Immunoenhancement Function of the Novel Hexapeptide (LVVLGH) from Thick-Shelled Mussel (Mytilus coruscus) on Immunodeficient Mice by Activating the NF-κB/MAPK Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Reagents, and Instruments
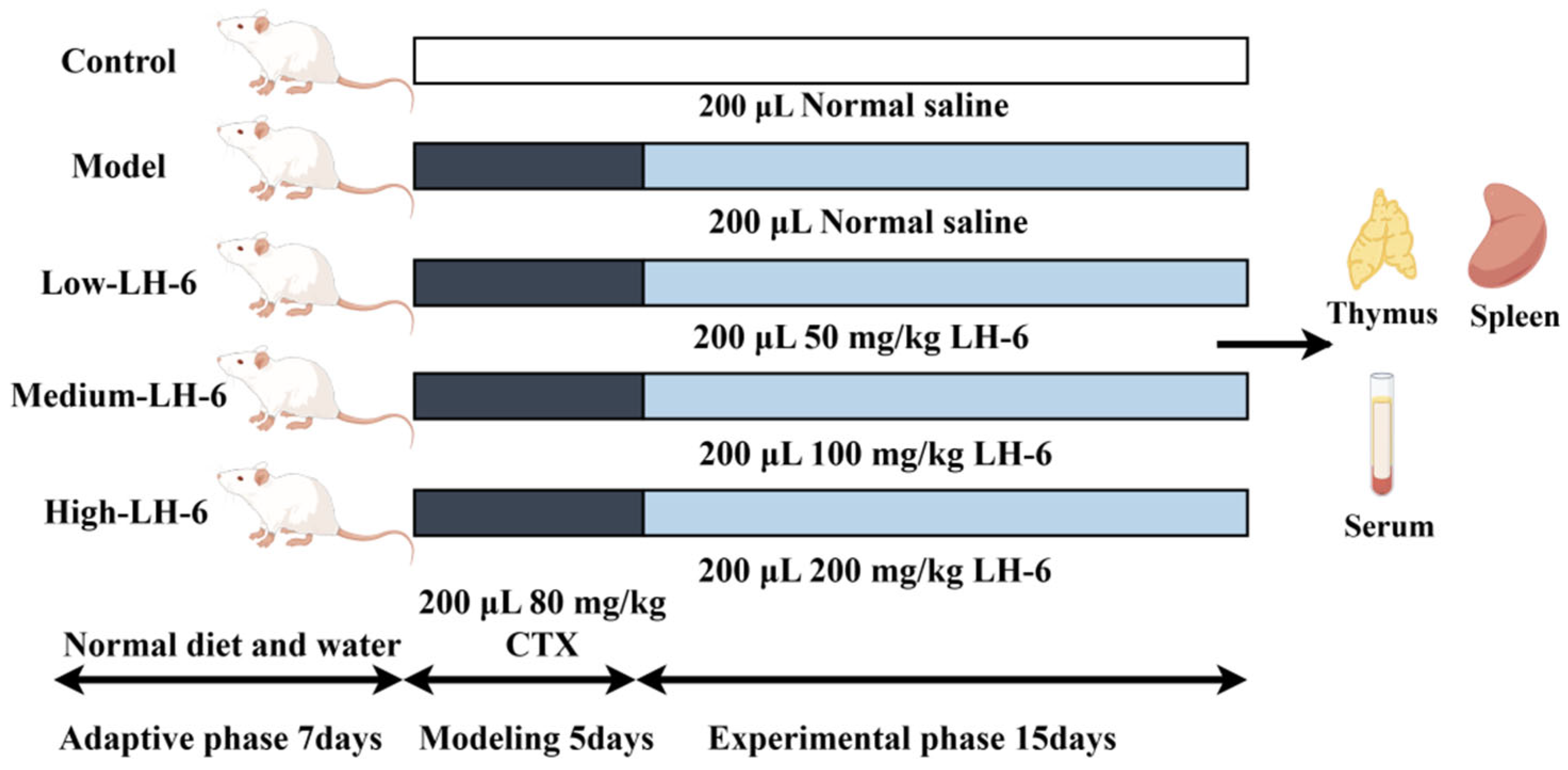
2.2. Animals and Treatments
2.3. Measurement of Body Weight and Thymus and Spleen Index
2.4. H&E Staining of Thymus and Spleen
2.5. Assay of Splenic Lymphocyte Proliferation
2.6. Determination of Phagocytosis and NO Synthesis in Macrophages
2.7. Assay of DTH
2.8. Measurement of Mouse Serum Hemolysin Half Hemolysin Value (HC50)
2.9. Assay of Natural Killer (NK) Cells
2.10. Determination of Cytokine and Immunoglobulins in Serum
2.11. Western Blot Analysis
2.12. Molecular Docking Analysis
2.13. Statistical Analysis
3. Results
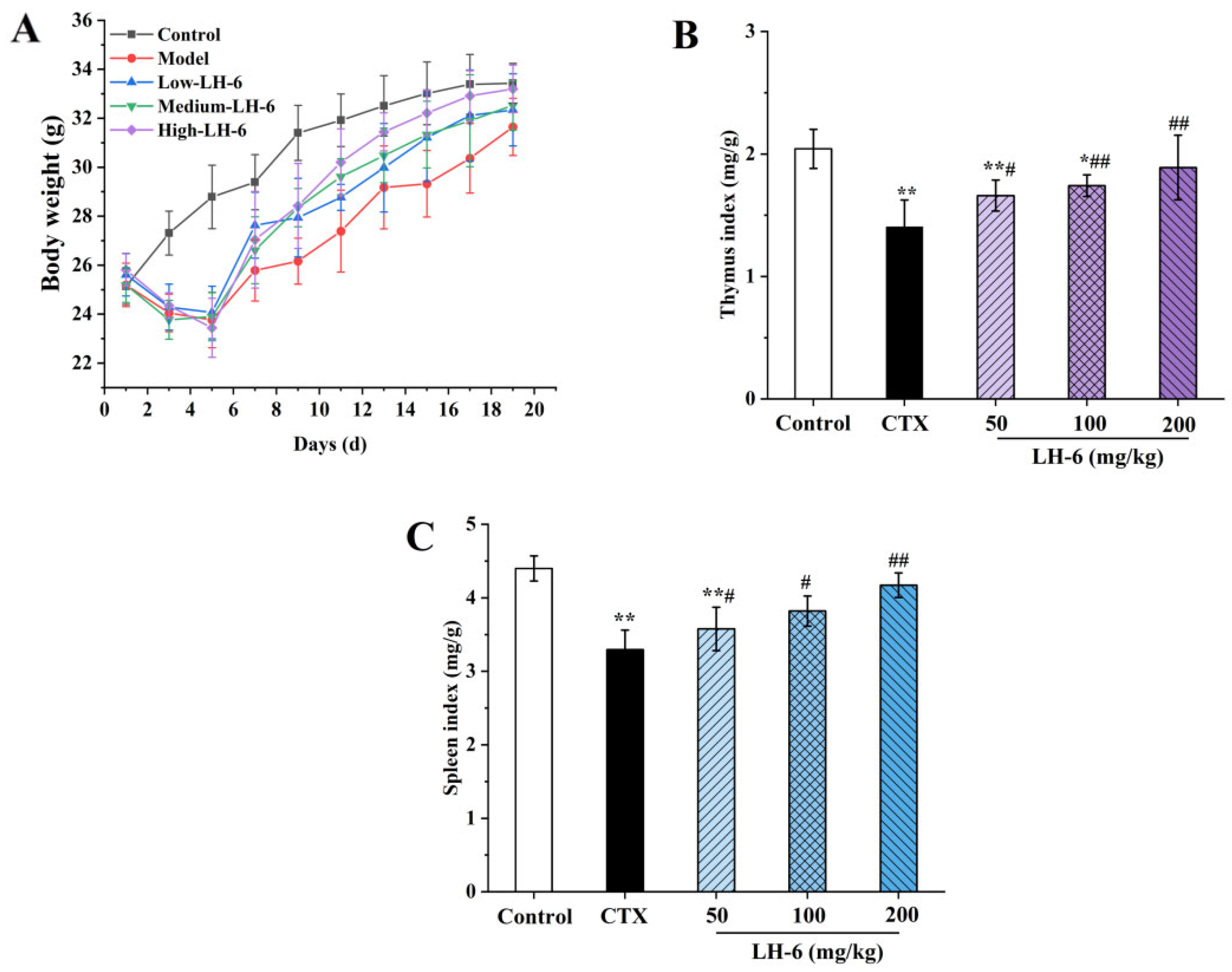
3.1. Effect of LH-6 on Body Weight and Thymus and Spleen Index
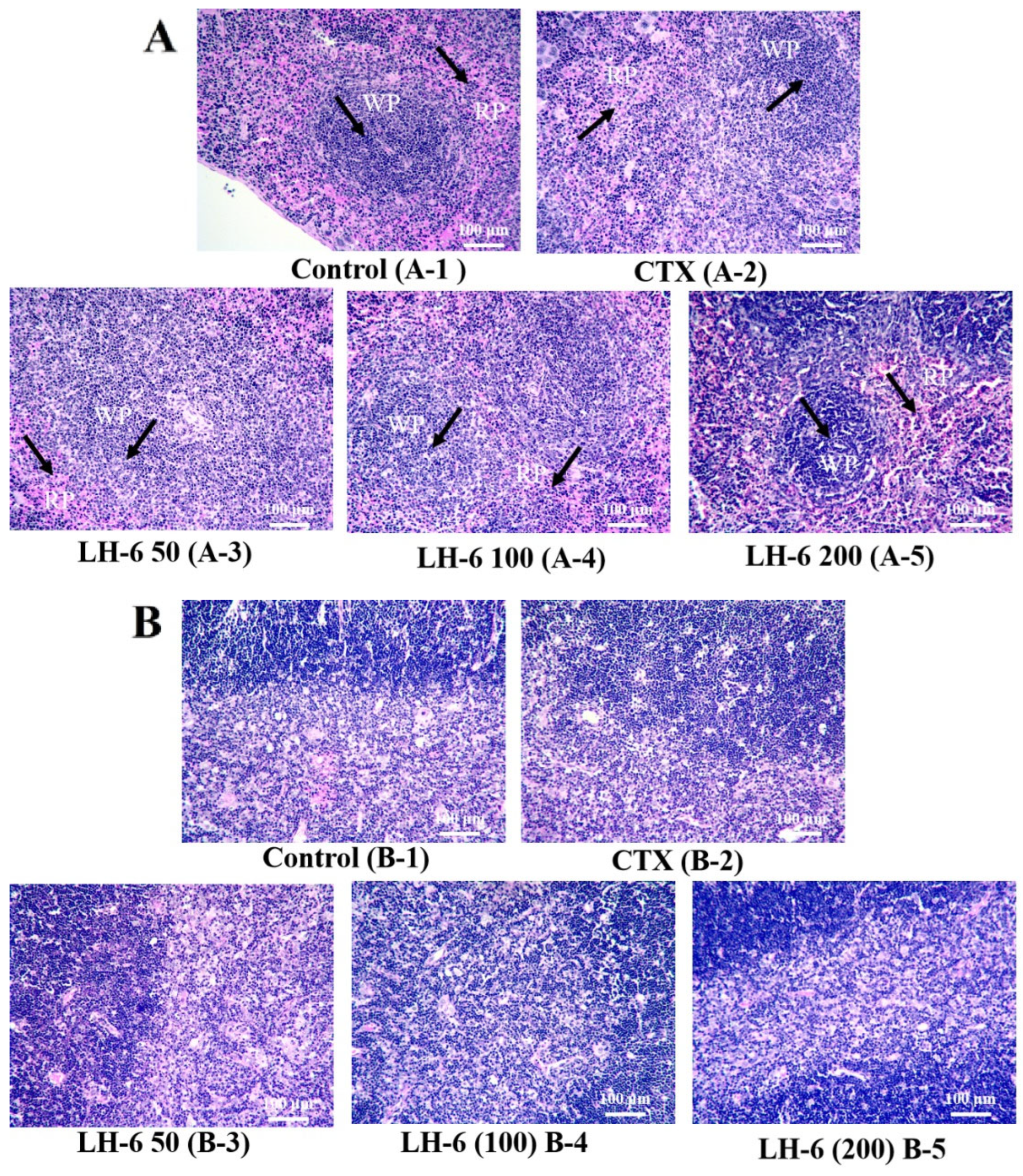
3.2. Histopathologic Analysis of Thymus and Spleen
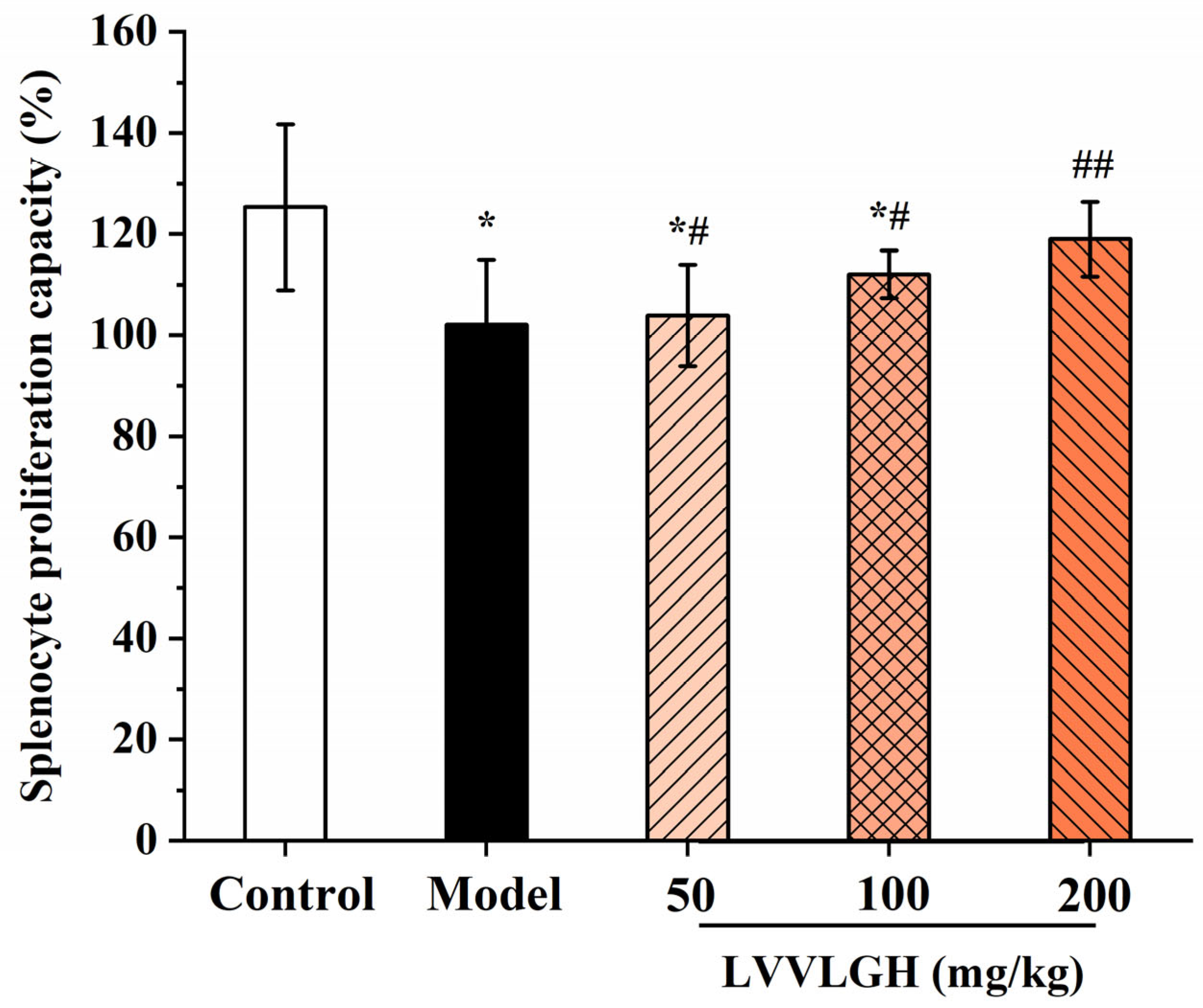
3.3. Effect of LH-6 on Spleen Lymphocytes
3.4. Effect of LH-6 on Phagocytic Activity and NO Secretion in Peritoneal Macrophages
3.5. Effect of LH-6 on DTH in Mice
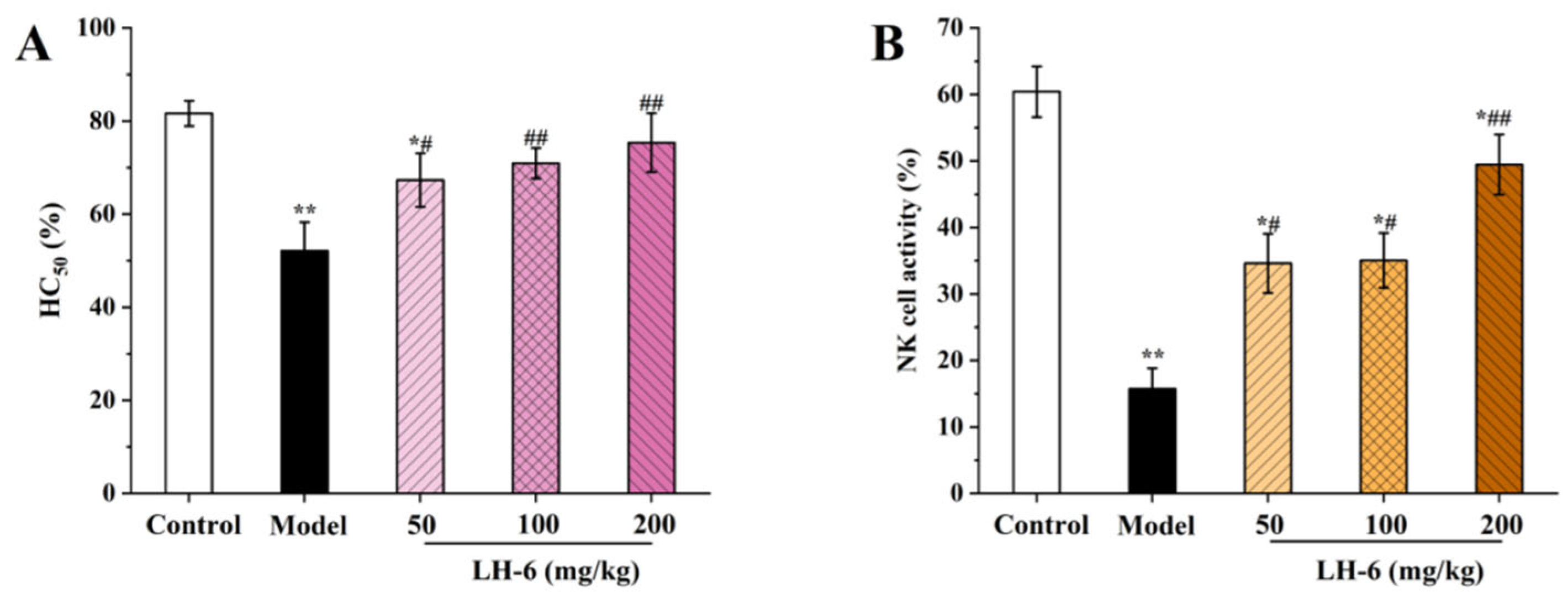
3.6. Effect of LH-6 on the Humoral Immune Response and NK Cell Killing Activity
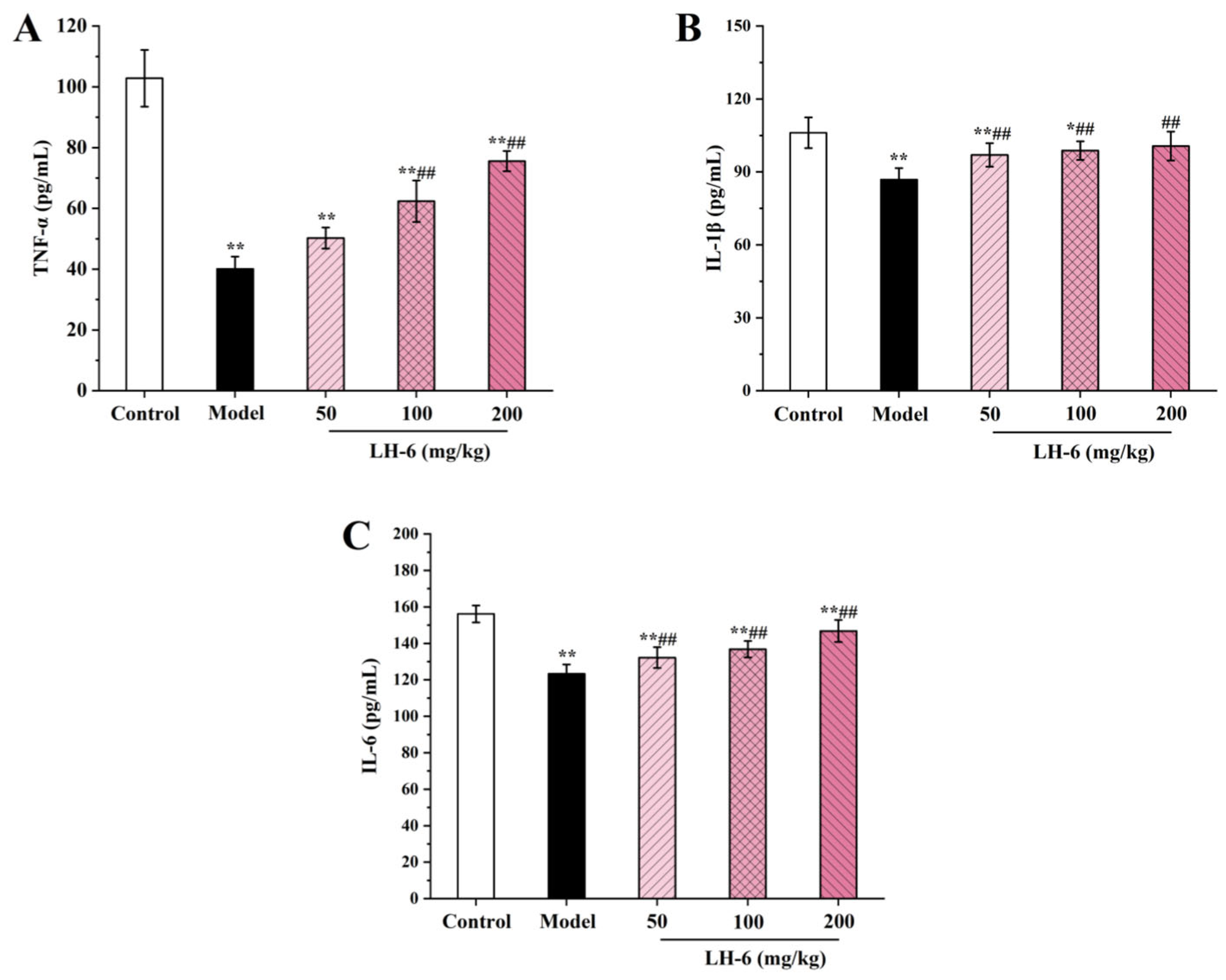
3.7. Effect of LH-6 on Serum Cytokines
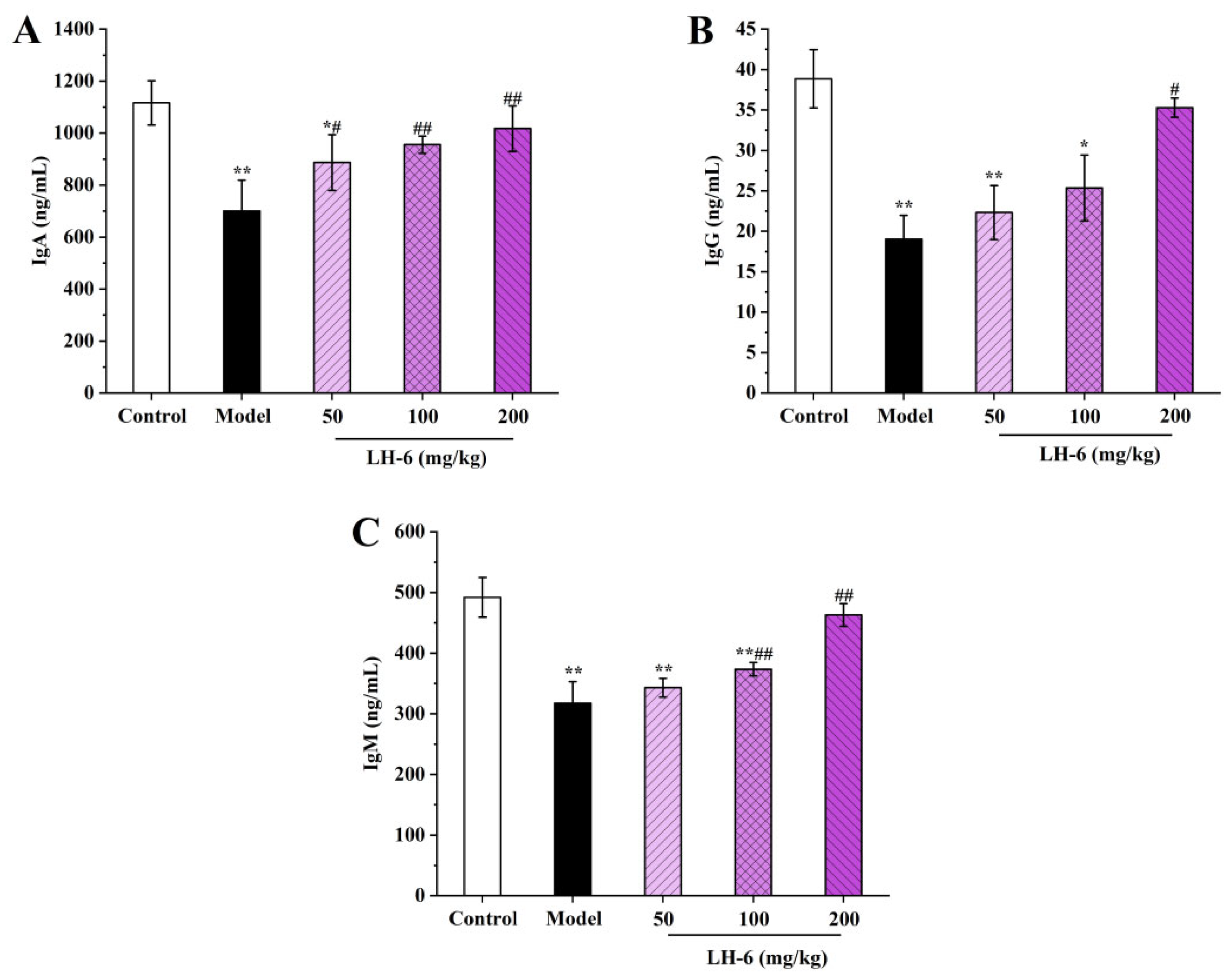
3.8. Effects of LH-6 on Serum Immunoglobulins Levels
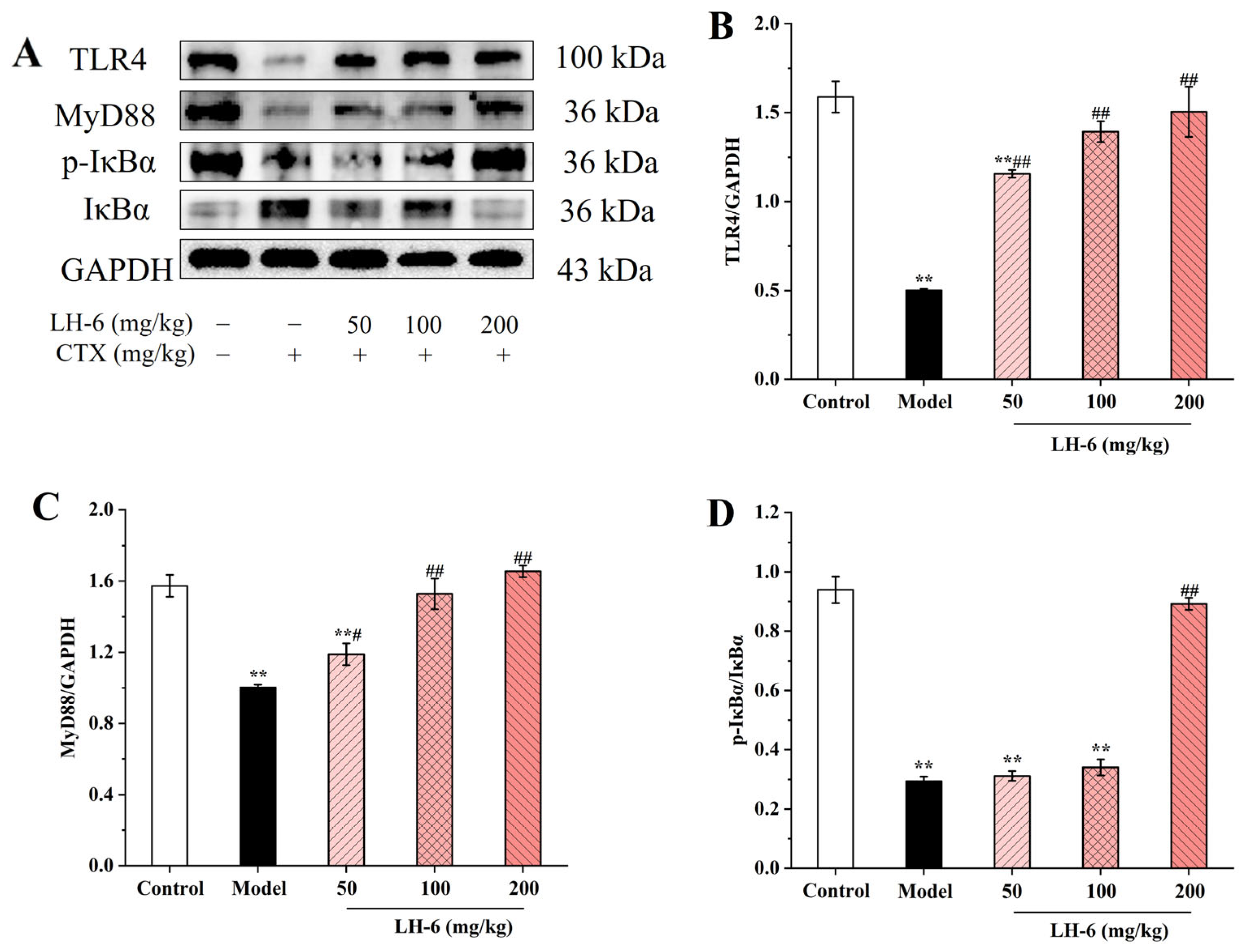
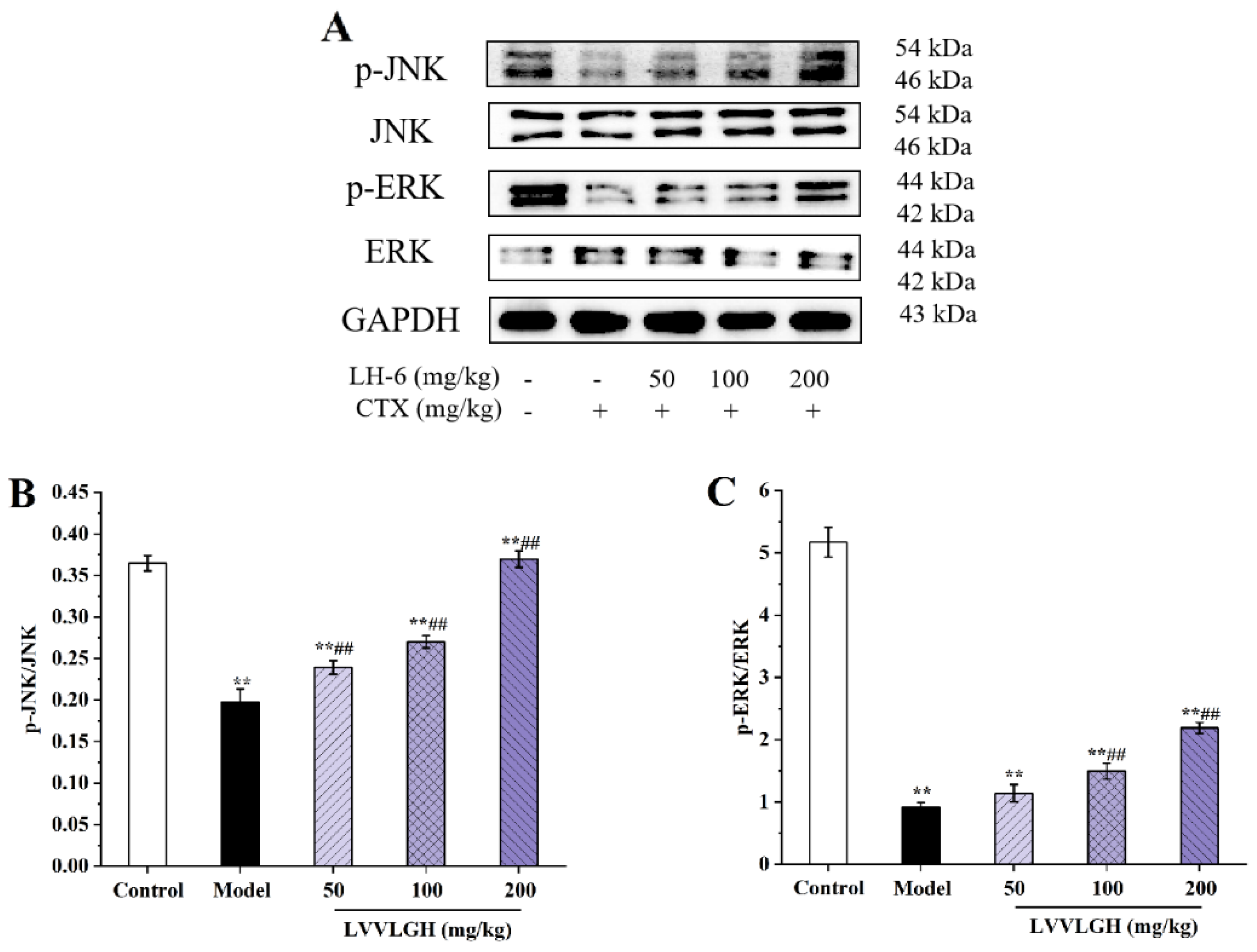
3.9. Effects of LH-6 on Expression of NF-κB and MAPK Pathway-Related Proteins in Spleen
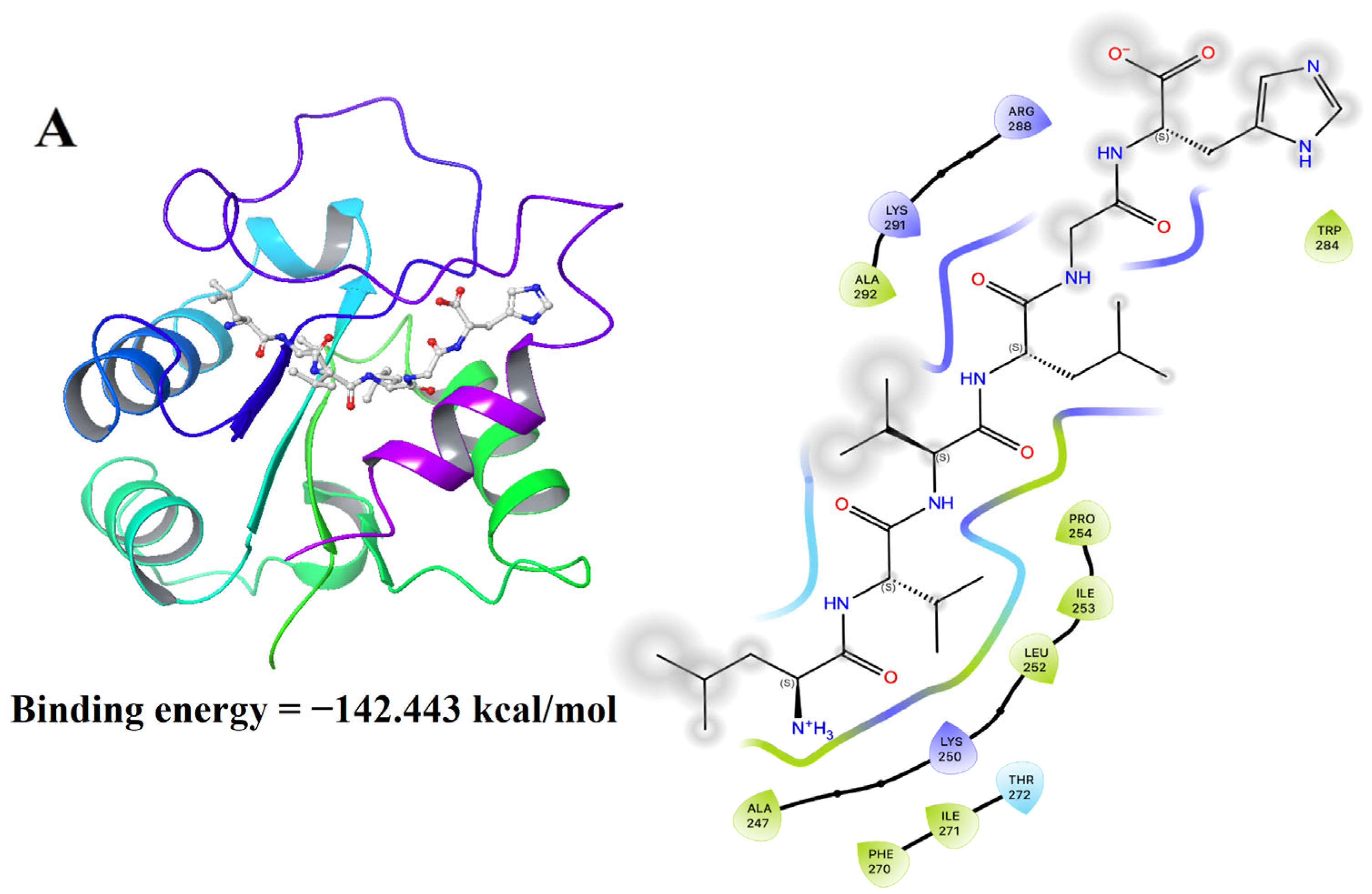
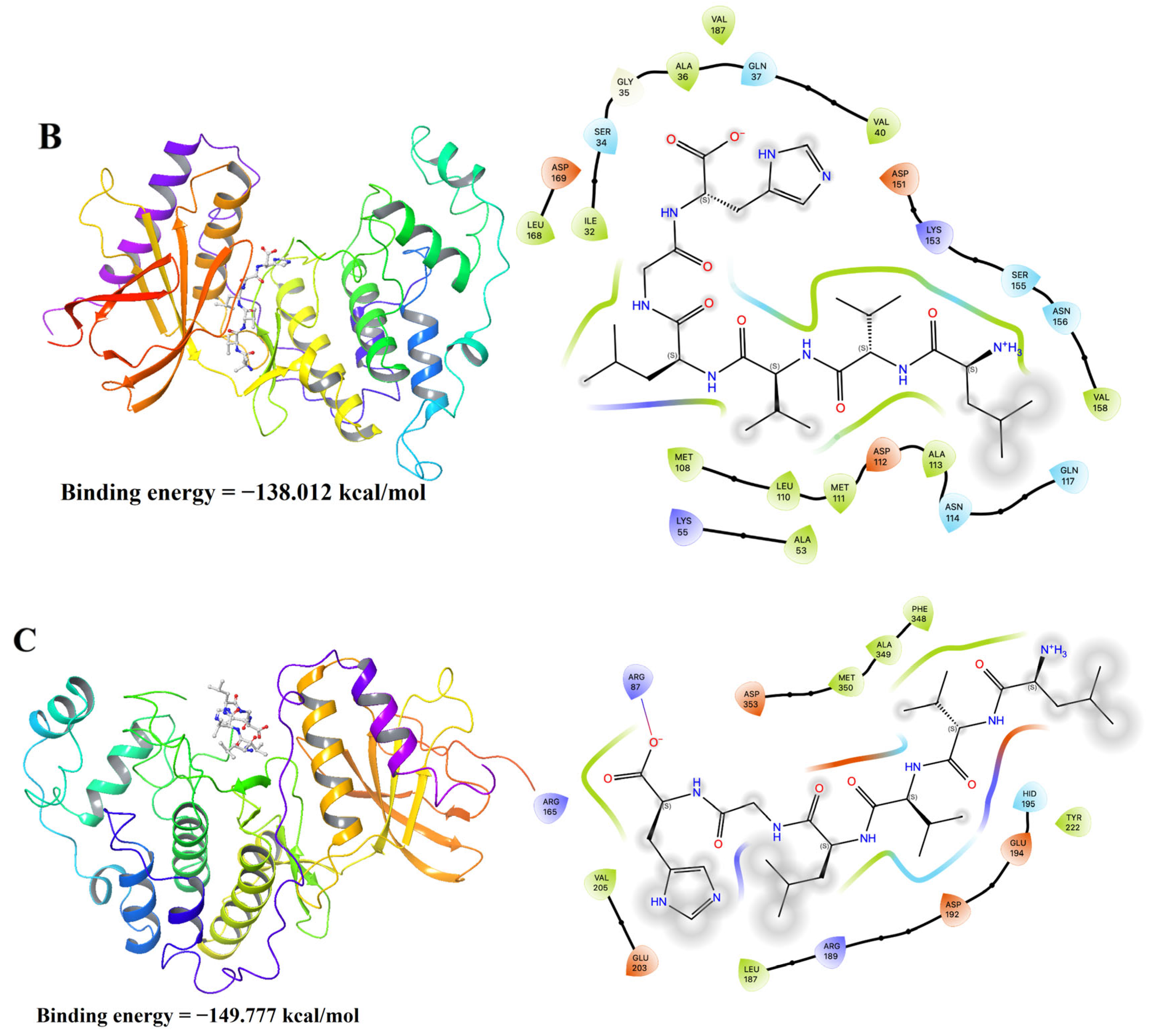
3.10. Molecular Docking Results of LH-6 with NF-κB and MAPK Pathways
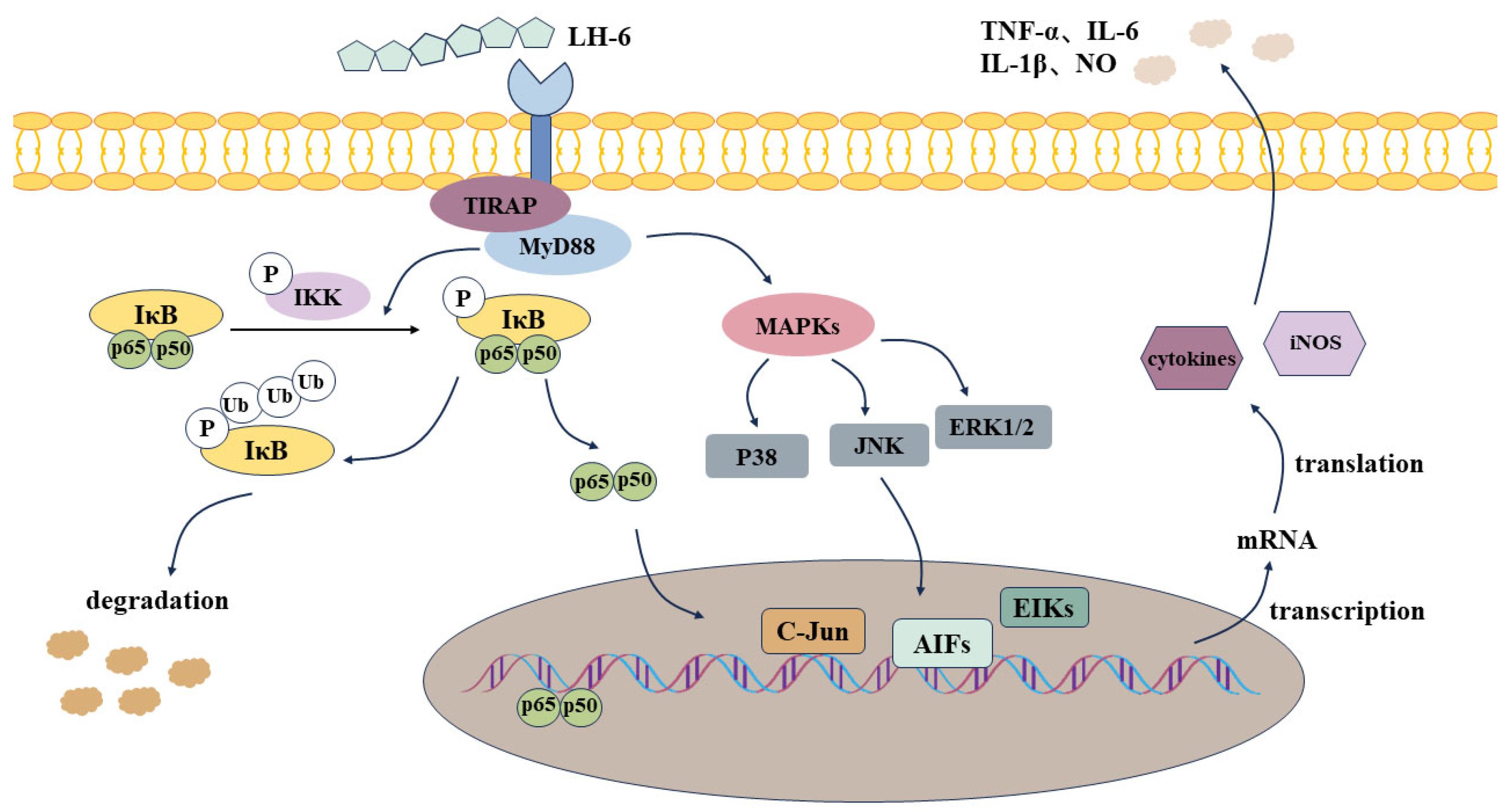
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, F.; Zhang, Z.; Ye, S.; Hong, X.; Jin, H.; Huang, F.; Ding, G. Immunoenhancement effects of pentadecapeptide derived from Cyclina sinensis on immune-deficient mice induced by cyclophosphamide. J. Funct. Foods 2019, 60, 103408. [Google Scholar] [CrossRef]
- Varadé, J.; Magadán, S.; González-Fernández, Á. Human immunology and immunotherapy: Main achievements and challenges. Cell Mol. Immunol. 2021, 18, 805–828. [Google Scholar] [CrossRef]
- Xu, D.H.; Xie, H.Y.; Li, Y.L.; Wang, L.; Qing, L.; Yu, S.; Zeng, H. Phyllanthus emblica polysaccharide (PEP) attenuates cyclophosphamide-induced immunosuppression through microbiota-dependent or -independent regulation of immune responses. J. Funct. Foods 2024, 114, 106065. [Google Scholar] [CrossRef]
- Yu, Y.; Mo, S.; Shen, M.; Chen, Y.; Yu, Q.; Li, Z.; Xie, J. Sulfated modification enhances the immunomodulatory effect of Cyclocarya paliurus polysaccharide on cyclophosphamide-induced immunosuppressed mice through MyD88-dependent MAPK/NF-κB and PI3K-Akt signaling pathways. Food Res. Int. 2021, 150, 110756. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; Abdel Ghaffar, F.R.; El-Elaimy, I.A.; Gouida, M.S.; Abd El Latif, H.M. Antitumor and immune-modulatory efficacy of dual-treatment based on levamisole and/or taurine in Ehrlich ascites carcinoma-bearing mice. Biomed. Pharmacother. 2018, 106, 43–49. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Cao, X. Regulation of type I interferon signaling in immunity and inflammation: A comprehensive review. J. Autoimmun. 2017, 83, 1–11. [Google Scholar] [CrossRef]
- Samadi, M.; Kamrani, A.; Nasiri, H.; Shomali, N.; Heris, J.A.; Shahabi, P.; Akbari, M. Cancer immunotherapy focusing on the role of interleukins: A comprehensive and updated study. Pathol. Res. Pract. 2023, 249, 154732. [Google Scholar] [CrossRef]
- Li, J.; Zhan, L.; Qin, C. The double-sided effects of Mycobacterium Bovis bacillus Calmette–Guérin vaccine. npj Vaccines 2021, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; He, K.; Dong, X.; Zhang, Z.; Wang, F.; Tang, Y.; Ding, G. Immunomodulatory activity of low molecular-weight peptides from Nibea japonica skin in cyclophosphamide-induced immunosuppressed mice. J. Funct. Foods 2020, 68, 103888. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.H. Recent developments on production, purification and biological activity of marine peptides. Food Res. Int. 2021, 147, 110468. [Google Scholar] [CrossRef]
- Saraswat, S.; Chugh, A. Engraulisin: A novel marine derived cell penetrating peptide with activity against drug resistant bacteria. BBA-Biomembr. 2024, 1866, 184255. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.Y.; Suo, S.K.; Wang, Y.M.; Chi, C.F.; Wang, B. Systematical investigation on anti-Fatigue function and underlying mechanism of high Fischer ratio oligopeptides from Antarctic krill on exercise-induced fatigue in mice. Mar. Drugs 2024, 22, 322. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, S.; Fan, F.; Tu, M.; Xu, Z.; Du, M. Identification and molecular mechanism of antithrombotic peptides from oyster proteins released in simulated gastro-intestinal digestion. Food Funct. 2019, 10, 5426–5435. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.Z.; Wang, J.; Wang, Y.M.; Zeng, Y.-H.; Chi, C.F.; Wang, B. Optimization of the preparation process and ameliorative efficacy in osteoporotic rats of peptide-calcium chelates from Skipjack tuna (Katsuwonus pelamis) meat. Foods 2024, 13, 2778. [Google Scholar] [CrossRef]
- Festa, M.; Sansone, C.; Brunet, C.; Crocetta, F.; Di Paola, L.; Lombardo, M.; Albini, A. Cardiovascular active peptides of marine origin with ACE inhibitory activities: Potential role as anti-hypertensive drugs and in prevention of SARS-CoV-2 infection. Int. J. Mol. Sci. 2020, 21, 8364. [Google Scholar] [CrossRef]
- Suo, S.K.; Zhao, Y.Q.; Wang, Y.M.; Pan, X.Y.; Chi, C.F.; Wang, B. Seventeen novel angiotensin converting enzyme (ACE) inhibitory peptides from protein hydrolysate of Mytilus edulis: Isolation, identification, molecular docking study, and protective function on HUVECs. Food Funct. 2022, 13, 7831–7846. [Google Scholar] [CrossRef]
- Librizzi, M.; Martino, C.; Mauro, M.; Abruscato, G.; Arizza, V.; Vazzana, M.; Luparello, C. Natural anticancer peptides from marine animal species: Evidence from in vitro cell model systems. Cancers 2024, 16, 36. [Google Scholar] [CrossRef]
- Kong, J.; Hu, X.M.; Cai, W.W.; Wang, Y.M.; Chi, C.F.; Wang, B. Bioactive peptides from Skipjack tuna cardiac arterial bulbs (II): Protective function on UVB-irradiated HaCaT cells through antioxidant and anti-apoptotic mechanisms. Mar. Drugs 2023, 21, 105. [Google Scholar] [CrossRef] [PubMed]
- Montuori, E.; de Pascale, D.; Lauritano, C. Recent discoveries on marine organism immunomodulatory activities. Mar. Drugs 2022, 20, 422. [Google Scholar] [CrossRef]
- Li, J.; Yang, L.; Li, G.; Liu, S.; Cao, W.; Lin, H.; Zheng, H. Low-molecular-weight oyster peptides ameliorate cyclophosphamide-chemotherapy side-effects in Lewis lung cancer mice by mitigating gut microbiota dysbiosis and immunosuppression. J. Funct. Foods 2022, 95, 105196. [Google Scholar] [CrossRef]
- Yuan, Z.; Yang, M.; Zhu, D.; Wu, D.; Cheng, S.; Wu, C.; Du, M. Immunomodulatory effect of ethanol-soluble oligopeptides from Atlantic cod (Gadus morhua). Food Sci. Hum. Well. 2023, 12, 1192–1203. [Google Scholar] [CrossRef]
- Zhao, R.; Jiang, X.X.; Zhao, Q.L.; Ye, H.W.; Lin, Y.; Huang, J.; Tang, Y.P. Immunoenhancing effects of Cyclina sinensis pentadecapeptide through modulation of signaling pathways in mice with cyclophosphamide-induced immunosuppression. Mar. Drugs 2022, 20, 560. [Google Scholar] [CrossRef]
- Qian, J.; Deng, F.; Shumway, S.E.; Hu, M.; Wang, Y. The thick-shell mussel Mytilus coruscus: Ecology, physiology, and aquaculture. Aquaculture 2024, 580, 740350. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, S.; Zeng, Y.; He, K.; Luo, Y.; Yu, F. Antioxidant peptides from Mytilus coruscus on H2O2-induced human umbilical vein endothelial cell stress. Food Biosci. 2020, 38, 100762. [Google Scholar] [CrossRef]
- Liu, L.; He, M.; Yang, Z.; Wang, H.; Zhang, X.; He, J.; Liao, Z. Myticofensin, a novel antimicrobial peptide family identified from Mytilus coruscus. Fish Shellfish Immun. 2022, 131, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, F.J.; Wang, X.M.; Zhao, G.H.; Cai, D.; Yu, J.H.; Zhou, D.Y. Preparation and hepatoprotective activities of peptides derived from mussels (Mytilus edulis) and clams (Ruditapes philippinarum). Mar. Drugs 2022, 20, 719. [Google Scholar] [CrossRef]
- He, K.; Zeng, Y.; Tian, H.; Zhang, Z.; Zhang, H.; Huang, F.; Yu, F. Macrophage immunomodulatory effects of low molecular weight peptides from Mytilus coruscus via NF-κB/MAPK signaling pathways. J. Funct. Foods 2021, 83, 104562. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, Y.; Zeng, Y.; Yang, X.; Yu, F.; Wang, B. Immunomodulatory peptides from thick-shelled mussel (Mytilus coruscus): Isolation, identification, molecular docking and immunomodulatory effects on RAW264.7 cells. Food Biosci. 2024, 59, 103874. [Google Scholar] [CrossRef]
- Yang, X.; Yang, L.; Pan, D.; Liu, H.; Xia, H.; Wang, S.; Sun, G. Wheat peptide protects against ethanol-induced gastric mucosal damage through downregulation of TLR4 and MAPK. J. Funct. Foods 2020, 75, 104271. [Google Scholar] [CrossRef]
- Zhang, Y.; He, P.; Pan, L.; Lin, Z.; Yang, J.; Wu, H.; Zhang, M. Immunomodulatory effect of earthworm protein autolysates on cyclophosphamide (CTX) induced immunosuppressed mice. Food Biosci. 2023, 56, 103297. [Google Scholar] [CrossRef]
- Cleypool, C.G.J.; Mackaaij, C.; Verlinde-Schellekens, S.A.M.W.; Bleys, R.L.A.W. A comparative anatomical and histological study on the presence of an apical splenic nerve in mice and humans. J. Anat. 2022, 240, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Lu, X.; Liu, Y.; Zhang, Q.; Wang, F.; Jin, M.; Wang, Y. Protective effects of Bacillus amyloliquefaciens-derived nonapeptide in cyclophosphamide-induced immunosuppressed mice. J. Funct. Foods 2024, 119, 106273. [Google Scholar] [CrossRef]
- Pasala, P.K.; Reddy, L.S.S.; Silvia, N.; Reddy, Y.D.; Sampath, A.; Dorababu, N.; Rudrapal, M. Molecular docking and in vivo immunomodulatory activity of Albizia procera bark on doxorubicin induced immunosuppressive rats. J. King Saud Univ. Sci. 2022, 34, 101828. [Google Scholar] [CrossRef]
- Gao, D.; Liu, Z.; Liu, F.; Chen, L.; Wang, W.; Ma, J.; Hou, J. Study of the immunoregulatory effect of Lactobacillus rhamnosus 1.0320 in immunosuppressed mice. J. Funct. Foods 2021, 79, 104423. [Google Scholar] [CrossRef]
- Jang, S.H.; Oh, M.S.; Baek, H.I.; Ha, K.C.; Lee, J.Y.; Jang, Y.S. Silk peptide treatment potentiates natural killer cell activity in vitro and induces natural killer cell maturation and activation in mouse splenocytes. Pharm. Biol. 2019, 57, 369–379. [Google Scholar] [CrossRef]
- Huang, L.; Shen, M.; Wu, T.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Mesona chinensis Benth polysaccharides protect against oxidative stress and immunosuppression in cyclophosphamide-treated mice via MAPKs signal transduction pathways. Int. J. Biol. Macromol. 2020, 152, 766–774. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, L.; Gao, C.; Chen, W.; Vong, C.T.; Yao, P.; Wang, Y. Astragali radix (Huangqi): A promising edible immunomodulatory herbal medicine. J. Ethnopharmacol. 2020, 258, 112895. [Google Scholar] [CrossRef]
- Wang, F.; Lou, J.; Gao, X.; Zhang, L.; Sun, F.; Wang, Z.; Qin, Z. Spleen-targeted nanosystems for immunomodulation. Nano Today 2023, 52, 101943. [Google Scholar] [CrossRef]
- Li, J.; Tao, W.; Zhou, W.; Xing, J.; Luo, M.; Lu, S.; Yang, Y. Dendrobium officinale leaf polysaccharide has a dual effect of alleviating the syndromes of immunosuppressed mice and modulating immune system of normal mice. J. Funct. Foods 2024, 113, 105974. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez-Pomares, L. Physiological roles of macrophages. Pflug. Arch. Eur. J. Phy. 2017, 469, 365–374. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- de Abreu Mello, A.; Motta Portal, T.; Allodi, S.; Nunes da Fonseca, R.; Monteiro de Barros, C. Adrenoreceptor phylogeny and novel functions of nitric oxide in ascidian immune cells. J. Invertebr. Pathol. 2024, 203, 108057. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.W., Jr.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Alin. Immun. 2010, 125, S41–S52. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Tar. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Huang, P.; Han, J.; Hui, L. MAPK signaling in inflammation-associated cancer development. Protein Cell 2010, 1, 218–226. [Google Scholar] [CrossRef]
- Tang, S.; Li, C.; Suo, Z.; Xu, Y.; Wei, K.; Zhao, L.; Li, X. Neuroprotective effects of savinin on LPS-Induced neuroinflammation in vivo via regulating MAPK/NF-κB pathway and NLRP3 inflammasome activation. Molecules 2023, 28, 1575. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Dasgupta, A.; Mitragotri, S. Transdermal immunomodulation: Principles, advances and perspectives. Adv. Drug Deliver. Rev. 2018, 127, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Abd El Salam, A.S.G.; Samaha, M.M.; Abd Elrazik, N.A. Cytoprotective effects of cinnamaldehyde and adipoRon against cyclophosphamide-induced cardio-renal toxicity in rats: Insights into oxidative stress, inflammation, and apoptosis. Int. Immunopharmacol. 2023, 124, 111044. [Google Scholar] [CrossRef]
- Bosticardo, M.; Notarangelo, L.D. Human thymus in health and disease: Recent advances in diagnosis and biology. Semin. Immunol. 2023, 66, 101732. [Google Scholar] [CrossRef]
- Martinez-Micaelo, N.; González-Abuín, N.; Mulero, M.; Pinent, M.; Ardévol, A.; Blay, M. Procyanidins and docosahexaenoic acid suppress inflammation and boost immune system in cafeteria diet-fed rats. J. Funct. Foods 2015, 15, 61–71. [Google Scholar] [CrossRef]
- Zeng, Y.; Hu, X.; Yu, Z.; Wang, F.; Zhang, Z.; He, K.; Yu, F. Immune enhancement and antioxidant effects of low molecular-weight peptides derived from Nibea japonica muscles on immune-deficient mice induced by cyclophosphamide. Process Biochem. 2021, 102, 42–50. [Google Scholar] [CrossRef]
- Zhou, R.; Teng, L.; Zhu, Y.; Zhang, C.; Yang, Y.; Chen, Y. Preparation of Amomum longiligulare polysaccharides 1-PLGA nanoparticle and its immune enhancement ability on RAW264.7 cells. Int. Immunopharmacol. 2021, 99, 108053. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, E.; Nam, J.O. Bioconverted extract of Sophorae fructus modulates the innate immune response in RAW264.7 macrophages and mouse splenocytes. J. Funct. Foods 2024, 116, 106202. [Google Scholar] [CrossRef]
- Muntasell, A.; Ochoa, M.C.; Cordeiro, L.; Berraondo, P.; López-Díaz de Cerio, A.; Cabo, M.; Melero, I. Targeting NK-cell checkpoints for cancer immunotherapy. Curr. Opin. Immunol. 2017, 45, 73–81. [Google Scholar] [CrossRef]
- Tran, T.H.M.; Mi, X.J.; Huh, J.E.; Aditi Mitra, P.; Kim, Y.J. Cirsium japonicum var. maackii fermented with Pediococcus pentosaceus induces immunostimulatory activity in RAW 264.7 cells, splenocytes and CTX-immunosuppressed mice. J. Funct. Foods 2023, 102, 105449. [Google Scholar]
- Cai, L.; Lv, Y.; Yan, Q.; Guo, W. Cytokines: The links between bone and the immune system. Injury 2024, 55, 111203. [Google Scholar] [CrossRef]
- Yatim, K.M.; Lakkis, F.G. A brief journey through the immune system. Clin. J. Am. Soc. Nephro. 2015, 10, 1274–1281. [Google Scholar] [CrossRef]
- Liu, J.P.; Wang, J.; Zhou, S.X.; Huang, D.C.; Qi, G.H.; Chen, G.T. Ginger polysaccharides enhance intestinal immunity by modulating gut microbiota in cyclophosphamide-induced immunosuppressed mice. Int. J. Biol. Macromol. 2022, 223, 1308–1319. [Google Scholar] [CrossRef]
- Szczypka, M.; Sobieszczańska, A.; Suszko-Pawłowska, A.; Lis, M. Selegiline and clomipramine effects on lymphocyte subsets, regulatory T cells and sheep red blood cell (SRBC)-induced humoral immune response after in vivo administration in mice. Eur. J. Pharmacol. 2020, 887, 173560. [Google Scholar] [CrossRef]
- Gu, B.; You, J.; Li, Y.; Duan, C.; Fang, M. Enteric-coated garlic supplement markedly enhanced normal mice immunocompetence. Eur. Food Res. Technol. 2010, 230, 627–634. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, D.; Cheng, L.; Zhao, D.; Ye, H.; Zheng, S.; Chen, S. Xiaowugui decoction alleviates experimental rheumatoid arthritis by suppressing Rab5a-mediated TLR4 internalization in macrophages. Phytomedicine 2024, 132, 155762. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. NF-κB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef]
- Dong, X.M.; Suo, S.K.; Wang, Y.M.; Zeng, Y.H.; Chi, C.F.; Wang, B. High Fischer ratio oligopeptides from Antarctic krill: Ameliorating function and mechanism to alcoholic liver injury through regulating AMPK/Nrf2/IκBα pathways. J. Funct. Foods 2024, 122, 106537. [Google Scholar] [CrossRef]

| Group | Initial/(mm) | Final/(mm) | Toe Swelling/(mm) |
|---|---|---|---|
| Control Model | 2.45 ± 0.07 2.06 ± 0.08 | 2.96 ± 0.09 2.32 ± 0.06 | 0.51 ± 0.07 0.26 ± 0.03 ** |
| Low-LH-6 Medium-LH-6 High-LH-6 | 2.29 ± 0.05 2.31 ± 0.09 2.27 ± 0.09 | 2.61 ± 0.11 2.68 ± 0.07 2.70 ± 0.17 | 0.32 ± 0.05 ** 0.37 ± 0.04 *# 0.43 ± 0.11 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zeng, Y.; Que, F.; Fu, S.; Xu, L.; Yu, F.; Wang, B. Immunoenhancement Function of the Novel Hexapeptide (LVVLGH) from Thick-Shelled Mussel (Mytilus coruscus) on Immunodeficient Mice by Activating the NF-κB/MAPK Pathway. Foods 2025, 14, 1865. https://doi.org/10.3390/foods14111865
Yang X, Zeng Y, Que F, Fu S, Xu L, Yu F, Wang B. Immunoenhancement Function of the Novel Hexapeptide (LVVLGH) from Thick-Shelled Mussel (Mytilus coruscus) on Immunodeficient Mice by Activating the NF-κB/MAPK Pathway. Foods. 2025; 14(11):1865. https://doi.org/10.3390/foods14111865
Chicago/Turabian StyleYang, Xu, Yu Zeng, Fandi Que, Shiqing Fu, Li Xu, Fangmiao Yu, and Bin Wang. 2025. "Immunoenhancement Function of the Novel Hexapeptide (LVVLGH) from Thick-Shelled Mussel (Mytilus coruscus) on Immunodeficient Mice by Activating the NF-κB/MAPK Pathway" Foods 14, no. 11: 1865. https://doi.org/10.3390/foods14111865
APA StyleYang, X., Zeng, Y., Que, F., Fu, S., Xu, L., Yu, F., & Wang, B. (2025). Immunoenhancement Function of the Novel Hexapeptide (LVVLGH) from Thick-Shelled Mussel (Mytilus coruscus) on Immunodeficient Mice by Activating the NF-κB/MAPK Pathway. Foods, 14(11), 1865. https://doi.org/10.3390/foods14111865






