Action | References |
|---|---|---|---|
| Cinnamaldehyde | Aspergillus spp., Fusarium oxysporum, Penicillium digitatum, Phytophthora capsici | Increased oxidative stress, cell membrane damage, disrupted fatty acid, polysaccharide and leucine metabolism | [26,29,39,40,42,45] |
| Cinnamic acid | Botrytis cinerea, Fusarium spp., Pyricularia grisea | Disruption of organelle function, induction of cell death | [12,167] |
| Carvacrol | Fusarium oxysporum, Cladosporium spp., Alternaria alternata | Damage to cell membrane, leakage of intracellular components | [58,168] |
| Resveratrol | Ralstonia solanacearum, Xanthomonas oryzae | Damage to bacterial membrane, inhibition of metabolism | [65,66] |
| Eugenol | Aspergillus spp., Penicillium spp. | Destruction of cell membrane, inhibition of protein synthesis | [90,91] |
| Substance | Polymer Matrix | Impact on Food Shelf Life | Antimicrobial Effect | References |
|---|---|---|---|---|
| Cinnamaldehyde | Polylactide (PLA), sodium alginate | Extend bread freshness by 21 days, protect raw beef and strawberries | Inhibition of growth of Penicillium spp., Aspergillus niger, E. coli, S. aureus | [110,111,112] |
| Cinnamic acid | Sodium alginate and pectin starch, PLA | Reduction of bacterial contamination of raw beef by 84% | Antibacterial activity against E. coli and L. innocua | [114,115,116] |
| Carvacrol | Cellulose acetate | Extend freshness of pork ham 2.8 times | Antimicrobial activity against Weissella viridescens and Pseudomonas fluorescens | [127] |
| Resveratrol | Poliethylene (PE) | Delay fat oxidation, extend meat shelf life | Antimicrobial activity against S. aureus, Campylobacter spp. | [137,140] |
| Eugenol | Zein, PLA | Increase film flexibility, extend pork shelf life | Inhibiting the growth of E. coli and S. aureus | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dembińska, K.; Shinde, A.H.; Pejchalová, M.; Richert, A.; Swiontek Brzezinska, M. The Application of Natural Phenolic Substances as Antimicrobial Agents in Agriculture and Food Industry. Foods 2025, 14, 1893. https://doi.org/10.3390/foods14111893
Dembińska K, Shinde AH, Pejchalová M, Richert A, Swiontek Brzezinska M. The Application of Natural Phenolic Substances as Antimicrobial Agents in Agriculture and Food Industry. Foods. 2025; 14(11):1893. https://doi.org/10.3390/foods14111893
Chicago/Turabian StyleDembińska, Katarzyna, Ambika H. Shinde, Marcela Pejchalová, Agnieszka Richert, and Maria Swiontek Brzezinska. 2025. "The Application of Natural Phenolic Substances as Antimicrobial Agents in Agriculture and Food Industry" Foods 14, no. 11: 1893. https://doi.org/10.3390/foods14111893
APA StyleDembińska, K., Shinde, A. H., Pejchalová, M., Richert, A., & Swiontek Brzezinska, M. (2025). The Application of Natural Phenolic Substances as Antimicrobial Agents in Agriculture and Food Industry. Foods, 14(11), 1893. https://doi.org/10.3390/foods14111893








