Nutritional Composition of Four Edible Grasshopper Species Frequently Consumed in Madagascar: Insights for Nutritional Contribution and Alternative Insect Farming
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grasshoppers
2.2. Forage Collection
2.3. Experimental Design
2.4. Comparison of Production Parameters
2.5. Sample Collection and Preparation for Nutrition Analysis
2.6. Proximate Analysis of Kikuyu Grass, Vary Mena Rice Tillers, Rice Bran, Paracinema tricolor, Oxya hyla, Eyprepocnemis smaragdipes, and Acrida madecassa Grasshoppers
2.7. Mineral Composition Analysis of Kikuyu Grass, Vary Mena Rice Tillers, Rice Bran, Paracinema tricolor, Oxya hyla, Eyprepocnemis smaragdipes, and Acrida madecassa Grasshoppers
2.8. Determination of the Fatty Acid Profile of Paracinema tricolor, Oxya hyla, Eyprepocnemis smaragdipes, and Acrida madecassa Grasshoppers
2.9. Amino Acid Composition Determination of Paracinema tricolor, Oxya hyla, Eyprepocnemis smaragdipes, and Acrida madecassa Grasshoppers
2.10. Determination of the Vitamins of Paracinema tricolor, Oxya hyla, Eyprepocnemis smaragdipes, and Acrida madecassa Grasshoppers
2.11. Statistical Analysis
3. Results
3.1. Proximate Composition and Mineral Element Concentrations of Kikuyu Grass Forage, Vary Mena Rice Forage, and Rice Bran.
3.2. Comparison of Production Parameters of Paracinema tricolor, Oxya hyla, Eyprepocnemis smaragdipes, and Acrida madecassa Edible Grasshoppers
3.3. Proximate Composition of Paracinema tricolor, Oxya hyla, Eyprepocnemis smaragdipes, and Acrida madecassa Grasshoppers
3.4. Mineral Elements in Paracinema tricolor, Oxya hyla, Eyprepocnemis smaragdipes, and Acrida madecassa Grasshoppers
3.5. Fatty Acids in Paracinema tricolor, Oxya hyla, Eyprepocnemis smaragdipes, and Acrida madecassa Grasshoppers
3.6. Amino Acids in Paracinema tricolor, Oxya hyla, Eyprepocnemis smaragdipes, and Acrida madecassa Grasshoppers
3.7. Vitamins in Paracinema tricolor, Oxya hyla, Eyprepocnemis smaragdipes, and Acrida madecassa Grasshoppers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magara, H.J.O.; Solofondranohatra, C.L.; Hugel, S.; Fisher, L.B. Weeds and agro by-products for sustainable farming of edible field cricket, Gryllus madagascarensis (Orthoptera: Gryllidae). PLoS ONE 2025, 20, e0313083. [Google Scholar] [CrossRef] [PubMed]
- UN. World Population Prospects. Summary of Results. New York: United Nations. 2023. Available online: https://www.worldometers.info/world-population/madagascar-population/ (accessed on 18 February 2025).
- UN. Climate-Affected Madagascar Adapts to New Reality: A UN Resident Coordinator Blog. 2024. Available online: https://news.un.org (accessed on 23 March 2025).
- World Bank. A Ten-Year Program to Combat Chronic Malnutrition in Madagascar. 2018. Available online: https://www.worldbank.org/en/news/feature/2018/03/08/a-ten-year-program-to-combat-chronic-malnutrition-in-madagascar (accessed on 23 March 2025).
- Vaivada, T.; Akseer, N.; Akseer, S.; Somaskandan, A.; Stefopulos, M.; Bhutta, Z.A. Stunting in childhood: An overview of global burden, trends, determinants, and drivers of decline. Am. J. Clin. Nutr. 2020, 112 (Suppl. S2), 777S–791S. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food systems for our future: Joining forces for a zero hunger world. In Proceedings of the 16th Global Forum for Food and Agriculture (GFFA), Berlin, Germany, 17–20 January 2024; FAO Regional Office for Europe and Central Asia: Budapest, Hungary, 2024; pp. 1–3. [Google Scholar]
- Jongema, Y. List of Edible Insects of the World; Wageningen University & Research: Wageningen, The Netherlands, 2017; pp. 1–75. [Google Scholar]
- Magara, H.J.O.; Niassy, S.; Ayieko, M.A.; Mukundamago, M.; Egonyu, J.P.; Tanga, C.M.; Kimathi, E.K.; Ongere, J.O.; Fiaboe, K.K.M.; Hugel, S.; et al. Edible Crickets (Orthoptera) Around the world: Distribution, nutritional value, and other benefits—A review. Front. Nutr. 2021, 7, 537915. [Google Scholar] [CrossRef] [PubMed]
- van Itterbeeck, J.; Pelozuelo, L. How many edible insect species are there? A not so simple question. Diversity 2022, 14, 143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Otieno, M.H.J.; Ayieko, M.A.; Niassy, S.; Salifu, D.; Abdelmutalab, A.G.; Fathiya, K.M.; Subramanian, S.; Fiaboe, K.K.M.; Roos, N.; Ekesi, S.; et al. Integrating temperature-dependent life table data into Insect Life Cycle Model for predicting the potential distribution of Scapsipedus icipe Hugel & Tanga. PLoS ONE 2019, 14, e0222941. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Zhou, S.; Duan, H.; Guo, J.; Yan, W. Nutritional composition, health benefits, and application value of edible insects: A review. Foods 2022, 11, 3961. [Google Scholar] [CrossRef]
- Fisher, B.; Hugel, S. Edible insects traditions and uses on Madagascar. In The New Natural History of Madagascar; Princeton University Press: Princeton, NJ, USA, 2022; pp. 218–230, 252–264. [Google Scholar] [CrossRef]
- Agaba, J. Grasshoppers: Africa’s Untapped Food Source. 2022. Available online: https://allianceforscience.org/blog/2022/05/grasshoppers-africas-untapped-food-source/ (accessed on 28 February 2025).
- Tchibozo, S.; Lecoq, M. Edible Orthoptera from Africa: Preservation and promotion of traditional knowledge. Metaleptea 2017, 37, 24–29. [Google Scholar]
- Soliman, M.M.; El-Shazly, M. Bioaccumulation of heavy metals by grasshoppers and a mantid along a pollution gradient. Ecol. Balk. 2017, 9, 7–21. Available online: https://api.semanticscholar.org/CorpusID:222176555 (accessed on 1 March 2025).
- Paul, M.; Frederich, M.; Roel Uyttenbroeck, R.; Séverin Hatt, S.; Malik, P.; Lebecque, S.; Hamaidia, H.; Miazek, K.; Goffin, D.; Luc Willems, L.; et al. Grasshoppers as a food source? A review. Biotechnol. Agron. Soc. Environ. 2016, 20, 337–352. [Google Scholar] [CrossRef]
- Murugu, D.K.; Onyango, A.N.; Ndiritu, A.K.; Osuga, I.M.; Xavier, C.; Nakimbugwe, D.; Tanga, C.M. From farm to fork: Crickets as alternative source of protein, minerals, and vitamins. Front. Nutr. 2021, 8, 704002. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mao, C.; Li, X.; Jiang, L.; Zhang, W.; Li, M.; Liu, H.; Fang, Y.; Liu, S.; Yang, G.; et al. Edible insects: A new sustainable nutritional resource worth promoting. Foods 2023, 12, 4073. [Google Scholar] [CrossRef] [PubMed]
- Magara, H.J.O. Assessment of Cricket Species Composition, Feed Substrates, Optimal Temperature and Nutritional Content of Edible Cricket Scapsipedus icipe. Ph.D. Thesis, JOOUST, Bondo, Kenya, 2020. [Google Scholar]
- Magara, H.J.O.; Tanga, C.M.; Ayieko, M.A.; Hugel, S.; Mohamed, S.A.; Khamis, F.M.; Salifu, D.; Niassy, S.; Sevgan, S.; Fiaboe, K.K.M.; et al. Performance of newly described native edible cricket Scapsipedus icipe (Orthoptera: Gryllidae) on various diets of relevance for farming. J. Econ. Entomol. 2019, 112, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Magara, H.J.O.; Tanga, C.M.; Fisher, L.B.; Azrag, A.G.A.; Niassy, S.; Egonyu, J.P.; Hugel, S.; Roos, N.; Ayieko, M.A.; Sevgan, S.; et al. Impact of temperature on the bionomics and geographical range margins of the two-spotted field cricket Gryllus bimaculatus in the world: Implications for its mass farming. PLoS ONE 2024, 19, e0300438. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; İnal, F. The nutritional value of grasshoppers and locusts: A review. Ann. Anim. Sci. 2025, 25, 455–465. [Google Scholar] [CrossRef]
- Baiyegunhi, L.J.S.; Oppong, B.B.; Senyolo, G.M. Mopane worm (Imbrasia belina) and rural household food security in Limpopo province, South Africa. Food Secur. 2016, 8, 153–165. [Google Scholar] [CrossRef]
- van Huis, A. Insects as food and feed, a new emerging agricultural sector: A review. J. Insects Food Feed. 2020, 6, 27–44. [Google Scholar] [CrossRef]
- Bessot, J.C.; Pauli, G.; Kerschen, C.; Moreau, G.; Hirth, C. Allergie respiratoire au criquet migrateur. Rev. Française D’allergologie Et D’immunologie Clin. 1978, 18, 19–24. [Google Scholar] [CrossRef]
- Grasshopper: From Farm to Table. 2024. Available online: www.youtube.com (accessed on 22 March 2025).
- Cosgrove, E. Commercial Grasshopper Farmer Hargol Foodtech Raises $600k Seed Round. 2017. Available online: https://agfundernews.com/grasshopper-farmer-hargol-foodtech-raises-600k-seed-round (accessed on 1 April 2025).
- Magara, H.J.O.; Hugel, S.; Fisher, B.L. Effect of feed on the growth performance, nutrition content and cost of raising the field cricket (Gryllus madagascarensis) as a sustainable nutrient source in Madagascar. Foods 2024, 13, 3139. [Google Scholar] [CrossRef]
- Grabowski, N.T.; Tchibozo, S.; Abdulmawjood, A.; Acheuk, F.; Guerfali, M.M.; Sayed, W.A.A.; Plötz, M. Edible insects in Africa in terms of food, wildlife resource, and pest management legislation. Foods 2020, 9, 502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morelli, T.; Smith, A.; Mancini, A.; Balko, E.; Borgerson, C.; Dolch, R.; Farris, Z.; Federman, S.; Golden, C.; Holmes, S.; et al. The fate of Madagascar’s rainforest habitat. Nat. Clim. Change 2020, 10, 89–96. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemist: Washington, DC, USA, 2009; Volume 2. [Google Scholar]
- Boulos, S.; Tännler, A.; Nyström, L. Nitrogen-to-protein conversion factors for edible insects on the Swiss market: T. molitor, A. domesticus, and L. migratoria. Front. Nutr. 2020, 7, 89. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Method of Analysis, 18th ed.; Association of Officiating Analytical Chemists: Washington, DC, USA, 2005; pp. 935.14–992.24. [Google Scholar]
- Osman, A.; Osafo, E.L.K.; Atto-Kotoku, V.; Yunus, A.A.; Simpa Anim-Jnr, A.; Yawo Akwetey, W.; Anti, C. Carcass characteristics and meat quality of adult Sahelian does fed a basal diet of Brachiaria decumbens grass supplemented with probiotics and concentrates. Cogent Food Agric. 2023, 9, 2225259. [Google Scholar] [CrossRef]
- ISO 12966-2:2011; Animal and Vegetable Fats and Oils-Gas Chromatography of Fatty Acid Methyl Esters. 1st ed. ISO: Geneva, Switzerland, 2011.
- AOAC (Association of Official Analytical Chemists). Official Method 996.06 Fat (Total, Saturated, and Unsaturated) in Foods: Hydrolytic Extraction Gas Chromatographic Method. In Official Methods of Analysis of AOAC INTERNATIONAL, 22nd ed.; Latimer, G.W., Ed.; AOAC Publications: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Cheseto, X.; Kachigamba, D.L.; Ekesi, S.; Ndung’u, M.; Teal, P.E.A.; Beck, J.J.; Torto, B. Identification of the ubiquitous antioxidant tripeptide glutathione as a fruit fly semiochemical. J. Agric. Food Chem. 2017, 65, 8560–8568. [Google Scholar] [CrossRef]
- Jermacz, I.; Maj, J.; Morzycki, J.W.; Wojtkielewicz, A. GC-MS analysis of β-carotene ethenolysis products and their synthesis as potentially active vitamin A analogues. Toxicol. Mech. Methods 2008, 18, 469–471. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing, R version 4.4.2; R Core Team: Vienna, Austria, 2024; Available online: https://www.r-project.org/ (accessed on 28 March 2025).
- USDA; Agricultural Research Service; Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, Release 28 (Slightly Revised). 2018. Available online: https://ndb.nal.usda.gov/ndb/foods/show/4732?fgcd (accessed on 16 February 2025).
- Gao, Y.; Zhao, Y.-J.; Xu, M.-L.; Shi, S.-S. Clanis bilineata tsingtauica: A sustainable edible insect resource: A review. Sustainability 2021, 13, 12533. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S. Protein intake and energy balance. Regul. Pept. 2008, 149, 67–69. [Google Scholar] [CrossRef]
- Rachel, L.B.; Helen, H.; Saloni, K.; Joanna, E.C.; Keval, C.; Herbert, H.; Carel, W.L.E.; Louise, T.; Jimmy, D.B.; Dominic, J.W. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006, 4, 223–233. [Google Scholar] [CrossRef]
- René, R.; Jean-Philippe, B. Dietary protein and bone health. J. Bone Miner. Res. 2009, 19, 527–553. [Google Scholar] [CrossRef]
- Kerstetter, J.E.; Kenny, A.M.; Insogna, K.L. Dietary protein and skeletal health: A review of recent human research. Curr. Opin. Lipidol. 2011, 22, 16–20. [Google Scholar] [CrossRef]
- Altorf-van der Kuil, W.; Engberink, M.F.; Brink, E.J.; van Baak, M.A.; Bakker, S.J.; Navis, G.; van’t Veer, P.; Geleijnse, J.M. Dietary protein and blood pressure: A systematic review. PLoS ONE 2010, 5, e12102. [Google Scholar] [CrossRef]
- Evans, E.M.; Mojtahedi, M.C.; Thorpe, M.P.; Valentine, R.J.; Kris-Etherton, P.M.; Layman, D.K. Effects of protein intake and gender on body composition changes: A randomized clinical weight loss trial. Nutr. Metab. 2012, 20, 55. [Google Scholar] [CrossRef]
- Rizzoli, R.; Reginster, J.Y.; Arnal, J.F.; Bautmans, I.; Beaudart, C.; Bischoff-Ferrari, H.; Biver, E.; Boonen, S.; Brandi, M.L.; Chines, A.; et al. Quality of life in sarcopenia and frailty. Calcif. Tissue Int. 2013, 93, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Kinyuru, J.N. Nutrient content and lipid characteristics of desert locust (Schistoscerca gregaria) swarm in Kenya. Int. J. Trop. Insect Sci. 2021, 41, 1993–1999. [Google Scholar] [CrossRef]
- Roggenkamp, H.; Kumagai, H.; Wanasundara, J.P.D. Rice protein and rice protein products. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: New York, NY, USA, 2017; pp. 47–65. [Google Scholar] [CrossRef]
- Stull, V.J. Impacts of insect consumption on human health. J. Insects Food Feed. 2021, 7, 695–713. [Google Scholar] [CrossRef]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; De Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- UNICEF. UNICEF Data: Monitoring the Situation of Children and Women. 2023. Available online: https://data.unicef.org/country/mdg/ (accessed on 28 March 2025).
- Pasiakos, S.M.; McLellan, T.M.; Lieberman, H.R. The effects of protein supplements on muscle mass, strength, and aerobic and anaerobic power in healthy adults: A systematic review. Sports Med. 2015, 45, 111–131. [Google Scholar] [CrossRef]
- Brown, T.J.; Brainard, J.; Song, F.; Wang, X.; Abdelhamid, A.; Hooper, L.; PUFAH Group. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 Diabetes mellitus: Systematic review and meta-analysis of randomised controlled trials. BMJ 2019, 21, l4697. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Isaac, A.; Jesse, C.C.; Jacqueline, A.Y.; Fiifi, A.E.; Jia, J.L.; Marina, A.T.; Elaine, R. Edible insect powder for enrichment of bakery products–A review of nutritional, physical characteristics and acceptability of bakery products to consumers. Future Foods 2023, 8, 100251. [Google Scholar] [CrossRef]
- Adámková, A.; Mlček, J.; Kouřimská, L.; Borkovcová, M.; Bušina, T.; Adámek, M.; Bednářová, M.; Krajsa, J. Nutritional potential of selected insect species reared on the island of Sumatra. Int. J. Environ. Res. Public Health 2017, 14, 521. [Google Scholar] [CrossRef]
- Anankware, J.P.; Roberts, B.J.; Cheseto, X.; Osuga, I.; Savolainen, V.; Collins, C.M. The nutritional profiles of five important edible insect species from West Africa—An analytical and literature synthesis. Front. Nutr. 2021, 8, 792941. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; van der Poel, A.F.B. Effects of diet on the chemical composition of migratory locusts (Locusta migratoria). J. Insect Physiol. 2011, 57, 1171–1178. [Google Scholar] [CrossRef]
- FAO/WHO. Carbohydrates in Human Nutrition: A Joint FAO/WHO Expert Consultation Report; Food and Agriculture Organization: Rome, Italy, 1998; Volume 66, pp. 1–140. [Google Scholar] [PubMed]
- Harika, R.; Faber, M.; Samuel, F.; Mulugeta, A.; Kimiywe, J.; Eilander, A. Are low intakes and deficiencies in iron, Vitamin A, zinc, and iodine of public health concern in Ethiopian, Kenyan, Nigerian, and South African children and adolescents? Food Nutr. Bull. 2017, 38, 405–427. [Google Scholar] [CrossRef] [PubMed]
- Cormick, G.; Belizán, J.M. Calcium intake and health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef] [PubMed]
- Siriamornpun, S.; Thammapat, P. Chapter 16: Insects as a delicacy and a nutritious food in Thailand. In Using Food Science and Technology to Improve Nutrition and Promote National Development: Selected Case Studies; Robertson, G.L., Lupien, J.R., Eds.; International Union of Food Science and Technology: Bangkok, Thailand, 2007; pp. 1–12. [Google Scholar]
- Ohta, T.; Ido, A.; Kusano, K.; Miura, C.; Miura, T. A novel polysaccharide in insects activates the innate immune system in mouse macrophage RAW264 cells. PLoS ONE 2014, 9, e114823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramos-Elorduy, J. Tienen los insectos propiedades terapéuticas. In Memorias del 15 Congreso Internacional de Medicina Tradicional y Alternativas Terapéuticas; Academia Mexicana de Medicina Tradicional: Mexico DF, Mexico, 2001; pp. 135–136. [Google Scholar]
- Ademolu, K.O.; Idowu, A.B.; Olatunde, G.O. Nutritional value assessment of variegated grasshopper, Zonocerus variegatus (L.) (Acridoidea: Pygomorphidae), during post-embryonic development. Afr. Entomol. 2010, 18, 360–364. [Google Scholar] [CrossRef]
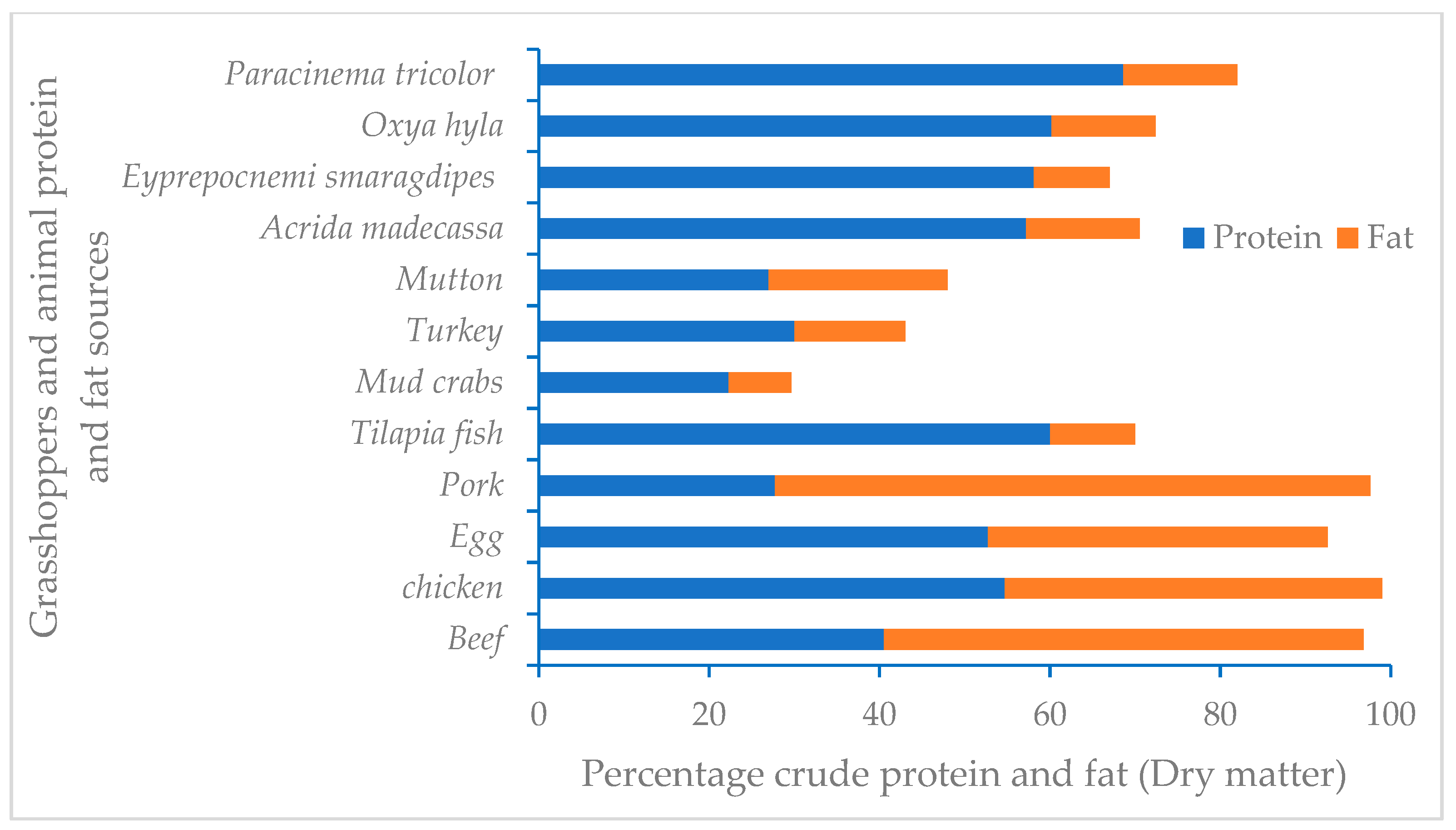
| Nutrient Components and Unts | Kikuyu Grass Forage | Vary Mena Rice Forage | Rice Bran |
|---|---|---|---|
| Proximate | |||
| Moisture (%) | 77.80 ± 0.92 | 76.20 ± 0.75 | 11.40 ± 0.39 |
| Protein (g/kg DM) | 200.00 ± 0.45 | 180.00 ± 3.50 | 130.00 ± 2.15 |
| Fats (g/kg DM) | 20.00 ± 1.22 | 18.00 ± 1.01 | 155.00 ± 2.05 |
| Ash (g/kg DM) | 90.00 ± 2.5 | 85.00 ± 2.20 | 72.00 ± 1.84 |
| Crude fibre (g/kg DM) | 280.00 ± 7.10 | 250.00 ± 6.20 | 77.00 ± 1.98 |
| Carbohydrate (g/kg DM) | 332.20 ± 9.02 | 390.80 ± 8.50 | 554.60 ± 3.35 |
| Mineral elements | |||
| Calcium (g/kg DM) | 4.20 ± 0.20 | 3.80 ± 0.20 | 1.32 ± 0.02 |
| Phosphorus (g/kg DM) | 2.10 ± 0.10 | 2.01 ± 0.08 | 12.48 ± 0.45 |
| Magnesium (g/kg DM) | 1.01 ± 0.05 | 0.92 ± 0.04 | 5.85 ± 0.30 |
| Potassium (g/kg DM) | 18.03 ± 0.71 | 17.22 ± 0.50 | 8.68 ± 0.32 |
| Sodium (g/kg DM) | 0.80 ± 0.04 | 0.72 ± 0.03 | 0.28 ± 0.01 |
| Iron (mg/kg DM) | 120.00 ± 6.02 | 115.00 ± 4.58 | 108.00 ± 5.25 |
| Copper (mg/kg DM) | 5.50 ± 0.40 | 5.02 ± 0.30 | 6.25 ± 0.20 |
| Zinc (mg/kg DM) | 22.00 ± 0.72 | 20.04 ± 0.60 | 33.54 ± 0.58 |
| Manganese (mg/kg DM) | 70.00 ± 3.00 | 65.00 ± 3.10 | 45.50 ± 0.40 |
| Grasshopper Species | Survival Rate to Adult (%) | Development Time to Adult (Days) | Feed Consumed (g) | Feed Conversion Ratio | Biomass Yield (g) | Fecundity (Eggs per Female) | Hatchability (%) |
|---|---|---|---|---|---|---|---|
| Paracinema tricolor | 90.04 ± 0.18 a | 41.14 ± 0.32 a | 1050.40 ± 0.54 a | 1.84 ± 0.01 b | 573.99 ± 1.13 b | 276.42 ± 0.51 a | 92.22 ± 0.17 a |
| Oxya hyla | 85.12 ± 0.36 b | 42.02 ± 0.29 b | 1071.01 ± 5.34 b | 2.58 ± 0.11 d | 403.56 ± 4.28 d | 260.39 ± 1.21 b | 90.10 ± 0.22 b |
| Eyprepocnemis smaragdipes | 77.72 ± 0.32 d | 46.20 ± 0.34 c | 1151.00 ± 4.30 c | 2.23 ± 0.01 c | 515.93 ± 2.06 c | 218.64 ± 0.51 d | 85.98 ± 0.17 d |
| Acrida madecassa | 80.76 ± 0.53 c | 48.42 ± 0.25 d | 1217.20 ± 6.20 | 1.44 ± 0.01 a | 846.26 ± 5.50 a | 240.03 ± 0.71 c | 88.06 ± 0.20 c |
| F | 212.90 | 132.90 | 257.7 | 83.23 | 371.60 | 1014.00 | 199.70 |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| df | 3, 16 | 3, 16 | 3, 16 | 3, 16 | 3, 16 | 3, 16 | 3, 16 |
| Grasshopper | Nutrient | ||||||
|---|---|---|---|---|---|---|---|
| Moisture (%) | Protein (%) | Lipids (%) | Fibre (%) | Ash (%) | Carbohydrate (%) | Energy (Kcal/kg) | |
| Paracinema tricolor | 1.95 ± 0.16 a | 68.58 ± 0.49 a | 13.38 ± 0.74 a | 3.89 ± 0.07 c | 9.76 ± 0.07 a | 2.44 ± 0.02 c | 4044.90 ± 45.56 a |
| Oxya hyla | 2.54 ± 0.23 b | 60.22 ± 0.94 b | 12.21 ± 0.19 a | 4.34 ± 0.09 b | 5.77 ± 0.38 d | 14.92 ± 0.06 a | 4104.90 ± 16.31 a |
| Eyprepocnemis smaragdipes | 8.95 ± 0.28 d | 58.09 ± 0.09 b | 8.88 ± 0.11 b | 4.46 ± 0.04 b | 7.14 ± 0.16 c | 12.48 ± 0.32 b | 3611.77 ± 11.80 b |
| Acrida madecassa | 7.86 ± 0.12 c | 57.15 ± 0.52 c | 13.34 ± 1.59a | 8.37 ± 0.08 a | 8.37 ± 0.56 b | 4.91 ± 0.02 c | 3627.17 ± 164.65 b |
| p-value | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| F-value | 874.20 | 362.90 | 17.16 | 2379.30 | 74.17 | 327.20 | 28.3 |
| df | 3, 8 | 3, 8 | 3, 8 | 3, 8 | 3, 8 | 3, 8 | 3, 8 |
| RDI (g/100g DM) by USDA for | |||||||
| Children 5 to 10 years | NR | 24 | 70 | 4 | 15 | 220 | 1800 |
| Adult males | NR | 46 | 95 | 6 | 24 | 230 | 2550 |
| Adult females | NR | 56 | 70 | 6 | 24 | 230 | 200 |
| Grasshopper Species | Mineral Elements (mg/100 g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Magnesium (Mg) | Iron (Fe) | Calcium (Ca) | Copper (Cu) | Phosphorus (P) | Zinc (Zn) | Potassium (K) | Manganese (Mn) | Sodium (Na) | |
| Paracinema tricolor | 123.40 ± 3.34 a | 20.39 ± 0.19 a | 366.17 ± 15.67 a | 2.62 ± 0.01 b | 859.77 ± 14.71 a | 29.80 ± 0.81 a | 1210.62 ± 17.37 a | 12.57 ± 0.06 a | 264.70 ± 5.77 a |
| Oxya hyla | 103.56 ± 3.88 b | 7.67 ± 0.65 c | 148.12 ± 10.10 b | 5.80 ± 0.44 a | 314.68 ± 7.39 c | 16.75 ± 0.25 c | 1014.31 ± 22.49 b | 3.53 ± 0.15 c | 151.95 ± 6.11 b |
| Eyprepocnemis smaragdipes | 105.72 ± 7.63 b | 19.07 ± 0.37 b | 36.78 ± 4.09 d | 1.82 ± 0.06 c | 765.51 ± 24.52 b | 23.75 ± 3.02 b | 844.38 ± 23.56 c | 7.22 ± 0.41 b | 40.75 ± 1.15 c |
| Acrida madecassa | 74.21 ± 4.14 c | 4.95 ± 0.34 d | 65.21 ± 4.20 c | 1.79 ± 0.08 c | 216.28 ± 9.08 | 14.85 ± 0.79 c | 825.56 ± 26.14 c | 0.81 ± 0.05 d | 82.93 ± 4.16 c |
| F | 49.10 | 989.90 | 698.40 | 211.50 | 1247.00 | 53.69 | 188.50 | 1544.00 | 25.96 |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | =0.0002 |
| df | 3, 8 | 3, 8 | 3, 8 | 3, 8 | 3, 8 | 3, 8 | 3, 8 | 3, 8 | 3, 8 |
| Mutton | 24.00 | 2.00 | 22.00 | 0.13 | 232.00 | 4.63 | 336.00 | 0.02 | 403.00 |
| Turkey | 30.00 | 1.10 | 14.00 | 0.09 | 263.00 | 2.43 | 239.00 | 0.03 | 103.00 |
| Mud crab | 47.65 | 1.21 | 24.61 | 0.91 | 296.00 | 3.80 | 29.50 | 0.03 | 26.85 |
| Tilapia fish | 34.00 | 0.69 | 16.70 | 0.08 | 392.00 | 0.41 | 380.00 | 0.81 | 56.00 |
| Pork | 25.9 | 1.40 | 37.90 | 0.10 | 323.00 | 3.20 | 504.30 | 0.01 | 83.70 |
| Eggs | 50.30 | 7.30 | 54.00 | 0.30 | 122.00 | 5.40 | 578.60 | 0.01 | 595.40 |
| Chicken | 58.80 | 2.60 | 32.20 | 1.40 | 172.00 | 3.90 | 555.70 | 0.11 | 205.80 |
| Beef | 39.80 | 4.30 | 18.70 | 0.20 | 263.00 | 8.40 | 624.70 | 0.02 | 138.00 |
| RDI (mg/100 g DM) by the USDA for Children | |||||||||
| 0–3 years | 20–50 | 7 | 700 | 0.4–1 | 100–460 | 2–3 | 2000 | 0.003–1.2 | 1200 |
| 4–8 years | 65 | 10 | 1000 | 1–1.5 | 500 | 4 | 2300 | 1.5 | 1500 |
| 9–13 years | 100–135 | 8–10 | 1000–1300 | 1–2 | 1250 | 4–6 | 2500 | 1.66 | 1800 |
| Adults | 320–429 | 10–18 | 1000–1200 | 1.5–3.0 | 700 | 8–11 | 2300–3400 | 1.8–2.3 | 1500 |
| Fatty Acid | Paracinema tricolor | Oxya hyla | Eyprepocnemis smaragdipes | Acrida madecassa | p-Value | F-Value | df |
|---|---|---|---|---|---|---|---|
| Lauric acid (C12:0) | 0.06± 0.01 c | 0.42 ± 0.05 a | 0.30 ± 0.01 b | 0.03 ± 0.01 c | 163.90 | 0.0001 | 3, 8 |
| Myristic acid (C14:0) | 1.10 ± 0.25 b | 3.32 ± 0.43 a | 0.56 ± 0.02 c | 0.42 ± 0.04 c | 87.71 | 0.0001 | 3, 8 |
| Pentadecanoic acid (C15:0) | 0.26 ± 0.07 b | 0.55 ± 0.11 a | 0.15± 0.01 b | 0.29 ± 0.03 b | 18.25 | 0.0006 | 3, 8 |
| Palmitic acid (C16:0) | 11.59 ± 0.93 c | 13.74 ± 0.55 b | 10.55 ± 0.55 c | 17.26 ± 0.45 a | 63.11 | 0.0001 | 3, 8 |
| Margaric acid (C17:0) | 1.38 ± 0.36 a | 1.14 ± 0.08 a | 0.42 ± 0.03 b | 0.41 ± 0.06 b | 21.35 | 0.0004 | 3, 8 |
| Stearic acid (C18:0) | 10.25 ± 0.04 b | 11.30 ± 0.86 a | 10.10 ± 0.40 b | 8.09 ± 0.20 c | 23.18 | 0.0003 | 3, 8 |
| Arachidic acid (C20:0) | 1.57 ± 0.26 b | 1.54 ± 0.15 b | 1.30 ± 0.06 b | 2.28 ± 0.06 a | 21.95 | 0.0003 | 3, 8 |
| Behenic acid (C22:0) | 1.06 ± 0.07 a | 0.52 ± 0.01 b | 0.40 ± 0.02 b | 1.00 ± 0.02 a | 24.14 | 0.0001 | 3, 8 |
| Lignoceric acid (C24:0) | 0.04 ± 0.01 c | 0.85 ± 0.01 b | 1.31 ± 0.01 a | 0.02 ± 0.01 d | 12,100.00 | 0.0001 | 3, 8 |
| Alpha-linolenic acid (C 18:3(n-3)) | 0.50 ± 0.10 a | 0.40 ± 0.10 a | 0.34 ± 0.01 a | 0.32 ± 0.01 a | 3.88 | 0.0555 | 3, 8 |
| Linoleic acid (C18:2(n-6)) | 43.73 ± 0.70 a | 39.06 ± 0.38 b | 38.92 ± 0.66 b | 39.65 ± 0.61 b | 43.20 | 0.0001 | 3, 8 |
| Oleic acid (C18:1(n-9)) | 28.46 ± 0.40 c | 27.16 ± 0.64 d | 35.65 ± 0.55 a | 30.23 ± 0.34 b | 168.70 | 0.0001 | 3, 8 |
| Monounsaturated fatty acids (MUFAs) | 28.46 ± 0.40 c | 27.16 ± 0.64 d | 35.65 ± 0.55 a | 30.23 ± 0.34 b | 168.70 | 0.0001 | 3, 8 |
| Polyunsaturated fatty acids (PUFAs) | 44.23 ± 0.04 a | 39.46 ± 0.37 b | 39.26 ± 0.62 b | 39.97 ± 0.64 b | 46.06 | 0.0001 | 3, 8 |
| Saturated fatty acids (SFAs) | 27.31 ± 0.34 c | 33.38 ± 0.45 a | 25.09 ± 0.71 d | 29.80 ± 0.52 b | 57.86 | 0.0001 | 3, 8 |
| Essential fatty acids (EFAs) | 44.23 ± 0.04 a | 39.46 ± 0.37 b | 39.26 ± 0.62 b | 39.97 ± 0.64 b | 46.06 | 0.0001 | 3, 8 |
| PUFA/SFA | 1.62 | 1.18 | 1.56 | 1.34 | |||
| Amino Acid | Paracinema tricolor | Oxya hyla | Eyprepocnemis smaragdipes | Acrida madecassa | p-Value | F-Value | df | RDA by USDA for | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Essential amino acids | Infants (3–4 months) | Children (~2 years) | Children (10–12 years) | Adults | |||||||
| Histidine | 14.60 ± 0.20 a | 5.04 ± 0.08 b | 4.79 ± 0.10 b | 4.75 ± 0.01 b | 0.0001 | 581.70 | 3, 8 | 1.6 | 1.9 | 1.9 | 1.1 |
| Isoleucine | 59.28 ± 0.04 a | 45.24 ± 0.25 b | 43.74 ± 0.09 c | 43.11 ± 0.10 d | 0.0001 | 9037.00 | 3, 8 | 4.0 | 2.8 | 2.8 | 1.3 |
| Lysine | 24.83 ± 0.31 a | 16.06 ± 0.10 b | 15.58 ± 0.13 c | 15.31 ± 0.17 c | 0.0001 | 1650.00 | 3, 8 | 6.0 | 5.8 | 4.4 | 1.6 |
| Methionine | 23.02 ± 0.03 a | 21.39 ± 0.04 b | 20.67 ± 0.06 b | 20.91 ± 0.77 b | 0.0002 | 22.70 | 3, 8 | NR | NR | NR | NR |
| Valine | 39.81 ± 0.69 c | 42.82 ± 0.08 a | 41.30 ± 0.10 b | 40.67 ± 0.06 b | 0.0001 | 43.05 | 3, 8 | 5.4 | 3.5 | 2.5 | 1.3 |
| Phenylalanine | 34.75 ± 0.22 a | 33.80 ± 0.27 b | 32.65 ± 0.19 c | 32.15 ± 0.12 d | 0.0001 | 96.13 | 3, 8 | NR | NR | NR | NR |
| Leucine | 98.45 ± 0.23 a | 81.94 ± 0.59 b | 78.70 ± 0.24 c | 77.17 ± 0.15 d | 0.0001 | 2375.00 | 3, 8 | 9.3 | 6.6 | 4.4 | 1.9 |
| Nonessential amino acid | |||||||||||
| Arginine | 18.60 ± 0.07 a | 14.36 ± 0.31 b | 14.02 ± 0.16 bc | 13.75 ± 0.05 c | 0.0001 | 481.20 | 3, 8 | NR | NR | NR | NR |
| Glutamine | 2.81 ± 0.04 a | 1.83 ± 0.03 b | 1.75 ± 0.02 c | 1.71 ± 0.02 c | 0.0001 | 1391.00 | 3, 8 | NR | NR | NR | NR |
| Glutamic acid | 13.03 ± 0.07 a | 11.72 ± 0.07 b | 11.31 ± 0.06 c | 11.14 ± 0.07 d | 0.0001 | 559.50 | 3, 8 | NR | NR | NR | NR |
| Proline | 26.29 ± 0.14 d | 31.54 ± 0.06 a | 30.44 ± 0.07 b | 30.03 ± 0.10 c | 0.0001 | 1605.00 | 3, 8 | NR | NR | NR | NR |
| Tyrosine | 32.49 ± 0.13 a | 25.26 ± 0.35 b | 24.49 ± 0.05 c | 24.09 ± 0.10 d | 0.0001 | 1266.00 | 3, 8 | NR | NR | NR | NR |
| Hydroxyproline | 12.05 ± 0.20 a | 9.83 ± 0.42 b | 9.33 ± 0.14 c | 9.22 ± 0.03 c | 0.0001 | 90.9 3 | 3, 8 | NR | NR | NR | NR |
| Grasshopper Species | Vitamin A (μg/100) | Vitamin E (IU/kg) | Vitamin C (Ascorbic Acid) (mg/100 g) | Vitamin B1 (Thiamine) (mg/100 g) | Vitamin B2 (Riboflavin) (mg/100 g) | Vitamin B3 (Niacin) (mg/100 g) | Vitamin B9 (Folic Acid) (mg/100 g) |
|---|---|---|---|---|---|---|---|
| Paracinema tricolor | 334.21 ± 0.06 a | 47.61 ± 0.06 a | 0.16 ± 0.02 a | 0.83 ± 0.06 a | 1.50 ± 0.64 a | 3.34 ± 0.06 a | 1.49 ± 0.10 a |
| Oxya hyla | 293.42 ± 0.31 b | 41.79 ± 0.04 b | 0.13 ± 0.02 ab | 0.70 ± 0.03 b | 1.64 ± 0.06 a | 2.92 ± 0.07 b | 1.29 ± 0.06 b |
| Eyprepocnemis smaragdipes | 283.09 ± 0.12 c | 40.39 ± 0.06 c | 0.11 ± 0.02 b | 0.66 ± 0.02 b | 1.60 ± 0.05 a | 2.79 ± 0.04 c | 1.16 ± 0.05 bc |
| Acrida madecassa | 278.70 ± 0.42 d | 39.70 ± 0.07 d | 0.11 ± 0.02 b | 0.65 ± 0.03 b | 1.58 ± 0.05 a | 1.75 ± 0.03 d | 1.07 ± 0.03 c |
| F | 2679.00 | 875.30 | 4.63 | 16.61 | 0.102 | 486.30 | 21.60 |
| p | <0.0001 | <0.0001 | =0.0369 | =0.0009 | =0.9570 | <0.0001 | =0.0003 |
| df | 3, 8 | 3, 8 | 3, 8 | 3, 8 | 3, 8 | 3, 8 | 3, 8 |
| Recommended daily intake (RDI) | 500.00–700.00 μg | 7.50–10.00 mg | 45.00 mg | 1.20–1.30 mg | 1.10–1.30 mg | 14.00–16.00 mg | 0.40 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magara, H.J.O.; Hugel, S.; Fisher, B.L. Nutritional Composition of Four Edible Grasshopper Species Frequently Consumed in Madagascar: Insights for Nutritional Contribution and Alternative Insect Farming. Foods 2025, 14, 1848. https://doi.org/10.3390/foods14111848
Magara HJO, Hugel S, Fisher BL. Nutritional Composition of Four Edible Grasshopper Species Frequently Consumed in Madagascar: Insights for Nutritional Contribution and Alternative Insect Farming. Foods. 2025; 14(11):1848. https://doi.org/10.3390/foods14111848
Chicago/Turabian StyleMagara, Henlay J. O., Sylvain Hugel, and Brian L. Fisher. 2025. "Nutritional Composition of Four Edible Grasshopper Species Frequently Consumed in Madagascar: Insights for Nutritional Contribution and Alternative Insect Farming" Foods 14, no. 11: 1848. https://doi.org/10.3390/foods14111848
APA StyleMagara, H. J. O., Hugel, S., & Fisher, B. L. (2025). Nutritional Composition of Four Edible Grasshopper Species Frequently Consumed in Madagascar: Insights for Nutritional Contribution and Alternative Insect Farming. Foods, 14(11), 1848. https://doi.org/10.3390/foods14111848







