Fortification of Bread with Carob Extract: A Comprehensive Study on Dough Behavior and Product Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Ingredients
- Wheat flour was supplied by the local mill (Kikindski mlin AD, Kikinda, Serbia) and had following chemical composition: moisture 11.8%, ash 0.55% d.w., protein 12.7% d.w., and wet gluten content 27.0%;
- Carob pulp flour originating from Croatia was purchased from local distributer Vega ADM (Senta, Serbia);
- Bakers’ yeast (Lesaffre, Budapest, Hungary);
- Salt (So product, Stara Pazova, Serbia).
2.2. Preparation of Carob Extract
2.3. Characterization of Carob Extracts
2.3.1. Chemical Profile
2.3.2. Antioxidant Activity
2.4. Rheological Properties of Dough
2.4.1. Empirical Measurements
2.4.2. Fundamental Measurements
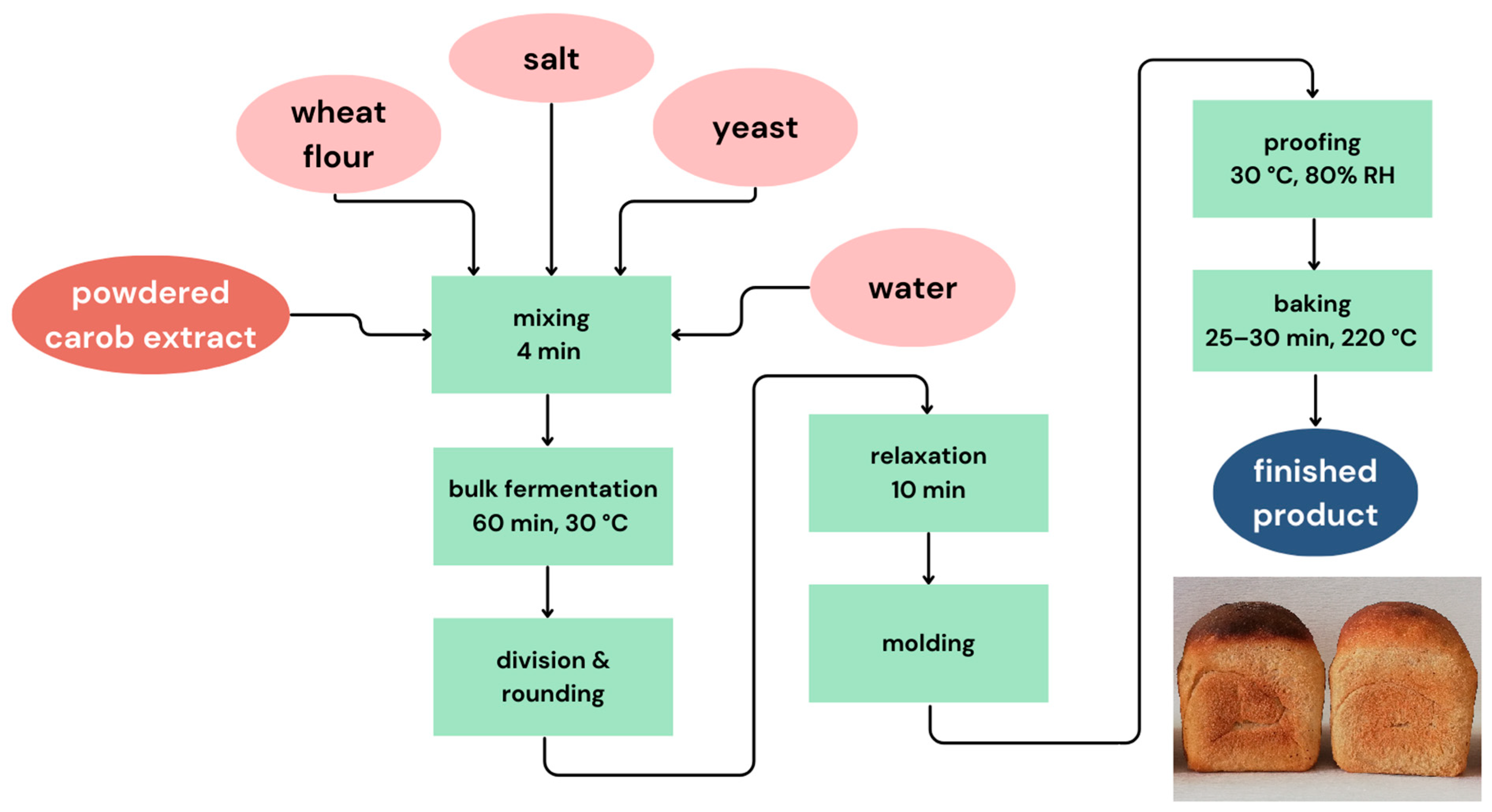
2.5. Laboratory Baking Test
2.6. Technological Quality of Bread
2.6.1. Specific Volume
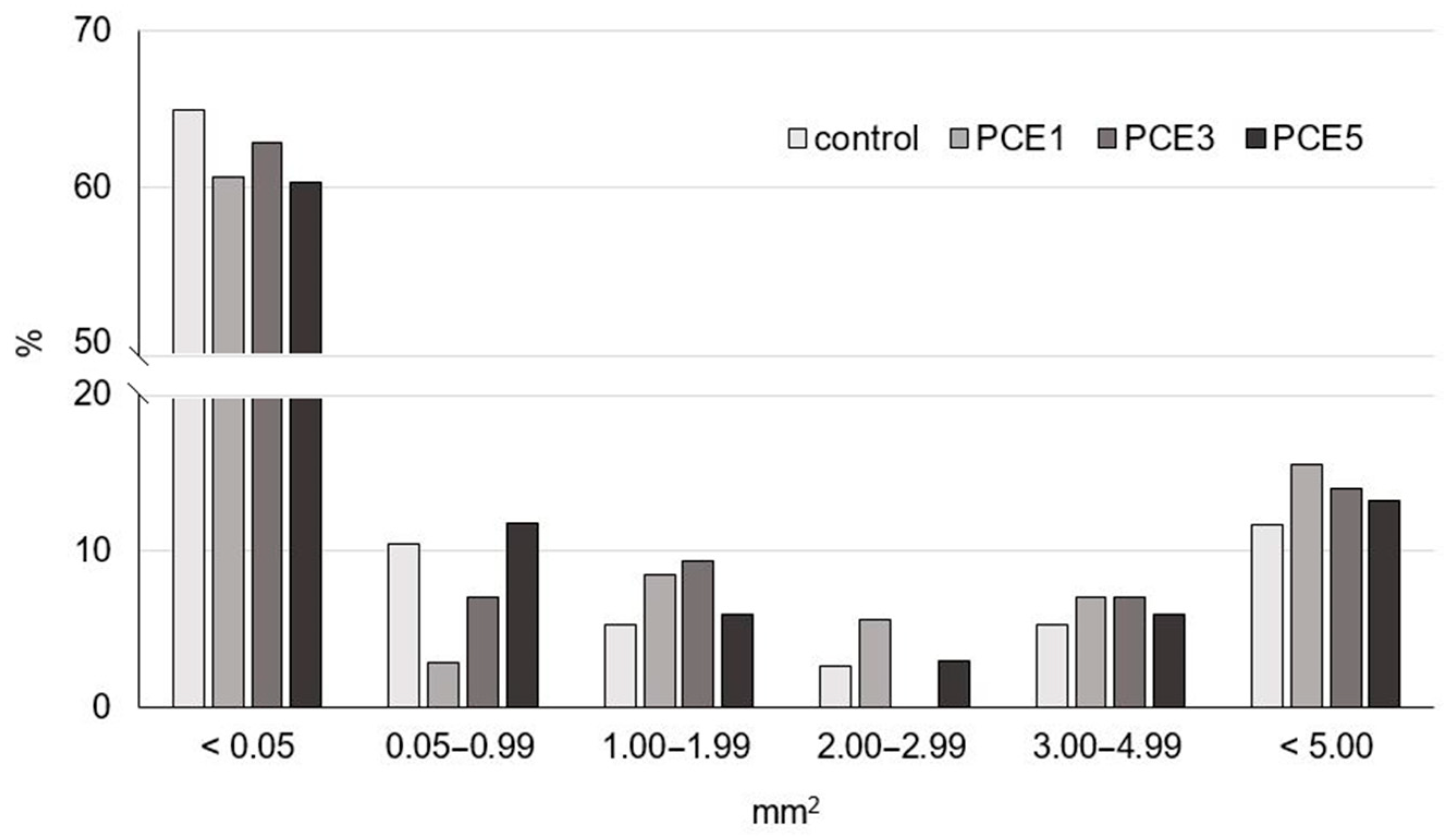
2.6.2. Crumb Structure by Digital Image Analysis
2.6.3. Texture Profile Analysis
2.6.4. Bread Color
2.6.5. Sensory Properties
2.7. Chemical Profile of Bread
2.8. Statistical Analysis
3. Results
3.1. Characterization of CE and PCE
3.2. Rheological Properties of Dough with PCE
3.2.1. Empirical Rheological Parameters
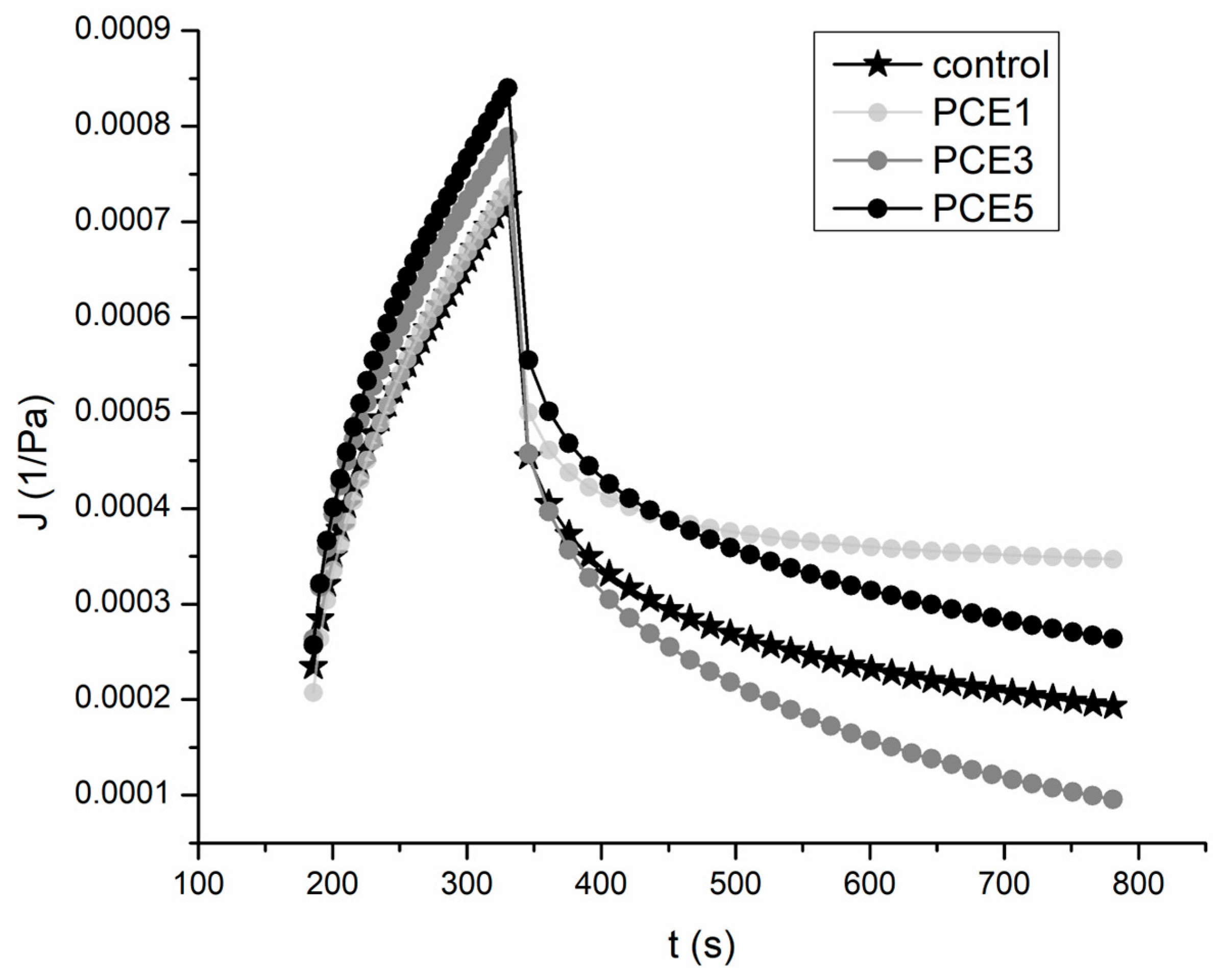
3.2.2. Fundamental Rheological Parameters
3.3. Bread Quality with the Addition of PCE
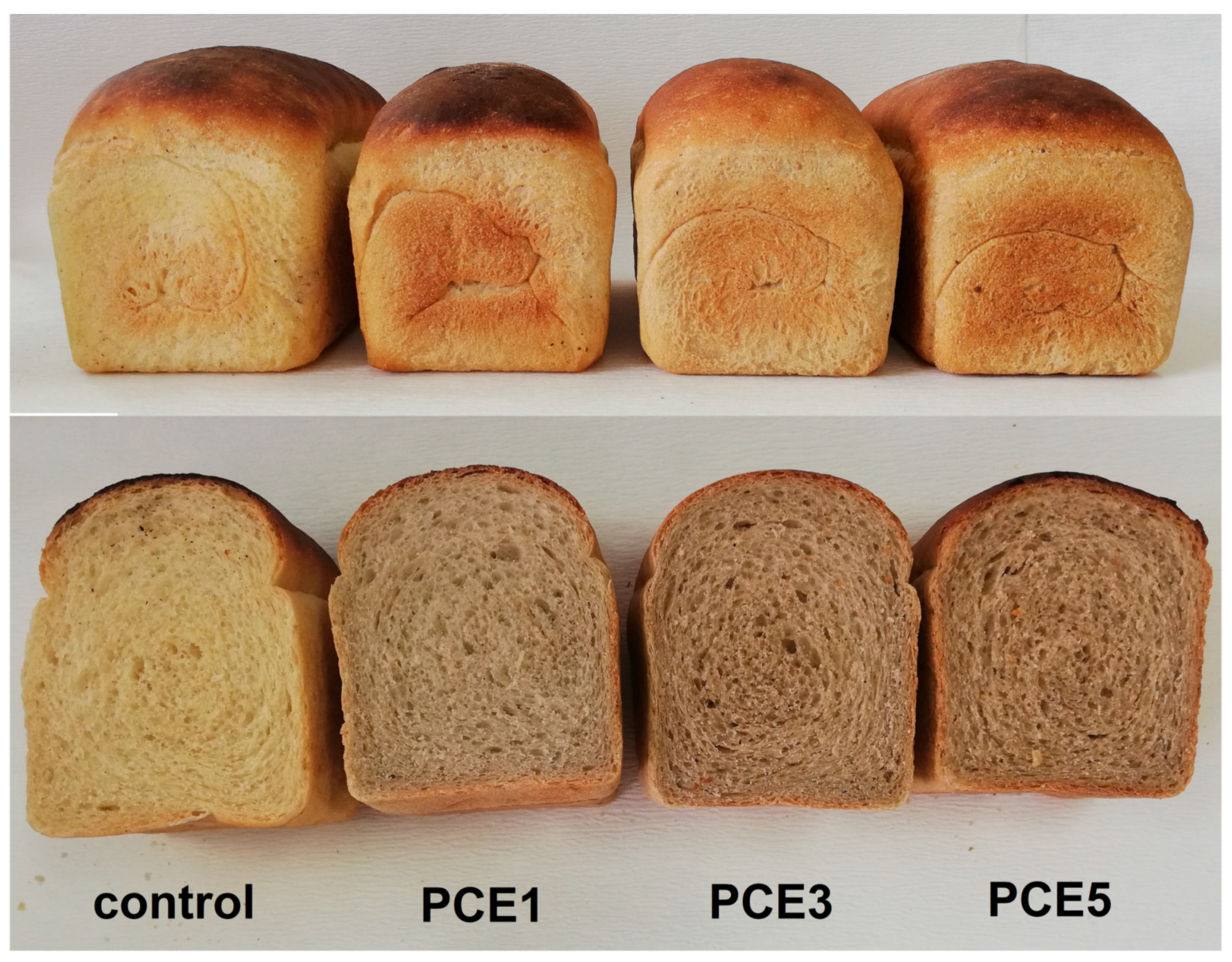
3.3.1. Physical and Textural Characteristics
3.3.2. Sensory Characteristics
3.3.3. Nutritional and Chemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miletić, I.; Šobajić, S.; Đorđević, B. Functional Foods and Their Role in the Improvement of Health Status. J. Med. Biochem. 2008, 27, 367–370. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Excipient Foods: Designing Food Matrices That Improve the Oral Bioavailability of Pharmaceuticals and Nutraceuticals. Food Funct. 2014, 5, 1320–1333. [Google Scholar] [CrossRef] [PubMed]
- Kapsak, W.R.; Rahavi, E.B.; Childs, N.M.; White, C. Functional Foods: Consumer Attitudes, Perceptions, and Behaviors in a Growing Market. J. Am. Diet. Assoc. 2011, 111, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Rader, J.I.; Weaver, C.M.; Angyal, G. Total Folate in Enriched Cereal-Grain Products in the United States Following Fortification. Food Chem. 2000, 70, 275–289. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; Gallagher, E. Recent Advances in the Development of High-Fibre Baked Products. Trends Food Sci. Technol. 2012, 28, 4–14. [Google Scholar] [CrossRef]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, C.S. The Effects of Dietary Fibre Addition on the Quality of Common Cereal Products. J. Cereal Sci. 2013, 58, 216–227. [Google Scholar] [CrossRef]
- Fendri, L.B.; Chaari, F.; Maaloul, M.; Kallel, F.; Abdelkafi, L.; Chaabouni, S.E.; Ghribi-Aydi, D. Wheat Bread Enrichment by Pea and Broad Bean Pods Fibers: Effect on Dough Rheology and Bread Quality. LWT-Food Sci. Technol. 2016, 73, 584–591. [Google Scholar] [CrossRef]
- Rosell, C.M. Bread Fortification. In Bread and Its Fortification. Nutrition and Health Benefits; Rosell, C.M., Bajerska, J., El Sheikha, A.F., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 163–186. [Google Scholar]
- Rodríguez-Solana, R.; Romano, A.; Moreno-Rojas, J.M. Carob Pulp: A Nutritional and Functional By-Product Worldwide Spread in the Formulation of Different Food Products and Beverages. A Review. Processes 2021, 9, 1146. [Google Scholar] [CrossRef]
- Brassesco, M.E.; Brandão, T.R.S.; Silva, C.L.M.; Pintado, M. Carob Bean (Ceratonia siliqua L.): A New Perspective for Functional Food. Trends Food Sci. Technol. 2021, 114, 310–322. [Google Scholar] [CrossRef]
- Esposito, L.; Casolani, N.; Ruggeri, M.; Spizzirri, U.G.; Aiello, F.; Chiodo, E.; Martuscelli, M.; Restuccia, D.; Mastrocola, D. Sensory Evaluation and Consumers’ Acceptance of a Low Glycemic and Gluten-Free Carob-Based Bakery Product. Foods 2024, 13, 2815. [Google Scholar] [CrossRef]
- Buzzanca, C.; D’Amico, A.; Pistorio, E.; Di Stefano, V.; Melilli, M.G. Carob-Based Functional Beverages: Nutritional Value and Health Properties. Beverages 2025, 11, 1. [Google Scholar] [CrossRef]
- Salinas, M.V.; Carbas, B.; Brites, C.; Puppo, M.C. Influence of Different Carob Flours (Ceratonia siliqua L.) on Wheat Dough Performance and Bread Quality. Food Bioprocess Technol. 2015, 8, 1561–1570. [Google Scholar] [CrossRef]
- Šoronja-Simović, D.; Smole-Možina, S.; Raspor, P.; Maravić, N.; Zahorec, J.; Luskar, L.; Šereš, Z. Carob Flour and Sugar Beet Fiber as Functional Additives in Bread. Acta Period. Technol. 2016, 47, 83–93. [Google Scholar] [CrossRef]
- Rico, D.; Alonso De Linaje, A.; Herrero, A.; Asensio-Vegas, C.; Miranda, J.; Martínez-Villaluenga, C.; De Luis, D.A.; Martin-Diana, A.B. Carob By-Products and Seaweeds for the Development of Functional Bread. J. Food Process. Preserv. 2018, 42, e13700. [Google Scholar] [CrossRef]
- Issaoui, M.; Flamini, G.; Delgado, A. Sustainability Opportunities for Mediterranean Food Products through New Formulations Based on Carob Flour (Ceratonia siliqua L.). Sustainability 2021, 13, 8026. [Google Scholar] [CrossRef]
- Zahorec, J.; Šoronja-Simović, D.; Petrović, J.; Šereš, Z.; Pavlić, B.; Božović, D.; Perović, L.; Martić, N.; Bulut, S.; Kocić-Tanackov, S. Development of Functional Bread: Exploring the Nutritional, Bioactive, and Microbial Potential of Carob (Ceratonia siliqua L.) Pulp Powder. Processes 2024, 12, 2882. [Google Scholar] [CrossRef]
- Miś, A.; Krekora, M.; Niewiadomski, Z.; Dziki, D.; Nawrocka, A. Water Redistribution between Model Bread Dough Components during Mixing. J. Cereal Sci. 2020, 95, 103035. [Google Scholar] [CrossRef]
- De Steur, H.; Gellynck, X.; Storozhenko, S.; Liqun, G.; Lambert, W.; Van Der Straeten, D.; Viaene, J. Willingness-to-Accept and Purchase Genetically Modified Rice with High Folate Content in Shanxi Province, China. Appetite 2010, 54, 118–125. [Google Scholar] [CrossRef]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Bonikowski, R.; Balawejder, M. Optimization of Extraction Process of Antioxidant Compounds from Yellow Onion Skin and Their Use in Functional Bread Production. LWT 2020, 117, 108614. [Google Scholar] [CrossRef]
- Kessler, J.C.; Vieira, V.; Martins, I.M.; Manrique, Y.A.; Ferreira, P.; Calhelha, R.C.; Afonso, A.; Barros, L.; Rodrigues, A.E.; Dias, M.M. Chemical and Organoleptic Properties of Bread Enriched with Rosmarinus officinalis L.: The Potential of Natural Extracts Obtained through Green Extraction Methodologies as Food Ingredients. Food Chem. 2022, 384, 132514. [Google Scholar] [CrossRef]
- Đurović, S.; Vujanović, M.; Radojković, M.; Filipović, J.; Filipović, V.; Gašić, U.; Tešić, Ž.; Mašković, P.; Zeković, Z. The Functional Food Production: Application of Stinging Nettle Leaves and Its Extraction in the Baking of a Bread. Food Chem. 2020, 312, 126091. [Google Scholar] [CrossRef] [PubMed]
- Zahorec, J.; Šoronja-Simović, D.; Petrović, J.; Šereš, Z.; Pavlić, B.; Sterniša, M.; Smole Možina, S.; Ačkar, Đ.; Šubarić, D.; Jozinović, A. The Effect of Carob Extract on Antioxidant, Antimicrobial and Sensory Properties of Bread. Appl. Sci. 2024, 14, 3603. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Celano, R.; Campone, L.; Rastrelli, L. Critical Analysis of Green Extraction Techniques Used for Botanicals: Trends, Priorities, and Optimization Strategies—A Review. TrAC Trends Anal. Chem. 2024, 173, 117627. [Google Scholar] [CrossRef]
- Čepo, D.V.; Jug, M.; Grdić Rajković, M.; Jablan, J. Formulation of a Nutraceutical Derived from Carob: β-Cyclodextrin Encapsulation of Antioxidants from Carob Pod. J. Food Nutr. Res. 2017, 56, 48–60. [Google Scholar]
- Ydjedd, S.; Bouriche, S.; López-Nicolás, R.; Sánchez-Moya, T.; Frontela-Saseta, C.; Ros-Berruezo, G.; Rezgui, F.; Louaileche, H.; Kati, D.-E. Effect of In Vitro Gastrointestinal Digestion on Encapsulated and Nonencapsulated Phenolic Compounds of Carob (Ceratonia siliqua L.) Pulp Extracts and Their Antioxidant Capacity. J. Agric. Food Chem. 2017, 65, 827–835. [Google Scholar] [CrossRef]
- Wang, J.; Martínez-Hernández, A.; de Lamo-Castellví, S.; Romero, M.-P.; Kaade, W.; Ferrando, M.; Güell, C. Low-Energy Membrane-Based Processes to Concentrate and Encapsulate Polyphenols from Carob Pulp. J. Food Eng. 2020, 281, 109996. [Google Scholar] [CrossRef]
- Zahorec, J.; Šoronja-Simović, D.; Kocić-Tanackov, S.; Bulut, S.; Martić, N.; Bijelić, K.; Božović, D.; Pavlić, B. Carob Pulp Flour Extract Obtained by a Microwave-Assisted Extraction Technique: A Prospective Antioxidant and Antimicrobial Agent. Separations 2023, 10, 465. [Google Scholar] [CrossRef]
- Martić, N.; Zahorec, J.; Stilinović, N.; Andrejić-Višnjić, B.; Pavlić, B.; Kladar, N.; Šoronja-Simović, D.; Šereš, Z.; Vujčić, M.; Horvat, O.; et al. Hepatoprotective Effect of Carob Pulp Flour (Ceratonia siliqua L.) Extract Obtained by Optimized Microwave-Assisted Extraction. Pharmaceutics 2022, 14, 657. [Google Scholar] [CrossRef]
- AACC International. AACCI Method 54-21.02 Physical Dough Tests Methods: Rheological Behavior of Flour by Farinograph: Constant Flour Weight Procedure. In Approved Methods of the American Association of Cereal Chemists, 11th ed.; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- AACC International. AACCI Method 54-10.01 Physical Dough Tests Methods: Extensigraph Method, General. In Approved Methods of the American Association of Cereal Chemists, 11th ed.; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Selaković, A.; Nikolić, I.; Dokić, L.; Šoronja-Simović, D.; Šimurina, O.; Zahorec, J.; Šereš, Z. Enhancing Rheological Performance of Laminated Dough with Whole Wheat Flour by Vital Gluten Addition. LWT–Food Sci. Technol. 2021, 138, 110604. [Google Scholar] [CrossRef]
- AACC International. AACCI Method 10-05 Baking Quality Methods: Guidelines for Measurement of Volume by Rapeseed Displacement. In Approved Methods of the American Association of Cereal Chemists, 11th ed.; AACC International: St. Paul, MN, USA, 2008. [Google Scholar]
- Chiavaro, E.; Vittadini, E.G.P.; Musci, M.; Bianchi, F.; Curti, E. Shelf-Life Stability of Artisanally and Industrially Produced Durum Wheat Sourdough Bread (“Altamura Bread”). LWT–Food Sci. Technol. 2008, 41, 58–70. [Google Scholar] [CrossRef]
- CIE. International Commission on Illumination, Colorimetry: Official Recommendation of the International Commission on Illumination; Publication CIE No. (E-1.31); Bureau Central de la CIE: Paris, France, 1976. [Google Scholar]
- Kaluđerski, G.; Filipović, N. Metode Ispitivanja Kvaliteta Žita, Brašna i Gotovih Proizvoda; Tehnološki Fakultet: Novi Sad, Serbia, 1998. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis of the Association of Official Analytical Chemists International, 17th ed.; Association of Official Analytical Communities: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Continuous and Pulsed Ultrasound-Assisted Extraction of Carob’s Antioxidants: Processing Parameters Optimization and Identification of Polyphenolic Composition. Ultrason. Sonochem. 2021, 76, 105630. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Mellinas, C.; Garrigós, M.C.; Balart, R.; Torres-Giner, S. Optimization of Microwave-Assisted Extraction of Phenolic Compounds with Antioxidant Activity from Carob Pods. Food Anal. Methods 2019, 12, 2480–2490. [Google Scholar] [CrossRef]
- Goulas, V.; Georgiou, E. Utilization of Carob Fruit as Sources of Phenolic Compounds with Antioxidant Potential: Extraction Optimization and Application in Food Models. Foods 2020, 9, 20. [Google Scholar] [CrossRef]
- Buchner, N.; Krumbein, A.; Rohn, S.; Kroh, L.W. Effect of Thermal Processing on the Flavonols Rutin and Quercetin. Rapid Commun. Mass Spectrom. 2006, 20, 3229–3235. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of Heat Processing on Thermal Stability and Antioxidant Activity of Six Flavonoids. J. Food Process. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Miyazaki, M.; Maeda, T.; Morita, N. Effect of Various Dextrin Substitutions for Wheat Flour on Dough Properties and Bread Qualities. Food Res. Int. 2004, 37, 59–65. [Google Scholar] [CrossRef]
- Pycia, K.; Jaworska, G.; Telega, J.; Sudoł, I.; Kuźniar, P. Effect of Adding Potato Maltodextrins on Baking Properties of Triticale Flour and Quality of Bread. LWT–Food Sci. Technol. 2018, 96, 199–204. [Google Scholar] [CrossRef]
- Sivam, S.A.; Sun-Waterhouse, D.; Quek, S.; Perera, O.C. Properties of Bread Dough with Added Fiber Polysaccharides and Phenolic Antioxidants: A Review. J. Food Sci. 2010, 75, R163–R174. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Quek, S.Y.; Perera, C.O. Physicochemical Properties of Bread Dough and Finished Bread with Added Pectin Fiber and Phenolic Antioxidants. J. Food Sci. 2011, 76, C97–C107. [Google Scholar] [CrossRef]
- AbuDujayn, A.A.; Mohamed, A.A.; Alamri, M.S.; Hussain, S.; Ibraheem, M.A.; Qasem, A.A.A.; Shamlan, G.; Alqahtani, N.K. Relationship between Dough Properties and Baking Performance of Panned Bread: The Function of Maltodextrins and Natural Gums. Molecules 2022, 28, 1. [Google Scholar] [CrossRef] [PubMed]
- Han, H.M.; Koh, B.K. Effect of Phenolic Acids on the Rheological Properties and Proteins of Hard Wheat Flour Dough and Bread. J. Sci. Food Agric. 2011, 91, 2495–2499. [Google Scholar] [CrossRef] [PubMed]
- Dapčević Hadnađev, T.; Pojić, M.; Hadnađev, M.; Torbica, A. The Role of Empirical Rheology on Flour Quality Control. In Wide Spectra of Quality Control; Akyar, I., Ed.; InTech: Rijeka, Croatia, 2011; pp. 335–360. [Google Scholar]
- Dobraszczyk, B.J.; Morgenstern, M.P. Rheology and the Breadmaking Process. J. Cereal Sci. 2003, 38, 229–245. [Google Scholar] [CrossRef]
- Lopes-Da-Silva, J.A.; Santos, D.M.; Freitas, A.; Brites, C.; Gil, A.M. Rheological and Nuclear Magnetic Resonance (NMR) Study of the Hydration and Heating of Undeveloped Wheat Doughs. J. Agric. Food Chem. 2007, 55, 5636–5644. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N. Relationship of Polymeric Proteins and Empirical Dough Rheology with Dynamic Rheology of Dough and Gluten from Different Wheat Varieties. Food Hydrocoll. 2013, 33, 342–348. [Google Scholar] [CrossRef]
- Bonnand-Ducasse, M.; Della Valle, G.; Lefebvre, J.; Saulnier, L. Effect of Wheat Dietary Fibres on Bread Dough Development and Rheological Properties. J. Cereal Sci. 2010, 52, 200–206. [Google Scholar] [CrossRef]
- Leroy, V.; Pitura, K.M.; Scanlon, M.G.; Page, J.H. The Complex Shear Modulus of Dough over a Wide Frequency Range. J. Non-Newton. Fluid Mech. 2010, 165, 475–478. [Google Scholar] [CrossRef]
- Hadnađev, T.R.D.; Dokić, L.; Hadnađev, M.; Pojić, M.; Torbica, A. Rheological and Breadmaking Properties of Wheat Flours Supplemented with Octenyl Succinic Anhydride–Modified Waxy Maize Starches. Food Bioprocess Technol. 2013, 7, 235–247. [Google Scholar] [CrossRef]
- Marchetti, L.; Cardós, M.; Campana, L.; Ferrero, C. Effect of Glutens of Different Quality on Dough Characteristics and Breadmaking Performance. LWT–Food Sci. Technol. 2012, 46, 224–231. [Google Scholar] [CrossRef]
- Bravo-Nuñez, A.; Sahagún, M.; Martínez, P.; Gómez, M. Incorporation of Gluten and Hydrolysed Gluten Proteins Has Different Effects on Dough Rheology and Cookie Characteristics. Int. J. Food Sci. Technol. 2018, 53, 1452–1458. [Google Scholar] [CrossRef]
- Ghannam, M.T.; Esmail, M.N. Rheological Properties of Carboxymethyl Cellulose. J. Appl. Polym. Sci. 1997, 64, 289–301. [Google Scholar] [CrossRef]
- Skendi, A.; Biliaderis, C.G.; Papageorgiou, M.; Izydorczyk, M.S. Effects of Two Barley β-Glucan Isolates on Wheat Flour Dough and Bread Properties. Food Chem. 2010, 119, 1159–1167. [Google Scholar] [CrossRef]
- Karolini-Skaradzińska, Z.; Czubaszek, A.; Stanisławska, M.; Szewców, P. Zmiany Właściwości Wypiekowych Mąki Pszennej Pod Wpływem Dodatku Maltodekstryn. Żywność Nauka Technol. Jakość 2012, 19, 108–121. [Google Scholar]
- Filipović, N.; Djurić, M.; Gyura, J. The Effect of the Type and Quantity of Sugar-Beet Fibers on Bread Characteristics. J. Food Eng. 2007, 78, 1047–1053. [Google Scholar] [CrossRef]
- Filipović, V.S.; Filipović, J.S.; Vučurović, V.M.; Radovanović, V.B.; Košutić, M.B.; Novković, N.Đ.; Vukelić, N.B. The Effect of Yeast Extract Addition on Bread Quality Parameters. J. Serb. Chem. Soc. 2020, 85, 737–750. [Google Scholar] [CrossRef]
- Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional Components of Carob Fruit: Linking the Chemical and Biological Space. Int. J. Mol. Sci. 2016, 17, 1875. [Google Scholar] [CrossRef]

| Parameter | CE 1 | PCE |
|---|---|---|
| Polyphenols content | ||
| TPC (mg GAE/100 g) | 1609.92 ± 56.15 a | 1545.78 ± 74.42 a |
| TFC (mg CE/100 g) | 271.92 ± 5.73 a | 267.72 ± 6.25 a |
| Antioxidant activity | ||
| DPPH (μM TE/g) | 99.02 ± 0.60 a | 92.34 ± 0.15 b |
| FRAP (μM Fe2+/g) | 50.45 ± 5.36 a | 46.16 ± 2.66 a |
| ABTS (μM TE/g) | 110.55 ± 0.97 a | 102.90 ± 0.03 b |
| Compound (µg/g) | ||
| trans-cinnamic acid | <LOD 2 | <LOD |
| caffeic acid | 259.3 ± 13.0 a | 240.2 ± 12.0 a |
| chlorogenic acid | 83.6 ± 4.2 a | 73.9 ± 3.7 a |
| p-coumaric acid | <LOD | <LOD |
| ferulic acid | <LOD | <LOD |
| gallic acid | 2371.9 ± 355.8 a | 2475.5 ± 371.3 a |
| rosmarinic acid | <LOD | <LOD |
| quercetin | <LOQ | <LOQ |
| quercitrin | 153.5 ± 7.7 a | 74.9 ± 3.7 b |
| rutin | 233.1 ± 18.6 a | 94.9 ± 7.6 b |
| Sample 1 | Water Absorption (%) | Development Time (min) | Stability (min) | Degree of Softening (FU) |
|---|---|---|---|---|
| control | 56.03 ± 0.08 c | 2.0 ± 0.2 a | 0.5 ± 0.3 a | 45.0 ± 0.5 d |
| PCE1 | 56.17 ± 0.11 c | 2.3 ± 0.3 a | 0.5 ± 0.2 a | 35.0 ± 1.5 b |
| PCE3 | 55.20 ± 0.16 b | 2.0 ± 0.1 a | 1.0 ± 0.1 b | 40.0 ± 1.0 c |
| PCE5 | 54.67 ± 0.12 a | 9.0 ± 0.2 b | 1.5 ± 0.3 c | 30.0 ± 1.0 a |
| Energy (cm2) | Resistance (EU) | Extensibility (mm) | R/E 2 | |
| control | 124.5 ± 0.84 a | 400 ± 10 a | 167.5 ± 3.0 b | 2.39 ± 0.17 a |
| PCE1 | 136.5 ± 1.06 b | 420 ± 15 ab | 165.5 ± 4.2 b | 2.54 ± 0.29 a |
| PCE3 | 140.0 ± 0.50 c | 450 ± 20 b | 160.0 ± 4.5 ab | 2.81 ± 0.42 a |
| PCE5 | 143.8 ± 0.97 d | 455 ± 10 b | 158.5 ± 2.3 a | 2.87 ± 0.21 a |
| Sample * | G0′ (kPa) | n | R2 |
|---|---|---|---|
| control | 9.17 ± 0.08 a | 0.234 ± 0.002 a | 0.9999 |
| PCE1 | 10.15 ± 0.04 b | 0.243 ± 0.001 b | 0.9999 |
| PCE3 | 10.02 ± 0.11 b | 0.266 ± 0.001 d | 1.000 |
| PCE5 | 13.90 ± 0.05 c | 0.255 ± 0.004 c | 1.000 |
| Parameter 2 | Control | PCE1 1 | PCE3 | PCE5 |
|---|---|---|---|---|
| Creep phase | ||||
| J0 (10−4·Pa−1) | 2.35 ± 0.05 b | 2.07 ± 0.13 a | 2.63 ± 0.02 d | 2.57 ± 0.01 c |
| η0 (105·Pas) | 4.55 ± 0.20 b | 4.49 ± 0.27 b | 4.19 ± 0.21 ab | 3.94 ± 0.16 a |
| J1 (10−4·Pa−1) | 2.40 ± 0.05 a | 2.43 ± 0.11 a | 2.60 ± 0.06 b | 2.77 ± 0.03 b |
| Jmax (10−4·Pa−1) | 7.27 ± 0.03 a | 7.36 ± 0.32 a | 7.89 ± 0.09 b | 8.40 ± 0.13 c |
| λ (s) | 143.8 a | 143.8 a | 143.8 a | 143.8 a |
| R2 | 0.9973 | 0.9974 | 0.9967 | 0.9967 |
| Recovery phase | ||||
| J0 (10−4·Pa−1) | 4.54 ± 0.14 a | 5.01 ± 0.08 b | 4.62 ± 0.11 a | 5.55 ± 0.02 c |
| η0 (105·Pas) | 6.37 ± 0.02 c | 2.25 ± 0.16 a | 2.46 ± 0.20 a | 2.96 ± 0.11 b |
| J1 (10−4·Pa−1) | 0.64 ± 0.01 a | 1.15 ± 0.12 c | 1.05 ± 0.01 c | 0.87 ± 0.10 b |
| λ (s) | 339.6 a | 339.8 a | 339.6 a | 339.6 a |
| R2 | 0.9019 | 0.9915 | 0.9872 | 0.9888 |
| Sample * | Specific Volume (cm3/g) | Pore Density (1/cm2) | Average Pore Surface Area (mm2) | Proportion of Pore Surface Area (%) | Proportion of Pore Walls Surface Area (%) |
|---|---|---|---|---|---|
| control | 3.04 ± 0.12 b | 19.3 | 2.46 | 47.31 | 52.69 |
| PCE1 | 2.87 ± 0.15 ab | 17.5 | 2.44 | 45.35 | 54.65 |
| PCE3 | 2.74 ± 0.05 a | 17.8 | 2.57 | 46.72 | 53.29 |
| PCE5 | 2.74 ± 0.01 a | 17.0 | 2.76 | 47.00 | 53.00 |
| Sample * | Hardness (kg) | Springiness | Resilience | Cohesiveness | Chewiness (kg) |
|---|---|---|---|---|---|
| control | 1.660 ± 0.465 a | 0.708 ± 0.091 a | 0.383 ± 0.027 a | 0.749 ± 0.062 a | 2.376 ± 0.319 b |
| PCE1 | 1.444 ± 0.529 a | 0.712 ± 0.093 a | 0.377 ± 0.029 a | 0.767 ± 0.078 a | 1.135 ± 0.374 a |
| PCE3 | 1.441 ± 0.243 a | 0.720 ± 0.089 a | 0.387 ± 0.028 a | 0.745 ± 0.058 a | 1.288 ± 0.691 a |
| PCE5 | 1.435 ± 0.280 a | 0.697 ± 0.094 a | 0.377 ± 0.021 a | 0.764 ± 0.059 a | 1.483 ± 0.235 a |
| Sample * | Crust Color | ||
|---|---|---|---|
| L* | a* | b* | |
| control | 70.42 ± 1.11 b | 3.26 ± 0.97 a | 28.96 ± 0.96 a |
| PCE1 | 64.78 ± 1.10 ab | 6.26 ± 0.85 bc | 30.02 ± 0.71 a |
| PCE3 | 59.86 ± 1.81 a | 8.28 ± 1.59 c | 29.57 ± 1.71 a |
| PCE5 | 57.71 ± 5.15 a | 8.73 ± 1.10 c | 29.98 ± 2.09 a |
| Crumb color | |||
| L* | a* | b* | |
| control | 65.82 ± 1.97 b | −1.29 ± 0.07 a | 16.06 ± 0.66 b |
| PCE1 | 61.83 ± 2.18 ab | 0.40 ± 0.12 b | 15.95 ± 0.70 b |
| PCE3 | 58.79 ± 1.81 a | 2.25 ± 0.21 c | 16.04 ± 1.01 b |
| PCE5 | 57.74 ± 1.23 a | 3.14 ± 0.06 d | 17.27 ± 0.45 b |
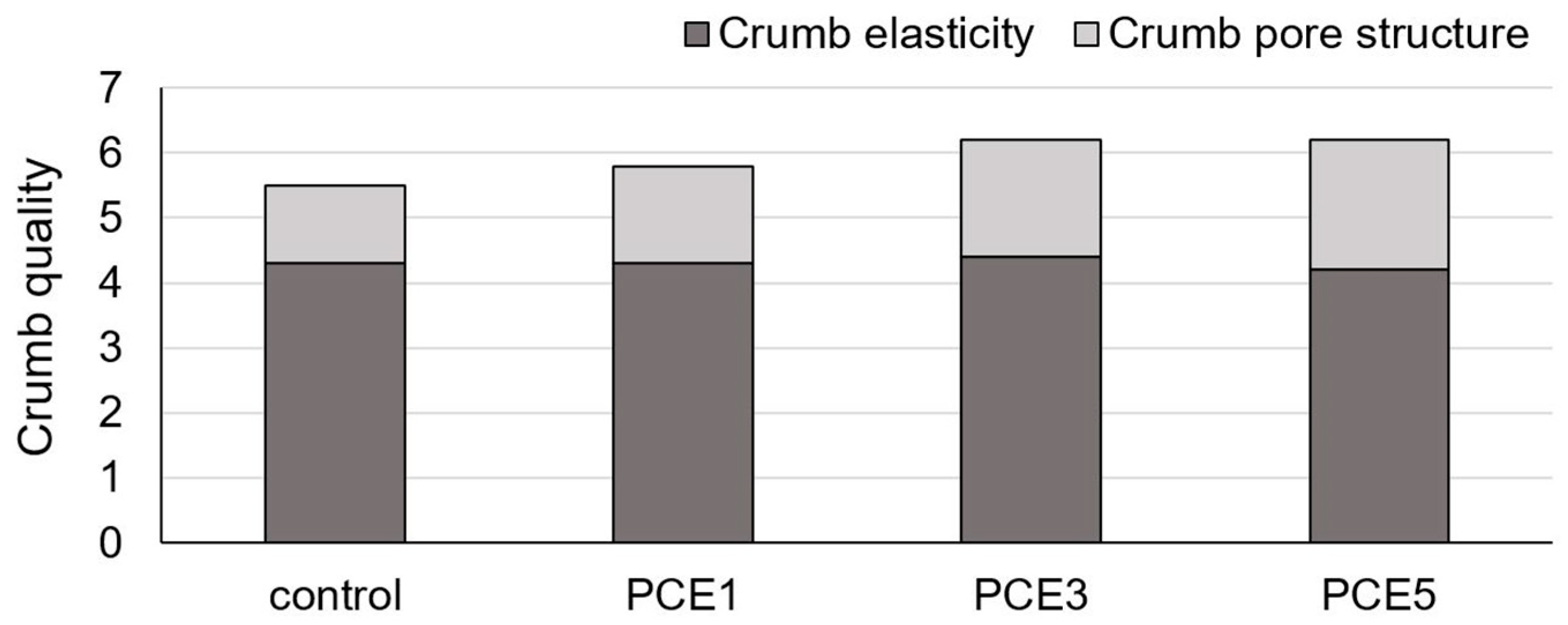
| Sample * | External Appearence | Crumb Structure | Smell | Taste | Total Score | Quality Category |
|---|---|---|---|---|---|---|
| control | 20.0 | 24.0 | 14.0 | 21.0 | 79.0 | Very good |
| PCE1 | 20.0 | 24.0 | 18.0 | 27.0 | 89.0 | Very good |
| PCE3 | 20.0 | 27.0 | 20.0 | 30.0 | 97.0 | Excellent |
| PCE5 | 20.0 | 27.0 | 20.0 | 30.0 | 97.0 | Excellent |
| Parameter | Control | PCE5 * | ||
|---|---|---|---|---|
| Dietary fibers content | ||||
| Total dietary fibers (g/100 g) | 1.18 ± 0.12 a | 2.65 ± 0.09 b | ||
| Insoluble dietary fibers (g/100 g) | 0.49 ± 0.17 a | 0.65 ± 0.22 a | ||
| Soluble dietary fibers (g/100 g) | 0.69 ± 0.18 a | 2.00 ± 0.18 b | ||
| control (crumb) | control (crumb + crust) | PCE5 (crumb) | PCE5 (crumb + crust) | |
| Polyphenols content | ||||
| TPC (mg GAE/100 g) | 11.52 ± 1.45 a | 12.67 ± 0.88 a | 46.37 ± 3.37 b | 60.53 ± 3.77 c |
| TFC (mg CE/100 g) | 38.29 ± 1.76 a | 45.45 ± 1.09 b | 50.17 ± 0.58 c | 58.11 ± 1.80 d |
| Antioxidant activity | ||||
| DPPH (μM TE/100 g) | 32.92 ± 4.32 a | 40.85 ± 1.14 b | 164.15 ± 4.85 c | 181.48 ± 5.75 d |
| FRAP (μM Fe2+/100 g) | 154.95 ± 6.69 a | 157.66 ± 4.71 a | 440.89 ± 4.45 b | 533.32 ± 18.54 c |
| ABTS (μM TE/100 g) | 17.09 ± 9.08 a | 27.40 ± 9.10 a | 214.85 ± 12.28 b | 225.94 ± 6.21 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahorec, J.; Šoronja-Simović, D.; Petrović, J.; Nikolić, I.; Pavlić, B.; Bijelić, K.; Bojanić, N.; Fišteš, A. Fortification of Bread with Carob Extract: A Comprehensive Study on Dough Behavior and Product Quality. Foods 2025, 14, 1821. https://doi.org/10.3390/foods14101821
Zahorec J, Šoronja-Simović D, Petrović J, Nikolić I, Pavlić B, Bijelić K, Bojanić N, Fišteš A. Fortification of Bread with Carob Extract: A Comprehensive Study on Dough Behavior and Product Quality. Foods. 2025; 14(10):1821. https://doi.org/10.3390/foods14101821
Chicago/Turabian StyleZahorec, Jana, Dragana Šoronja-Simović, Jovana Petrović, Ivana Nikolić, Branimir Pavlić, Katarina Bijelić, Nemanja Bojanić, and Aleksandar Fišteš. 2025. "Fortification of Bread with Carob Extract: A Comprehensive Study on Dough Behavior and Product Quality" Foods 14, no. 10: 1821. https://doi.org/10.3390/foods14101821
APA StyleZahorec, J., Šoronja-Simović, D., Petrović, J., Nikolić, I., Pavlić, B., Bijelić, K., Bojanić, N., & Fišteš, A. (2025). Fortification of Bread with Carob Extract: A Comprehensive Study on Dough Behavior and Product Quality. Foods, 14(10), 1821. https://doi.org/10.3390/foods14101821










