Physiological and Molecular Mechanisms of Light-Induced Greening in Potatoes: A Path to Food Safety
Abstract
1. Introduction
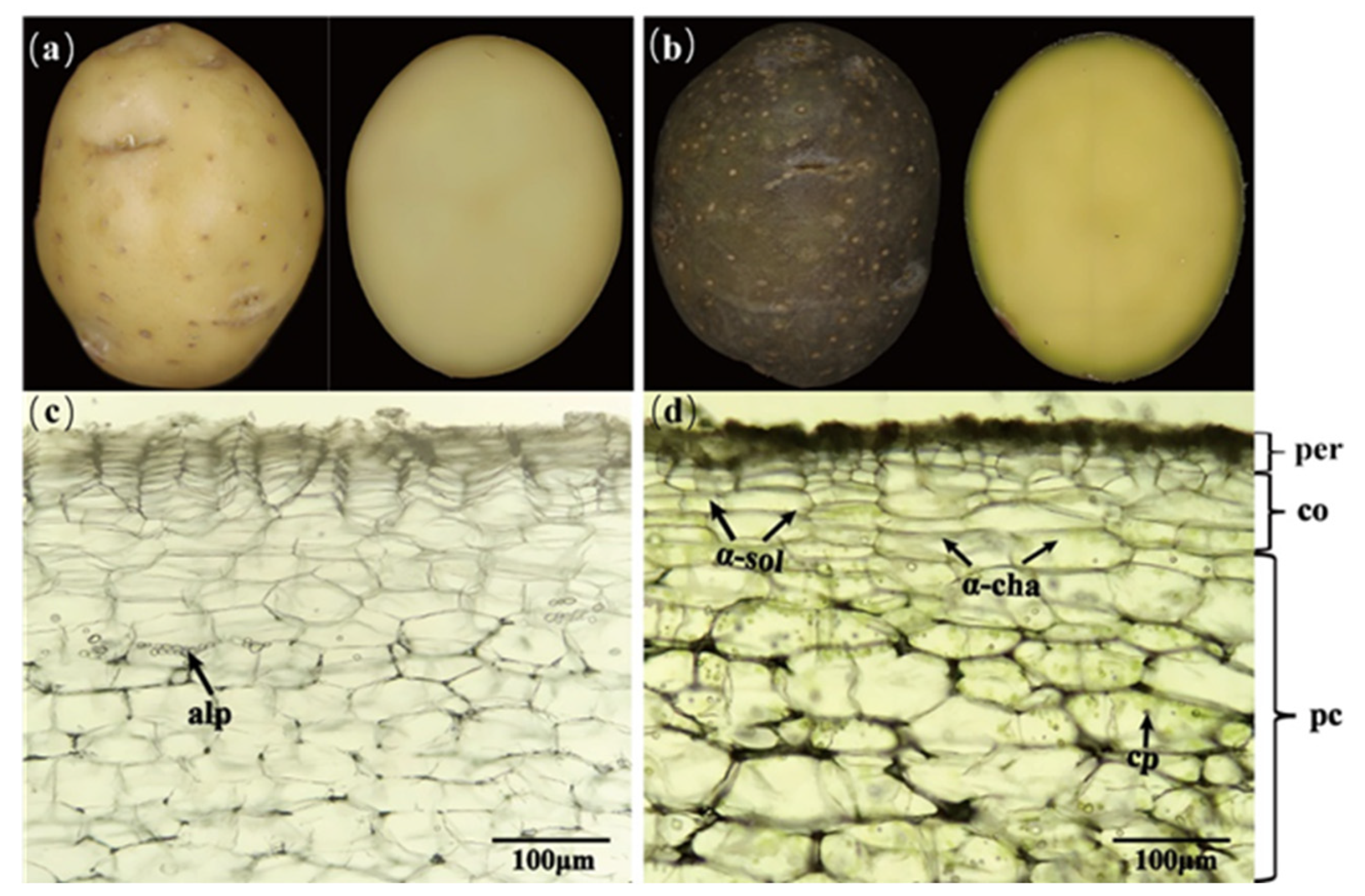
2. The Effects of Different Light Conditions on Chlorophyll and SGA Levels in Potatoes
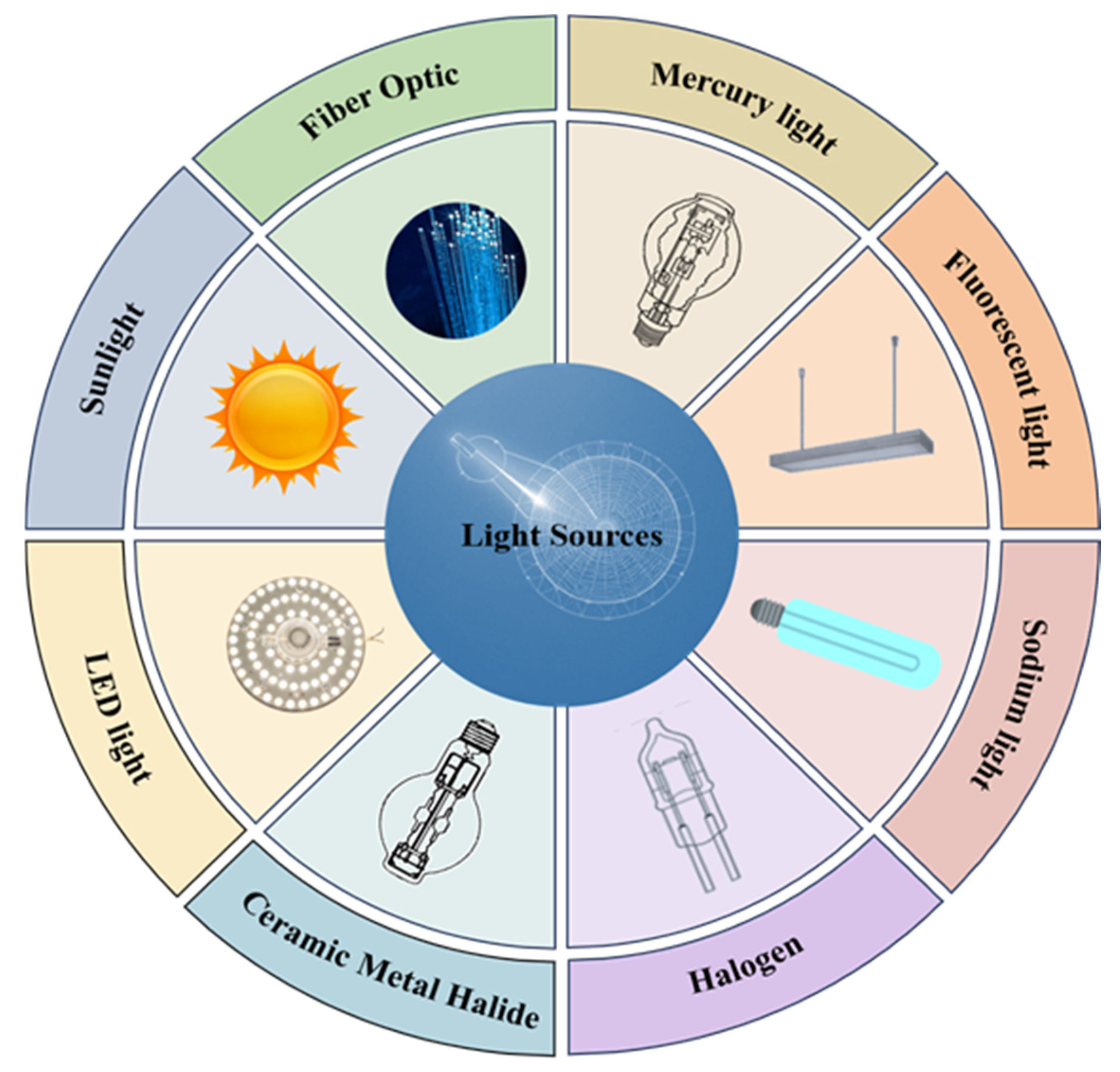
2.1. Light Sources
2.2. Light Wavelengths
2.3. Light Intensity
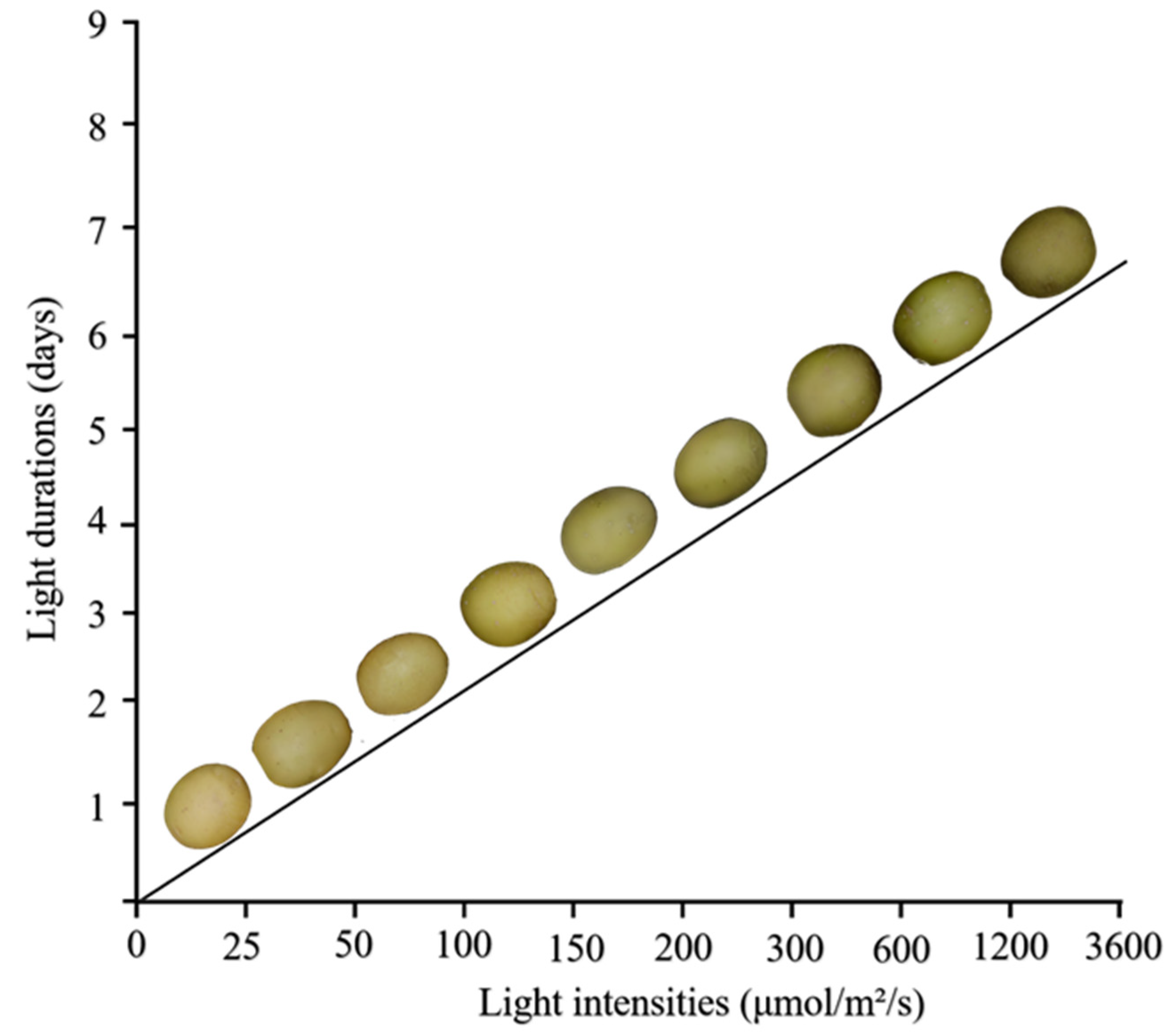
2.4. Light Duration
3. Potato Cultivars to Tolerate Greening
4. Molecular Mechanisms of Light-Induced Biosynthesis of Chlorophyll and SGAs
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birch, P.R.J.; Bryan, G.; Fenton, B.; Gilroy, E.M.; Hein, I.; Jones, J.T.; Prashar, A.; Taylor, M.A.; Torrance, L.; Toth, I.K. Crops that feed the world 8: Potato: Are the trends of increased global production sustainable? Food Secur. 2012, 4, 477–508. [Google Scholar] [CrossRef]
- Faostat. Available online: https://www.fao.org/faostat (accessed on 14 July 2023).
- Grunenfelder, L. Physiological Studies of Light-Induced Greening in Fresh Market Potatoes. Master’s Thesis, Washington State University, Pullman, WA, USA, 2005. [Google Scholar]
- Townsend, J.N. Potato Field Greening and Response to Potassium Fertilization in the Columbia Basin. Ph.D. Thesis, Washington State University, Pullman, WA, USA, 2021. [Google Scholar]
- French-Brooks, J. Reducing Supply Chain and Consumer Potato Waste; Research Report; WRAP: Banbury, UK, 2012; p. 50. Available online: http://www.wrap.org.uk/ (accessed on 31 December 2023).
- Bamberg, J.; Moehninsi; Navarre, R.; Suriano, J. Variation for tuber greening in the diploid wild potato solanum microdontum. Am. J. Potato Res. 2015, 92, 435–443. [Google Scholar] [CrossRef]
- Muraja-Fras, J.; Krsnik-Rasol, M.; Wrischer, M. Plastid transformation in greening potato tuber tissue. J. Plant Physiol. 1994, 144, 58–63. [Google Scholar] [CrossRef]
- Anstis, P.; Northcote, D. Development of chloroplasts from amyloplasts in potato tuber discs. New Phytol. 1973, 72, 449–463. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Merkle-Lehman, D.L.; Kung, S.D. Light-induced transformation of amyloplasts into chloroplasts in potato tubers. Plant Physiol. 1984, 75, 142–145. [Google Scholar] [CrossRef]
- Mochizuki, N.; Tanaka, R.; Grimm, B.; Masuda, T.; Moulin, M.; Smith, A.G.; Tanaka, A.; Terry, M.J. The cell biology of tetrapyrroles: A life and death struggle. Trends Plant Sci. 2010, 15, 488–498. [Google Scholar] [CrossRef]
- Pavlista, A.D. Green Potatoes: The Problem and the Solution; Cooperative Extension, Institute of Agriculture and Natural Resources, University of Nebraska–Lincoln: Lincoln, NE, USA, 2001. [Google Scholar]
- Dhalsamant, K.; Singh, C.B.; Lankapalli, R. A review on greening and glycoalkaloids in potato tubers: Potential solutions. J. Agric. Food Chem. 2022, 70, 13819–13831. [Google Scholar] [CrossRef] [PubMed]
- Dao, L.; Friedman, M. Chlorophyll, chlorogenic acid, glycoalkaloid, and protease inhibitor content of fresh and green potatoes. J. Agric. Food Chem. 1994, 42, 633–639. [Google Scholar] [CrossRef]
- Edwards, E.J.; Cobb, A.H. Effect of temperature on glycoalkaloid and chlorophyll accumulation in potatoes (Solanum tuberosum L. cv. King Edward) stored at low photon flux density, including preliminary modeling using an artificial neural network. J. Agric. Food Chem. 1997, 45, 1032–1038. [Google Scholar] [CrossRef]
- Edwards, E.J.; Saint, R.E.; Cobb, A.H. Is there a link between greening and light-enhanced glycoalkaloid accumulation in potato (Solanum tuberosum L.) tubers? J. Sci. Food Agric. 1998, 76, 327–333. [Google Scholar] [CrossRef]
- Okamoto, H.; Ducreux, L.J.M.; Allwood, J.W.; Hedley, P.E.; Wright, A.; Gururajan, V.; Terry, M.J.; Taylor, M.A. Light regulation of chlorophyll and glycoalkaloid biosynthesis during tuber greening of potato S. tuberosum. Front. Plant Sci. 2020, 11, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Maga, J.A. Glycoalkaloids in solanaceae. Food Rev. Int. 1994, 10, 385–418. [Google Scholar] [CrossRef]
- Sinden, S.; Deahl, K.; Aulenbach, B. Effect of glycoalkaloids and phenolics on potato flavor. J. Food Sci. 1976, 41, 520–523. [Google Scholar] [CrossRef]
- Takagi, K.; Toyoda, M.; Fujiyama, Y.; Saito, Y. Effect of cooking on the contents of α-chaconine and α-solanine in potatoes. J. Food Hyg. Soc. Jpn. 1990, 31, 67–73_61. [Google Scholar] [CrossRef]
- Morris, S.C.; Lee, T.H. The toxicity and teratogenicity of solanaceae glycoalkaloids, particularly those of the potato (Solanum tuberosum L.): A review. Food Aust. 1984, 36, 118–124. [Google Scholar]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; Nielsen, E.; et al. Risk assessment of glycoalkaloids in feed and food, in particular in potatoes and potato-derived products. EFSA J. 2020, 18, e06222. [Google Scholar] [PubMed]
- Mensinga, T.T.; Sips, A.J.A.M.; Rompelberg, C.J.M.; van Twillert, K.; Meulenbelt, J.; van den Top, H.J.; van Egmond, H.P. Potato glycoalkaloids and adverse effects in humans: An ascending dose study. Regul. Toxicol. Pharm. 2005, 41, 66–72. [Google Scholar] [CrossRef]
- Mekapogu, M.; Sohn, H.-B.; Kim, S.-J.; Lee, Y.-Y.; Park, H.-M.; Jin, Y.-I.; Hong, S.-Y.; Suh, J.-T.; Kweon, K.; Jeong, J.-C.; et al. Effect of light quality on the expression of glycoalkaloid biosynthetic genes contributing to steroidal glycoalkaloid accumulation in potato. Am. J. Potato Res. 2016, 93, 264–277. [Google Scholar] [CrossRef]
- Percival, G. The influence of light upon glycoalkaloid and chlorophyll accumulation in potato tubers (Solanum tuberosum L.). Plant Sci. 1999, 145, 99–107. [Google Scholar] [CrossRef]
- Olsen, N.L.; Brandt, T.; Price, W.J. The impact of retail light source on greening of russet burbank potato tubers. Am. J. Potato Res. 2017, 95, 123–129. [Google Scholar] [CrossRef]
- Grunenfelder, L.A.; Knowles, L.O.; Hiller, L.K.; Knowles, N.R. Glycoalkaloid development during greening of fresh market potatoes (Solanum tuberosum L.). J. Agric. Food Chem. 2006, 54, 5847–5854. [Google Scholar] [CrossRef] [PubMed]
- Conner, H.W. Effect of light on solanine synthesis in the potato tuber. Plant Physiol. 1937, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.M.D.; Toledo, M.C.F.; Garcia, L.C. Effect of light and temperature on the formation of glycoalkaloids in potato tubers. Food Control. 2007, 18, 503–508. [Google Scholar] [CrossRef]
- Percival, G.; Harrison, J.; Dixon, G. The influence of temperature on light enhanced glycoalkaloid synthesis in potato. Ann. Appl. Biol. 1993, 123, 141–153. [Google Scholar] [CrossRef]
- Percival, G.; Dixon, G.; Sword, A. Glycoalkaloid concentration of potato tubers following continuous illumination. J. Sci. Food Agric. 1994, 66, 139–144. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Hughes, D.; Howard, F. Effect of color and intensity of fluorescent lights and application of chemicals and waxes on chlorophyll development of White Rose potatoes. Am. Potato J. 1960, 37, 229–236. [Google Scholar] [CrossRef]
- Liljemark, A.; Widoff, E. Greening and solanine development of white potatoes in various types of consumer packages. Am. J. Potato Res. 1960, 28, 589–602. [Google Scholar]
- Petermann, J.B.; Morris, S.C. The spectral responses of chlorophyll and glycoalkaloid synthesis in potato tubers (Solanum tuberosum). Plant Sci. 1985, 39, 105–110. [Google Scholar] [CrossRef]
- Tanios, S.; Eyles, A.; Corkrey, R.; Tegg, R.S.; Thangavel, T.; Wilson, C.R. Quantifying risk factors associated with light-induced potato tuber greening in retail stores. PLoS ONE 2020, 15, e0235522. [Google Scholar] [CrossRef]
- Patil, B.; Salunkhe, O.; Singh, B. Metabolism of solanine and chlorophyll in potato tubers as affected by light and specific chemicals. J. Food Sci. 1971, 36, 474–476. [Google Scholar] [CrossRef]
- Griffiths, D.; Dale, M.; Bain, H. The effect of cultivar, maturity and storage on photo-induced changes in the total glycoalkaloid and chlorophyll contents of potatoes (Solanum tuberosum L.). Plant Sci. 1994, 98, 103–109. [Google Scholar] [CrossRef]
- Larsen, E. Investigations on Cause and Prevention of Greening of Potato Tubers; Idaho Agricultural Experiment Station Research; Agricultural Experiment Station of the University of Idaho: Moscow, Idaho, 1949; Bulletin. No. 1940; p. 16. [Google Scholar]
- Folsom, D. Permanence of greening of potato tubers. Am. Potato J. 1947, 24, 336–340. [Google Scholar] [CrossRef]
- Martin, S.K.; Sheppard, R.L. Effect of different packaging materials and light exposure times on chlorophyll concentration in 2 cultivars of potato. N. Z. J. Agric. Res. 1983, 11, 63–67. [Google Scholar] [CrossRef]
- Şengül, M.; Keleş, F.; Keleş, M.S. The effect of storage conditions (temperature, light, time) and variety on the glycoalkaloid content of potato tubers and sprouts. Food Control. 2004, 15, 281–286. [Google Scholar] [CrossRef]
- Baur, S.; Bellé, N.; Hausladen, H.; Wurzer, S.; Brehm, L.; Stark, T.D.; Hücklhoven, R.; Hofmann, T.; Dawid, C. Quantitation of toxic steroidal glycoalkaloids and newly iIdentified saponins in post-harvest light-stressed potato (Solanum tuberosum L.) Varieties. J. Agric. Food Chem. 2022, 70, 8300–8308. [Google Scholar] [CrossRef] [PubMed]
- Reeves, A.F. Varietal differences in potato tuber greening. Am. Potato J. 1988, 65, 651–658. [Google Scholar] [CrossRef]
- Salunkhe, D.; Wu, M.; Jadhav, S. Effects of light and temperature on the formation of solanine in potato slices. J. Food Sci. 1972, 37, 969–970. [Google Scholar] [CrossRef]
- Heyes, D.J.; Hunter, C.N. Making light work of enzyme catalysis: Protochlorophyllide oxidoreductase. Trends Biochem. Sci. 2005, 30, 642–649. [Google Scholar] [CrossRef]
- Haverkort, A.; Linnemann, A.; Struik, P.; Wiskerke, J. On processing potato 3: Survey of performances, productivity and losses in the supply chain. Potato Res. 2023, 66, 385–427. [Google Scholar] [CrossRef]
- Mancinelli, A.L.; Rabino, I. The “high irradiance responses” of plant photomorphogenesis. Bot. Rev. 1978, 44, 129–180. [Google Scholar] [CrossRef]
- Lutz, J.; Findlen, H.; Ramsey, G. Quality of red river valley potatoes in various types of consumer packages. Am. Potato J. 1951, 28, 589–602. [Google Scholar] [CrossRef]
- Percival, G.; Dixon, G.R.; Sword, A. Glycoalkaloid concentration of potato tubers following exposure to daylight. J. Sci. Food Agric. 1996, 71, 59–63. [Google Scholar] [CrossRef]
- Rymuza, K.; Gugała, M.; Zarzecka, K.; Sikorska, A.; Findura, P.; Malaga-Toboła, U.; Kapela, K.; Radzka, E. The effect of light exposures on the content of hharmful substances in edible potato tuber. Agriculture 2020, 10, 139. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, Y.; Zhao, X.; Wang, E.; Liu, T.; Zhang, H.; Liu, T.; Botao, S. The transcription factor StMYB113 regulates light-induced greening by modulating steroidal glycoalkaloid biosynthesis in potatoes (Solanum tuberosum L.). Hortic. Adv. 2024, 2, 7. [Google Scholar] [CrossRef]
- Yaronskaya, E.; Grimm, B. The pathway from 5-aminolevulinic acid to protochlorophyllide and protoheme. Chlorophylls Bacteriochlorophylls Biochem. Biophys. Funct. Appl. 2006, 25, 173–188. [Google Scholar]
- Tanaka, R.; Tanaka, A. Tetrapyrrole biosynthesis in higher plants. Annu. Rev. Plant Biol. 2007, 58, 321–346. [Google Scholar] [CrossRef]
- Richter, A.S.; Nägele, T.; Grimm, B.; Kaufmann, K.; Schroda, M.; Leister, D.; Kleine, T. Retrograde signaling in plants: A critical review focusing on the GUN pathway and beyond. Plant Commun. 2023, 4, 100511. [Google Scholar] [CrossRef]
- Shepherd, M.; Reid, J.D.; Hunter, C.N. Purification and kinetic characterization of the magnesium protoporphyrin IX methyltransferase from Synechocystis PCC6803. Biochem. J. 2003, 371, 351–360. [Google Scholar] [CrossRef]
- Tottey, S.; Block, M.A.; Allen, M.; Westergren, T.; Albrieux, C.; Scheller, H.V.; Merchant, S.; Jensen, P.E. Arabidopsis CHL27, located in both envelope and thylakoid membranes, is required for the synthesis of protochlorophyllide. Proc. Natl. Acad. Sci. USA 2003, 100, 16119–16124. [Google Scholar] [CrossRef]
- Apel, K.; Santel, H.J.; Redlinger, T.E.; Falk, H. The protochlorophyllide holochrome of barley (Hordeum vulgare L.). Eur. J. Biochem. 2005, 111, 251–258. [Google Scholar] [CrossRef]
- Nagata, N.; Tanaka, R.; Satoh, S.; Tanaka, A. Identification of a vinyl reductase gene for chlorophyll synthesis in arabidopsis thaliana and implications for the evolution of prochlorococcus species. Plant Cell 2005, 17, 233–240. [Google Scholar] [CrossRef]
- Keller, Y.; Bouvier, F.; d’Harlingue, A.; Camara, B. Metabolic compartmentation of plastid prenyllipid biosynthesis. Eur. J. Biochem. 2001, 251, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Oster, U.; Tanaka, R.; Tanaka, A.; Rüdiger, W. Cloning and functional expression of the gene encoding the key enzyme for chlorophyll b biosynthesis (CAO) from arabidopsis thaliana. Plant J. 2001, 21, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, R.; Umemoto, N.; Mizutani, M. Recent advances in steroidal glycoalkaloid biosynthesis in the genus solanum. Plant Biotechnol. 2023, 40, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Grzech, D.; Smit, S.J.; Alam, R.M.; Boccia, M.; Nakamura, Y.; Hong, B.; Barbole, R.; Heinicke, S.; Kunert, M.; Seibt, W.; et al. Incorporation of nitrogen in antinutritional solanum alkaloid biosynthesis. Nat. Chem. Biol. 2024, 21, 131–142. [Google Scholar] [CrossRef]
- McCue, K.F.; Breksa, A.; Vilches, A.; Belknap, W.R. Modification of potato steroidal glycoalkaloids with silencing RNA constructs. Am. J. Potato Res. 2017, 95, 9–14. [Google Scholar] [CrossRef]
- Akiyama, R.; Watanabe, B.; Nakayasu, M.; Lee, H.J.; Kato, J.; Umemoto, N.; Muranaka, T.; Saito, K.; Sugimoto, Y.; Mizutani, M. The biosynthetic pathway of potato solanidanes diverged from that of spirosolanes due to evolution of a dioxygenase. Nat. Commun. 2021, 12, 1300. [Google Scholar] [CrossRef]
- Paik, I.; Huq, E. Plant photoreceptors: Multi-functional sensory proteins and their signaling networks. Semin. Cell Dev. Biol. 2019, 92, 114–121. [Google Scholar] [CrossRef]
- Sharma, S.; Sanyal, S.K.; Sushmita, K.; Chauhan, M.; Sharma, A.; Anirudhan, G.; Veetil, S.K.; Kateriya, S. Modulation of phototropin signalosome with artificial illumination holds great potential in the development of climate-smart crops. Curr. Genom. 2021, 22, 181–213. [Google Scholar] [CrossRef]
- Pham, V.N.; Kathare, P.K.; Huq, E. Phytochromes and phytochrome interacting factors. Plant Physiol. 2018, 176, 1025–1038. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, R.; Zeng, X.Y.; Lee, S.; Ye, L.H.; Tian, S.L.; Zhang, Y.J.; Busch, W.; Zhou, W.B.; Zhu, X.G.; et al. GLK transcription factors accompany ELONGATED HYPOCOTYL5 to orchestrate light-induced seedling development in Arabidopsis. Plant Phys. 2024, 194, 2400–2421. [Google Scholar] [CrossRef] [PubMed]
- Paik, I.; Chen, F.; Ngoc Pham, V.; Zhu, L.; Kim, J.; Huq, E. A phyB-PIF1-SPA1 kinase regulatory complex promotes photomorphogenesis inArabidopsis. Nat. Commun. 2019, 10, 4216. [Google Scholar] [CrossRef]
- Lu, X.-D.; Zhou, C.-M.; Xu, P.-B.; Luo, Q.; Lian, H.-L.; Yang, H.-Q. Red-light-dependent interaction of phyB with SPA1 promotes COP1–SPA1 dissociation and photomorphogenic development in arabidopsis. Mol. Plant 2015, 8, 467–478. [Google Scholar] [CrossRef]
- Leivar, P.; Tepperman, J.M.; Monte, E.; Calderon, R.H.; Liu, T.L.; Quail, P.H. Definition of early transcriptional circuitry involved in light-induced reversal of PIF-imposed repression of photomorphogenesis in young Arabidopsis seedlings. Plant Cell 2009, 21, 3535–3553. [Google Scholar] [CrossRef]
- Shin, J.; Kim, K.; Kang, H.; Zulfugarov, I.S.; Bae, G.; Lee, C.-H.; Lee, D.; Choi, G. Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 2009, 106, 7660–7665. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Ortiz, G.; Johansson, H.; Lee, K.P.; Bou-Torrent, J.; Stewart, K.; Steel, G.; Rodríguez-Concepción, M.; Halliday, K.J. The HY5-PIF regulatory module coordinates light and temperature control of photosynthetic gene transcription. PLoS Genet. 2014, 10, e1004416. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.-M.; Qiu, Y.; Van Buskirk, E.K.; Yang, E.J.; Chen, M. Light-regulated gene repositioning in arabidopsis. Nat. Commun. 2014, 5, 3027. [Google Scholar] [CrossRef]
- Wang, W.; Tang, W.; Ma, T.; Niu, D.; Jin, J.B.; Wang, H.; Lin, R. A pair of light signaling factors FHY3 and FAR1 regulates plant immunity by modulating chlorophyll biosynthesis. J. Integr. Plant Biol. 2015, 58, 91–103. [Google Scholar] [CrossRef]
- Swinnen, G.; De Meyer, M.; Pollier, J.; Molina-Hidalgo, F.J.; Ceulemans, E.; Venegas-Molina, J.; De Milde, L.; Fernández-Calvo, P.; Ron, M.; Pauwels, L.; et al. The basic helix–loop–helix transcription factors MYC1 and MYC2 have a dual role in the regulation of constitutive and stress-inducible specialized metabolism in tomato. New Phytol. 2022, 236, 911–928. [Google Scholar] [CrossRef]
- Yu, G.; Li, C.; Zhang, L.; Zhu, G.; Munir, S.; Shi, C.; Zhang, H.; Ai, G.; Gao, S.; Zhang, Y. An allelic variant of GAME9 determines its binding capacity with the GAME17 promoter in the regulation of steroidal glycoalkaloid biosynthesis in tomato. J. Exp. Bot. 2020, 71, 2527–2536. [Google Scholar] [CrossRef]
- Mathews, S. Evolutionary Studies Illuminate the Structural-Functional Model of Plant Phytochromes. Plant Cell 2010, 22, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, H.; Liu, W.; Wang, Y.; Zeng, F. Effect of red light on the expression of the phytochrome gene family and the accumulation of glycoside alkaloids in potatoes. Foods 2023, 12, 4194. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Virgin, H.I.; Sundqvist, C. Pigment formation in potato tubers (Solanum tuberosum L.) exposed to light followed by darkness. Physiol. Plant 1992, 86, 587–592. [Google Scholar] [CrossRef]
 Positive regulation.
Positive regulation.  The dotted line needs to be verified by testing.
The dotted line needs to be verified by testing.
 Positive regulation.
Positive regulation.  The dotted line needs to be verified by testing.
The dotted line needs to be verified by testing.

 Positive regulation.
Positive regulation.  Negative regulation.
Negative regulation.  Mechanisms are unclear and need further validation by experiments.
Mechanisms are unclear and need further validation by experiments.
 Positive regulation.
Positive regulation.  Negative regulation.
Negative regulation.  Mechanisms are unclear and need further validation by experiments.
Mechanisms are unclear and need further validation by experiments.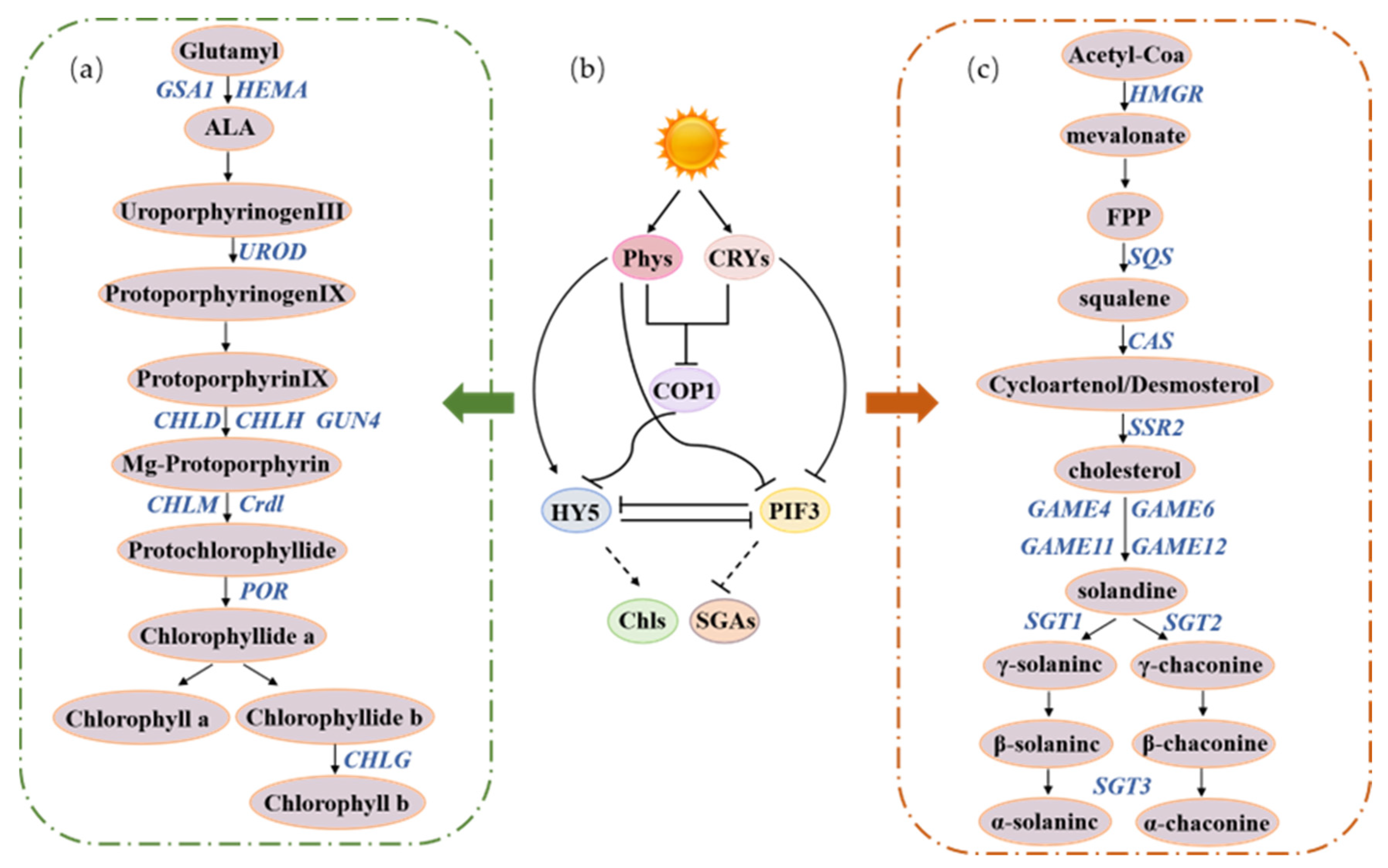
| Influencing Factors | Crucial Role | References | |
|---|---|---|---|
| Light conditions | Light sources | The effects of various light sources on greening rate and chlorophyll synthesis in greening tubers. | [6,24,25,26] |
| The accumulation of SGAs of greening tubers when exposed to various light sources. | [24,27,28,29,30] | ||
| Light wavelengths | The effects of various light wavelengths on greening rate and chlorophyll synthesis in greening tubers. | [16,31,32,33,34] | |
| The accumulation of SGAs of greening tubers when exposed to various light wavelengths. | [14,16,31,32,33,34] | ||
| Light intensity | The effects of various light intensity on greening rate and chlorophyll synthesis in greening tubers. | [8,31,32,35] | |
| The accumulation of SGAs of greening tubers when exposed to various light intensity. | [3,26,32,34,36] | ||
| Light durations | The effects of various light durations on greening rate and chlorophyll synthesis in greening tubers. | [14,37,38,39] | |
| The accumulation of SGAs of greening tubers when exposed to various light durations. | [33,39,40,41] | ||
| Cultivars | Pigment accumulation | Accumulation of pigmentation in the tuber periderm reduced greening change. | [3,26,41,42] |
| Suberin of tuber periderm | Cultivars susceptible to greening are strongly associated with higher suberin deposition in the tuber periderm. | [26] |
| Cultivars | Light Conditions | Treatment Duration | SGA Concentration (mg/100 g FW or DW) | Characteristics | References |
|---|---|---|---|---|---|
| Pentland Hawk | Fluorescent light/natural light | 21 days | 25.41 (fluorescent); 761 mg/kg FW (natural light) | White-skinned variety | [29,48] |
| Kerrs Pink | Fluorescent light/sodium light | 21 days | 22.49 (fluorescent) | Pink-skinned variety | [29,36] |
| Desiree | Fluorescent light/sodium light | 10–21 days | Low accumulation (exceeding 10 mg/100 g FW after 10 days) | Red-skinned variety | [24,36] |
| White Rose | Fluorescent light/retail LED | 10 days | 76–138 (periderm, DW) | Extremely sensitive to greening | [26] |
| Yukon Gold | Ceramic metal halide light | 10 days | 44–110 (periderm, DW) | Yellow-skinned variety | [26] |
| Russet Norkotah | Fiber optic/halogen light | 10 days | 37.3–98.2 (periderm, DW) | Russet-skinned variety | [26] |
| Dark Red Norland | Fluorescent light | 10 days | 38–159 (periderm, DW) | Red-skinned variety | [26] |
| Albatros | LED light | 1–16 days | 12.4–17.1 (stable, FW) | Variety with high baseline SGAs | [41] |
| Solo | LED light | 16 days | 14.7 (peak, FW) | Moderately sensitive | [41] |
| Brodick | Natural light | 48 h | 3× SGAs increase compared to Ailsa | Variety with high SGA accumulation rate | [36] |
| GL76B/102 | Fluorescent light | 48 h | 28 mg/100 g FW (65% increase vs. Desiree) | Low initial SGAs variety | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zao, X.; Li, W.; Cheng, L.; Yu, B.; Sa, G. Physiological and Molecular Mechanisms of Light-Induced Greening in Potatoes: A Path to Food Safety. Foods 2025, 14, 1798. https://doi.org/10.3390/foods14101798
Zao X, Li W, Cheng L, Yu B, Sa G. Physiological and Molecular Mechanisms of Light-Induced Greening in Potatoes: A Path to Food Safety. Foods. 2025; 14(10):1798. https://doi.org/10.3390/foods14101798
Chicago/Turabian StyleZao, Xiaohua, Wenli Li, Lixiang Cheng, Bin Yu, and Gang Sa. 2025. "Physiological and Molecular Mechanisms of Light-Induced Greening in Potatoes: A Path to Food Safety" Foods 14, no. 10: 1798. https://doi.org/10.3390/foods14101798
APA StyleZao, X., Li, W., Cheng, L., Yu, B., & Sa, G. (2025). Physiological and Molecular Mechanisms of Light-Induced Greening in Potatoes: A Path to Food Safety. Foods, 14(10), 1798. https://doi.org/10.3390/foods14101798





