Influence of Lactiplantibacillus plantarum and Saccharomyces cerevisiae Individual and Collaborative Inoculation on Flavor Characteristics of Rose Fermented Beverage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Determination of Physicochemical Parameters
2.4. Determination of Total Phenols and Total Flavonoids
2.5. Determination of Free Amino Acids (FAAs) and Organic Acids
2.6. Determination of VOCs by HS-SPME-GC-MS
2.7. Qualitative and Quantitative Analysis
2.8. Analysis of E-Nose
2.9. Data Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics Analysis
3.2. Free Amino Acids and Organic Acids Analysis
3.3. Electronic Nasal Analysis
3.4. Volatile Compounds Analyzed by HS-SPME-GC-MS
3.5. Classification Comparison of Volatile Compounds in Rose Beverage
3.6. ROAV Analysis
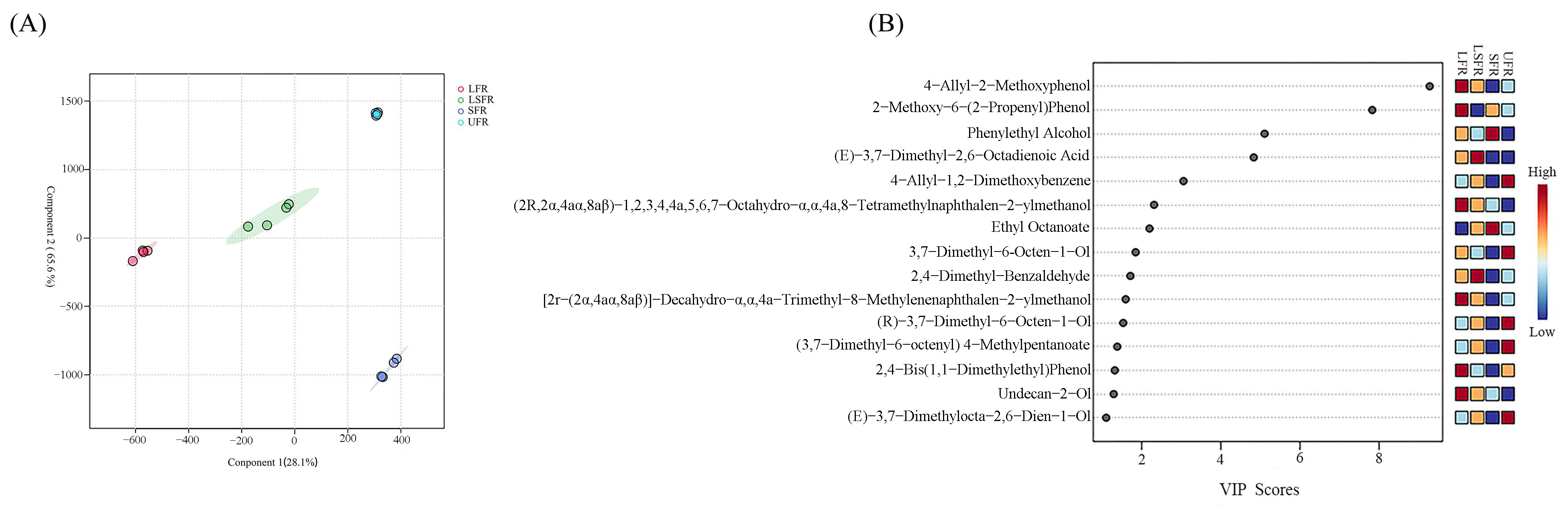
3.7. Identification of Flavor Markers Using PLS-DA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakhtiar, Z.; Eghlima, G.; Hatami, M.; Mirjalili, M.H. Quantification of fatty acids in seed oil and important bioactive compounds in Iranian Rosa canina L. ecotypes for potential cosmetic and medicinal uses. Sci. Rep. 2023, 13, 22721. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Tian, X.; He, L.; Li, C.; Tao, H.; Wang, X.; Qiao, S.; Zeng, X. Effects of tandem fermentation of edible mushroom and L. plantarum on sensory, polysaccharide, vitamin C, and γ-aminobutyric acid of Rosa roxburghii Tratt and coix seed beverage. Food Chem. 2023, 20, 101041. [Google Scholar] [CrossRef]
- Peña, F.; González, F.; Jiménez-Aspee, F.; Bustamante, L.; Ruiz, A. Stability of bioactive compounds and antioxidant activity in rosehip juice (Rosa spp.). Molecules 2024, 29, 2448. [Google Scholar] [CrossRef] [PubMed]
- Önder, D. Variation in antioxidant capacity, antioxidant activity and mineral composition during flower development of oil-bearing rose (Rosa damascena Mill.). Sci. Rep. 2023, 13, 17255. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, L.X.; Han, Q.D.; Dong, G.Z.; Wang, B.; Zhang, J.F.; Lei, S.M.; Liu, Y.G. Quality assessment of rose tea with different drying methods based on physicochemical properties, HS-SPME-GC-MS, and GC-IMS. J. Food Sci. 2023, 88, 1378–1391. [Google Scholar] [CrossRef]
- Cui, W.H.; Du, X.Y.; Zhong, M.C.; Fang, W.; Suo, Z.Q.; Wang, D.; Dong, X.; Jiang, X.D.; Hu, J.Y. Complex and reticulate origin of edible roses (Rosa, Rosaceae) in China. Hortic. Res. 2022, 9, uhab051. [Google Scholar] [CrossRef]
- Sknepnek, A.; Tomić, S.; Miletić, D.; Lević, S.; Čolić, M.; Nedović, V.; Nikšić, M. Fermentation characteristics of novel Coriolus versicolor and Lentinus edodes kombucha beverages and immunomodulatory potential of their polysaccharide extracts. Food Chem. 2021, 342, 128344. [Google Scholar] [CrossRef]
- Angelino, D.; Rosi, A.; Vici, G.; Dello Russo, M.; Pellegrini, N.; Martini, D.; On Behalf of the Sinu Young Working Group. Nutritional quality of plant-based drinks sold in italy: The food labelling of italian products (FLIP) Study. Foods 2020, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Cuvas-Limon, R.B.; Nobre, C.; Cruz, M.; Rodriguez-Jasso, R.M.; Ruíz, H.A.; Loredo-Treviño, A.; Texeira, J.A.; Belmares, R. Spontaneously fermented traditional beverages as a source of bioactive compounds: An overview. Crit. Rev. Food Sci. Nutr. 2021, 61, 2984–3006. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Erol, Z.; Rugji, J.; Taşçı, F.; Kahraman, H.A.; Toppi, V.; Musa, L.; Di Giacinto, G.; Bahmid, N.A.; Mehdizadeh, M.; et al. An overview of fermentation in the food industry—Looking back from a new perspective. Bioresour. Bioprocess. 2023, 10, 85. [Google Scholar] [CrossRef]
- Sanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, Y.; Guan, Z.; Liu, R.; Lu, Z.; Chai, L.; Xu, H.; Shi, J.; Zhang, X.; Xu, Z. Lactic acid bacteria-mediated transformation of black tea extract: Insights into metabolite profile and sensory characteristics. Food Biosci. 2025, 66, 106299. [Google Scholar] [CrossRef]
- Wang, D.; Deng, Y.; Chen, X.; Wang, K.; Zhao, L.; Wang, Z.; Liu, X.; Hu, Z. Elucidating the effects of Lactobacillus plantarum fermentation on the aroma profiles of pasteurized litchi juice using multi-scale molecular sensory science. Curr. Res. Food Sci. 2023, 6, 100481. [Google Scholar] [CrossRef]
- Liu, Z.; Xiong, S.; Hardie, W.J.; Huang, X.; Liu, Q.; Yang, J.; Li, J.; Liu, X.; Xiao, M.; Xiong, T.; et al. Transcriptomics and metabolomics reveal effects of Lactiplantibacillus plantarum NCU116 fermentation on carrot flavor. Food Biosci. 2025, 64, 105898. [Google Scholar] [CrossRef]
- Abla, M.; Cai, Y.; Gao, L.; Wu, J.; Yang, L. Changes in the antioxidant and anti-inflammatory activities of Rosa rugosa “Mohong” during fermentation. Heliyon 2024, 10, e25982. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Castro, M.; Feller, J.F. Review on sensor array-based analytical technologies for quality control of food and beverages. Sensors 2023, 23, 4017. [Google Scholar] [CrossRef]
- Shalit, M.; Guterman, I.; Volpin, H.; Bar, E.; Tamari, T.; Menda, N.; Adam, Z.; Zamir, D.; Vainstein, A.; Weiss, D.; et al. Volatile ester formation in roses. Identification of an acetyl-coenzyme a. geraniol/citronellol acetyltransferase in developing rose petals. Plant Physiol. 2003, 131, 1868–1876. [Google Scholar] [CrossRef]
- Baydar, H.; Schulz, H.; Kruger, H.; Erbas, S.; Kineci, S. Influences of fermentation time, hydro-distillation time and fractions on essential oil composition of damask rose (Rosa damascene Mill.). J. Essent. Oil-Bear. Plants 2013, 11, 224–232. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, X.; Huang, Q.; Zhang, L.; Liu, X.; Liu, R.; Lu, Q. Antioxidant and anti-inflammatory activities of rape bee pollen after fermentation and their correlation with chemical components by ultra-performance liquid chromatography-quadrupole time of flight mass spectrometry-based untargeted metabolomics. Food Chem. 2023, 409, 135342. [Google Scholar] [CrossRef]
- Tronchoni, J.; Curiel, J.A.; Morales, P.; Torres-Pérez, R.; Gonzalez, R. Early transcriptional response to biotic stress in mixed starter fermentations involving Saccharomyces cerevisiae and Torulaspora delbrueckii. Int. J. Food Microbiol. 2017, 241, 60–68. [Google Scholar] [CrossRef]
- Li, J.; Xu, H.; Li, H.; Xie, Y.; Ding, K.; Xu, S.; Wang, Z.; Wang, R.; Yi, C.; Ding, S. Co-fermentation of Lactiplantibacillus and Streptococcusccus enriches the key-contribution volatile and non-volatile components of jujube juice. Food Res. Int. 2024, 196, 115093. [Google Scholar] [CrossRef] [PubMed]
- García, C.; Rendueles, M.; Díaz, M. Liquid-phase food fermentations with microbial consortia involving lactic acid bacteria: A review. Food Res. Int. 2019, 119, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.N.; Afrin, S.; Humayun, S.; Ahmed, M.M.; Saha, B.K. Identification and growth characterization of a novel strain of Saccharomyces boulardii isolated from soya paste. Front. Nutr. 2020, 7, 27. [Google Scholar] [CrossRef]
- Li, K.; Han, G.; Liu, L.; Zhao, Y.; Liu, T.; Wang, H.; Xu, X. Flavor formation mechanisms based on phospholipid fermentation simulation system in oyster juice co-fermented by Lactiplantibacillus plantarum and Saccharomyces cerevisiae. Food Chem. 2025, 465, 142109. [Google Scholar] [CrossRef]
- Bai, J.; He, L.Z.; Zhang, J.F.; Gu, X.Y.; Wu, B.Q.; Wang, A.L.; Zhu, Y.; Zhang, J.Y.; Zhao, Y.S.; Yuan, J.; et al. Influences of Lactiplantibacillus plantarum and Saccharomyces cerevisiae fermentation on the nutritional components, flavor property and lipid-lowering effect of highland barley. J. Future Foods 2024, 4, 258–266. [Google Scholar] [CrossRef]
- Fei, C.; Xue, Q.; Li, W.; Xu, Y.; Mou, L.; Li, W.; Lu, T.; Yin, W.; Li, L.; Yin, F. Variations in volatile flavour compounds in crataegi fructus roasting revealed by e-nose and HS-GC-MS. Front. Nutr. 2023, 9, 1035623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Cheng, J.; Hong, Q.; Dong, W.; Chen, X.; Wu, G.; Zhang, Z. Identification of changes in the volatile compounds of robusta coffee beans during drying based on HS-SPME/GC-MS and e-nose analyses with the aid of chemometrics. LWT-Food Sci. Technol. 2022, 161, 113317. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Jin, J.; Li, H.; Chen, F.; Fei, Y.; Wang, Y. Characterization of the flavor profile and dynamic changes in chinese traditional fish sauce (yu-lu) based on electronic nose, SPME-GC-MS and HS-GC-IMS. Food Res. Int. 2024, 192, 114772. [Google Scholar] [CrossRef]
- Hao, Y.; Li, J.; Zhao, Z.; Xu, W.; Wang, L.; Lin, X.; Hu, X.; Li, C. Flavor characteristics of shanlan rice wines fermented for different time based on HS-SPME-GC-MS-O, HS-GC-IMS, and electronic sensory analyses. Food Chem. 2024, 432, 137150. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Lou, H.; Li, Y.; Khashaba, R.; Zhao, R. Understanding the correlation between formation of flavor compounds and dominant bacteria during Luoyang mung bean sour fermentation. Food Biosci. 2024, 60, 104371. [Google Scholar] [CrossRef]
- Wang, T.; Xiao, M.; Tang, C.; Qi, J.; Li, Y.; Li, X. Transcriptomics and metabolomics provide the difference of unique odor between natural and cultivated Ophiocordyceps sinensis and the biosynthetic pathway of key volatile compounds. Food Biosci. 2025, 66, 106200. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Chang, L. Effects of lactic acid bacteria fermentation on the phytochemicals content, taste and aroma of blended edible rose and shiitake beverage. Food Chem. 2023, 405, 134722. [Google Scholar] [CrossRef]
- Suresh, S.; Radha, K.V. Effect of a mixed substrate on phytase production by Rhizopus oligosporus MTCC 556 using solid state fermentation and determination of dephytinization activities in food grains. Food Sci. Biotechnol. 2015, 24, 551–559. [Google Scholar] [CrossRef]
- Zhou, M.; Zheng, X.; Zhu, H.; Li, L.; Zhang, L.; Liu, M.; Liu, Z.; Peng, M.; Wang, C.; Li, Q.; et al. Effect of Lactobacillus plantarum enriched with organic/inorganic selenium on the quality and microbial communities of fermented pickles. Food Chem. 2021, 365, 130495. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Fontes, V.M.; Colombo Pimentel, T.; Martins da Silva, A.B.; Suely Madruga, M.; Magnani, M.; Dos Santos Lima, M. An improved method for determining free amino acids by RP-HPLC/DAD with o-phthalaldehyde derivatization: Method evaluation in beers and wines. Food Chem. 2024, 435, 137591. [Google Scholar] [CrossRef]
- Tlais, A.Z.A.; Da Ros, A.; Filannino, P.; Vincentini, O.; Gobbetti, M.; Di Cagno, R. Biotechnological re-cycling of apple by-products: A reservoir model to produce a dietary supplement fortified with biogenic phenolic compounds. Food Chem. 2021, 336, 127616. [Google Scholar] [CrossRef]
- Hao, J.; Tan, J.; Zhang, Y.; Gu, X.; Zhu, G.; Wang, S.; Li, J. Sewage sludge-derived nutrients and biostimulants stimulate rice leaf photosynthesis and root metabolism to enhance carbohydrate, nitrogen and antioxidants accumulation. Chemosphere 2024, 352, 141335. [Google Scholar] [CrossRef]
- Jin, X.; Chen, W.; Chen, H.; Chen, W.; Zhong, Q. Comparative evaluation of the antioxidant capacities and organic acid and volatile contents of mango slurries fermented with six different probiotic microorganisms. J. Food Sci. 2018, 83, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, S.; Tu, T.; Li, Y.; Niu, M.; Tong, Y.; Yang, Y.; Xu, T.; Zhao, J.; Shen, C.; et al. Free amino acids identification and process optimization in greengage wine fermentation and flavor formation. J. Food Sci. 2023, 88, 988–1003. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, Z.; Zhang, Z.H.; He, R.; Zhao, X.; Gao, X. Improving the flavour of enzymatically hydrolysed beef liquid by sonication. Foods 2023, 12, 4460. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Wang, S.; Liu, Y.; Li, L.; Gao, M. Non-targeted metabolomics analyze dough fermented by S. cerevisiae and L. plantarum to reveal the formation of flavor substances of bread. LWT-Food Sci. Technol. 2023, 15, 114538. [Google Scholar] [CrossRef]
- Chen, D.; Chia, J.Y.; Liu, S.Q. Impact of addition of aromatic amino acids on non-volatile and volatile compounds in lychee wine fermented with Saccharomyces cerevisiae merit.ferm. Int. J. Food Microbiol. 2014, 170, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S.; Xia, Y.; Wang, G.; Ni, L.; Zhang, H.; Ai, L. Effects of different lactic acid bacteria on the characteristic flavor profiles of chinese rice wine. J. Sci. Food Agri 2024, 104, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Sun, J.; Lassabliere, B.; Yu, B.; Liu, S. Green tea fermentation with Saccharomyces boulardii CNCM I-745 and Lactiplantibacillus plantarum 299v. LWT-Food Sci. Technol. 2022, 157, 113081. [Google Scholar] [CrossRef]
- Önder, S.; Tonguç, M.; Erbaş, S.; Önder, D.; Mutlucan, M. Investigation of phenological, primary and secondary metabolites changes during flower developmental of Rosa damascena. Plant Physiol. Biochem. 2022, 192, 20–34. [Google Scholar] [CrossRef]
- Cirlini, M.; Ricci, A.; Galaverna, G.; Lazzi, C. Application of lactic acid fermentation to elderberry juice: Changes in acidic and glucidic fractions. LWT-Food Sci. Technol. 2020, 118, 108779. [Google Scholar] [CrossRef]
- Wakamatsu, M.; Tani, T.; Taguchi, H.; Matsuoka, M.; Kida, K.; Akamatsu, T. Ethanol production from d-lactic acid by lactic acid-assimilating Saccharomyces cerevisiae NAM34-4C. J. Biosci. Bioeng. 2013, 116, 85–90. [Google Scholar] [CrossRef]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of short-chain fatty acids in inflammatory bowel disease. Nutrients 2023, 15, 4466. [Google Scholar] [CrossRef]
- Duan, W.; Huang, Y.; Xiao, J.; Zhang, Y.; Tang, Y. Determination of free amino acids, organic acids, and nucleotides in 29 elegant spices. Food Sci. Nutr. 2020, 8, 3777–3792. [Google Scholar] [CrossRef]
- Gean, E.I.; Ciucure, C.T.; Apetrei, C. Electrochemical sensors coupled with multivariate statistical analysis as screening tools for wine authentication issues: A review. Chemosensors 2020, 8, 59. [Google Scholar] [CrossRef]
- Kuerban, D.; Lu, J.; Huangfu, Z.; Wang, L.; Qin, Y.; Zhang, M. Optimization of fermentation conditions and metabolite profiling of grape juice fermented with lactic acid bacteria for improved flavor and bioactivity. Foods 2023, 12, 2407. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, B.; Fu, Y.; Shi, Y.G.; Chen, F.L.; Guan, H.N.; Liu, L.L.; Zhang, C.Y.; Zhu, P.Y.; Liu, Y.; et al. HS-GC-IMS with PCA to analyze volatile flavor compounds across different production stages of fermented soybean whey tofu. Food Chem. 2021, 346, 128880. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, A.; Yadav, A.K. Volatile constituents of essential oil and rose water of damask rose (Rosa damascena Mill.) cultivars from north indian hills. Nat. Prod. Rep. 2011, 25, 1577–1584. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, D.; Huang, G.; Jiang, X.; Fang, K.; Wang, Q.; Ni, E.; Li, B.; Pan, C.; Li, H.; et al. Identification and characterization of the key volatile flavor compounds in black teas from distinct regions worldwide. J. Food Sci. 2022, 87, 3433–3446. [Google Scholar] [CrossRef]
- Yan, X.; Tian, Y.; Zhao, F.; Wang, R.; Zhou, H.; Zhang, N.; Wang, Y.; Shan, Z.; Zhang, C. Analysis of the key aroma components of pu’er tea by synergistic fermentation with three beneficial microorganisms. Food Chem. X 2023, 21, 101048. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Qian, J.; Xu, Q.; Guo, Y.; Yao, W.; Qian, H.; Cheng, Y. Integration of cross-correlation analysis and untargeted flavoromics reveals. Food Biosci. 2024, 59, 103855. [Google Scholar] [CrossRef]
- Han, X.; Peng, Q.; Yang, H.; Hu, B.; Shen, C.; Tian, R.X. Influence of different carbohydrate sources on physicochemical properties and metabolites of fermented greengage (Prunus mume) wines. LWT-Food Sci. Technol. 2022, 121, 108929. [Google Scholar] [CrossRef]
- Liang, S.; Wang, F.; Granato, D.; Zhong, X.; Xiao, A.F.; Ye, Q.; Li, L.; Zou, C.; Yin, J.F.; Xu, Y.Q. Effect of β-glucosidase on the aroma of liquid-fermented black tea juice as an ingredient for tea-based beverages. Food Chem. 2023, 402, 134201. [Google Scholar] [CrossRef]
- Chen, X.M.; Kobayashi, H.; Sakai, M.; Hirata, H.; Asai, T.; Ohnishi, T.; Baldermann, S.; Watanabe, N. Functional characterization of rose phenylacetaldehyde reductase (par), an enzyme involved in the biosynthesis of the scent compound 2-phenylethanol. J. Plant Physiol. 2011, 168, 88–95. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Li, C.; Li, L.; Yang, D.; Wang, Y.; Chen, S.; Wang, D.; Wu, Y. Novel insight into flavor and quality formation in naturally fermented low-salt fish sauce based on microbial metabolism. Food Res. Int. 2023, 166, 112586. [Google Scholar] [CrossRef]
- Wang, R.; Sun, J.; Lassabliere, B.; Yu, B.; Liu, S.Q. β-Glucosidase activity of cyberlindnera (Williopsis) saturnus var. mrakii NCYC 2251 and its fermentation effect on green tea aroma compounds. LWT-Food Sci Technol. 2021, 151, 112184. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with functional properties: An approach to increase safety and shelf-life of fermented foods. Biomed. Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. Bioprocesses for 2-phenylethanol and 2-phenylethyl acetate production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 9991–10004. [Google Scholar] [CrossRef] [PubMed]
- Quan, Q.; Liu, W.; Guo, J.; Ye, M.; Zhang, J. Effect of six lactic acid bacteria strains on physicochemical characteristics, antioxidant activities and sensory properties of fermented orange juices. Foods 2022, 11, 1920. [Google Scholar] [CrossRef]
- Januszek, M.; Satora, P.; Wajda, Ł.; Tarko, T. Saccharomyces bayanus enhances volatile profile of apple brandies. Molecules 2020, 25, 3127. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Zhu, T.; Wang, J.; Huang, M.; Wei, J.; Ye, H.; Wu, J.; Zhang, J.; Meng, N. Characterization of the key odorants and their content variation in niulanshan baijiu with different storage years using flavor sensory omics analysis. Food Chem. 2021, 376, 131851. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, S.; Xu, X.; Lao, F.; Wu, J. Volatile and non-volatile profiles in jujube pulp co-fermented with lactic acid bacteria. LWT-Food Sci. Technol. 2022, 154, 112772. [Google Scholar] [CrossRef]
- Long, Z.; Zhao, S.; Xu, X.; Du, W.; Chen, Q.; Hu, S. Dynamic changes in flavor and microbiota in traditionally fermented bamboo shoots (Chimonobambusa szechuanensis (rendle) keng f.). Foods 2023, 12, 3035. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wen, R.; Wang, J.; Zou, T.; Zhang, D.; Hou, Y.; Song, H.; Li, H.; Ma, H.; Shi, J. Identification of key aroma-active compounds in long shelf-life yoghurt and milk produced by different pre-sterilisation processes of raw milk using SBSE-GC × GC–O–MS. Int. J. Food Sci. Technol. 2024, 59, 3667–3678. [Google Scholar] [CrossRef]
- Zhang, R.L.; Song, X.R.; Liu, W.X.; Gao, X.F. Mixed fermentation of Chlorella pyrenoidosa and Bacillus velezensis SW-37 by optimization. LWT-Food Sci. Technol. 2023, 175, 114448. [Google Scholar] [CrossRef]
- Pan, C.; Shi, S.; Yang, X.; Xiang, H.; Wang, D.; Zhao, Y.; Ouyang, Q. Effect of water migration on changes of quality and volatile compounds in frozen penaeus monodon. Food Chem. 2024, 457, 140425. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Kan, H.; Li, Y.; Liu, Y.; Qiu, X. Analysis of volatile components in Rosa roxburghii Tratt. and Rosa sterilis using headspace-solid-phase microextraction-gas chromatography-mass spectrometry. Molecules 2023, 28, 7879. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Song, Y.; Zhu, J.; Xu, X.; Pang, B.; Jin, H.; Jiang, C.; Liu, Y.; Shi, J. Potential application of CHS and 4CL genes from grape endophytic fungus in production of naringenin and resveratrol and the improvement of polyphenol profiles and flavour of wine. Food Chem. 2021, 347, 128972. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.; Wüst, M. Volatile phenols-important contributors to the aroma of plant-derived foods. Molecules 2020, 25, 4529. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Xue, J.; Cai, X.D.; Guo, J.; Li, B.; Wu, S. Assessment of the key aroma compounds in rose-based products. J. Food Drug Anal. 2016, 24, 471–476. [Google Scholar] [CrossRef]
- Acebrón, I.; Plaza-Vinuesa, L.; de Las Rivas, B.; Muñoz, R.; Cumella, J.; Sánchez-Sancho, F.; Mancheño, J.M. Structural basis of the substrate specificity and instability in solution of a glycosidase from Lactobacillus plantarum. Biochim. Biophys. Acta Proteins Proteom. 2017, 1865, 1227–1236. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, C.; Wang, M.; Chen, J.; Chang, H.; Chang, Z.; Zhou, J.; Yue, M.; Zhang, W.; Zhang, Q.; et al. Effect of temperature regulation on microbial community, volatile flavours, amino acid profiles, and iridoid glycosides during noni (Morinda citrifolia L.) fruit fermentation. Food Chem. 2025, 462, 140966. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, K.; Liu, Q.; Liu, H.; Wang, H.; Sun, F.; Kong, B. Impact and correlation of fermentation temperature on the bacterial community, flavor characteristics, and proteolysis of Harbin dry sausages. Food Chem. 2025, 464, 141703. [Google Scholar] [CrossRef]
- Gu, Z.; Jin, Z.; Schwarz, P.; Rao, J.; Chen, B. Uncovering aroma boundary compositions of barley malts by untargeted and targeted flavoromics with HS-SPME-GC-MS/olfactometry. Food Chem. 2022, 394, 133541. [Google Scholar] [CrossRef]
- Liu, H.; Xu, X.; Cui, H.; Xu, J.; Yuan, Z.; Liu, J.; Li, C.; Li, J.; Zhu, D. Plant-based fermented beverages and key emerging processing technologies. Food Rev. Int. 2023, 39, 5844–5863. [Google Scholar] [CrossRef]
- Yuan, Y.H.; Mu, D.D.; Guo, L.; Wu, X.F.; Chen, X.S.; Li, X.J. From flavor to function: A review of fermented fruit drinks, their microbial profiles and health benefits. Food Res. Int. 2024, 196, 115095. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Xia, N.; Wang, Q.Z.; Chen, D.W. Identification of the non-volatile taste-active components in Crab Sauce. Foods 2019, 8, 324. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, W.; Li, G.; Xie, R.; He, J.; Hao, Y.; Li, Q.; Yu, X.; Lin, H.; Sun, Y. Analysis of flavor quality of jiulong dabaicha white tea. Food Sci. 2025, 46, 201–210. [Google Scholar]
- Zhang, C.; Wang, X.; Liu, Y.; Wang, J.; Xie, J. Characteristics of meat flavoring prepared using hydrolyzed plant protein mix by three different heating processes. Food Chem. 2024, 446, 138853. [Google Scholar] [CrossRef]





| Indexes | LFR | SFR | LSFR | UFR |
|---|---|---|---|---|
| pH | 3.31 ± 0.006 d | 4.41 ± 0.020 c | 3.56 ± 0.006 b | 4.89 ± 0.006 a |
| Total sugar (g/L) | 7.5 ± 0.301 c | 13.37 ± 1.367 b | 4.09 ± 0.784 d | 36.52 ± 0.410 a |
| Viable counts (log CFU/mL, 0 h) Viable counts (log CFU/mL, 24 h) | 6.48 ± 0.010 a 8.46 ± 0.012 a | 6.79 ± 0.018 b 7.37 ± 0.085 b | 6.52 ± 0.023 a 8.46 ± 0.053 a | 0 c 0 c |
| Total amino acid content (mg/L) | 1628.38 ± 23.810 d | 165.27 ± 4.193 c | 1255.23 ± 22.288 b | 209.97 ± 9.053 a |
| Chromaticity strength | 1.02 ± 0.022 a | 0.87 ± 0.009 c | 0.91 ± 0.004 b | 0.98 ± 0.003 a |
| L* | 57.10 ± 0.590 c | 62.51 ± 0.076 b | 61.57 ± 0.368 a | 61.53 ± 0.284 a |
| a* | 53.31 ± 0.856 d | 30.22 ± 0.012 c | 44.19 ± 0.387 b | 27.51 ± 0.234 a |
| b* | 28.74 ± 0.236 d | 38.85 ± 0.480 c | 27.99 ± 0.176 b | 45.39 ± 0.320 a |
| Total phenols (mg/mL) | 2.64 ± 0.050 ab | 2.47 ± 0.020 b | 2.75 ± 0.020 a | 2.22 ± 0.06 0 c |
| Total flavonoids (mg/mL) | 0.25 ± 0.010 a | 0.23 ± 0.006 a | 0.25 ± 0.004 a | 0.2 ± 0.010 b |
| Compounds | CAS | Odor Description | Threshold (mg/kg) | OAV | |||
|---|---|---|---|---|---|---|---|
| LSFR | LFR | SFR | UFR | ||||
| 4−Allyl−2−Methoxyphenol | 97−53−0 | Strong aroma of cloves | 0.00 | 117,381.10 | 193,529.17 | 2015.22 | 31417.69 |
| (R)−3,7−Dimethyl−6−Octen−1−Ol | 106−22−9 | Rose | 0.01 | 18,876.00 | 23,610.79 | 935.95 | 12790.26 |
| 3,7−Dimethylocta−1,6−Dien−3−Ol | 78−70−6 | Lilies of the valley, wood, rose, and fruity | 0.00 | 17,389.15 | 36,328.51 | 1178.91 | 18379.08 |
| 3,7−Dimethyl−6−Octen−1−Ol | 1117−61−9 | Rose | 0.01 | 14,921.49 | 24,945.78 | 868.02 | 11672.07 |
| (E)−3,7−Dimethylocta−2,6−Dien−1−Ol | 106−25−2 | Sweet rose aroma | 0.01 | 13,456.40 | 15,013.88 | 58.74 | 8357.00 |
| Phenylethyl Alcohol | 60−12−8 | Floral, rosy, honey | 0.05 | 2559.54 | 5029.25 | 2810.90 | 499.52 |
| 7−Methyl−3−Methyleneocta−1,6−Diene | 123−35−3 | Sweet citrus | 0.02 | 2144.04 | 1872.15 | 35.87 | 1805.65 |
| Nonan−1−Ol | 143−08−8 | Rose, citrus | 0.00 | 1716.28 | 2594.72 | — | 131.15 |
| 2,4−Bis(1,1−Dimethylethyl)Phenol | 96−76−4 | Phenolic | 0.03 | 1436.80 | 2289.11 | 263.22 | 851.26 |
| 2−Methoxy−2−methylbutane | 994−05−8 | Camphor | 0.02 | 1092.30 | 27.48 | 1760.40 | 16.64 |
| 3−Methylbutyl Acetate | 123−92−2 | Banana | 0.00 | 755.66 | 104.92 | 5617.35 | 99.04 |
| (4R)−4−Isopropenyl−1−Methylcyclohexene | 5989−54−8 | Fresh orange aroma | 0.03 | 449.31 | 343.20 | 19.09 | 288.16 |
| Nonanal | 124−19−6 | Fatty, oily | 0.00 | 405.60 | 395.93 | 93.82 | 1762.02 |
| 2−Phenylethyl acetate | 103−45−7 | Sweet rose aroma, fruity | 0.10 | 361.50 | 104.82 | 57.94 | 72.80 |
| Ethyl hexanoate | 123−66−0 | Fruity, wine | 0.00 | 303.98 | — | 21991.37 | — |
| 2−(Methylamino)Benzoic Acid Methyl Ester | 85−91−6 | Mild, slightly sweet | 0.02 | 224.69 | 520.52 | 199.08 | 497.03 |
| 1−Methyl−4−(1−Methylethenyl)Cyclohexene | 555−10−2 | Pepper and citrus aroma | 0.04 | 182.44 | 143.30 | 5.69 | 114.21 |
| (2Z)−3,7−Dimethylocta−2,6−Dien−1−Ol | 106−25−2 | Rose and orange aroma | 0.08 | 175.04 | 117.53 | 2.84 | 34.58 |
| Undecan−2−Ol | 1653−30−1 | Citrus fruity aroma | 0.07 | 165.33 | 300.89 | 15.31 | 1.37 |
| Methyl 2−Hydroxybenzoat | 118−61−6 | Holly | 0.06 | 67.80 | 74.13 | 2.06 | 12.71 |
| α−Terpineol | 98−55−5 | Fresh, green, woody | 0.30 | 51.44 | 62.64 | 10.96 | 26.00 |
| 4−Allyl−1,2−Dimethoxybenzene | 93−15−2 | Aroma of cloves | 1.25 | 39.99 | 47.50 | 5.43 | 48.76 |
| (Z)−3,7−Dimethyl−2,6−octadienylethanoate | 105−87−3 | Sweet, floral, slightly fruity | 0.10 | 32.96 | — | — | 2.04 |
| (5S)−5−Isopropyl−2−Methylcyclohexa−1,3−Diene | 2243−33−6 | Green, woody, peppery notes | 0.20 | 27.46 | 20.39 | <1 | 19.29 |
| Nonan−2−Ol | 628−99−9 | Fruity | 0.28 | 17.20 | 34.50 | 1.37 | 3.78 |
| Decan−1−Ol | 112−30−1 | Rose and orange flower aroma | 0.20 | 12.25 | 4.40 | 1.20 | — |
| Hexan−1−Ol | 111−27−3 | Fruity | 0.20 | 6.54 | 11.56 | <1 | <1 |
| Octanoic Acid | 124−07−2 | Sweat, cheese | 5.00 | 3.23 | <1 | 3.24 | <1 |
| Nonanoic Acid | 112−05−0 | Unpleasant smell | 1.50 | 3.10 | 5.00 | — | <1 |
| Benzaldehyde | 100−52−7 | Almond, burnt sugar | 0.30 | 2.04 | 3.43 | <1 | 18.89 |
| Benzyl Alcohol | 100−51−6 | Floral, sweet | 5.50 | 1.73 | 2.83 | — | — |
| Ethyl Octanoate | 106−32−1 | Pineapple | 0.65 | 1.45 | <1 | 75.37 | <1 |
| 5−Isopropyl−2−Methylphenol | 89−83−8 | Musk, grass, spices | 1.50 | <1 | 2.74 | <1 | 1.46 |
| (E)−β−Ionone | 14901−07−6 | Pleasant, sweet citrus | 0.47 | — | <1 | <1 | 4.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Chao, Y.; Huang, C.; Li, X.; Yi, Z.; Zhu, Z.; Yan, L.; Ding, Y.; Peng, Y.; Xie, C. Influence of Lactiplantibacillus plantarum and Saccharomyces cerevisiae Individual and Collaborative Inoculation on Flavor Characteristics of Rose Fermented Beverage. Foods 2025, 14, 1868. https://doi.org/10.3390/foods14111868
Zhou Y, Chao Y, Huang C, Li X, Yi Z, Zhu Z, Yan L, Ding Y, Peng Y, Xie C. Influence of Lactiplantibacillus plantarum and Saccharomyces cerevisiae Individual and Collaborative Inoculation on Flavor Characteristics of Rose Fermented Beverage. Foods. 2025; 14(11):1868. https://doi.org/10.3390/foods14111868
Chicago/Turabian StyleZhou, Yingjun, Yinying Chao, Chengzi Huang, Xiaochun Li, Zhuhu Yi, Zuohua Zhu, Li Yan, Yu Ding, Yuande Peng, and Chunliang Xie. 2025. "Influence of Lactiplantibacillus plantarum and Saccharomyces cerevisiae Individual and Collaborative Inoculation on Flavor Characteristics of Rose Fermented Beverage" Foods 14, no. 11: 1868. https://doi.org/10.3390/foods14111868
APA StyleZhou, Y., Chao, Y., Huang, C., Li, X., Yi, Z., Zhu, Z., Yan, L., Ding, Y., Peng, Y., & Xie, C. (2025). Influence of Lactiplantibacillus plantarum and Saccharomyces cerevisiae Individual and Collaborative Inoculation on Flavor Characteristics of Rose Fermented Beverage. Foods, 14(11), 1868. https://doi.org/10.3390/foods14111868






