Comparative Study by HPTLC of Selected Capparis spinosa Samples (Buds and Leaves) from the Cycladic Islands in Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extracts Preparation
2.2. Solvents and Reagents
2.3. HPTLC Profiling and Quantification
2.4. Calibration and Validation
2.5. Total Phenolic Content
2.6. Antioxidant Activity
2.7. Antimicrobial Activity
2.8. Statistical Analysis
3. Results
3.1. Extraction
3.2. HPTLC Analysis
3.3. Total Phenolic Content
3.4. Antioxidant Activity
3.5. Antimicrobial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fici, S. A Taxonomic Revision of the Capparis spinosa Group (Capparaceae) from the Mediterranean to Central Asia. Phytotaxa 2014, 174, 1–24. [Google Scholar] [CrossRef]
- Capparis spinosa L.|Plants of the World Online. Kew Science. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:146789-1 (accessed on 17 February 2025).
- Chedraoui, S.; Abi-Rizk, A.; El-Beyrouthy, M.; Chalak, L.; Ouaini, N.; Rajjou, L. Capparis spinosa L. in A Systematic Review: A Xerophilous Species of Multi Values and Promising Potentialities for Agrosystems Under the Threat of Global Warming. Front. Plant Sci. 2017, 8, 1845. [Google Scholar] [CrossRef] [PubMed]
- Caper-Capparis Spinosa. Available online: https://hort.purdue.edu/newcrop/CropFactSheets/caper.html (accessed on 17 February 2025).
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Plant of the Millennium, Caper (Capparis spinosa L.), Chemical Composition and Medicinal Uses. Bull. Natl. Res. Cent. 2021, 45, 131. [Google Scholar] [CrossRef]
- Sher, H.; Alyemeni, M.N. Ethnobotanical and Pharmaceutical Evaluation of Capparis spinosa L, Validity of Local Folk and Unani System of Medicine. J. Med. Plants Res. 2010, 4, 1751–1756. [Google Scholar] [CrossRef]
- Osbaldeston, T.A.; Wood, R.P.A. De Materia Medica: Being an Herbal with Many Other Medicinal Materials: Written in Greek in the First Century of the Common Era: A New Indexed Version in Modern English; IBIDIS: Johannesburg, South Africa, 2000; p. 932. [Google Scholar]
- Inocencio, C.; Rivera, D.; Alcaraz, F.; Tomás-Barberán, F.A. Flavonoid Content of Commercial Capers (Capparis spinosa, C. sicula and C. orientalis) Produced in Mediterranean Countries. Eur. Food Res. Technol. 2000, 212, 70–74. [Google Scholar] [CrossRef]
- Proestos, C.; Boziaris, I.S.; Nychas, G.J.E.; Komaitis, M. Analysis of Flavonoids and Phenolic Acids in Greek Aromatic Plants: Investigation of Their Antioxidant Capacity and Antimicrobial Activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
- Mohaddab, M.; Genva, M.; Fakiri, M.; El-Goumi, Y.; Zeroual, A.; Fauconnier, M.L. Capparis spinosa: A Rich Source of Phenolic Compounds—A Comprehensive Review of Its Phytochemistry, Health Benefits, and Biotechnological Applications. Biocatal. Agric. Biotechnol. 2024, 61, 103409. [Google Scholar] [CrossRef]
- Kdimy, A.; El Yadini, M.; Guaadaoui, A.; Bourais, I.; El Hajjaji, S.; Le, H.V. Phytochemistry, Biological Activities, Therapeutic Potential, and Socio-Economic Value of the Caper Bush (Capparis spinosa L.). Chem. Biodivers. 2022, 19, e202200300. [Google Scholar] [CrossRef]
- Gull, T.; Anwar, F.; Sultana, B.; Alcayde, M.A.C.; Nouman, W. Capparis Species: A Potential Source of Bioactives and High-Value Components: A Review. Ind. Crops. Prod. 2015, 67, 81–96. [Google Scholar] [CrossRef]
- Lo Bosco, F.; Guarrasi, V.; Moschetti, M.; Germanà, M.A.; Butera, D.; Corana, F.; Papetti, A. Nutraceutical Value of Pantelleria Capers (Capparis spinosa L.). J. Food Sci. 2019, 84, 2337–2346. [Google Scholar] [CrossRef]
- Khatib, M.; Pieraccini, G.; Innocenti, M.; Melani, F.; Mulinacci, N. An Insight on the Alkaloid Content of Capparis spinosa L. Root by HPLC-DAD-MS, MS/MS and 1H QNMR. J. Pharm. Biomed. Anal. 2016, 123, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Moghaddasian, B.; Asli, D.E.; Eghdami, A. Anoosh Determination of Rutin Content in Caper (Capparis spinosa) by Three Analytical Methods. Sch. Res. Libr. Ann. Biol. Res. 2012, 3, 4303–4306. [Google Scholar]
- Shinde, D.B.; Chavan, M.J.; Wakte, P.S. HPTLC in Herbal Drug Quantification. In High-Performance Thin-Layer Chromatography (HPTLC); Springer: Berlin/Heidelberg, Germany, 2011; pp. 117–139. [Google Scholar] [CrossRef]
- Pawar, H.; Ghule, B.; Sahu, A.; Takale, N.; Kotagale, N. High-Performance Thin-Layer Chromatography Method Development and Validation for Quantification of Rutin in Different Parts of Capparis zeylanica Linn. Plant. J. Plant Chrome-Mod TLC 2024, 37, 137–149. [Google Scholar] [CrossRef]
- Doshi, G.M.; Pawar, M.K.; Chavda, K.H. Quantification of Rutin and Quercetin by HPTLC/HPLC and In Vitro Immunomodulatory and Anticancer Activities of Capparis moonii Fruits Extracts. Int. J. Basic. Clin. Pharmacol. 2018, 7, 153–161. [Google Scholar] [CrossRef][Green Version]
- Ganos, C.; Aligiannis, N.; Chinou, I.; Naziris, N.; Chountoulesi, M.; Mroczek, T.; Graikou, K. Rindera graeca (Boraginaceae) Phytochemical Profile and Biological Activities. Molecules 2020, 25, 3625. [Google Scholar] [CrossRef]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH Harmonised Tripartite Guideline Validation of Analytical Procedures: Text and Methodology q2(r1). Available online: https://www.fda.gov/media/152208/download (accessed on 13 April 2025).
- Varvouni, E.F.; Zengin, G.; Graikou, K.; Ganos, C.; Mroczek, T.; Chinou, I. Phytochemical Analysis and Biological Evaluation of the Aerial Parts from Symphytum Anatolicum Boiss. and Cynoglottis Barrelieri (All.) Vural & Kit Tan (Boraginaceae). Biochem. Syst. Ecol. 2020, 92, 104128. [Google Scholar] [CrossRef]
- Mollica, A.; Zengin, G.; Locatelli, M.; Stefanucci, A.; Mocan, A.; Macedonio, G.; Carradori, S.; Onaolapo, O.; Onaolapo, A.; Adegoke, J.; et al. Anti-Diabetic and Anti-Hyperlipidemic Properties of Capparis spinosa L.: In Vivo and In Vitro Evaluation of Its Nutraceutical Potential. J. Funct. Foods 2017, 35, 32–42. [Google Scholar] [CrossRef]
- Rodrigo, M.; Lazaro, M.J.; Alvarruiz, A.; Giner, V. Composition of Capers (Capparis spinosa): Influence of Cultivar, Size and Harvest Date. J. Food Sci. 1992, 57, 1152–1154. [Google Scholar] [CrossRef]
- Siracusa, L.; Kulisic-Bilusic, T.; Politeo, O.; Krause, I.; Dejanovic, B.; Ruberto, G. Phenolic Composition and Antioxidant Activity of Aqueous Infusions from Capparis spinosa L. and Crithmum maritimum L. before and after Submission to a Two-Step In Vitro Digestion Model. J. Agric. Food Chem. 2011, 59, 12453–12459. [Google Scholar] [CrossRef]
- Sonmezdag, A.S.; Kelebek, H.; Selli, S. Characterization of Aroma-Active Compounds, Phenolics, and Antioxidant Properties in Fresh and Fermented Capers (Capparis spinosa) by GC-MS-Olfactometry and LC-DAD-ESI-MS/MS. J. Food Sci. 2019, 84, 2449–2457. [Google Scholar] [CrossRef]
- Mohebali, N.; Shahzadeh Fazeli, S.A.; Ghafoori, H.; Farahmand, Z.; MohammadKhani, E.; Vakhshiteh, F.; Ghamarian, A.; Farhangniya, M.; Sanati, M.H. Effect of Flavonoids Rich Extract of Capparis spinosa on Inflammatory Involved Genes in Amyloid-Beta Peptide Injected Rat Model of Alzheimer’s Disease. Nutr. Neurosci. 2018, 21, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, Z.; Aghel, N.; Keyghobadi, H. Rutin from Different Parts of Capparis spinosa Growing Wild in Khuzestan/Iran. Pak. J. Biol. Sci. 2008, 11, 768–772. [Google Scholar] [CrossRef]
- Aksay, O.; Selli, S.; Kelebek, H. LC-DAD-ESI-MS/MS-Based Assessment of the Bioactive Compounds in Fresh and Fermented Caper (Capparis spinosa) Buds and Berries. Food Chem. 2021, 337, 127959. [Google Scholar] [CrossRef]
- Jiménez-López, J.; Ruiz-Medina, A.; Ortega-Barrales, P.; Llorent-Martínez, E.J. Phytochemical Profile and Antioxidant Activity of Caper Berries (Capparis spinosa L.): Evaluation of the Influence of the Fermentation Process. Food Chem. 2018, 250, 54–59. [Google Scholar] [CrossRef]
- Azad, M.; Mohammadi, P.; Bohlooli, S.; Mostafalou, S. Protective Effect of Capparis spinosa Hydroalcoholic Extract on the Integrity of Rat Pancreatic Islets. J. Adv. Med. Biomed. Res. 2020, 28, 2676–6264. [Google Scholar] [CrossRef]
- Tlili, N.; Khaldi, A.; Triki, S.; Munné-Bosch, S. Phenolic Compounds and Vitamin Antioxidants of Caper (Capparis spinosa). Plant Foods Hum. Nutr. 2010, 65, 260–265. [Google Scholar] [CrossRef]
- Khanavi, M.; Ara, L.; Khavassi, N.; Hajimehdipoor, H. Capparis spinosa: A Comparative Study of Raw and Processed Fruits. J. Med. Plants 2020, 19, 91–99. [Google Scholar] [CrossRef]
- Stefanucci, A.; Zengin, G.; Locatelli, M.; Macedonio, G.; Wang, C.K.; Novellino, E.; Mahomoodally, M.F.; Mollica, A. Impact of Different Geographical Locations on Varying Profile of Bioactives and Associated Functionalities of Caper (Capparis spinosa L.). Food Chem. Toxicol. 2018, 118, 181–189. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of Fermentation on the Antioxidant Activity in Plant-Based Foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.F. Phytochemical and Pharmacological Properties of Capparis Spinosa as a Medicinal Plant. Nutrients 2018, 10, 116. [Google Scholar] [CrossRef]
- Germano, M.P.; De Pasquale, R.; D’Angelo, V.; Catania, S.; Silvari, V.; Costa, C. Evaluation of Extracts and Isolated Fraction from Capparis spinosa L. Buds as an Antioxidant Source. J. Agric. Food Chem. 2002, 50, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and Anti-Inflammatory Activities of Quercetin and Its Derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Al-Azawi, A.H.; Kassim Ghaima, K.; Salih, H.H. Phytochemical, Antibacterial and Antioxidant Activities of Capparis spinosa L. Cultivated in Iraq. Biosci. Rep. 2018, 15, 2611–2618. [Google Scholar]
- Oudah, S.K.; Al-Salih, R.M.H.; Gusar, S.H.; Roomi, A.B. Study of the role of polyphenolic extract of Capparis spinosa l. leaves as acute toxicity and antibacterial agent. Plant Arch. 2019, 19, 3821–3829. [Google Scholar]
- Do, T.K.T.; Reich, E. Insights into the evolution and future of high-performance thin-layer chromatography in routine quality control: A review. J. Planar Chromatogr. 2023, 36, 317–325. [Google Scholar] [CrossRef]
- Do, T.K.T.; Trettin, I.; Reich, E. Standardized HPTLC for Reproducible Chemical Fingerprinting. Methods Mol. Biol. 2025, 2895, 47–61. [Google Scholar] [CrossRef]
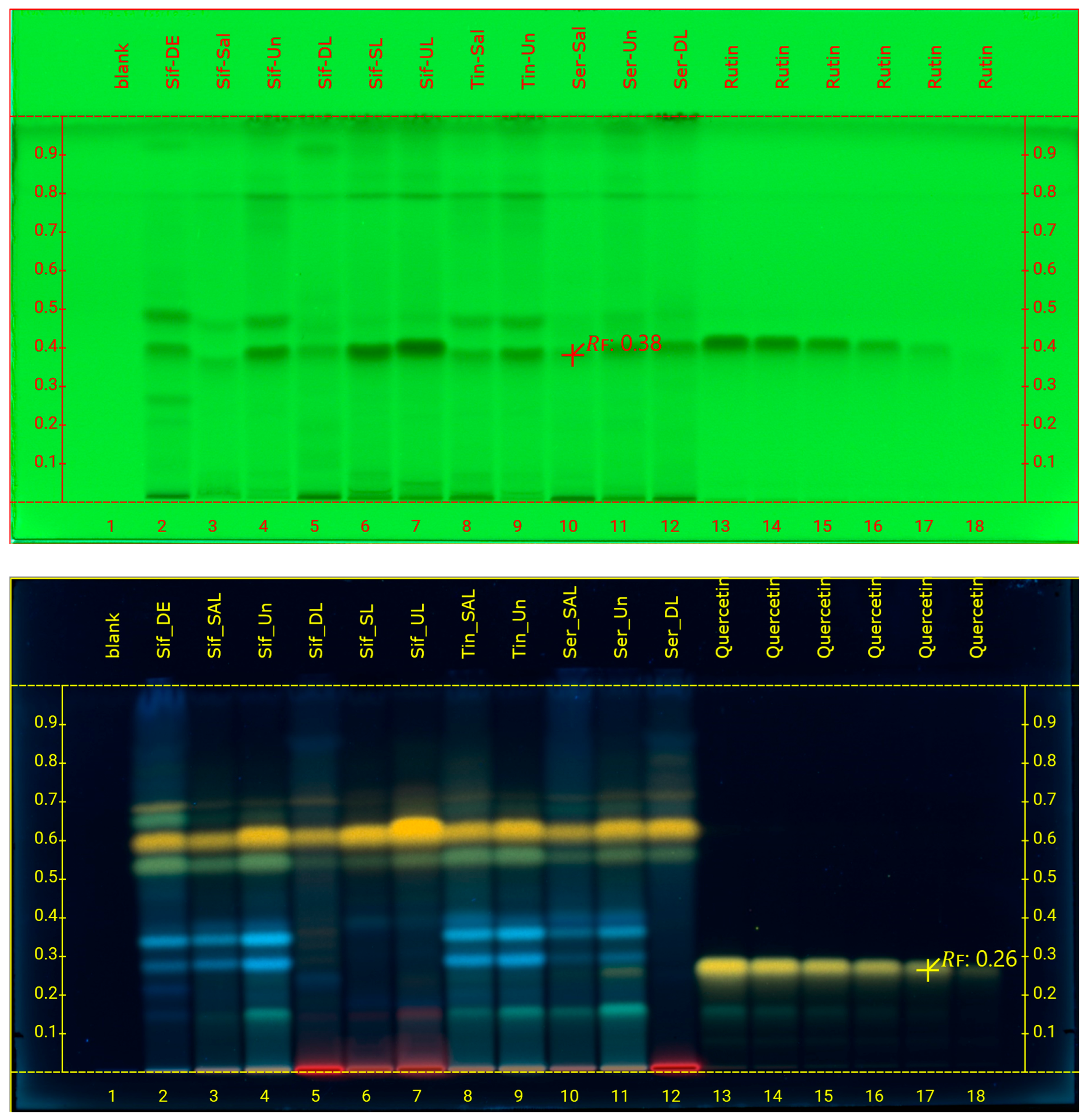
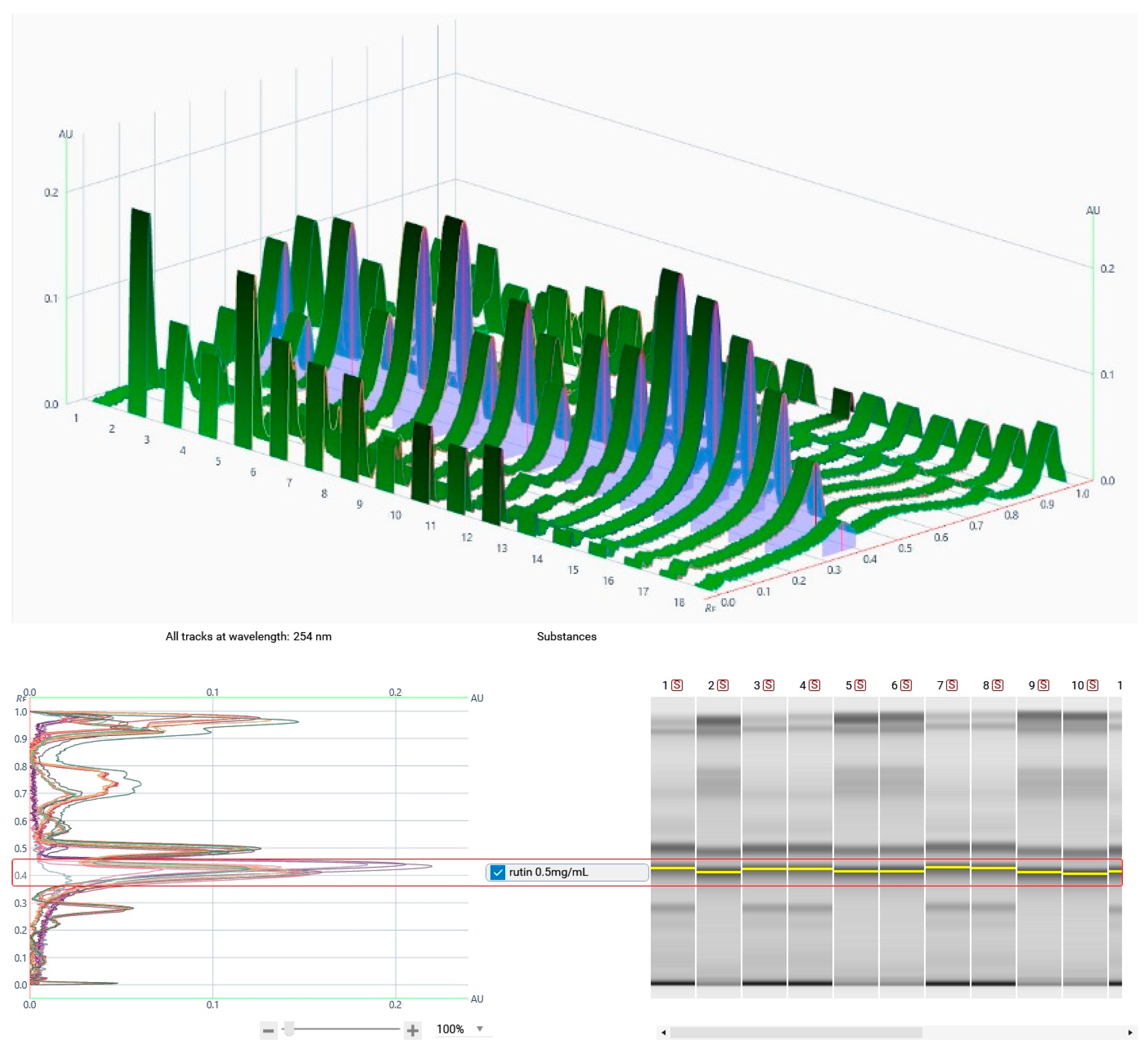

| Sample | Code | Weight Of Methanolic Extracts (g) | %Yield |
|---|---|---|---|
| Sifnos dry buds | Sif-DE | 2.69 | 53.8 |
| Sifnos salted buds | Sif-Sal | 2.06 | 41.2 |
| Sifnos desalted buds | Sif-Un | 1.00 | 20.0 |
| Sifnos dry leaves | Sif-DL | 0.90 | 18.0 |
| Sifnos salted leaves | Sif-SL | 1.64 | 32.8 |
| Sifnos desalted leaves | Sif-UL | 0.60 | 12.0 |
| Serifos salted buds | Ser-Sal | 2.69 | 53.8 |
| Serifos desalted buds | Ser-Un | 1.37 | 27.4 |
| Serifos dry leaves | Ser-DL | 0.96 | 19.2 |
| Tinos salted buds | Tin-Sal | 2.96 | 59.2 |
| Tinos desalted buds | Tin-Un | 1.33 | 26.6 |
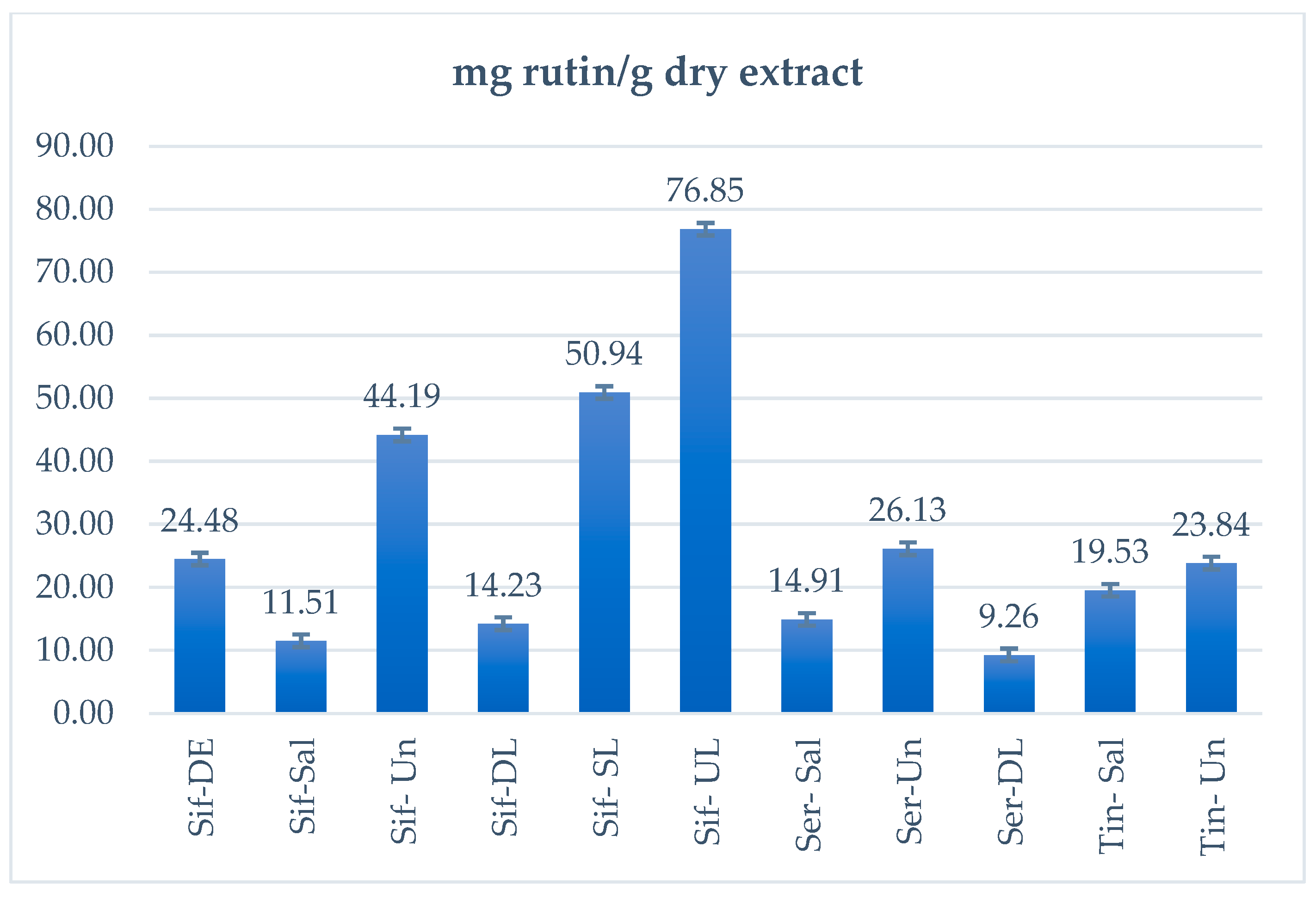
| Sample | μg Rutin/Band | SD | mg Rutin/g Dry Extract |
|---|---|---|---|
| Sif-DE | 2.45 | 0.13 | 24.48 |
| Sif-Sal | 1.15 | 0.04 | 11.51 |
| Sif-Un | 4.42 | 0.23 | 44.19 |
| Sif-DL | 1.42 | 0.06 | 14.23 |
| Sif-SL | 5.09 | 0.26 | 50.94 |
| Sif-UL | 7.69 | 0.29 | 76.85 |
| Ser-Sal | 1.49 | 0.06 | 14.91 |
| Ser-Un | 2.61 | 0.14 | 26.13 |
| Ser-DL | 0.93 | 0.02 | 9.26 |
| Tin-Sal | 1.95 | 0.10 | 19.53 |
| Tin-Un | 2.38 | 0.13 | 23.84 |
| Sample | μg Quercetin/Band | SD | mg Quercetin/g Dry Extract |
| Ser-Un | 0.288 | 0.01 | 2.88 |
| Chemical Compound | Sample | Added Amount per Band (μg) | HPTLC Recovery (% ± RSD) |
|---|---|---|---|
| rutin | Sif-DE | 1.25 | 82.16 ± 0.10 |
| 0.75 | 82.67 ± 0.30 | ||
| 0.25 | 99.20 ± 0.48 | ||
| Tin-Un | 1.25 | 90.24 ± 0.01 | |
| 0.75 | 92.60 ± 0.11 | ||
| 0.25 | 97.60 ± 2.43 | ||
| quercetin | Ser-Un | 1.95 | 87.6 ± 1.18 |
| 0.45 | 109.38 ± 0.52 | ||
| 0.3 | 110.58 ± 2.27 |
| Sample | TPC mg GAE/g Extract ± Standard Deviation | |
|---|---|---|
| Buds | Sif-DE | 58.3 ± 2.0 |
| Sif-Sal | 18.6 ± 0.6 | |
| Sif-Un | 39.0 ± 2.8 | |
| Ser-Sal | 37.7 ± 2.1 | |
| Ser-Un | 48.8 ± 1.9 | |
| Tin-Sal | 11.7 ± 0.0 | |
| Tin-Un | 32.1 ± 0.9 | |
| Leaves | Sif-DL | 62.4 ± 1.1 |
| Sif-SL | 12.5 ± 2.3 | |
| Sif-UL | 27.4 ± 0.7 | |
| Ser-DL | 50.9 ± 1.4 |
| Sample | % Inhibition | SD | % Inhibition | SD | % Inhibition | SD | |
|---|---|---|---|---|---|---|---|
| 200 μg/mL | 100 μg/mL | 50 μg/mL | |||||
| Buds | Sif-DE | 31.4 | 1.1 | 18.5 | 0.2 | 10.2 | 0.9 |
| Sif-Sal | 13.4 | 0.6 | 7.1 | 0.9 | 4.4 | 0.7 | |
| Sif-Un | 18.3 | 0.6 | 9.4 | 0.6 | 5.7 | 0.8 | |
| Ser-Sal | 16.7 | 0.6 | 10.4 | 0.6 | 5.7 | 0.5 | |
| Ser-Un | 26.0 | 0.5 | 15.7 | 2.5 | 8.5 | 0.6 | |
| Tin-Sal | 8.0 | 0.6 | 3.5 | 0.6 | 1.7 | 0.6 | |
| Tin-Un | 20.0 | 0.9 | 11.2 | 0.4 | 5.4 | 0.7 | |
| Leaves | Sif-DL | 35.2 | 0.3 | 20.7 | 0.9 | 12.2 | 0.8 |
| Sif-SL | 12.0 | 0.8 | 8.3 | 0.7 | 3.3 | 0.3 | |
| Sif-UL | 16.4 | 0.4 | 7.9 | 0.4 | 3.7 | 0.6 | |
| Ser-DL | 30.1 | 1.0 | 17.9 | 0.6 | 9.8 | 0.2 | |
| Sample | S. aureus | S. epidermidis | P. aeruginosa | K. pneumoniae | E. cloacae | E. coli | S. mutans | S. viridans | C. albicans | C. tropicalis | C. glabrata |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sif-DE | 0.32 ± 0.04 | 0.27 ± 0.03 | 0.92 ± 0.07 | 0.98 ± 0.13 | 1.12 ± 0.01 | 0.92 ± 0.13 | 0.36 ± 0.02 | 0.30 ± 0.01 | 0.88 ± 0.06 | 0.86 ± 0.03 | 0.39 ± 0.02 |
| Sif-Sal | 0.67 ± 0.01 | 0.65 ± 0.07 | 1.32 ± 0.05 | 1.28 ± 0.12 | 1.44 ± 0.04 | 1.38 ± 0.09 | 0.68 ± 0.02 | 0.64 ± 0.02 | 1.00 ± 0.13 | 0.89 ± 0.09 | 0.71 ± 0.04 |
| Sif-Un | 0.49 ± 0.06 | 0.47 ± 0.07 | 1.00 ± 0.09 | 1.16 ± 0.09 | 1.32 ± 0.08 | 1.20 ± 0.07 | 0.52 ± 0.02 | 0.48 ± 0.02 | 0.99 ± 0.10 | 0.76 ± 0.04 | 0.56 ± 0.02 |
| Sif-DL | 0.28 ± 0.07 | 0.21 ± 0.03 | 0.88 ± 0.08 | 0.95 ± 0.1 | 1.00 ± 0.08 | 0.86 ± 0.10 | 0.32 ± 0.04 | 0.25 ± 0.01 | 0.88 ± 0,07 | 0.48 ± 0.01 | 0.38 ± 0.03 |
| Sif-SL | 0.88 ± 0.03 | 0.83 ± 0.05 | 1.45 ± 0.06 | 1.57 ± 0.09 | 1.52 ± 0.04 | 1.44 ± 0.10 | 0.89 ± 0.06 | 0.82 ± 0.02 | 1.37 ± 0.10 | 1.07 ± 0.08 | 0.91 ± 0.05 |
| Sif-UL | 0.59 ± 0.10 | 0.56 ± 0.06 | 1.14 ± 0.08 | 1.25 ± 0.08 | 1.39 ± 0.04 | 1.33 ± 0.11 | 0.64 ± 0.04 | 0.61 ± 0.02 | 0.96 ± 0.10 | 0.79 ± 0.05 | 0.65 ± 0.02 |
| Ser-Sal | 0.52 ± 0.09 | 0.50 ± 0.07 | 1.15 ± 0.07 | 1.18 ± 0.04 | 1.35 ± 0.04 | 1.26 ± 0.05 | 0.56 ± 0.03 | 0.51 ± 0.02 | 0.94 ± 0.09 | 0.84 ± 0.05 | 0.58 ± 0.01 |
| Ser-Un | 0.39 ± 0.03 | 0.37 ± 0.03 | 0.96 ± 0.13 | 1.12 ± 0.04 | 1.15 ± 0.08 | 0.99 ± 0.05 | 0.43 ± 0.03 | 0.39 ± 0.01 | 0.92 ± 0.04 | 0.69 ± 0.01 | 0.47 ± 0.01 |
| Ser-DL | 0.36 ± 0.04 | 0.38 ± 0.01 | 0.98 ± 0.09 | 1.07 ± 0.05 | 1.10 ± 0.08 | 0.94 ± 0.06 | 0.40 ± 0.01 | 0.34 ± 0.04 | 0.85 ± 0.06 | 0.57 ± 0.02 | 0.43 ± 0.02 |
| Tin-Sal | 0.97 ± 0.13 | 0.93 ± 0.12 | 1.67 ± 0.08 | 1.59 ± 0.07 | 1.58 ± 0.11 | 1.49 ± 0.04 | 0.96 ± 0.02 | 0.90 ± 0.09 | 1.25 ± 0.07 | 1.12 ± 0.05 | 0.98 ± 0.05 |
| Tin-Un | 0.51 ± 0.06 | 0.49 ± 0.06 | 1.12 ± 0.08 | 1.19 ± 0.03 | 1.37 ± 0.13 | 1.25 ± 0.09 | 0.54 ± 0.01 | 0.50 ± 0.04 | 0.89 ± 0.10 | 0.65 ± 0.01 | 0.38 ± 0.01 |
| netilmicin | 3.75 × 10−3 ± 0.02 | 3.90 × 10−3 ± 0.03 | 7.25 × 10−3 ± 0.01 | 7.98 × 10−3 ± 0.02 | 7 × 10−3 ± 0.01 | 345 × 10−3 ± 0.03 | - | - | - | - | - |
| sanguinarine | 0.016 ± 0.03 | 0.012 ± 0.01 | - | - | - | ||||||
| 5-flucytosine | - | - | - | - | - | - | - | - | 0.15 × 10−3 ± 0.03 | 0.94 × 10−3 ± 0.01 | 9.82 × 10−3 ± 0.02 |
| amphotericin B | - | - | - | - | - | - | - | - | 1.23 × 10−3 ± 0.02 | 0.43 × 10−3 ± 0.01 | 0.46 × 10−3 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotiadou, E.; Cheilari, A.; Graikou, K.; Chinou, I. Comparative Study by HPTLC of Selected Capparis spinosa Samples (Buds and Leaves) from the Cycladic Islands in Greece. Foods 2025, 14, 1827. https://doi.org/10.3390/foods14101827
Fotiadou E, Cheilari A, Graikou K, Chinou I. Comparative Study by HPTLC of Selected Capparis spinosa Samples (Buds and Leaves) from the Cycladic Islands in Greece. Foods. 2025; 14(10):1827. https://doi.org/10.3390/foods14101827
Chicago/Turabian StyleFotiadou, Evgenia, Antigoni Cheilari, Konstantia Graikou, and Ioanna Chinou. 2025. "Comparative Study by HPTLC of Selected Capparis spinosa Samples (Buds and Leaves) from the Cycladic Islands in Greece" Foods 14, no. 10: 1827. https://doi.org/10.3390/foods14101827
APA StyleFotiadou, E., Cheilari, A., Graikou, K., & Chinou, I. (2025). Comparative Study by HPTLC of Selected Capparis spinosa Samples (Buds and Leaves) from the Cycladic Islands in Greece. Foods, 14(10), 1827. https://doi.org/10.3390/foods14101827









