Forecasting Foodborne Disease Risk Caused by Vibrio parahaemolyticus Using a SARIMAX Model Incorporating Sea Surface Environmental and Climate Factors: Implications for Seafood Safety in Zhejiang, China
Abstract
1. Introduction
2. Study Area and Research Methods
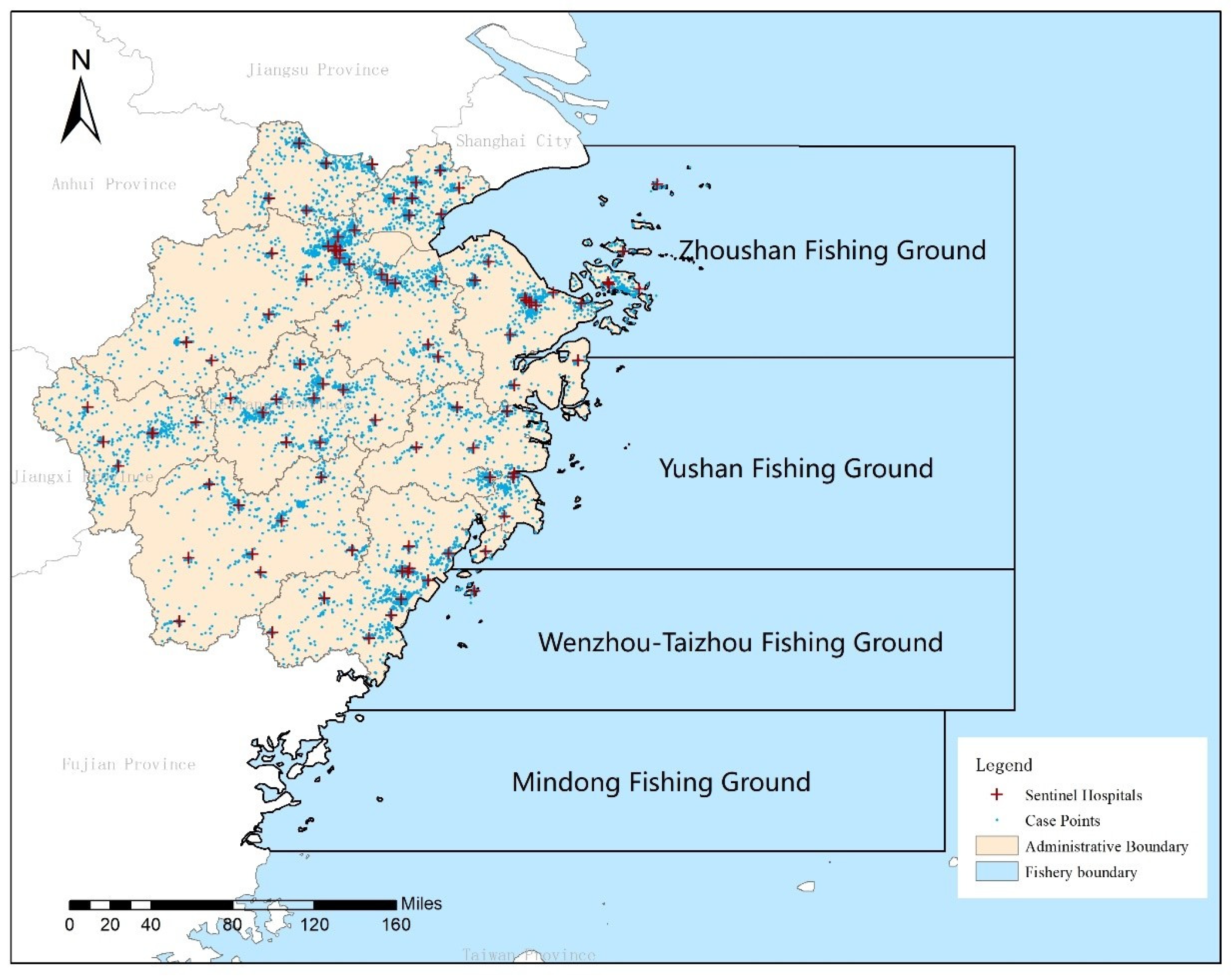
2.1. Study Area and Data Sources
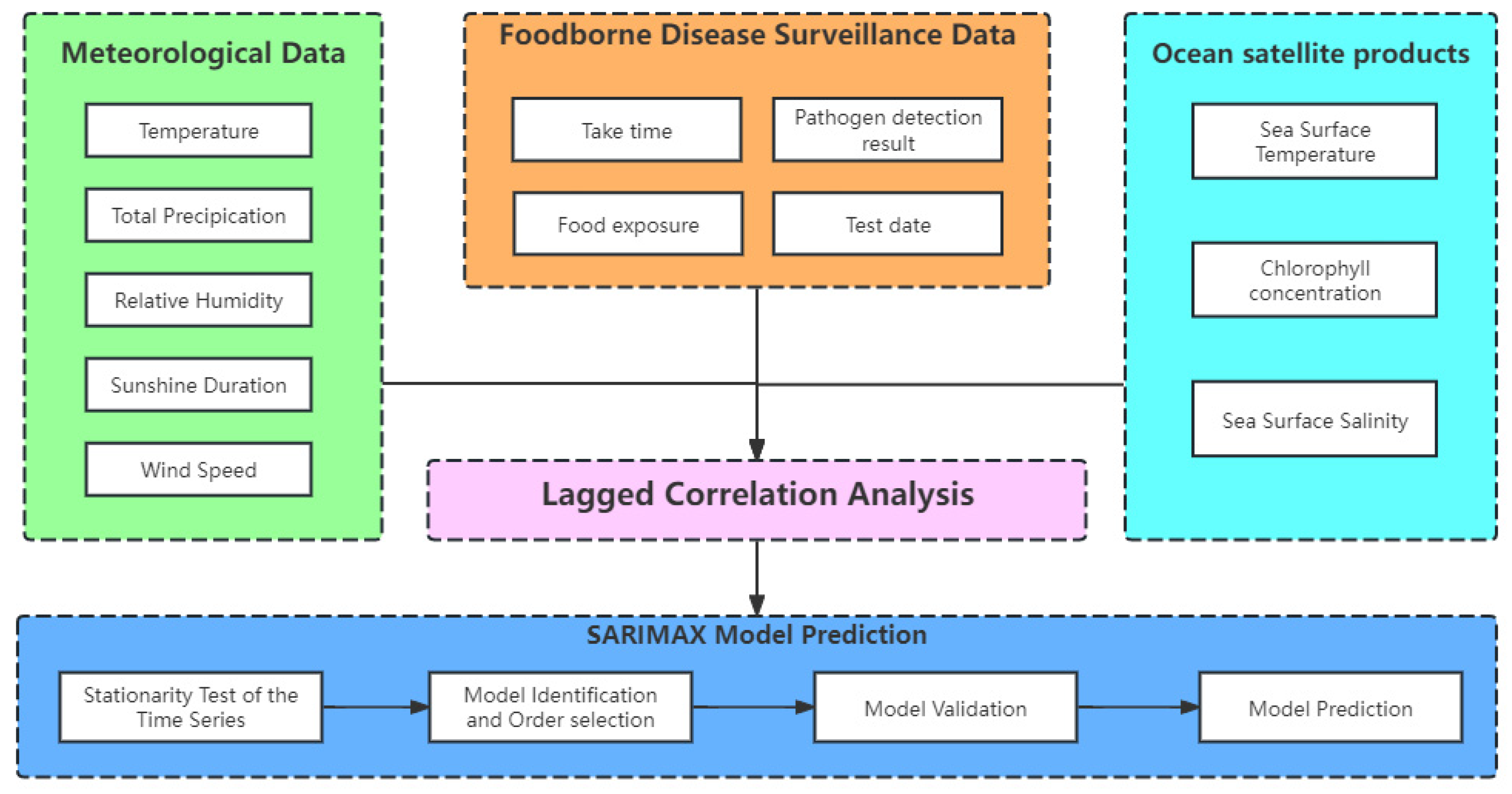
2.2. Analytical Framework and Data Sources
- Lag Correlation Analysis: Initially, a lag correlation analysis was performed to calculate the cross-correlation coefficients between meteorological data, marine satellite products, and V. parahaemolyticus detection data at various lag intervals. This step aimed to identify the meteorological and marine environmental factors influencing the detection rate of V. parahaemolyticus and to determine the respective lag periods for these influences.
- Multivariate Time Series Model Construction: Next, a multivariate time series model was developed. The sequential data were subjected to stationarity tests, and non-stationary sequences were differenced to achieve stationarity. The model parameters were identified and optimized using the autocorrelation function (ACF), partial autocorrelation function (PACF), and Bayesian Information Criteria (BIC) to fine-tune the SARIMAX model. The residuals of the model were carefully examined to ensure they adhered to white noise characteristics, which is a crucial assumption for the reliability of the model.
- Prediction and Evaluation: Finally, the established model was employed to predict the detection rates of V. parahaemolyticus, with its performance evaluated through appropriate metrics.
- Meteorological Data: This includes temperature, total precipitation, relative humidity, sunshine duration, and wind speed. These data were sourced from the European Centre for Medium-Range Weather Forecasts (ECMWF) (https://www.ecmwf.int/, accessed on 9 July 2024).
- Ocean Satellite Products: Sea surface temperature and chlorophyll levels were retrieved from NASA’s ocean data portal (https://oceandata.sci.gsfc.nasa.gov/, accessed on 19 July 2024), while sea surface salinity data were obtained from NASA’s Earth data portal (https://search.earthdata.nasa.gov/search, accessed on 28 July 2024).
- Foodborne Illness Data: Data on foodborne illnesses caused by V. parahaemolyticus were extracted from the Zhejiang Foodborne Disease Surveillance Reporting System. This dataset includes 182,311 cases and corresponding sample test results from 101 sentinel hospitals across the province, covering the years 2014 to 2018. Specific data points include the date, gender, age, address, occupation, and pathogen test results for each case. It should be noted that the dataset used in this study is based on confirmed clinical cases of foodborne illness caused by V. parahaemolyticus, as reported through sentinel hospitals. While seafood traceability and contamination testing are occasionally conducted during outbreak investigations, such environmental sampling data were not included in the present analysis. Our model thus focuses on predicting trends in clinical incidence rather than direct contamination levels in seafood products.
2.3. Multivariate Time Series Analysis
2.4. SARIMAX Model
2.4.1. Stationarity Test
2.4.2. Model Identification and Order Selection
2.4.3. Model Validation Method
2.4.4. Model Prediction Method
3. Results Analysis
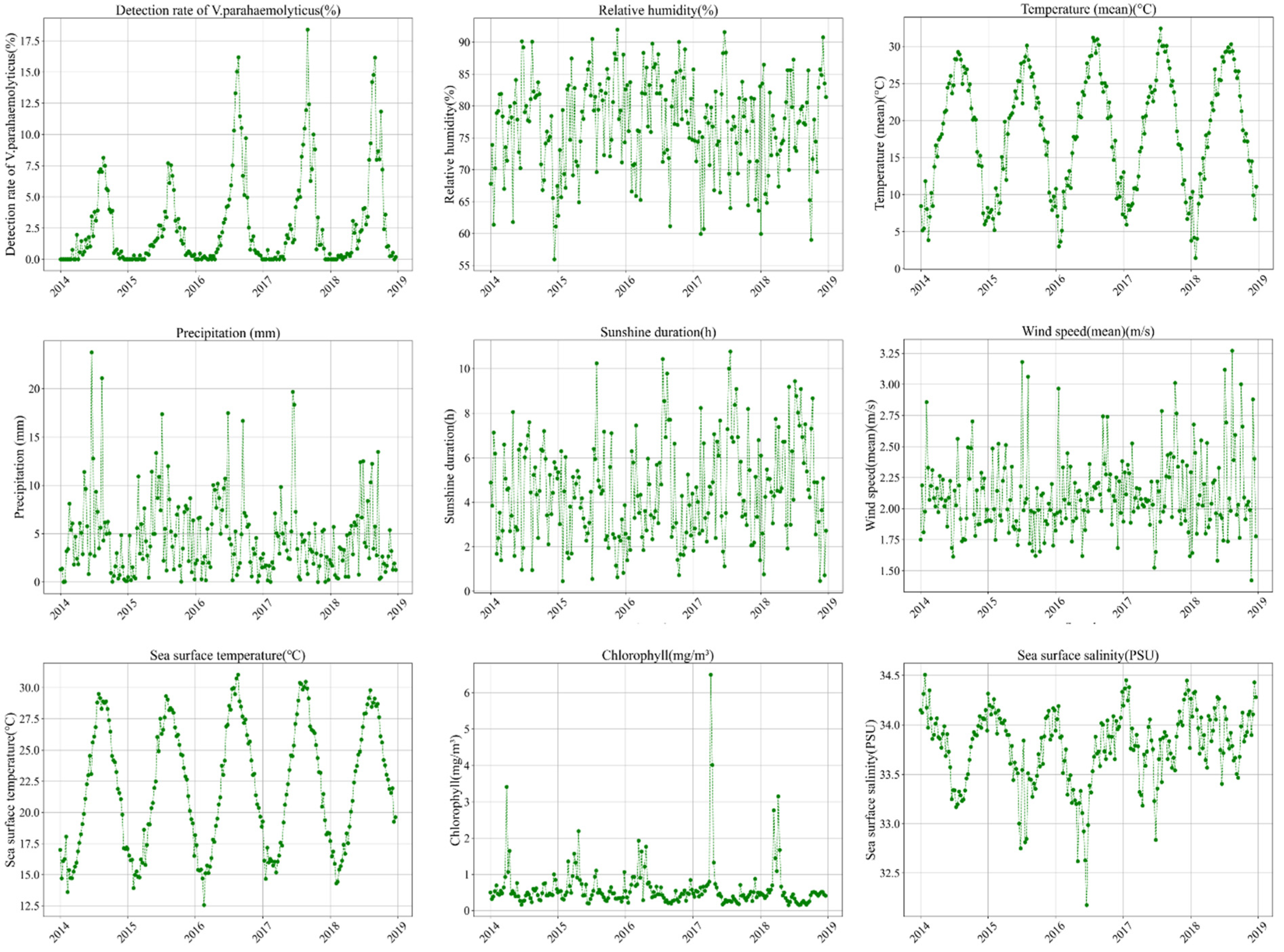
3.1. Multivariate Time Series
3.2. Lagged Correlation Analysis
- Meteorological Factors: Four meteorological variables showed positive lagged effects on the detection rate of V. parahaemolyticus, with varying time lags:
- Sunshine duration and air temperature exhibited a lag of 3 weeks;
- Total precipitation had a lag of 8 weeks;
- Relative humidity showed a lag of 7 weeks.
- Marine Factors: Among the marine environmental factors:
- Sunshine sea surface temperature demonstrated a positive lagged effect with a lag of 1 week;
- Sea surface salinity showed a negative lagged effect, with a lag of 8 weeks;
- Chlorophyll concentration and average wind speed did not show a significant lagged effect on the detection rate. However, a negative correlation was observed between chlorophyll concentration and the detection rate, while average wind speed exhibited a positive correlation.
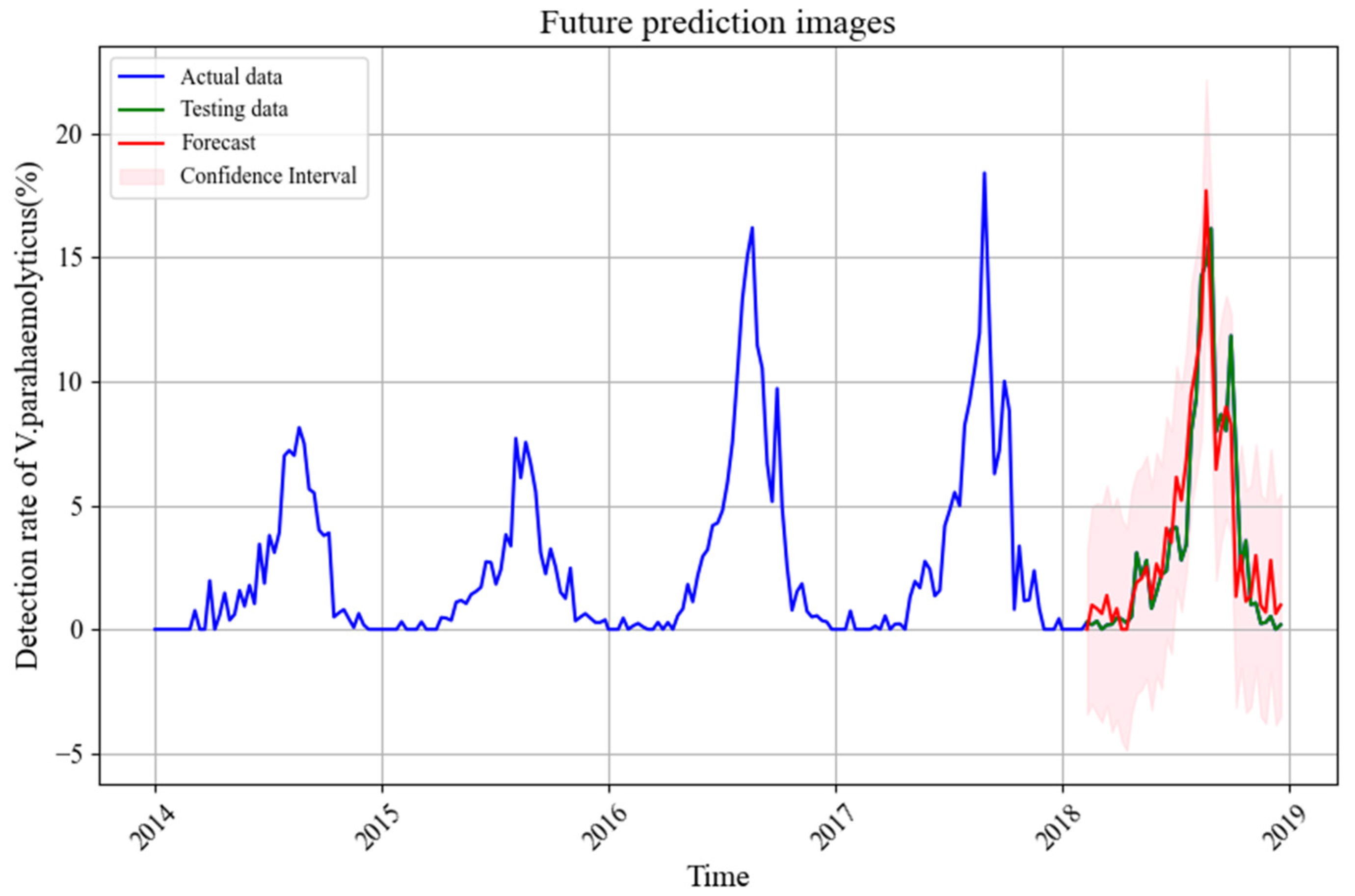
3.3. SARIMAX Model Prediction
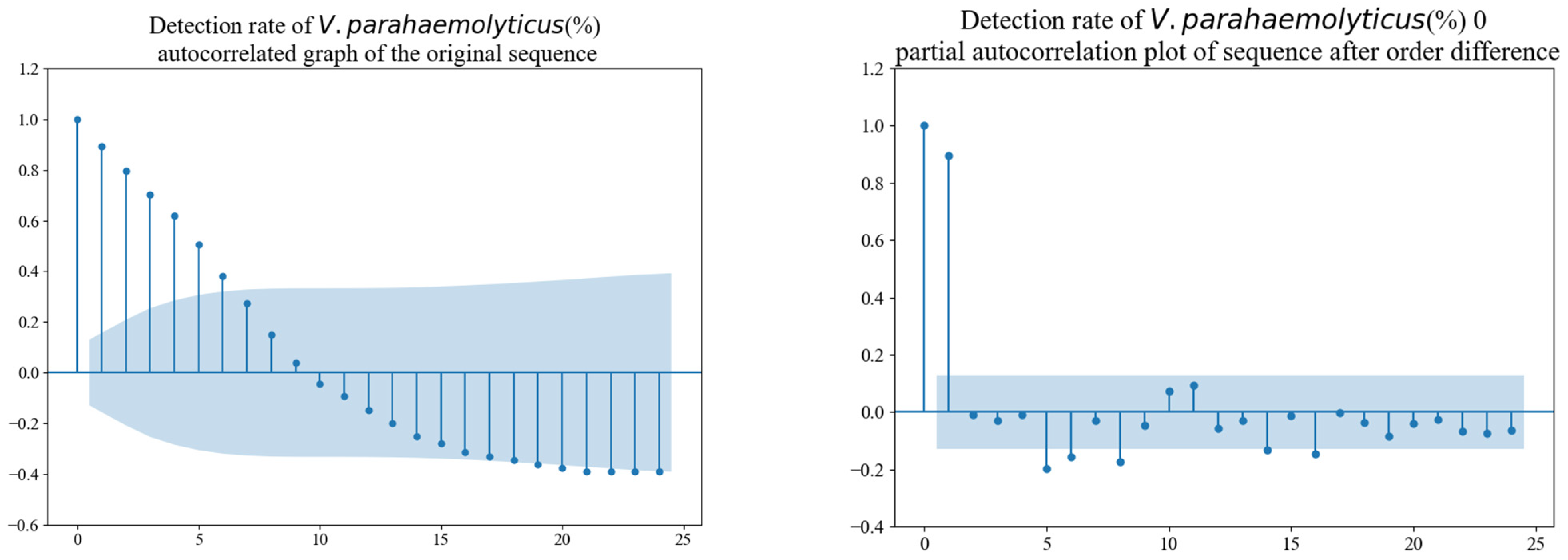
3.3.1. Stationarity Test of the Time Series
3.3.2. Parameter Testing and Model Construction
3.3.3. Model Validation
3.3.4. Model Prediction
4. Discussion
- Spatial Variations: This research primarily focused on temporal changes in disease incidence, neglecting spatial variations. Future studies should incorporate spatiotemporal analysis to enhance risk prediction and provide a more comprehensive understanding of disease dynamics.
- Expanded Predictive Variables: While this study considered meteorological and marine factors, future research should integrate additional variables, such as food consumption patterns, food exposure data, demographic factors (e.g., age structure), and fiscal and healthcare expenditures. Incorporating these factors would enable a more comprehensive understanding of bacterial foodborne disease incidence
- Geographical Specificity: This model is set under the conditions of subtropical monsoon climate and highly developed fisheries in Zhejiang Province, China (28–30° N). Not fully applicable to other coastal areas, model parameters need to be modified based on the marine climate environment of other regions.
- Lack of Direct Seafood Contamination Data: This study relied solely on clinical case data reported by sentinel hospitals. However, foodborne illness originates from pathogen contamination in seafood products, which is not captured in the current dataset. Future research should incorporate direct pathogen testing of seafood samples across various points in the supply chain to better validate temporal associations, identify contamination pathways, and improve the accuracy of early warning systems.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, R.K. Chapter 2—Foodborne infectious diseases. In Food Safety in the 21st Century; Gupta, R.K., Dudeja, Minhas, S., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 13–28. [Google Scholar]
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Chen, Y.; Yan, W.-X.; Zhou, Y.-J.; Zhen, S.-Q.; Zhang, R.-H.; Chen, J.; Liu, Z.-H.; Cheng, H.-Y.; Liu, H.; Duan, S.-G.; et al. Burden of self-reported acute gastrointestinal illness in China: A population-based survey. BMC Public Health 2013, 13, 456. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-N.; Liu, X.-M.; Chen, Q.; Liu, H.; Dai, Y.; Zhou, Y.-J.; Wen, J.; Tang, Z.-Z.; Chen, Y. Surveillance for foodborne disease outbreaks in China, 2003 to 2008. Food Control 2018, 84, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Lingling, M.; Xuexia, P.; Min, Z.; Junyan, Z.; Pu, G.; Junhang, P.; Li, Z. Contamination of Vibrio parahaemclyticus in Zhejiang province and risk assessment of Vibrio parahaemclyticus in shellfish. Chin. J. Zoonoses 2012, 28, 700–704. [Google Scholar] [CrossRef]
- Jiang, Y.; Chu, Y.; Xie, G.; Li, F.; Wang, L.; Huang, J.; Zhai, Y.; Yao, L. Antimicrobial resistance, virulence and genetic relationship of Vibrio parahaemolyticus in seafood from coasts of Bohai Sea and Yellow Sea, China. Int. J. Food Microbiol. 2019, 290, 116–124. [Google Scholar] [CrossRef]
- Morgado, M.E.; Brumfield, K.D.; Mitchell, C.; Boyle, M.M.; Colwell, R.R.; Sapkota, A.R. Increased incidence of vibriosis in Maryland, U.S.A., 2006–2019. Environ. Res. 2024, 244, 117940. [Google Scholar] [CrossRef]
- Ma, J.-Y.; Zhu, X.-K.; Hu, R.-G.; Qi, Z.-Z.; Sun, W.-C.; Hao, Z.-P.; Cong, W.; Kang, Y.-H. A systematic review, meta-analysis and meta-regression of the global prevalence of foodborne Vibrio spp. infection in fishes: A persistent public health concern. Mar. Pollut. Bull. 2023, 187, 114521. [Google Scholar] [CrossRef]
- Muzembo, B.A.; Kitahara, K.; Ohno, A.; Khatiwada, J.; Dutta, S.; Miyoshi, S.-I. Vibriosis in South Asia: A systematic review and meta-analysis. Int. J. Infect. Dis. 2024, 141, 106955. [Google Scholar] [CrossRef]
- Kocsis, T.; Magyar-Horváth, K.; Bihari, Z.; Kovács-Székely, I. Analysis of the correlation between the incidence of food-borne diseases and climate change in Hungary. Idojaras 2023, 127, 217–231. [Google Scholar] [CrossRef]
- Mirón, I.J.; Linares, C.; Díaz, J. The influence of climate change on food production and food safety. Environ. Res. 2023, 216, 114674. [Google Scholar] [CrossRef]
- Kim, J.G. Influence of Climatic Factors on the Occurrence of Vibrio parahaemolyticus Food Poisoning in the Republic of Korea. Climate 2024, 12, 25. [Google Scholar] [CrossRef]
- Venkatesan, P. Increase in Vibrio spp infections linked to climate change. Lancet Infect. Dis. 2024, 24, e18. [Google Scholar] [CrossRef]
- Martinez-Urtaza, J.; Bowers, J.C.; Trinanes, J.; DePaola, A. Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res. Int. 2010, 43, 1780–1790. [Google Scholar] [CrossRef]
- Ndraha, N.; Lin, H.-Y.; Lin, H.-J.; Hsiao, H.-I. Modeling the risk of Vibrio parahaemolyticus in oysters in Taiwan by considering seasonal variations, time periods, climate change scenarios, and post-harvest interventions. Microb. Risk Anal. 2023, 25, 100275. [Google Scholar] [CrossRef]
- Fletcher, G.C.; Cruz, C.D.; Hedderley, D.I. Vibrio parahaemolyticus: Predicting effects of storage temperature on growth in Crassostrea gigas harvested in New Zealand. Aquaculture 2024, 579, 740128. [Google Scholar] [CrossRef]
- Harrison, J.; Nelson, K.; Morcrette, H.; Morcrette, C.; Preston, J.; Helmer, L.; Titball, R.W.; Butler, C.S.; Wagley, S. The increased prevalence of Vibrio species and the first reporting of Vibrio jasicida and Vibrio rotiferianus at UK shellfish sites. Water Res. 2022, 211, 117942. [Google Scholar] [CrossRef]
- Hsiao, H.I.; Jan, M.S.; Chi, H.J. Impacts of Climatic Variability on Vibrio parahaemolyticus Outbreaks in Taiwan. Int. J. Environ. Res. Public Health 2016, 13, 188. [Google Scholar] [CrossRef]
- Baker, G.L. Food Safety Impacts from Post-Harvest Processing Procedures of Molluscan Shellfish. Foods 2016, 5, 29. [Google Scholar] [CrossRef]
- Cheng, Y.M. Discussion on the Existing Problems and Improvement Countermeasures of Food Cold Chain Logistics. China Food Saf. Mag. 2025, 6, 177–179 + 183. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on nitrofurans and their metabolites in food. EFSA J. 2016, 13, 4140. [Google Scholar] [CrossRef]
- de Noordhout, C.M.; Devleesschauwer, B.; Haagsma, J.A.; Havelaar, A.H.; Bertrand, S.; Vandenberg, O.; Quoilin, S.; Brandt, P.T.; Speybroeck, N. Burden of salmonellosis, campylobacteriosis and listeriosis: A time series analysis, Belgium, 2012 to 2020. Eurosurveillance 2017, 22, 30615. [Google Scholar] [CrossRef]
- Simpson, R.B.; Zhou, B.; Naumova, E.N. Seasonal synchronization of foodborne outbreaks in the United States, 1996–2017. Sci. Rep. 2020, 10, 17500. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.J.; Gregory, J.; Mulvenna, V.; Segal, Y.; Sullivan, S.G. Effect of Temperature and Rainfall on Sporadic Salmonellosis Notifications in Melbourne, Australia 2000–2019: A Time-Series Analysis. Foodborne Pathog. Dis. 2022, 19, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Park, K.H.; Bahk, G.J. Combined influence of multiple climatic factors on the incidence of bacterial foodborne diseases. Sci. Total Environ. 2018, 610, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Peng, Z.; Zhou, Y.; Zhang, J. Time series analysis of foodborne diseases during 2012–2018 in Shenzhen, China. J. Consum. Prot. Food Saf. 2022, 17, 83–91. [Google Scholar] [CrossRef]
- Rojas, F.; Ibacache-Quiroga, C. A forecast model for prevention of foodborne outbreaks of non-typhoidal salmonellosis. PeerJ 2020, 8, e10009. [Google Scholar] [CrossRef]
- Liang, W.; Hu, A.; Hu, J.; Wang, Y. Estimating the tuberculosis incidence using a SARIMAX-NNARX hybrid model by integrating meteorological factors in Qinghai Province, China. Int. J. Biometeorol. 2023, 67, 55–65. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, B.; Zheng, Q.; Zhou, W. Algorithm of Trawler Fishing Effort Extraction Based on BeiDou Vessel Monitoring System Data. In Geo-Informatics in Resource Management and Sustainable Ecosystem; Bian, F., Xie, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 159–168. [Google Scholar]
- Shamsudin, S.N.; Rahman, M.H.F.; Taib, M.N.; Razak, W.R.W.A.; Ahmad, A.H.; Zain, M.M. Analysis between Escherichia coli growth and physical parameters in water using Pearson correlation. In Proceedings of the 2016 7th IEEE Control and System Graduate Research Colloquium (ICSGRC), Shah Alam, Malaysia, 8 August 2016; pp. 131–136. [Google Scholar] [CrossRef]
- Mao, Q.; Zhang, K.; Yan, W.; Cheng, C. Forecasting the incidence of tuberculosis in China using the seasonal auto-regressive integrated moving average (SARIMA) model. J. Infect. Public Health 2018, 11, 707–712. [Google Scholar] [CrossRef]
- Lau, K.; Dorigatti, I.; Miraldo, M.; Hauck, K. SARIMA-modelled greater severity and mortality during the 2010/11 post-pandemic influenza season compared to the 2009 H1N1 pandemic in English hospitals. Int. J. Infect. Dis. 2021, 105, 161–171. [Google Scholar] [CrossRef]
- Malki, A.; Atlam, E.-S.; Hassanien, A.E.; Ewis, A.; Dagnew, G.; Gad, I. SARIMA model-based forecasting required number of COVID-19 vaccines globally and empirical analysis of peoples’ view towards the vaccines. Alex. Eng. J. 2022, 61, 12091–12110. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, Y.; Li, Y.; Yu, C.; Bai, Y.; Wang, L.; Wang, Y. Estimating the Long-Term Epidemiological Trends and Seasonality of Hemorrhagic Fever with Renal Syndrome in China. Infect. Drug Resist. 2021, 14, 3849–3862. [Google Scholar] [CrossRef]
- Tian, C.W.; Wang, H.; Luo, X.M. Time-series modelling and forecasting of hand, foot and mouth disease cases in China from 2008 to 2018. Epidemiol. Infect. 2019, 147, e82. [Google Scholar] [CrossRef]
- Vagropoulos, S.I.; Chouliaras, G.I.; Kardakos, E.G.; Simoglou, C.K.; Bakirtzis, A.G. Comparison of SARIMAX, SARIMA, modified SARIMA and ANN-based models for short-term PV generation forecasting. In Proceedings of the 2016 IEEE International Energy Conference (ENERGYCON), Leuven, Belgium, 4–8 April 2016; pp. 1–6. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Ghosh, J.K. AIC, BIC and Recent Advances in Model Selection. In Philosophy of Statistics; Bandyopadhyay, P.S., Forster, M.R., Eds.; North-Holland: Amsterdam, The Netherlands, 2011; Volume 7, pp. 583–605. [Google Scholar] [CrossRef]
- Makridakis, S.; Wheelwright, S.C.; Hyndman, R.J. Forecasting Methods and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, H. An Ultra-short-term Adaptive Forecasting Method of Electricity Load Based on the SARIMA Model. J. Nanjing Inst. Technol. Nat. Sci. Ed. 2024, 22, 78–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, P.; Hiller, J.E. Climate variations and Salmonella infection in Australian subtropical and tropical regions. Sci. Total Environ. 2010, 408, 524–530. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, H.J.; Myung, G.E.; Choi, E.J.; Kim, I.A.; Jeong, Y.I.; Park, G.J.; Soh, S.M. Distribution of Pathogenic Vibrio Species in the Coastal Seawater of South Korea (2017–2018). Osong Public Health Res. Perspect. 2019, 10, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.J.; Munyenyembe, K.; Almagro-Moreno, S.; Ogbunugafor, C.B. Patterns of air pressure, wind speed, and temperature are correlated with an increased risk of clinical infection from Vibrio vulnificus in endemic areas. medRxiv 2022. [Google Scholar] [CrossRef]
- Misiou, O.; Koutsoumanis, K. Climate change and its implications for food safety and spoilage. Trends Food Sci. Technol. 2022, 126, 142–152. [Google Scholar] [CrossRef]
- King, N.J.; Pirikahu, S.; Fletcher, G.C.; Pattis, I.; Roughan, B.; Perchec Merien, A.-M. Correlations between environmental conditions and Vibrio parahaemolyticus or Vibrio vulnificus in Pacific oysters from New Zealand coastal waters. N. Z. J. Mar. Freshw. Res. 2021, 55, 393–410. [Google Scholar] [CrossRef]
- Konrad, S.; Paduraru, P.; Romero-Barrios, P.; Henderson, S.B.; Galanis, E. Remote sensing measurements of sea surface temperature as an indicator of Vibrio parahaemolyticus in oyster meat and human illnesses. Environ. Health 2017, 16, 92. [Google Scholar] [CrossRef]
- Haley, B.J.; Kokashvili, T.; Tskshvediani, A.; Janelidze, N.; Mitaishvili, N.; Grim, C.J.; Constantin de Magny, G.; Chen, A.J.; Taviani, E.; Eliashvili, T.; et al. Molecular diversity and predictability of Vibrio parahaemolyticus along the Georgian coastal zone of the Black Sea. Front. Microbiol. 2014, 5, 45. [Google Scholar] [CrossRef]
- Urquhart, E.A.; Jones, S.H.; Yu, J.W.; Schuster, B.M.; Marcinkiewicz, A.L.; Whistler, C.A.; Cooper, V.S. Environmental Conditions Associated with Elevated Vibrio parahaemolyticus Concentrations in Great Bay Estuary, New Hampshire. PLoS ONE 2016, 11, e0155018. [Google Scholar] [CrossRef]
- Esteves, K.; Hervio-Heath, D.; Mosser, T.; Rodier, C.; Tournoud, M.-G.; Jumas-Bilak, E.; Colwell, R.R.; Monfort, P. Rapid Proliferation of Vibrio parahaemolyticus, Vibrio vulnificus, and Vibrio cholerae during Freshwater Flash Floods in French Mediterranean Coastal Lagoons. Appl. Environ. Microbiol. 2015, 81, 7600–7609. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Urtaza, J.; Lozano-Leon, A.; Varela-Pet, J.; Trinanes, J.; Pazos, Y.; Garcia-Martin, O. Environmental determinants of the occurrence and distribution of Vibrio parahaemolyticus in the rias of Galicia, Spain. Appl. Environ. Microbiol. 2008, 74, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Mertz, D.; Kim, T.H.; Johnstone, J.; Lam, P.-P.; Science, M.; Kuster, S.P.; Fadel, S.A.; Tran, D.; Fernandez, E.; Bhatnagar, N.; et al. Populations at risk for severe or complicated influenza illness: Systematic review and meta-analysis. BMJ 2013, 347, f5061. [Google Scholar] [CrossRef] [PubMed]


| Variable | N | Mean | S.D. | Minimum | Maximum |
|---|---|---|---|---|---|
| V. parahaemolyticus Detection Rate (%) | 229 | 2.6964 | 3.7230 | 0.0000 | 18.3935 |
| Air Temperature (°C) | 229 | 18.2956 | 7.9318 | 1.4378 | 32.4123 |
| Total Precipitation (mm) | 229 | 4.5351 | 4.1187 | 0.0000 | 23.7683 |
| Relative Humidity (%) | 229 | 77.0814 | 7.4093 | 55.9477 | 91.9691 |
| Sunshine Duration (h) | 229 | 4.4894 | 2.2546 | 0.4533 | 10.7682 |
| Wind Speed (m/s) | 229 | 2.1106 | 0.3159 | 1.4226 | 3.2706 |
| Sea Surface Temperature (°C) | 229 | 22.0112 | 5.0304 | 12.5561 | 31.0386 |
| Chlorophyll Concentration (mg/m3) | 229 | 0.6051 | 0.6296 | 0.1528 | 6.4957 |
| Sea Water Salinity (psu,‰) | 229 | 33.7804 | 0.3810 | 32.1755 | 34.5074 |
| Variable | Test Statistic | p-Value | 1% Level | 5% Level | 10% Level |
|---|---|---|---|---|---|
| V. parahaemolyticus Detection Rate | −4.9433 | 0.0000 | −3.4603 | −2.8747 | −2.5738 |
| Air Temperature | −7.4811 | 0.0000 | −3.4611 | −2.8751 | −2.5740 |
| Total Precipitation | −5.4752 | 0.0000 | −3.4598 | −2.8745 | −2.5737 |
| Relative Humidity | −10.9417 | 0.0000 | −3.4594 | −2.8743 | −2.5736 |
| Sunshine Duration | −10.3799 | 0.0000 | −3.4594 | −2.8743 | −2.5736 |
| Wind Speed | −13.6713 | 0.0000 | −3.4594 | −2.8743 | −2.5736 |
| Sea Surface Temperature | −8.4994 | 0.0000 | −3.4610 | −2.8750 | −2.5740 |
| Chlorophyll Concentration | −10.8984 | 0.0000 | −3.4594 | −2.8743 | −2.5736 |
| Sea Water Salinity | −3.5205 | 0.0075 | −3.4596 | −2.8744 | −2.5736 |
| Independent Variable | Coefficient | S.E. | z | p > |z| | 0.025 | 0.975 |
|---|---|---|---|---|---|---|
| Air Temperature | 0.0265 | 0.141 | 0.188 | 0.851 | −0.249 | 0.302 |
| Total Precipitation | −0.0302 | 0.088 | −0.364 | 0.716 | −0.193 | 0.132 |
| Relative Humidity | 0.0323 | 0.046 | 0.706 | 0.480 | −0.057 | 0.122 |
| Sunshine Duration | 0.1332 | 0.133 | 1.005 | 0.315 | −0.127 | 0.393 |
| Wind Speed | 1.1290 | 0.468 | 2.411 | 0.016 | 0.211 | 2.047 |
| Sea Surface Temperature | 0.2971 | 0.243 | 1.224 | 0.221 | −0.179 | 0.773 |
| Chlorophyll Concentration | 0.0014 | 0.684 | 0.002 | 0.998 | −1.340 | 1.343 |
| Sea Water Salinity | −0.3035 | 0.704 | −0.431 | 0.666 | −1.683 | 1.076 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, R.; Liu, T.; Fang, L.; Chen, J.; Yao, S.; Lei, H.; Song, Y. Forecasting Foodborne Disease Risk Caused by Vibrio parahaemolyticus Using a SARIMAX Model Incorporating Sea Surface Environmental and Climate Factors: Implications for Seafood Safety in Zhejiang, China. Foods 2025, 14, 1800. https://doi.org/10.3390/foods14101800
Ma R, Liu T, Fang L, Chen J, Yao S, Lei H, Song Y. Forecasting Foodborne Disease Risk Caused by Vibrio parahaemolyticus Using a SARIMAX Model Incorporating Sea Surface Environmental and Climate Factors: Implications for Seafood Safety in Zhejiang, China. Foods. 2025; 14(10):1800. https://doi.org/10.3390/foods14101800
Chicago/Turabian StyleMa, Rong, Ting Liu, Lei Fang, Jiang Chen, Shenjun Yao, Hui Lei, and Yu Song. 2025. "Forecasting Foodborne Disease Risk Caused by Vibrio parahaemolyticus Using a SARIMAX Model Incorporating Sea Surface Environmental and Climate Factors: Implications for Seafood Safety in Zhejiang, China" Foods 14, no. 10: 1800. https://doi.org/10.3390/foods14101800
APA StyleMa, R., Liu, T., Fang, L., Chen, J., Yao, S., Lei, H., & Song, Y. (2025). Forecasting Foodborne Disease Risk Caused by Vibrio parahaemolyticus Using a SARIMAX Model Incorporating Sea Surface Environmental and Climate Factors: Implications for Seafood Safety in Zhejiang, China. Foods, 14(10), 1800. https://doi.org/10.3390/foods14101800







