Antiaging Effect of 2-O-β-D-Glucopyranosyl Ascorbic Acid Derived from Lycium barbarum L. Through Modulating the IIS Pathway and Gut Microbiota in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AA-2βG
2.3. Nematode Strains
2.4. Lifespan Analysis
2.5. C. elegans Fertility Assay
2.6. Assay of Lifespan Under Various Stressors
2.7. Measurement of Lipofuscin and Body Size in C. elegans
2.8. Body Bending and Pharyngeal Pumping Assays
2.9. Motility Measurement
2.10. Reactive Oxygen Species (ROS) Assay in C. elegans
2.11. Measurement of Biochemical Indexes in C. elegans
2.12. mRNA Relative Expression Analysis
2.12.1. Transcriptome Analysis
2.12.2. RNA Extraction and RT-qPCR Analysis
2.13. DAF-16::GFP Intracellular Localization
2.14. Gut Microbiota Analysis
2.15. Statistical Analysis
3. Results and Discussion
3.1. AA-2βG Extended Lifespan of C. elegans
3.2. AA-2βG Enhanced Stress Resistance in C. elegans

3.3. AA-2βG Reduced Lipofuscin and Showed No Effect on Body Size in C. elegans
3.4. AA-2βG Improved the Health Span of C. elegans
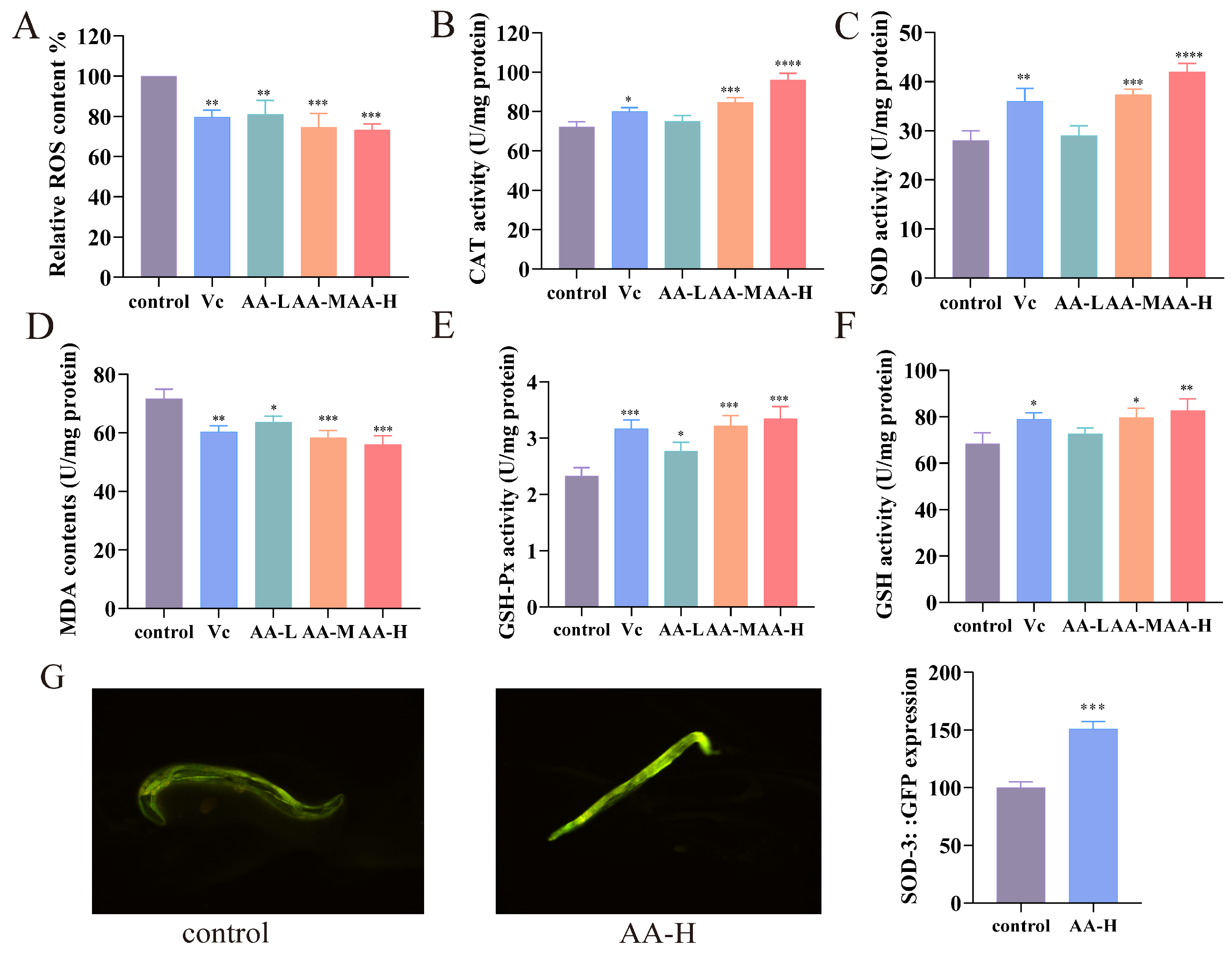
3.5. AA-2βG Enhanced Antioxidant Capacity in C. elegans
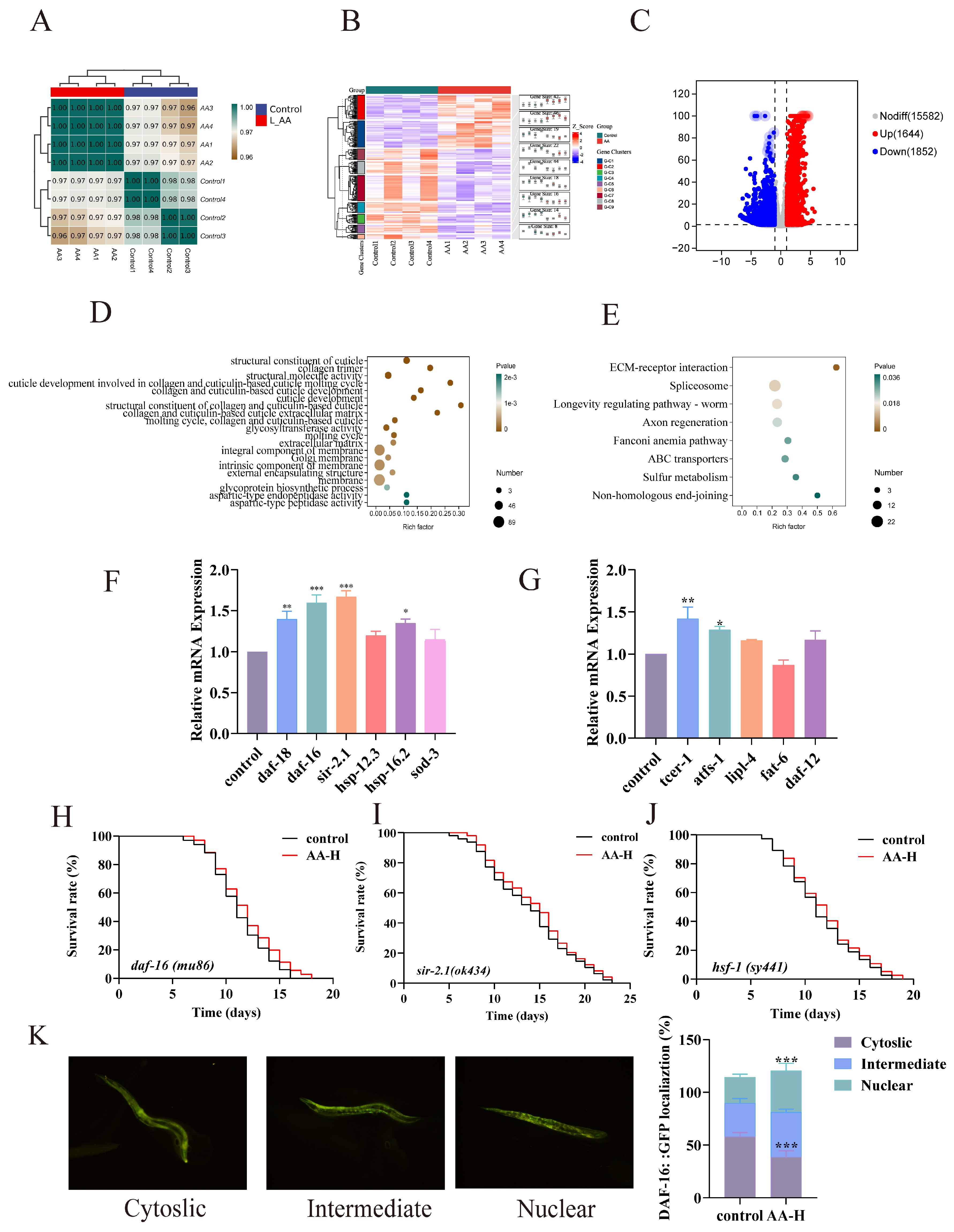
3.6. AA-2βG Modulated the Expression of Related mRNA in C. elegans
3.7. Genetic Requirements for AA-2βG-Mediated Antiaging Effects
3.8. Validation of Fluorescent Mutants
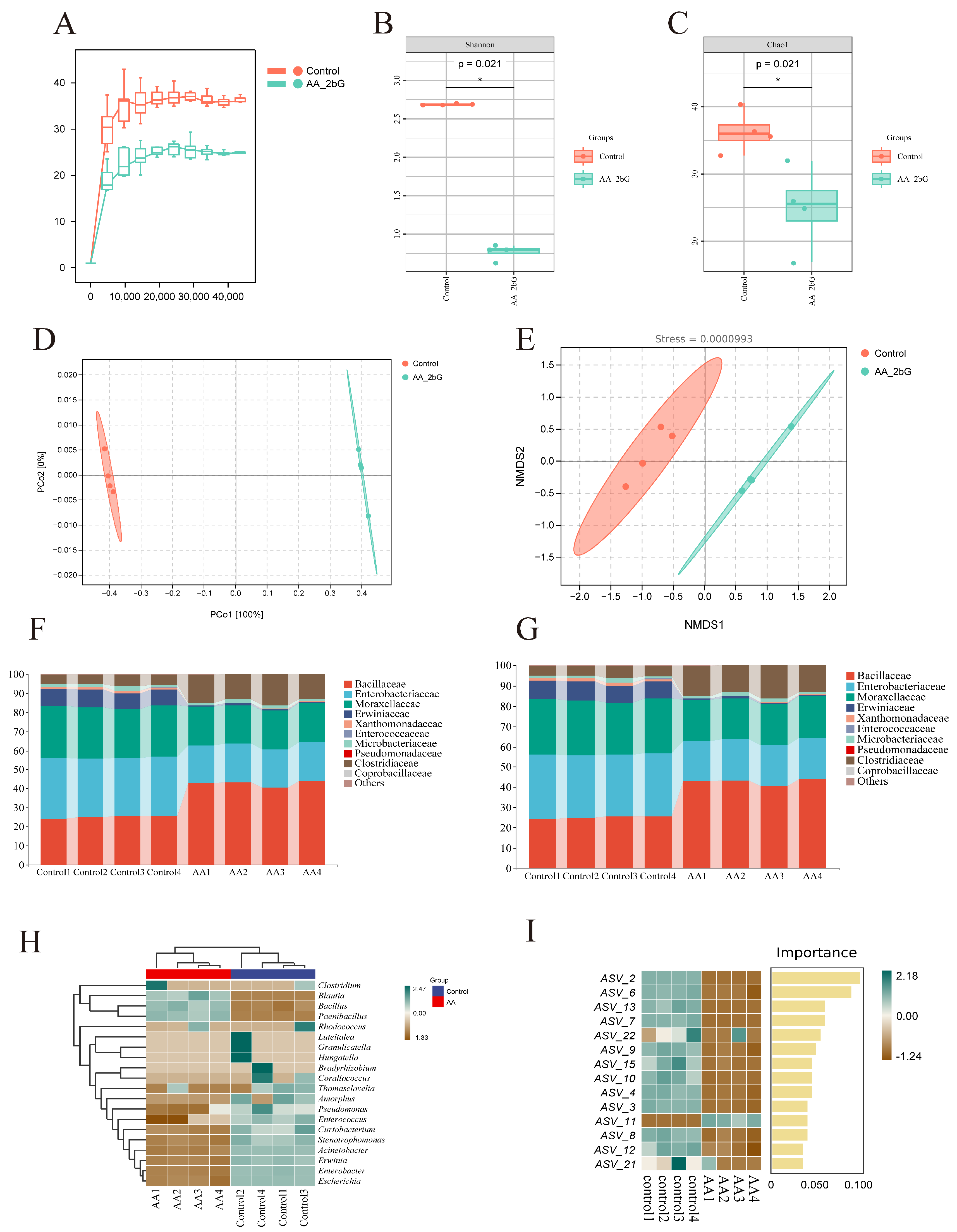
3.9. AA-2βG Regulated the Gut Microbiota of C. elegans
3.10. Functional Predictive Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borghesan, M.; Hoogaars, W.; Varela-Eirin, M.; Talma, N.; Demaria, M. A senescence-centric view of aging: Implications for longevity and disease. Trends Cell Biol. 2020, 30, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Partridge, L.; Fuentealba, M.; Kennedy, B.K. The quest to slow ageing through drug discovery. Nat. Rev. Drug Discov. 2020, 19, 513–553. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef] [PubMed]
- Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Djuriš, J.D.; Ilić, T.D.; Kostić, A.Ž.; Pešić, M.B. Health benefits and applications of goji berries in functional food products development: A review. Antioxidants 2022, 11, 248. [Google Scholar] [CrossRef]
- Ma, R.-H.; Zhang, X.-X.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. Research progress of Lycium barbarum L. as functional food: Phytochemical composition and health benefits. Curr. Opin. Food Sci. 2022, 47, 100871. [Google Scholar] [CrossRef]
- Miranda, M.R.; Vestuto, V.; Amodio, G.; Manfra, M.; Pepe, G.; Campiglia, P. Antitumor mechanisms of Lycium barbarum fruit: An overview of in vitro and in vivo potential. Life 2024, 14, 420. [Google Scholar] [CrossRef]
- Toyoda-Ono, Y.; Maeda, M.; Nakao, M.; Yoshimura, M.; Sugiura-Tomimori, N.; Fukami, H. 2-O-(b-D-Glucopyranosyl) ascorbic acid, a novel ascorbic acid analogue isolated from Lycium fruit. J. Agric. Food Chem. 2004, 52, 2092–2096. [Google Scholar] [CrossRef]
- Davey, M.W.; Montagu, M.V.; Inze, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Zhang, X.; Liu, J.; Hao, Y.; Yang, X.; Wang, Y. Comparative evaluation of the antioxidant effects of the natural vitamin C analog 2-O-b-D-glucopyranosyl-L-ascorbic acid isolated from Goji berry fruit. Arch. Pharm. Res. 2011, 34, 801–810. [Google Scholar] [CrossRef]
- Zhang, S.; Li, F.; Zhou, T.; Wang, G.; Li, Z. Caenorhabditis elegans as a useful model for studying aging mutations. Front. Endocrinol. 2020, 11, 554994. [Google Scholar] [CrossRef]
- Zečić, A.; Braeckman, B.P. DAF-16/FoxO in Caenorhabditis elegans and its role in metabolic remodeling. Cells 2020, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Li, S.T.; Zhao, H.Q.; Zhang, P.; Liang, C.Y.; Zhang, Y.P.; Hsu, A.L.; Dong, M.Q. DAF-16 stabilizes the aging transcriptome and is activated in mid-aged Caenorhabditis elegans to cope with internal stress. Aging Cell 2019, 18, e12896. [Google Scholar] [CrossRef] [PubMed]
- Ayuda-Durán, B.; González-Manzano, S.; Miranda-Vizuete, A.; Dueñas, M.; Santos-Buelga, C.; González-Paramás, A.M. Epicatechin modulates stress-resistance in C. elegans via insulin/IGF-1 signaling pathway. PLoS ONE 2019, 14, e0199483. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Somogyvári, M.; Sőti, C. Hsp90 stabilizes SIRT1 orthologs in mammalian cells and C. elegans. Int. J. Mol. Sci. 2018, 19, 3661. [Google Scholar] [CrossRef]
- Servello, F.A.; Apfeld, J. The heat shock transcription factor HSF-1 protects Caenorhabditis elegans from peroxide stress. Transl. Med. Aging 2020, 4, 88–92. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Huang, K.; Dong, W.; Liu, W.; Yan, Y.; Wan, P.; Peng, Y.; Xu, Y.; Zeng, X.; Cao, Y. 2-O-b-D-Glucopyranosyl-L-ascorbic acid, an ascorbic acid derivative isolated from the fruits of Lycium barbarum L., modulates gut microbiota and palliates colitis in dextran sodium sulfate-induced colitis in mice. J. Agric. Food Chem. 2019, 67, 11408–11419. [Google Scholar] [CrossRef]
- Dong, W.; Peng, Y.; Chen, G.; Xie, Z.; Xu, W.; Zhou, W.; Mi, J.; Lu, L.; Sun, Y.; Zeng, X. 2-O-b-D-Glucopyranosyl-L-ascorbic acid, an ascorbic acid derivative isolated from the fruits of Lycium barbarum L., ameliorates high fructose-induced neuroinflammation in mice: Involvement of gut microbiota and leaky gut. Food Sci. Hum. Wellness 2024, 13, 241–253. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Fan, H.; Billy, K.J.; Zhao, Y.; Zhan, X.; Yang, L.; Jia, Y. Effects of Lycium barbarum polysaccharides on health and aging of C. elegans depend on daf-12/daf-16. Oxidative Med. Cell. Longev. 2019, 2019, 6379493. [Google Scholar] [CrossRef]
- Harrington, S.; Knox, J.J.; Burns, A.R.; Choo, K.-L.; Au, A.; Kitner, M.; Haeberli, C.; Pyche, J.; D’Amata, C.; Kim, Y.-H. Egg-laying and locomotory screens with C. elegans yield a nematode-selective small molecule stimulator of neurotransmitter release. Commun. Biol. 2022, 5, 865. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Lai, M.; Li, Q.; Zhang, H.; Chen, Z.; Gong, S.; Liu, X.; Liu, B. Anti-oxidative and anti-aging effects of mannoprotein-rich yeast cell wall enzymatic hydrolysate by modulating gut microbiota and metabolites in Caenorhabditis elegans. Food Res. Int. 2023, 170, 112753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhu, J.; Liu, P.; Guo, R.; Yan, Y.; Mi, J.; Lu, L.; Cao, Y.; Zeng, X. Petunidin-3-O-[rhamnopyranosyl-(trans-p-coumaroyl)]-5-O-(b-D-glucopyranoside), the main anthocyanin from the fruits of Lycium ruthenicum murray, enhances the lifespan of Caenorhabditis elegans by activating DAF-16 and improving the gut microbiota. Food Biosci. 2024, 61, 104642. [Google Scholar] [CrossRef]
- Currey, H.N.; Liachko, N.F. Evaluation of motor impairment in C. elegans models of amyotrophic lateral sclerosis. J. Vis. Exp. 2021, 175, e62699. [Google Scholar] [CrossRef]
- Liang, L.; Yue, Y.; Zhong, L.; Liang, Y.; Shi, R.; Luo, R.; Zhao, M.; Cao, X.; Yang, M.; Du, J. Anti-aging activities of Rehmannia glutinosa Libosch. crude polysaccharide in Caenorhabditis elegans based on gut microbiota and metabonomic analysis. Int. J. Biol. Macromol. 2023, 253, 127647. [Google Scholar] [CrossRef]
- Wang, T.; Jing, M.; Zhang, T.; Zhang, Z.; Sun, Y.; Wang, Y. Tetramethylpyrazine nitrone TBN extends the lifespan of C. elegans by activating the Nrf2/SKN-1 signaling pathway. Biochem. Biophys. Res. Commun. 2022, 614, 107–113. [Google Scholar] [CrossRef]
- Yordy, J.; Kraus, C.; Hayward, J.J.; White, M.E.; Shannon, L.M.; Creevy, K.E.; Promislow, D.E.; Boyko, A.R. Body size, inbreeding, and lifespan in domestic dogs. Conserv. Genet. 2020, 21, 137–148. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, Y.; Wang, C.; Zhou, W.; Shu, Y.; Zhang, K.; Zeng, X.; Guo, R. Lonicera japonica polysaccharides improve longevity and fitness of Caenorhabditis elegans by activating DAF-16. Int. J. Biol. Macromol. 2023, 229, 81–91. [Google Scholar] [CrossRef]
- Zhou, K.I.; Pincus, Z.; Slack, F.J. Longevity and stress in Caenorhabditis elegans. Aging 2011, 3, 733. [Google Scholar] [CrossRef]
- Sandner, G.; Mueller, A.S.; Zhou, X.; Stadlbauer, V.; Schwarzinger, B.; Schwarzinger, C.; Wenzel, U.; Maenner, K.; van der Klis, J.D.; Hirtenlehner, S. Ginseng extract ameliorates the negative physiological effects of heat stress by supporting heat shock response and improving intestinal barrier integrity: Evidence from studies with heat-stressed Caco-2 cells, C. elegans and growing broilers. Molecules 2020, 25, 835. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.J.; Vashisth, M.K.; Ge, Y.; Dai, Q.; He, F.; Wang, X. Anti-inflammation and anti-aging mechanisms of mercaptopurine in vivo and in vitro. Biochem. Biophys. Res. Commun. 2023, 638, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Todorova, M.N.; Savova, M.S.; Mihaylova, L.V.; Georgiev, M.I. Icariin improves stress resistance and extends lifespan in Caenorhabditis elegans through hsf-1 and daf-2-driven hormesis. Int. J. Mol. Sci. 2023, 25, 352. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Azzini, E.; Zucca, P.; Varoni, E.M.; Kumar, N.V.A.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Peluso, I. Plant-derived bioactives and oxidative stress-related disorders: A key trend towards healthy aging and longevity promotion. Appl. Sci. 2020, 10, 947. [Google Scholar] [CrossRef]
- Takebayashi, J.; Yagi, Y.; Ishii, R.; Abe, S.; Yamada, K.; Tai, A. Antioxidant properties of 2-O-b-D-glucopyranosyl-L-ascorbic acid. Biosci. Biotechnol. Biochem. 2008, 72, 1558–1563. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, S.; Barbosa, G.O.; Bai, M.; Kornberg, T.B.; Ma, D.K. The conserved transmembrane protein TMEM-39 coordinates with COPII to promote collagen secretion and regulate ER stress response. PLoS Genet. 2021, 17, e1009317. [Google Scholar] [CrossRef]
- Hu, M.; Ling, Z.; Ren, X. Extracellular matrix dynamics: Tracking in biological systems and their implications. J. Biol. Eng. 2022, 16, 13. [Google Scholar] [CrossRef]
- Sherill-Rofe, D.; Raban, O.; Findlay, S.; Rahat, D.; Unterman, I.; Samiei, A.; Yasmeen, A.; Kaiser, Z.; Kuasne, H.; Park, M. Multi-omics data integration analysis identifies the spliceosome as a key regulator of DNA double-strand break repair. NAR Cancer 2022, 4, zcac013. [Google Scholar] [CrossRef]
- Wilkens, S. Structure and mechanism of ABC transporters. F1000Prime Rep. 2015, 7, 14. [Google Scholar] [CrossRef]
- Yen, K.; Narasimhan, S.D.; Tissenbaum, H.A. DAF-16/Forkhead box O transcription factor: Many paths to a single Fork (head) in the road. Antioxid. Redox Signal. 2011, 14, 623–634. [Google Scholar] [CrossRef]
- Lin, X.-X.; Sen, I.; Janssens, G.E.; Zhou, X.; Fonslow, B.R.; Edgar, D.; Stroustrup, N.; Swoboda, P.; Yates, J.R., 3rd; Ruvkun, G. DAF-16/FOXO and HLH-30/TFEB function as combinatorial transcription factors to promote stress resistance and longevity. Nat. Commun. 2018, 9, 4400. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, M.; Guarente, L. Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature 2011, 477, E1–E2. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Biró, J.B.; Ahmed, S.; Kovács, M.; Sigmond, T.; Hotzi, B.; Varga, M.; Vincze, V.V.; Mohammad, U.; Vellai, T. Age-dependent heat shock hormesis to HSF-1 deficiency suggests a compensatory mechanism mediated by the unfolded protein response and innate immunity in young Caenorhabditis elegans. Aging Cell 2024, 23, e14246. [Google Scholar] [CrossRef] [PubMed]
- Motola, D.L.; Cummins, C.L.; Rottiers, V.; Sharma, K.K.; Li, T.; Li, Y.; Suino-Powell, K.; Xu, H.E.; Auchus, R.J.; Antebi, A. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell 2006, 124, 1209–1223. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Savini, M.; Folick, A.K.; Hu, K.; Masand, R.; Graham, B.H.; Wang, M.C. Lysosomal signaling promotes longevity by adjusting mitochondrial activity. Dev. Cell 2019, 48, 685–696. [Google Scholar] [CrossRef]
- Amrit, F.R.G.; Steenkiste, E.M.; Ratnappan, R.; Chen, S.-W.; McClendon, T.B.; Kostka, D.; Yanowitz, J.; Olsen, C.P.; Ghazi, A. DAF-16 and TCER-1 facilitate adaptation to germline loss by restoring lipid homeostasis and repressing reproductive physiology in C. elegans. PLoS Genet. 2016, 12, e1005788. [Google Scholar] [CrossRef]
- Amrit, F.R.; Naim, N.; Ratnappan, R.; Loose, J.; Mason, C.; Steenberge, L.; McClendon, B.T.; Wang, G.; Driscoll, M.; Yanowitz, J.L. The longevity-promoting factor, TCER-1, widely represses stress resistance and innate immunity. Nat. Commun. 2019, 10, 3042. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’connor, E.M.; Cusack, S.; Harris, H.; Coakley, M.; Lakshminarayanan, B.; O’sullivan, O. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Dirksen, P.; Marsh, S.A.; Braker, I.; Heitland, N.; Wagner, S.; Nakad, R.; Mader, S.; Petersen, C.; Kowallik, V.; Rosenstiel, P. The native microbiome of the nematode Caenorhabditis elegans: Gateway to a new host-microbiome model. BMC Biol. 2016, 14, 38. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Y.; Imre, K.; Arslan-Acaroz, D.; Istanbullugil, F.R.; Fang, Y.; Ros, G.; Zhu, K.; Acaroz, U. Mechanisms of probiotic Bacillus against enteric bacterial infections. One Health Adv. 2023, 1, 21. [Google Scholar] [CrossRef]
- Donato, V.; Ayala, F.R.; Cogliati, S.; Bauman, C.; Costa, J.G.; Lenini, C.; Grau, R. Bacillus subtilis biofilm extends Caenorhabditis elegans longevity through downregulation of the insulin-like signalling pathway. Nat. Commun. 2017, 8, 14332. [Google Scholar] [CrossRef] [PubMed]
- Chanda, W.; Jiang, H.; Liu, S.-J. The Ambiguous correlation of Blautia with obesity: A systematic review. Microorganisms 2024, 12, 1768. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Madkour, M.; El-Saadony, M.T.; Reda, F.M. Paenibacillus polymyxa (LM31) as a new feed additive: Antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021, 276, 114920. [Google Scholar] [CrossRef]
- Cerqueira, G.M.; Peleg, A.Y. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life 2011, 63, 1055–1060. [Google Scholar] [CrossRef]
- Xiong, X.; Tian, S.; Yang, P.; Lebreton, F.; Bao, H.; Sheng, K.; Yin, L.; Chen, P.; Zhang, J.; Qi, W.; et al. Emerging enterococcus pore-forming toxins with MHC/HLA-I as receptors. Cell 2022, 185, 1157–1171. [Google Scholar] [CrossRef]
- Kirienko, N.V.; Cezairliyan, B.O.; Ausubel, F.M.; Powell, J.R. Pseudomonas aeruginosa PA14 pathogenesis in Caenorhabditis elegans. Methods Mol. Biol. 2014, 1149, 653–669. [Google Scholar] [CrossRef]
- Sun, R.; Jin, D.; Fei, F.; Xu, Z.; Cao, B.; Li, J. Mushroom polysaccharides from Grifola frondosa (Dicks.) Gray and Inonotus obliquus (Fr.) Pilat ameliorated dextran sulfate sodium-induced colitis in mice by global modulation of systemic metabolism and the gut microbiota. Front. Pharmacol. 2023, 14, 1172963. [Google Scholar] [CrossRef]
- Mutlu, A.S.; Duffy, J.; Wang, M.C. Lipid metabolism and lipid signals in aging and longevity. Dev. Cell 2021, 56, 1394–1407. [Google Scholar] [CrossRef]
- Johnson, A.A.; Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019, 18, e13048. [Google Scholar] [CrossRef]
- Dai, Z.-L.; Wu, G.; Zhu, W.-Y. Amino acid metabolism in intestinal bacteria: Links between gut ecology and host health. Front. Biosci. 2011, 16, 1768–1786. [Google Scholar] [CrossRef]
- Burhans, W.C.; Weinberger, M. DNA replication stress, genome instability and aging. Nucleic Acids Res. 2007, 35, 7545–7556. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, J.; Dong, W.; Zheng, J.; Han, B.; Zhang, Y.; Wang, J.; Zeng, X. Antiaging Effect of 2-O-β-D-Glucopyranosyl Ascorbic Acid Derived from Lycium barbarum L. Through Modulating the IIS Pathway and Gut Microbiota in Caenorhabditis elegans. Foods 2025, 14, 1875. https://doi.org/10.3390/foods14111875
Fang J, Dong W, Zheng J, Han B, Zhang Y, Wang J, Zeng X. Antiaging Effect of 2-O-β-D-Glucopyranosyl Ascorbic Acid Derived from Lycium barbarum L. Through Modulating the IIS Pathway and Gut Microbiota in Caenorhabditis elegans. Foods. 2025; 14(11):1875. https://doi.org/10.3390/foods14111875
Chicago/Turabian StyleFang, Jiayue, Wei Dong, Jingqian Zheng, Boxuan Han, Yuying Zhang, Jianing Wang, and Xiaoxiong Zeng. 2025. "Antiaging Effect of 2-O-β-D-Glucopyranosyl Ascorbic Acid Derived from Lycium barbarum L. Through Modulating the IIS Pathway and Gut Microbiota in Caenorhabditis elegans" Foods 14, no. 11: 1875. https://doi.org/10.3390/foods14111875
APA StyleFang, J., Dong, W., Zheng, J., Han, B., Zhang, Y., Wang, J., & Zeng, X. (2025). Antiaging Effect of 2-O-β-D-Glucopyranosyl Ascorbic Acid Derived from Lycium barbarum L. Through Modulating the IIS Pathway and Gut Microbiota in Caenorhabditis elegans. Foods, 14(11), 1875. https://doi.org/10.3390/foods14111875





