Beyond Color Extraction: How Pulsed Electric Fields and Sulfites Affect Phenolic and Volatile Compounds of Primitivo Red Wine
Abstract
1. Introduction
2. Materials and Methods
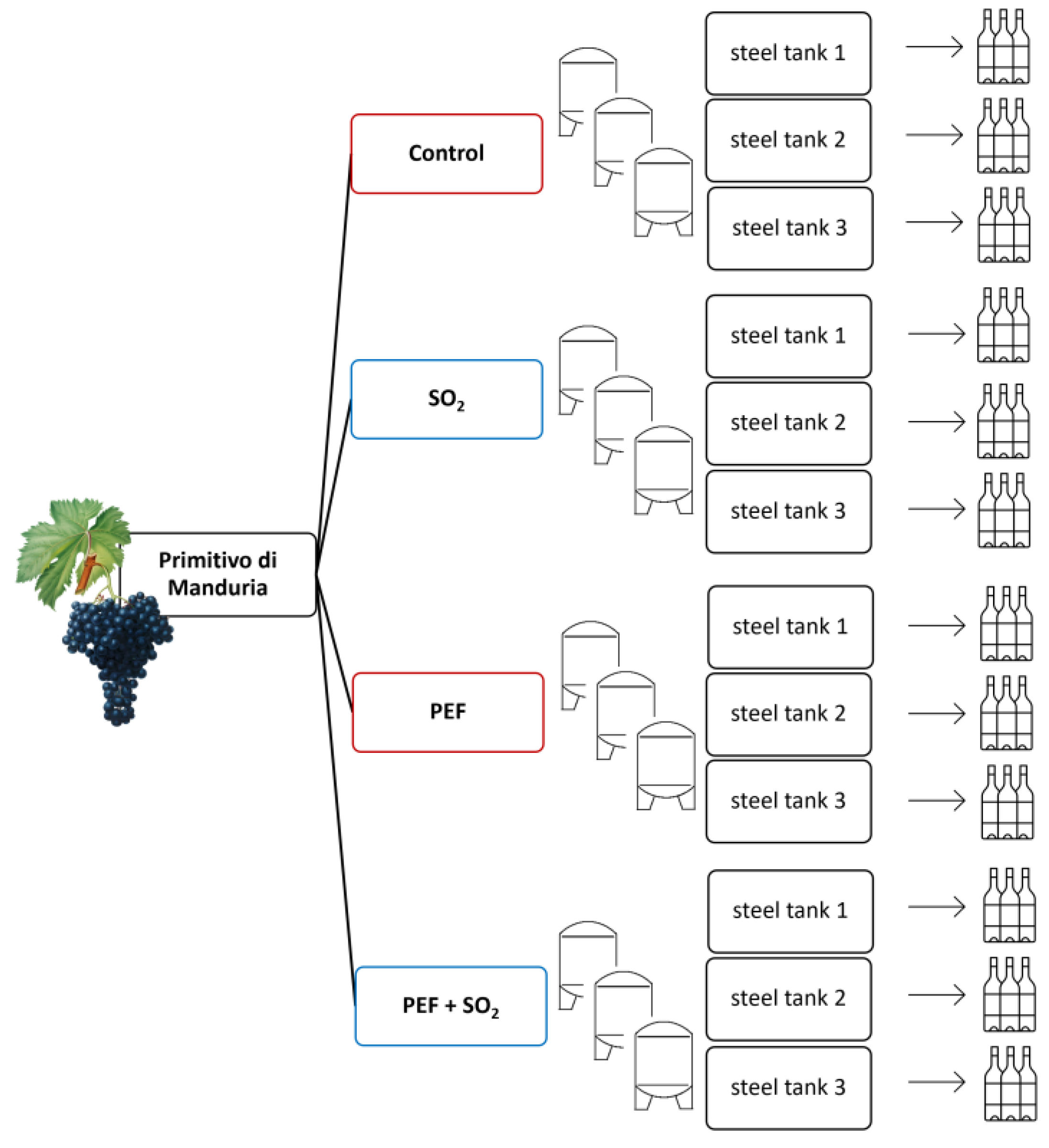
2.1. Grape Samples, PEF Equipment, and Processing
2.2. Must Characterization During Fermentation
2.2.1. Determination of Total Phenolic Content, Total Anthocyanins, Color Hue, and Color Intensity
2.3. Wine Characterization
2.3.1. General Oenological Parameters
2.3.2. Phenolic Composition and Color Indices
2.3.3. Antioxidant Activity Evaluation
2.3.4. Analysis of Phenolic Compounds by UHPLC-DAD-MS/MS
2.3.5. Analysis of Volatile Organic Compounds by SPME-GC/MS
2.3.6. Sensory Analysis
2.4. Statistical Analysis
3. Results and Discussion
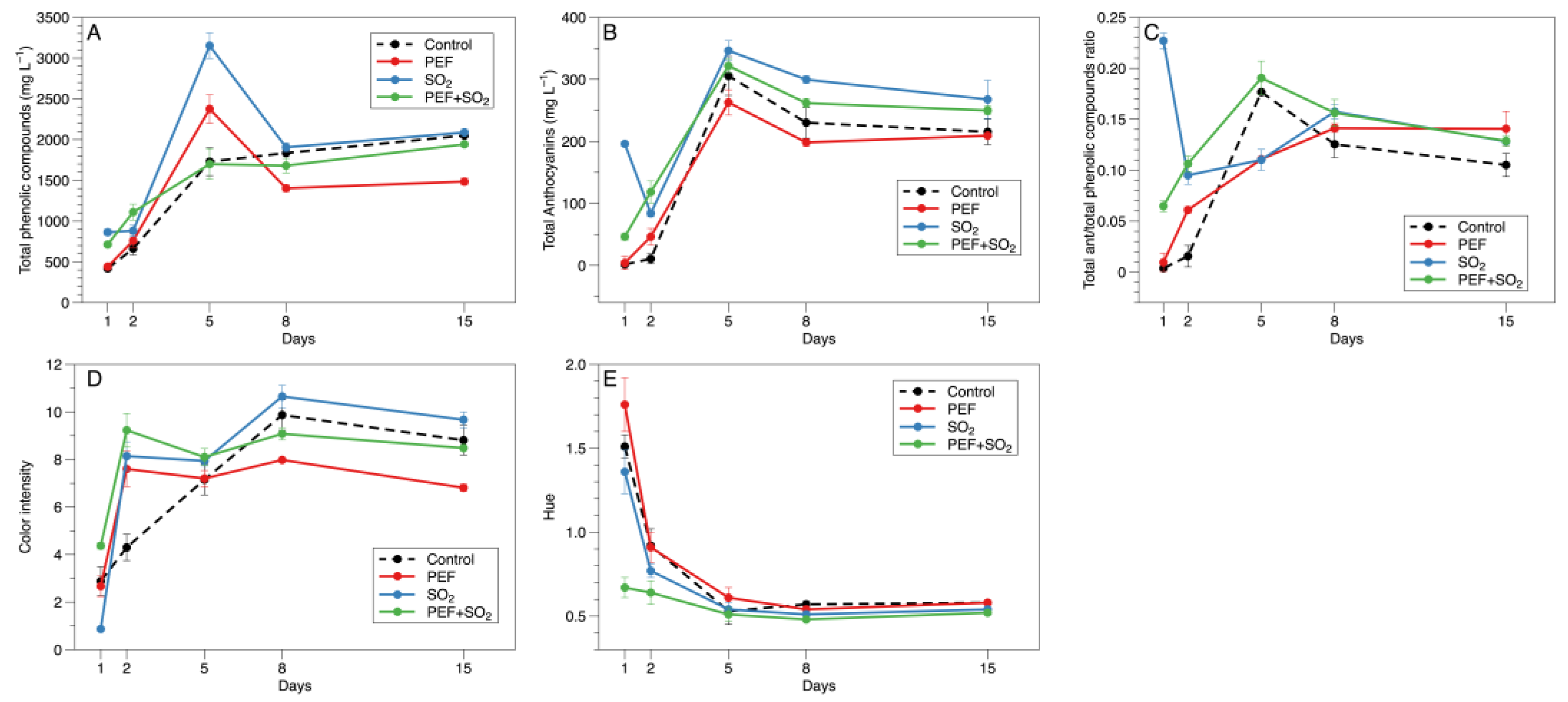
3.1. Color Trends During Maceration
3.2. Wine Characterization
3.2.1. Oenological Parameters
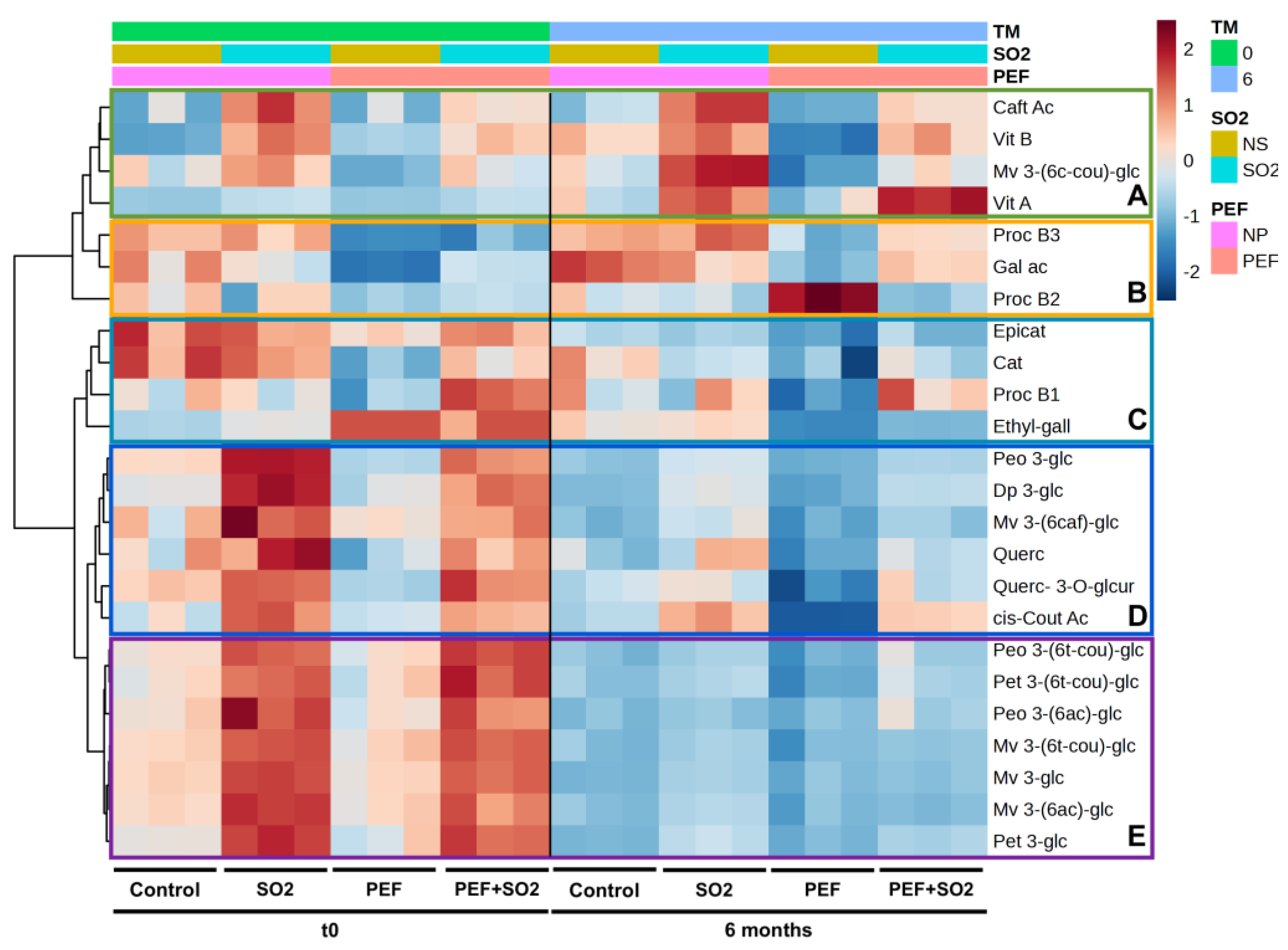
3.2.2. Phenolic Profile of Wines
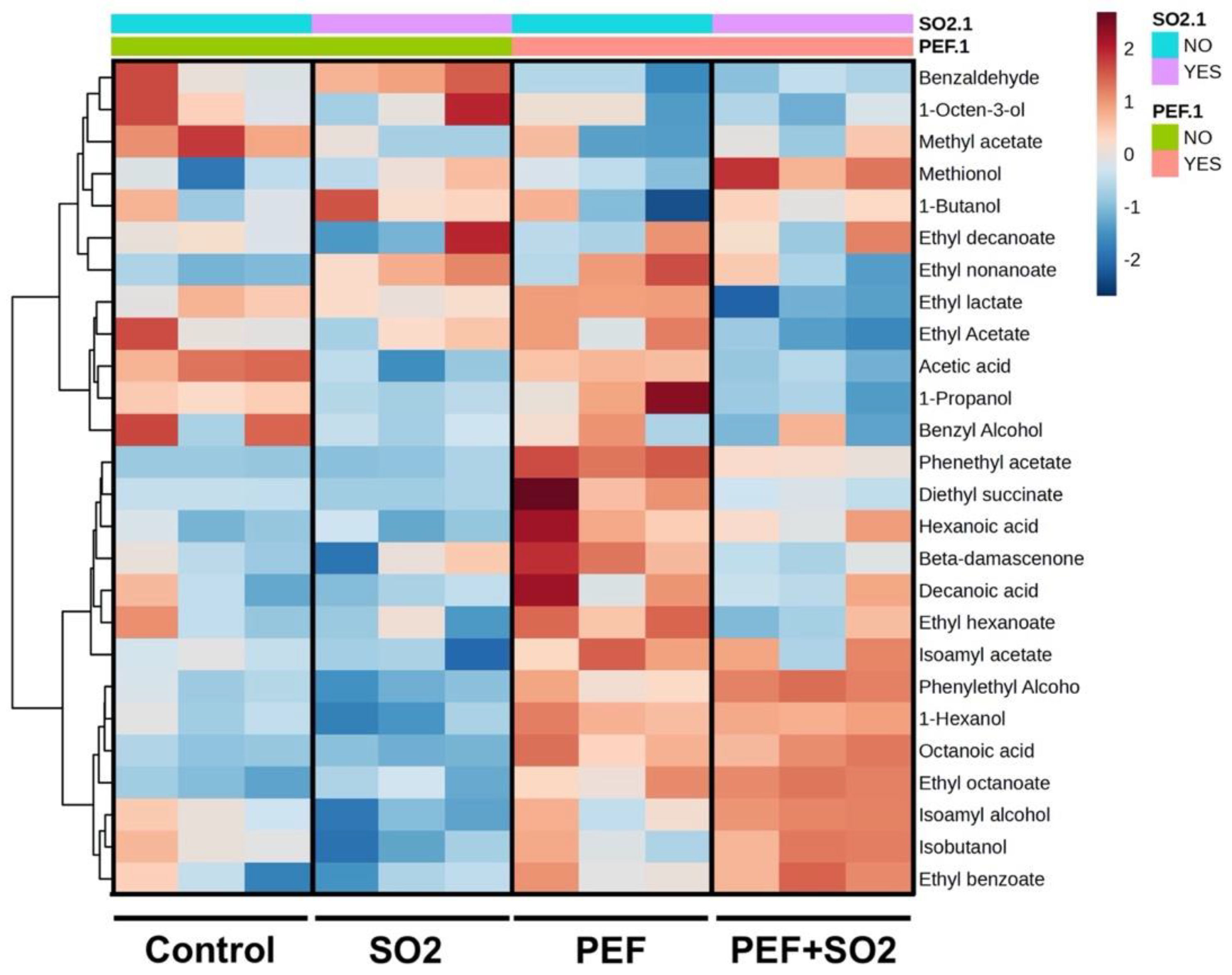
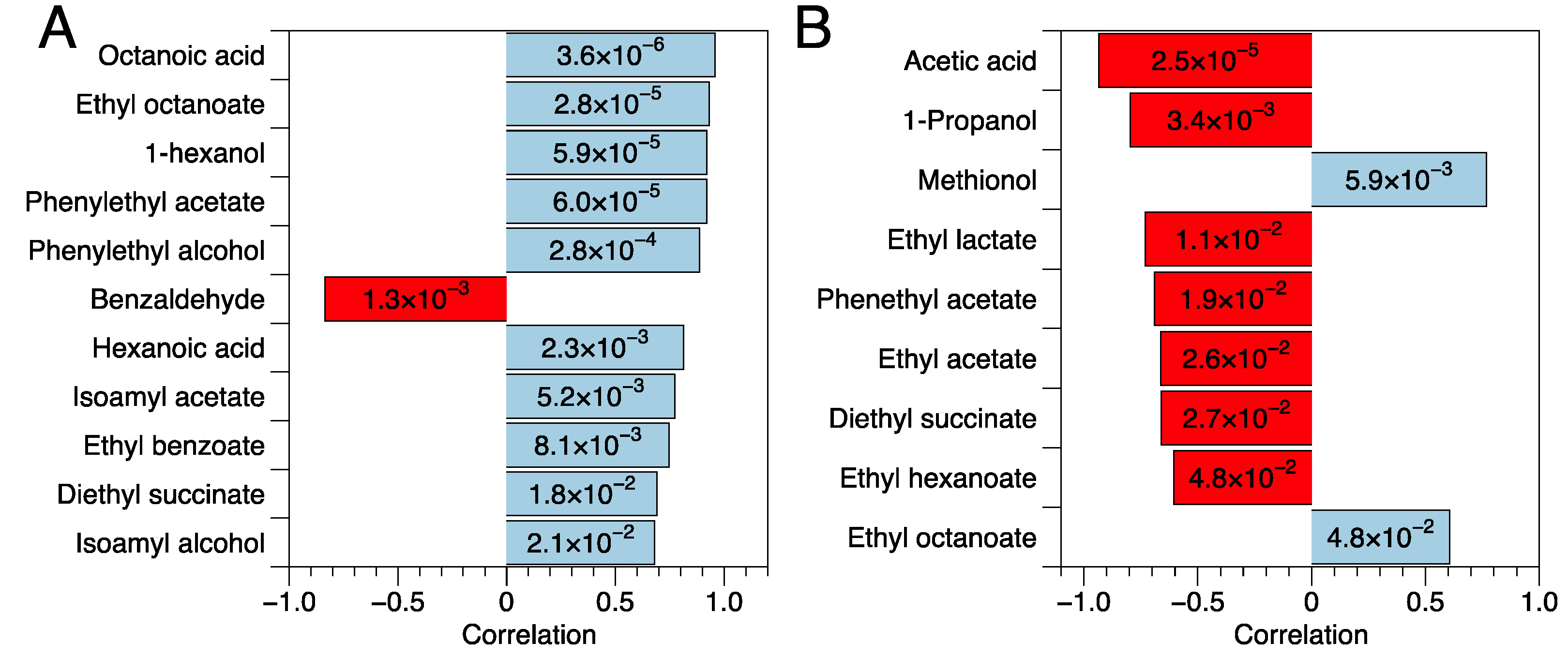
3.2.3. Volatile Profile
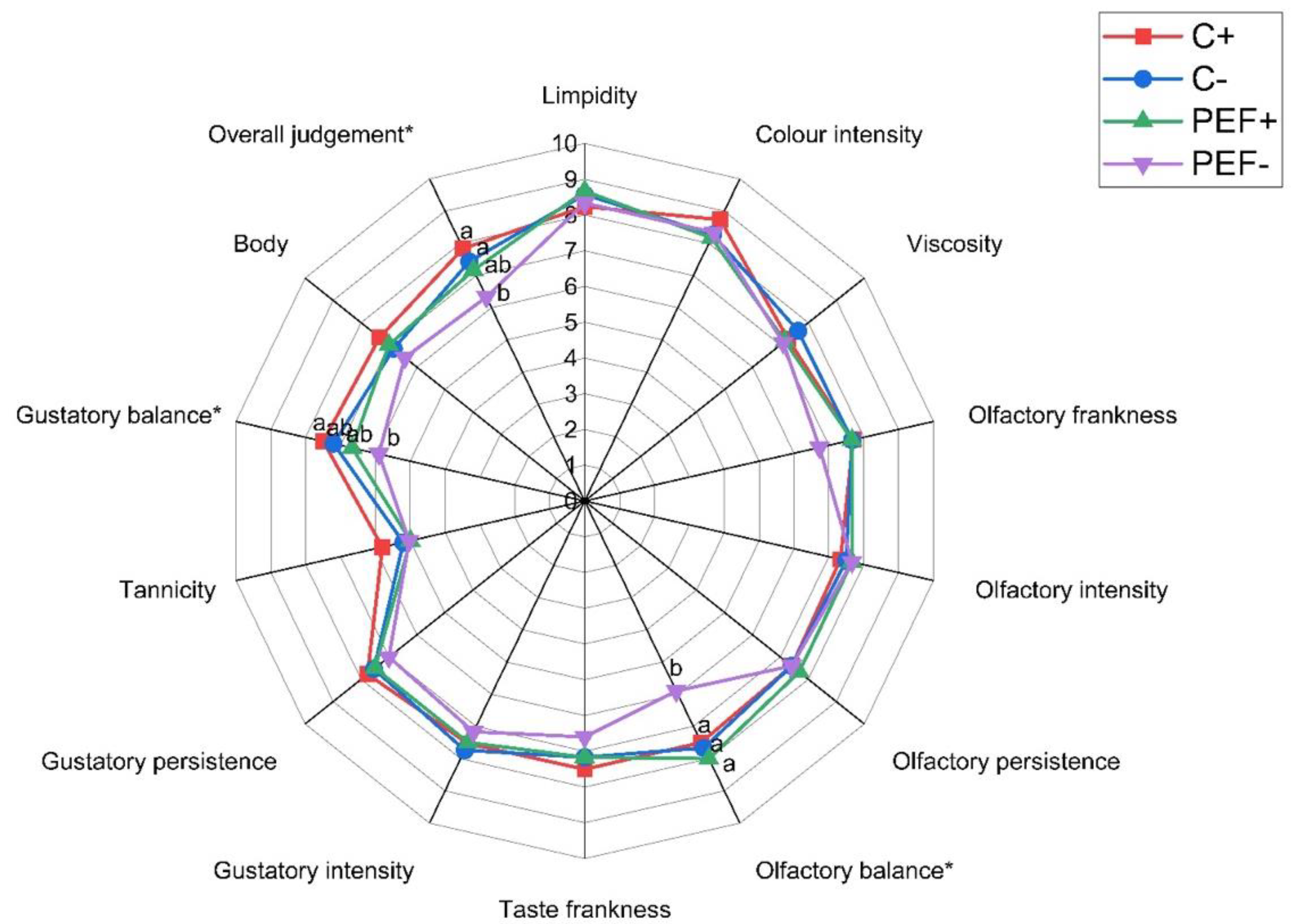
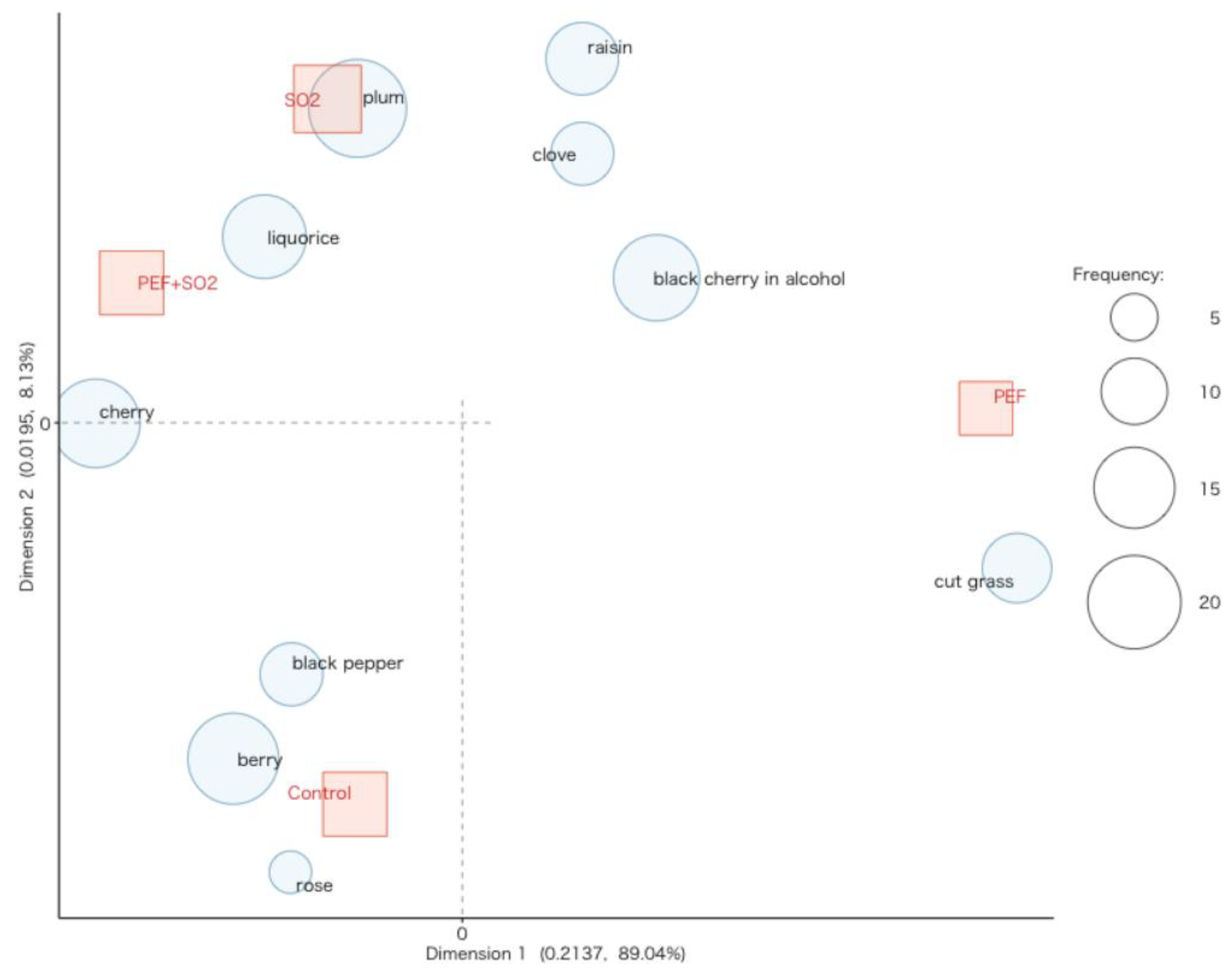
3.2.4. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krüger, R.T.; Alberti, A.; Nogueira, A. Current Technologies to Accelerate the Aging Process of Alcoholic Beverages: A Review. Beverages 2022, 8, 65. [Google Scholar] [CrossRef]
- Luchian, C.E.; Scutarașu, E.C.; Colibaba, L.C.; Motrescu, I.; Cotea, V.V. Non-Thermal and Thermal Physical Procedures—Optimistic Solutions in the Winemaking Industry. Appl. Sci. 2024, 14, 7537. [Google Scholar] [CrossRef]
- Ma, T.; Wang, J.; Wang, H.; Zhao, Q.; Zhang, F.; Ge, Q.; Li, C.; Gamboa, G.G.; Fang, Y.; Sun, X. Wine Aging and Artificial Simulated Wine Aging: Technologies, Applications, Challenges, and Perspectives. Food Res. Int. 2022, 153, 110953. [Google Scholar] [CrossRef]
- Maza, M.A.; Martínez, J.M.; Hernández-Orte, P.; Cebrián, G.; Sánchez-Gimeno, A.C.; Álvarez, I.; Raso, J. Influence of Pulsed Electric Fields on Aroma and Polyphenolic Compounds of Garnacha Wine. Food Bioprod. Process. 2019, 116, 249–257. [Google Scholar] [CrossRef]
- Noviello, M.; Paradiso, V.M.; Natrella, G.; Gambacorta, G.; Faccia, M.; Caponio, F. Application of Toasted Vine-Shoot Chips and Ultrasound Treatment in the Ageing of Primitivo Wine. Ultrason. Sonochemistry 2024, 104, 106826. [Google Scholar] [CrossRef]
- Silva, F.V.M.; Van Wyk, S. Emerging Non-Thermal Technologies as Alternative to SO2 for the Production of Wine. Foods 2021, 10, 2175. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Qureshi, M.I.; Khan, N.; Ahmad, M.H.; Liu, Z.; Aadil, R.M. Effective Valorization of Food Wastes and By-products through Pulsed Electric Field: A Systematic Review. J. Food Process Eng. 2021, 44, e13629. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Munir, M.A.; Naderipour, A.; Qureshi, M.I.; El-Din Bekhit, A.; Liu, Z.-W.; Aadil, R.M. Pulsed Electric Field: A Potential Alternative towards a Sustainable Food Processing. Trends Food Sci. Technol. 2021, 111, 43–54. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Munir, A.; Buntat, Z.; Ahmad, M.H.; Jusoh, Y.M.M.; Bekhit, A.E.-D.; Roobab, U.; Manzoor, M.F.; Aadil, R.M. Electrical Systems for Pulsed Electric Field Applications in the Food Industry: An Engineering Perspective. Trends Food Sci. Technol. 2020, 104, 1–13. [Google Scholar] [CrossRef]
- Brito, I.P.C.; Silva, E.K. Pulsed Electric Field Technology in Vegetable and Fruit Juice Processing: A Review. Food Res. Int. 2024, 184, 114207. [Google Scholar] [CrossRef]
- Naliyadhara, N.; Kumar, A.; Girisa, S.; Daimary, U.D.; Hegde, M.; Kunnumakkara, A.B. Pulsed Electric Field (PEF): Avant-Garde Extraction Escalation Technology in Food Industry. Trends Food Sci. Technol. 2022, 122, 238–255. [Google Scholar] [CrossRef]
- Roobab, U.; Inam-Ur-Raheem, M.; Khan, A.W.; Arshad, R.N.; Zeng, X.; Aadil, R.M. Innovations in High-Pressure Technologies for the Development of Clean Label Dairy Products: A Review. Food Rev. Int. 2023, 39, 970–991. [Google Scholar] [CrossRef]
- Cholet, C.; Delsart, C.; Petrel, M.; Gontier, E.; Grimi, N.; L’Hyvernay, A.; Ghidossi, R.; Vorobiev, E.; Mietton-Peuchot, M.; Gény, L. Structural and Biochemical Changes Induced by Pulsed Electric Field Treatments on Cabernet Sauvignon Grape Berry Skins: Impact on Cell Wall Total Tannins and Polysaccharides. J. Agric. Food Chem. 2014, 62, 2925–2934. [Google Scholar] [CrossRef]
- Martínez, J.M.; Cebrián, G.; Álvarez, I.; Raso, J. Release of Mannoproteins during Saccharomyces Cerevisiae Autolysis Induced by Pulsed Electric Field. Front. Microbiol. 2016, 7, 1435. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Maza, M.A.; Álvarez, I.; Raso, J. Pulsed Electric Fields Accelerate Release of Mannoproteins from Saccharomyces Cerevisiae during Aging on the Lees of Chardonnay Wine. Food Res. Int. 2019, 116, 795–801. [Google Scholar] [CrossRef]
- Maza, M.A.; Delso, C.; Álvarez, I.; Raso, J.; Martínez, J.M. Effect of Pulsed Electric Fields on Mannoproteins Release from Saccharomyces Cerevisiae during the Aging on Lees of Caladoc Red Wine. LWT 2020, 118, 108788. [Google Scholar] [CrossRef]
- González-Arenzana, L.; López-Alfaro, I.; Garde-Cerdán, T.; Portu, J.; López, R.; Santamaría, P. Microbial Inactivation and MLF Performances of Tempranillo Rioja Wines Treated with PEF after Alcoholic Fermentation. Int. J. Food Microbiol. 2018, 269, 19–26. [Google Scholar] [CrossRef]
- González-Arenzana, L.; Portu, J.; López, N.; Santamaría, P.; Gutiérrez, A.R.; López, R.; López-Alfaro, I. Pulsed Electric Field Treatment after Malolactic Fermentation of Tempranillo Rioja Wines: Influence on Microbial, Physicochemical and Sensorial Quality. Innov. Food Sci. Emerg. Technol. 2019, 51, 57–63. [Google Scholar] [CrossRef]
- Drosou, F.; Yang, E.; Marinea, M.; Dourtoglou, E.G.; Chatzilazarou, A.; Dourtoglou, V.G. An Assessment of Potential Applications with Pulsed Electric Field in Wines. BIO Web Conf. 2017, 9, 02010. [Google Scholar] [CrossRef]
- Puértolas, E.; Saldaña, G.; Álvarez, I.; Raso, J. Effect of Pulsed Electric Field Processing of Red Grapes on Wine Chromatic and Phenolic Characteristics during Aging in Oak Barrels. J. Agric. Food Chem. 2010, 58, 2351–2357. [Google Scholar] [CrossRef]
- Delso, C.; Berzosa, A.; Sanz, J.; Álvarez, I.; Raso, J. Microbial Decontamination of Red Wine by Pulsed Electric Fields (PEF) after Alcoholic and Malolactic Fermentation: Effect on Saccharomyces Cerevisiae, Oenococcus Oeni, and Oenological Parameters during Storage. Foods 2023, 12, 278. [Google Scholar] [CrossRef] [PubMed]
- Delso, C.; Ospina, S.; Berzosa, A.; Raso, J.; Álvarez-Lanzarote, I. Defining Winery Processing Conditions for the Decontamination of Must and Wine Spoilage Microbiota by Pulsed Electric Fields (PEF). Innov. Food Sci. Emerg. Technol. 2023, 89, 103478. [Google Scholar] [CrossRef]
- Puértolas, E.; López, N.; Condón, S.; Raso, J.; Álvarez, I. Pulsed Electric Fields Inactivation of Wine Spoilage Yeast and Bacteria. Int. J. Food Microbiol. 2009, 130, 49–55. [Google Scholar] [CrossRef]
- Morata, A.; González, C.; Tesfaye, W.; Loira, I.; Suárez-Lepe, J.A. Maceration and Fermentation. In Red Wine Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 35–49. ISBN 978-0-12-814399-5. [Google Scholar]
- Morata, A.; Escott, C.; Loira, I.; López, C.; Palomero, F.; González, C. Emerging Non-Thermal Technologies for the Extraction of Grape Anthocyanins. Antioxidants 2021, 10, 1863. [Google Scholar] [CrossRef]
- López-Giral, N.; López, R.; Santamaría, P.; González-Arenzana, L.; Garde-Cerdán, T. Phenolic and Colour Characteristics of Must and Wine Obtained from Red Grapes Treated by Pulsed Electric Fields. Efficacy of PEF to Reduce Maceration Time in Elaboration of Red Wines. Eur. Food Res. Technol. 2023, 249, 273–282. [Google Scholar] [CrossRef]
- Maza, M.A.; Pereira, C.; Martínez, J.M.; Camargo, A.; Álvarez, I.; Raso, J. PEF Treatments of High Specific Energy Permit the Reduction of Maceration Time during Vinification of Caladoc and Grenache Grapes. Innov. Food Sci. Emerg. Technol. 2020, 63, 102375. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D. Summary for Policymakers. In Climate Change 2013—The Physical Science Basis; Intergovernmental Panel On Climate Change, Ed.; Cambridge University Press: Cambridge, UK, 2014; pp. 1–30. ISBN 978-1-107-05799-9. [Google Scholar]
- Razungles, A. Extraction Technologies and Wine Quality. In Managing Wine Quality; Elsevier: Amsterdam, The Netherlands, 2010; pp. 589–630. ISBN 978-1-84569-798-3. [Google Scholar]
- Arcena, M.R.; Leong, S.Y.; Then, S.; Hochberg, M.; Sack, M.; Mueller, G.; Sigler, J.; Kebede, B.; Silcock, P.; Oey, I. The Effect of Pulsed Electric Fields Pre-Treatment on the Volatile and Phenolic Profiles of Merlot Grape Musts at Different Winemaking Stages and the Sensory Characteristics of the Finished Wines. Innov. Food Sci. Emerg. Technol. 2021, 70, 102698. [Google Scholar] [CrossRef]
- Comuzzo, P.; Voce, S.; Grazioli, C.; Tubaro, F.; Marconi, M.; Zanella, G.; Querzè, M. Pulsed Electric Field Processing of Red Grapes (Cv. Rondinella): Modifications of Phenolic Fraction and Effects on Wine Evolution. Foods 2020, 9, 414. [Google Scholar] [CrossRef]
- El Darra, N.; Rajha, H.N.; Ducasse, M.-A.; Turk, M.F.; Grimi, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Effect of Pulsed Electric Field Treatment during Cold Maceration and Alcoholic Fermentation on Major Red Wine Qualitative and Quantitative Parameters. Food Chem. 2016, 213, 352–360. [Google Scholar] [CrossRef]
- López-Giral, N.; González-Arenzana, L.; González-Ferrero, C.; López, R.; Santamaría, P.; López-Alfaro, I.; Garde-Cerdán, T. Pulsed Electric Field Treatment to Improve the Phenolic Compound Extraction from Graciano, Tempranillo and Grenache Grape Varieties during Two Vintages. Innov. Food Sci. Emerg. Technol. 2015, 28, 31–39. [Google Scholar] [CrossRef]
- López, N.; Puértolas, E.; Condón, S.; Álvarez, I.; Raso, J. Application of Pulsed Electric Fields for Improving the Maceration Process during Vinification of Red Wine: Influence of Grape Variety. Eur. Food Res. Technol. 2008, 227, 1099–1107. [Google Scholar] [CrossRef]
- Delso, C.; Berzosa, A.; Sanz, J.; Álvarez, I.; Raso, J. Pulsed Electric Field Processing as an Alternative to Sulfites (SO2) for Controlling Saccharomyces Cerevisiae Involved in the Fermentation of Chardonnay White Wine. Food Res. Int. 2023, 165, 112525. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; E Wrolstad, R.; Collaborators; Eisele, T.; Giusti, M.M.; Hofsommer, H.; Koswig, S.; A Krueger, D.; Kupina, S.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Glories, Y. La Couleur Des Vins Rouges. 2e Partie: Mesure, Origine et Interprétation. OENO One 1984, 18, 253–271. [Google Scholar] [CrossRef]
- Gambacorta, G.; Faccia, M.; Trani, A.; Lamacchia, C.; Gomes, T. Phenolic Composition and Antioxidant Activity of Southern Italian Monovarietal Virgin Olive Oils. Euro J. Lipid Sci. Technol. 2012, 114, 958–967. [Google Scholar] [CrossRef]
- Gambacorta, G.; Antonacci, D.; La Gatta, M.; Faccia, M.; La Gatta, B.; Pati, S.; Coletta, A.; La Notte, E. Phenolic Composition of Aglianico and Nero Di Troia Grapes and Wines as Affected by Cover Cropping and Irrigation. Ital. J. Food Sci. 2011, 23, 381–394. [Google Scholar]
- Sarneckis, C.J.; Dambergs, R.G.; Jones, P.; Mercurio, M.; Herderich, M.J.; Smith, P.A. Quantification of Condensed Tannins by Precipitation with Methyl Cellulose: Development and Validation of an Optimised Tool for Grape and Wine Analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Tarantino, A.; Difonzo, G.; Lopriore, G.; Disciglio, G.; Paradiso, V.M.; Gambacorta, G.; Caponio, F. Bioactive Compounds and Quality Evaluation of ‘Wonderful’ Pomegranate Fruit and Juice as Affected by Deficit Irrigation. J. Sci. Food Agric. 2020, 100, 5539–5545. [Google Scholar] [CrossRef]
- Ginjom, I.; D’Arcy, B.; Caffin, N.; Gidley, M. Phenolic Compound Profiles in Selected Queensland Red Wines at All Stages of the Wine-Making Process. Food Chem. 2011, 125, 823–834. [Google Scholar] [CrossRef]
- Marquez, A.; Dueñas, M.; Serratosa, M.P.; Merida, J. Identification by HPLC-MS of Anthocyanin Derivatives in Raisins. J. Chem. 2013, 2013, 274893. [Google Scholar] [CrossRef]
- Pérez-Navarro, J.; Izquierdo-Cañas, P.M.; Mena-Morales, A.; Martínez-Gascueña, J.; Chacón-Vozmediano, J.L.; García-Romero, E.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S. Phenolic Compounds Profile of Different Berry Parts from Novel Vitis Vinifera L. Red Grape Genotypes and Tempranillo Using HPLC-DAD-ESI-MS/MS: A Varietal Differentiation Tool. Food Chem. 2019, 295, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Topi, D.; Kelebek, H.; Guclu, G.; Selli, S. LC-DAD-ESI-MS/MS Characterization of Phenolic Compounds in Wines from Vitis Vinifera ‘ Shesh i Bardhë ’ and ‘ Vlosh ’ Cultivars. Food Process. Preserv. 2022, 46, e16157. [Google Scholar] [CrossRef]
- Zhang, X.-K.; Li, S.-Y.; Zhao, X.; Pan, Q.-H.; Shi, Y.; Duan, C.-Q. HPLC-MS/MS-Based Targeted Metabolomic Method for Profiling of Malvidin Derivatives in Dry Red Wines. Food Res. Int. 2020, 134, 109226. [Google Scholar] [CrossRef]
- Prezioso, I.; Fioschi, G.; Rustioni, L.; Mascellani, M.; Natrella, G.; Venerito, P.; Gambacorta, G.; Paradiso, V.M. Influence of Prolonged Maceration on Phenolic Compounds, Volatile Profile and Sensory Properties of Wines from Minutolo and Verdeca, Two Apulian White Grape Varieties. LWT 2024, 192, 115698. [Google Scholar] [CrossRef]
- Giacosa, S.; Río Segade, S.; Cagnasso, E.; Caudana, A.; Rolle, L.; Gerbi, V. SO2 in Wines. In Red Wine Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 309–321. ISBN 978-0-12-814399-5. [Google Scholar]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology, Vol. 1: The Microbiology of Wine and Vinifications, 2nd ed; John Wiley & Sons, Limited: West Sussex, UK, 2006; Volume 1. [Google Scholar]
- Osete-Alcaraz, A.; Gómez-Plaza, E.; Martínez-Pérez, P.; Weiller, F.; Schückel, J.; Willats, W.G.T.; Moore, J.P.; Ros-García, J.M.; Bautista-Ortín, A.B. The Influence of Hydrolytic Enzymes on Tannin Adsorption-Desorption onto Grape Cell Walls in a Wine-Like Matrix. Molecules 2021, 26, 770. [Google Scholar] [CrossRef]
- Osete-Alcaraz, A.; Gómez-Plaza, E.; Martínez-Pérez, P.; Weiller, F.; Schückel, J.; Willats, W.G.T.; Moore, J.P.; Ros-García, J.M.; Bautista-Ortín, A.B. The Impact of Carbohydrate-Active Enzymes on Mediating Cell Wall Polysaccharide-Tannin Interactions in a Wine-like Matrix. Food Res. Int. 2020, 129, 108889. [Google Scholar] [CrossRef]
- Pérez-Porras, P.; Bautista-Ortín, A.B.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Gómez-Plaza, E. The Generation of Suspended Cell Wall Material May Limit the Effect of Ultrasound Technology in Some Varietal Wines. Foods 2024, 13, 1306. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Du Toit, W. Cold Maceration Application in Red Wine Production and Its Effects on Phenolic Compounds: A Review. LWT 2018, 95, 200–208. [Google Scholar] [CrossRef]
- Delso, C.; Berzosa, A.; Sanz, J.; Álvarez, I.; Raso, J. Two-Step PEF Processing for Enhancing the Polyphenol Concentration and Decontaminating a Red Grape Juice. Foods 2022, 11, 621. [Google Scholar] [CrossRef]
- Pérez-Porras, P.; Gómez Plaza, E.; Martínez-Lapuente, L.; Ayestarán, B.; Guadalupe, Z.; Jurado, R.; Bautista-Ortín, A.B. High-Power Ultrasound in Enology: Is the Outcome of This Technique Dependent on Grape Variety? Foods 2023, 12, 2236. [Google Scholar] [CrossRef]
- Ricci, A.; Parpinello, G.; Versari, A. Recent Advances and Applications of Pulsed Electric Fields (PEF) to Improve Polyphenol Extraction and Color Release during Red Winemaking. Beverages 2018, 4, 18. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Fioschi, G.; Tripaldi, M.; Sanarica, L.; Pisarra, C.; Noviello, M.; Prezioso, I.; Gambacorta, G. Low-Pressure Continuous Dynamic Extraction from Oak Chips Combined with Passive Micro-Oxygenation to Tune Red Wine Properties. Heliyon 2024, 10, e36100. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y. Dealcoholized Wine: A Scoping Review of Volatile and Non-Volatile Profiles, Consumer Perception, and Health Benefits. Food Bioprocess Technol. 2024, 17, 3525–3545. [Google Scholar] [CrossRef]
- Akhtar, W.; Ceci, A.T.; Longo, E.; Marconi, M.A.; Lonardi, F.; Boselli, E. Dealcoholized Wine: Techniques, Sensory Impacts, Stability, and Perspectives of a Growing Industry. Comp. Rev. Food Sci. Food Safe 2025, 24, e70171. [Google Scholar] [CrossRef]
- Stirke, A.; Celiesiute-Germaniene, R.; Zimkus, A.; Zurauskiene, N.; Simonis, P.; Dervinis, A.; Ramanavicius, A.; Balevicius, S. The Link between Yeast Cell Wall Porosity and Plasma Membrane Permeability after PEF Treatment. Sci. Rep. 2019, 9, 14731. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; González-Arenzana, L.; López, N.; López, R.; Santamaría, P.; López-Alfaro, I. Effect of Different Pulsed Electric Field Treatments on the Volatile Composition of Graciano, Tempranillo and Grenache Grape Varieties. Innov. Food Sci. Emerg. Technol. 2013, 20, 91–99. [Google Scholar] [CrossRef]
- Delsart, C.; Cholet, C.; Ghidossi, R.; Grimi, N.; Gontier, E.; Gény, L.; Vorobiev, E.; Mietton-Peuchot, M. Effects of Pulsed Electric Fields on Cabernet Sauvignon Grape Berries and on the Characteristics of Wines. Food Bioprocess. Technol. 2014, 7, 424–436. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; Wiley: Chichester, UK, 2016; ISBN 978-1-118-62780-8. [Google Scholar]
- Prezioso, I.; Corcione, G.; Digiorgio, C.; Fioschi, G.; Paradiso, V.M. Ageing of Red Wine (Cv. Negroamaro) in Mediterranean Areas: Impact of Different Barrels and Apulian Traditional Amphorae on Phenolic Indices, Volatile Composition and Sensory Analysis. Foods 2025, 14, 650. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Calderón, F.; Suárez, J.A. Effects of pH, Temperature and SO2 on the Formation of Pyranoanthocyanins during Red Wine Fermentation with Two Species of Saccharomyces. Int. J. Food Microbiol. 2006, 106, 123–129. [Google Scholar] [CrossRef]
- Bakker, J.; Timberlake, C.F. Isolation, Identification, and Characterization of New Color-Stable Anthocyanins Occurring in Some Red Wines. J. Agric. Food Chem. 1997, 45, 35–43. [Google Scholar] [CrossRef]
- Morata, A.; Calderón, F.; González, M.C.; Gómez-Cordovés, M.C.; Suárez, J.A. Formation of the Highly Stable Pyranoanthocyanins (Vitisins A and B) in Red Wines by the Addition of Pyruvic Acid and Acetaldehyde. Food Chem. 2007, 100, 1144–1152. [Google Scholar] [CrossRef]
- Asenstorfer, R.E.; Markides, A.J.; Iland, P.G.; Jones, G.P. Formation of Vitisin A during Red Wine Fermentation and Maturation. Aust. J. Grape Wine Res. 2003, 9, 40–46. [Google Scholar] [CrossRef]
- Jouin, A.; Zeng, L.; Canosa, M.R.; Teissedre, P.-L.; Jourdes, M. Evolution of the Crown Procyanidins’ Tetramer during Winemaking and Aging of Red Wine. Foods 2022, 11, 3194. [Google Scholar] [CrossRef]
- Malien-Aubert, C.; Dangles, O.; Amiot, M.J. Influence of Procyanidins on the Color Stability of Oenin Solutions. J. Agric. Food Chem. 2002, 50, 3299–3305. [Google Scholar] [CrossRef]
- Escribano-Bailón, T.; Álvarez-García, M.; Rivas-Gonzalo, J.C.; Heredia, F.J.; Santos-Buelga, C. Color and Stability of Pigments Derived from the Acetaldehyde-Mediated Condensation between Malvidin 3- O -Glucoside and (+)-Catechin. J. Agric. Food Chem. 2001, 49, 1213–1217. [Google Scholar] [CrossRef]
- Bakker, J.; Bridle, P.; Bellworthy, S.J.; Garcia-Viguera, C.; Reader, H.P.; Watkins, S.J. Effect of Sulphur Dioxide and Must Extraction on Colour, Phenolic Composition and Sensory Quality of Red Table Wine. J. Sci. Food Agric. 1998, 78, 297–307. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Gil-Muñoz, R.; López-Roca, J.M.; Martı́nez-Cutillas, A.; Fernández-Fernández, J.I. Maintenance of Colour Composition of a Red Wine During Storage. Influence of Prefermentative Practices, Maceration Time and Storage. LWT-Food Sci. Technol. 2002, 35, 46–53. [Google Scholar] [CrossRef]
- Maza, M.A.; Martínez, J.M.; Delso, C.; Camargo, A.; Raso, J.; Álvarez, I. PEF-Dependency on Polyphenol Extraction during Maceration/Fermentation of Grenache Grapes. Innov. Food Sci. Emerg. Technol. 2020, 60, 102303. [Google Scholar] [CrossRef]
- Fourie, E.; Aleixandre-Tudo, J.L.; Mihnea, M.; Du Toit, W. Partial Least Squares Calibrations and Batch Statistical Process Control to Monitor Phenolic Extraction in Red Wine Fermentations under Different Maceration Conditions. Food Control. 2020, 115, 107303. [Google Scholar] [CrossRef]
- González-Neves, G.; Gil, G.; Barreiro, L. Influence of Grape Variety on the Extraction of Anthocyanins during the Fermentation on Skins. Eur. Food Res. Technol. 2008, 226, 1349–1355. [Google Scholar] [CrossRef]
- Saldaña, G.; Cebrián, G.; Abenoza, M.; Sánchez-Gimeno, C.; Álvarez, I.; Raso, J. Assessing the Efficacy of PEF Treatments for Improving Polyphenol Extraction during Red Wine Vinifications. Innov. Food Sci. Emerg. Technol. 2017, 39, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Natrella, G.; Noviello, M.; Trani, A.; Faccia, M.; Gambacorta, G. The Effect of Ultrasound Treatment in Winemaking on the Volatile Compounds of Aglianico, Nero Di Troia, and Primitivo Red Wines. Foods 2023, 12, 648. [Google Scholar] [CrossRef] [PubMed]
- Riffle, V.L.; Arredondo, J.A.; LoMonaco, I.; Appel, C.; Catania, A.A.; Dodson Peterson, J.C.; Casassa, L.F. Vine Age Affects Vine Performance, Grape and Wine Chemical and Sensory Composition of Cv. Zinfandel from California. Am. J. Enol. Vitic. 2022, 73, 277–293. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.-C.; Van Leeuwen, C.; Dubourdieu, D. Which Impact for β-Damascenone on Red Wines Aroma? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef]
- Romano, P.; Braschi, G.; Siesto, G.; Patrignani, F.; Lanciotti, R. Role of Yeasts on the Sensory Component of Wines. Foods 2022, 11, 1921. [Google Scholar] [CrossRef]
- Reynolds, A.G. Viticultural Practices and Their Effects on Grape and Wine Quality. In The World of Niagara Wine; Ripmeester, M., Mackintosh, P.G., Fullerton, C., Eds.; Wilfrid Laurier Press: Waterloo, ON, USA, 2013; pp. 185–212. ISBN 978-1-55458-405-5. [Google Scholar]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Review of Quality Factors on Wine Ageing in Oak Barrels. Trends Food Sci. Technol. 2006, 17, 438–447. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.-M.; Van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich Pathway for Fusel Alcohol Production: A Century of Research on Saccharomyces Cerevisiae Metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef]
- Lisanti, M.T.; Blaiotta, G.; Nioi, C.; Moio, L. Alternative Methods to SO2 for Microbiological Stabilization of Wine. Comp. Rev. Food Sci. Food Safe 2019, 18, 455–479. [Google Scholar] [CrossRef]
- Dennis, E.G.; Keyzers, R.A.; Kalua, C.M.; Maffei, S.M.; Nicholson, E.L.; Boss, P.K. Grape Contribution to Wine Aroma: Production of Hexyl Acetate, Octyl Acetate, and Benzyl Acetate during Yeast Fermentation Is Dependent upon Precursors in the Must. J. Agric. Food Chem. 2012, 60, 2638–2646. [Google Scholar] [CrossRef]
- Moio, L.; Ugliano, M.; Genovese, A.; Gambuti, A.; Pessina, R.; Piombino, P. Effect of Antioxidant Protection of Must on Volatile Compounds and Aroma Shelf Life of Falanghina (Vitis Vinifera L.) Wine. J. Agric. Food Chem. 2004, 52, 891–897. [Google Scholar] [CrossRef]
- Santos, C.V.A.; Pereira, C.; Martins, N.; Cabrita, M.J.; Gomes Da Silva, M. Different SO2 Doses and the Impact on Amino Acid and Volatile Profiles of White Wines. Beverages 2023, 9, 33. [Google Scholar] [CrossRef]
- Sonni, F.; Cejudo Bastante, M.J.; Chinnici, F.; Natali, N.; Riponi, C. Replacement of Sulfur Dioxide by Lysozyme and Oenological Tannins during Fermentation: Influence on Volatile Composition of White Wines. J. Sci. Food Agric. 2009, 89, 688–696. [Google Scholar] [CrossRef]
- Sonni, F.; Chinnici, F.; Natali, N.; Riponi, C. Pre-Fermentative Replacement of Sulphur Dioxide by Lysozyme and Oenological Tannins: Effect on the Formation and Evolution of Volatile Compounds during the Bottle Storage of White Wines. Food Chem. 2011, 129, 1193–1200. [Google Scholar] [CrossRef]
- Allamy, L.; Van Leeuwen, C.; Pons, A. Impact of Harvest Date on Aroma Compound Composition of Merlot and Cabernet-Sauvignon Must and Wine in a Context of Climate Change: A Focus on Cooked Fruit Molecular Markers. OENO One 2023, 57, 99–112. [Google Scholar] [CrossRef]
| At Bottling | 6 Months | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | SO2 | PEF + SO2 | PEF | Control | SO2 | PEF + SO2 | PEF | |
| E (% v/v) b | 16.51 ± 0.29 a | 16.7 ± 0.1 a | 15.77 ± 0.02 b | 15.38 ± 0.11 b | 16.53 ± 0.37 a | 16.88 ± 0.08 a | 15.87 ± 0.02 b | 15.51 ± 0.10 b |
| VA (g L−1) | 0.51 ± 0.04 bc | 0.44 ± 0.01 a b | 0.41 ± 0.00 c | 0.56 ± 0.05 a | 0.67 ± 0.02 a | 0.51 ± 0.02 a | 0.55 ± 0.08 a | 1.02 ± 0.41 a |
| TA (g L−1) | 4.95 ± 0.12 a b | 4.97 ± 0.05 a b | 5.12 ± 0.03 a | 4.81 ± 0.06 b | 4.69 ± 0.01 a | 4.77 ± 0.02 a | 4.73 ± 0.11 a | 5.46 ± 0.84 a |
| pH | 3.87+0.01 a | 3.86 ± 0.01 a b | 3.80 ± 0.01 c | 3.84 ± 0.01 b | 3.89 ± 0.02 a | 3.84 ± 0.02 a b | 3.79 ± 0.02 b | 3.78 ± 0.06 b |
| MA (g L−1) | 0.12 ± 0.02 bc | 0.19 ± 0.04 a b | 0.23 ± 0.03 a | 0.06 ± 0.01 c | 0.02 ± 0.02 a | 0.07 ± 0.01 a | 0.05 ± 0.05 a | 0.04 ± 0.02 a |
| LacA (g L−1) | 0.69 ± 0.01 a | 0.66 ± 0.03 a | 0.51 ± 0.02 b | 0.71 ± 0.09 a | 0.86 ± 0.03 a b | 0.67 ± 0.03 b | 0.69 ± 0.07 b | 1.59 ± 0.67 a |
| TarA (g L−1) | 2.41 ± 0.01 b | 2.59 ± 0.01 a | 2.32 ± 0.01 b | 2.31 ± 0.09 b | 2.06 ± 0.07 a b | 2.22 ± 0.08 a | 1.82 ± 0.04 c | 1.84 ± 0.14 bc |
| CitA (g L−1) | 0.14 ± 0.01 b | 0.15 ± 0.01 b | 0.17 ± 0.01 a | 0.13 ± 0.01 b | 0.09 ± 0.01 b | 0.13 ± 0.01 a b | 0.14 ± 0.02 a | 0.13 ± 0.02 a b |
| Ashes (g L−1) | 3.11 ± 0.06 b | 3.30 ± 0.01 a | 3.04 ± 0.03 bc | 3.01 ± 0.02 c | 3.12 ± 0.10 a | 3.18 ± 0.09 a | 2.85 ± 0.02 a b | 2.61 ± 0.28 b |
| Free SO2 (mg L−1) | 12.0 ± 1.0 b | 16.5 ± 0.5 a | 15.3 ± 0.6 a | 11.7 ± 0.6 b | 9.5 ± 0.7 b | 13.7 ± 1.2 a | 12.0 ± 1.0 a | 8.7 ± 0.6 b |
| Total SO2 (mg L−1) | 21.0 ± 1.0 b | 34.5 ± 0.5 a | 34.3 ± 2.3 a | 20.7 ± 0.6 b | 16.0 ± 3.0 b | 26.3 ± 2.5 a | 24.0 ± 1.7 a | 16.3 ± 1.5 b |
| TPC (mg L−1 gallic acid) | 2774.1 ± 70.8 a | 2777.7 ± 67.9 a | 2504.1 ± 46.7 b | 1970.8 ± 30.2 c | 2559.1 ± 26.4 a | 2932.2 ± 242.4 a | 2522.2 ± 111.8 a | 1941.9 ± 55.5 b |
| DPPH (µmol troloxeq L−1) | 9863.1 ± 111.4 a | 10,103.0 ± 104.5 a | 8368.2 ± 51.6 b | 6499.5 ± 272.6 c | 8979.3 ± 283.6 a | 9037.4 ± 69.6 a | 9110.6 ± 309.1 a | 7714.1 ± 721.1 a |
| ABTS (µmol troloxeq L−1) | 11,488.9 ± 375.4 a | 11,627.8 ± 677.9 a | 10,946.3 ± 373.4 a | 8725.9 ± 375.2 b | 11,975.9 ± 74.9 a | 11,731.5 ± 86.1 a | 11,998.1 ± 396.5 a | 8742.6 ± 456.8 b |
| A (mg L−1) | 360.2 ± 51.1 b | 445.5 ± 49.4 a b | 457.5 ± 19.4 a | 300.5 ± 6.7 c | 226.07 ± 11.1 b | 297.4 ± 4.0 a | 260.2 ± 32.4 a b | 188.5 ± 6.8 c |
| F (mg L−1 (+)-Catechin) | 1866.5 ± 84.5 a | 1870 ± 150.8 a | 1664.2 ± 74.1 a | 1339.6 ± 53.9 b | 1646.6 ± 51.5 a | 1681.3 ± 17.2 a | 1433.7 ± 58.0 b | 1114.0 ± 38.37 c |
| Pr (mg L−1 Cyanidin Chloride) | 1470.9 ± 142.7 a | 15,519 ± 38.2 a | 1481.1 ± 166.0 a | 1119.3 ± 40.8 b | 1664.0 ± 62 a | 1783.4 ± 162.1 a | 1641.7 ± 80.5 a | 1182.0 ± 38.3 b |
| Tan (mg L−1 Epicatechin) | 1569.6 ± 101.4 b | 1538.7 ± 23.8 b | 1847.9 ± 155.2 a | 1099.2 ± 29.1 c | 1625.8 ± 90.3 a | 1618.7 ± 142.5 a | 1292.5 ± 56.9 b | 984.6 ± 67.4 c |
| FRV (mg L−1 (+)-Catechin) | 764.9 ± 80.0 a | 628.9 ± 52.3 a b | 496.1 ± 593.4 a b | 498.2 ± 75.7 b | 620.5 ± 39.7 a | 667.3 ± 31.0 a | 373.7 ± 397.6 b | 364.6 ± 96.3 b |
| FRV/Pr | 0.52 ± 0.04 a | 0.41 ± 0.03 a | 0.40 ± 0.08 a | 0.44 ± 0.06 a | 0.37 ± 0.03 a b | 0.38 ± 0.02 a | 0.24 ± 0.02 b | 0.31 ± 0.09 a b |
| CI | 0.92 ± 0.03 b | 1.08 ± 0.02 a | 0.89 ± 0.04 b | 0.67 ± 0.03 c | 0.91 ± 0.04 b | 1.12 ± 0.05 a | 0.90 ± 0.07 b | 0.68 ± 0.02 c |
| T | 0.69 ± 0.01 a | 0.63 ± 0.01 b | 0.64 ± 0.01 b | 0.71 ± 0.01 a | 0.74 ± 0.01 a | 0.66 ± 0.01 b | 0.67 ± 0.01 b | 0.72 ± 0.01 a |
| DO420 (%) | 35.64 ± 0.06 b | 33.75 ± 0.39 b | 34.01 ± 0.18 a | 35.97 ± 0.45 a | 37.07 ± 0.05 a | 34.41 ± 0.28 b | 35.09 ± 0.38 b | 36.64 ± 0.58 a |
| DO520 (%) | 68.92 ± 1.37 b | 90.61 ± 4.12 a | 73.47 ± 2.41 b | 48.48 ± 2.02 c | 61.47 ± 2.61 b | 88.48 ± 5.89 a | 69.60 ± 7.32 b | 47.16 ± 1.86 c |
| DO620 (%) | 0.33 ± 0.02 b | 0.42 ± 0.01 a | 0.34 ± 0.03 b | 0.24 ± 0.02 c | 0.31 ± 0.02 b | 0.43 ± 0.02 a | 0.33 ± 0.03 b | 0.24 ± 0.02 c |
| dA (%) | 77.83 ± 0.09 b | 80.53 ± 0.44 a | 80.15 ± 0.23 a | 77.35 ± 0.81 b | 75.78 ± 0.09 b | 79.86 ± 0.36 a | 78.61 ± 0.49 a | 76.42 ± 1.17 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noviello, M.; Caponio, G.R.; Corcione, G.; Tarricone, L.; Milella, R.A.; Mazzone, F.; Mescia, L.; Lamacchia, C.M.; Makhlouf, F.Z.; Tripaldi, M.; et al. Beyond Color Extraction: How Pulsed Electric Fields and Sulfites Affect Phenolic and Volatile Compounds of Primitivo Red Wine. Foods 2025, 14, 1792. https://doi.org/10.3390/foods14101792
Noviello M, Caponio GR, Corcione G, Tarricone L, Milella RA, Mazzone F, Mescia L, Lamacchia CM, Makhlouf FZ, Tripaldi M, et al. Beyond Color Extraction: How Pulsed Electric Fields and Sulfites Affect Phenolic and Volatile Compounds of Primitivo Red Wine. Foods. 2025; 14(10):1792. https://doi.org/10.3390/foods14101792
Chicago/Turabian StyleNoviello, Mirella, Giusy Rita Caponio, Giuseppe Corcione, Luigi Tarricone, Rosa Anna Milella, Francesco Mazzone, Luciano Mescia, Claudio Maria Lamacchia, Fatima Zohra Makhlouf, Massimo Tripaldi, and et al. 2025. "Beyond Color Extraction: How Pulsed Electric Fields and Sulfites Affect Phenolic and Volatile Compounds of Primitivo Red Wine" Foods 14, no. 10: 1792. https://doi.org/10.3390/foods14101792
APA StyleNoviello, M., Caponio, G. R., Corcione, G., Tarricone, L., Milella, R. A., Mazzone, F., Mescia, L., Lamacchia, C. M., Makhlouf, F. Z., Tripaldi, M., Paradiso, V. M., Fioschi, G., & Squeo, G. (2025). Beyond Color Extraction: How Pulsed Electric Fields and Sulfites Affect Phenolic and Volatile Compounds of Primitivo Red Wine. Foods, 14(10), 1792. https://doi.org/10.3390/foods14101792









