Evaluation of Dietary Bioactive Agents Against Aflatoxin B1 and Ochratoxin A-Induced Duodenal Toxicity in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. In Vivo Study and Experimental Protocol
2.3. RNA Extraction
2.4. Reverse Transcription and qPCR Parameters
2.5. Statyistical Analysis
3. Results
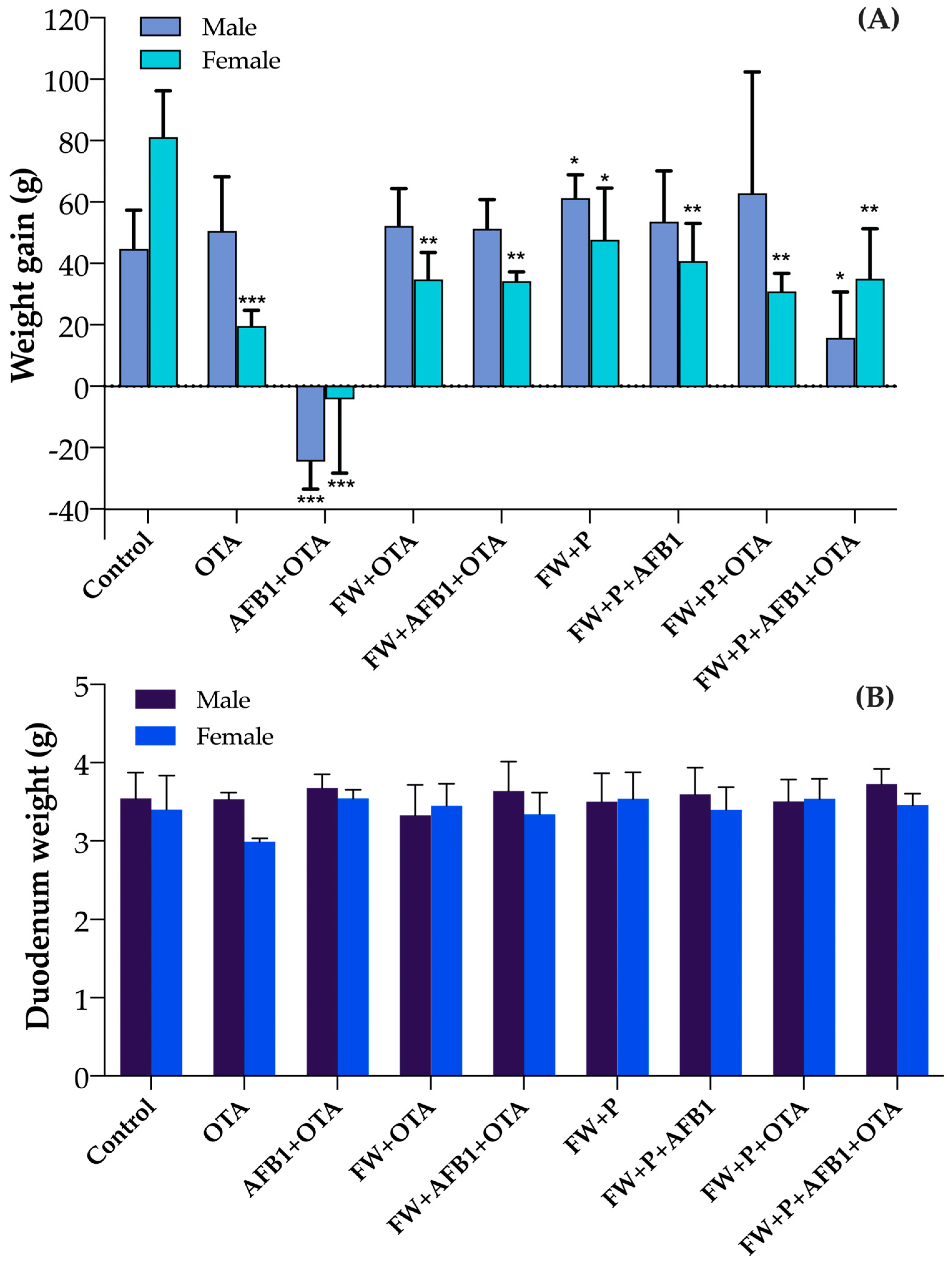
3.1. Body Weight Gain and Duodenum Variations in Rats
3.2. Differential Gene Expression of Apoptosis Key Genes Resulting from Exposure to OTA and FW
3.3. Differential Gene Expression of Apoptosis Key Genes Resulting from Exposure to AFB1 + OTA and FW
3.4. Differential Gene Expression of Apoptosis Key Genes Resulting from Exposure to FW + P
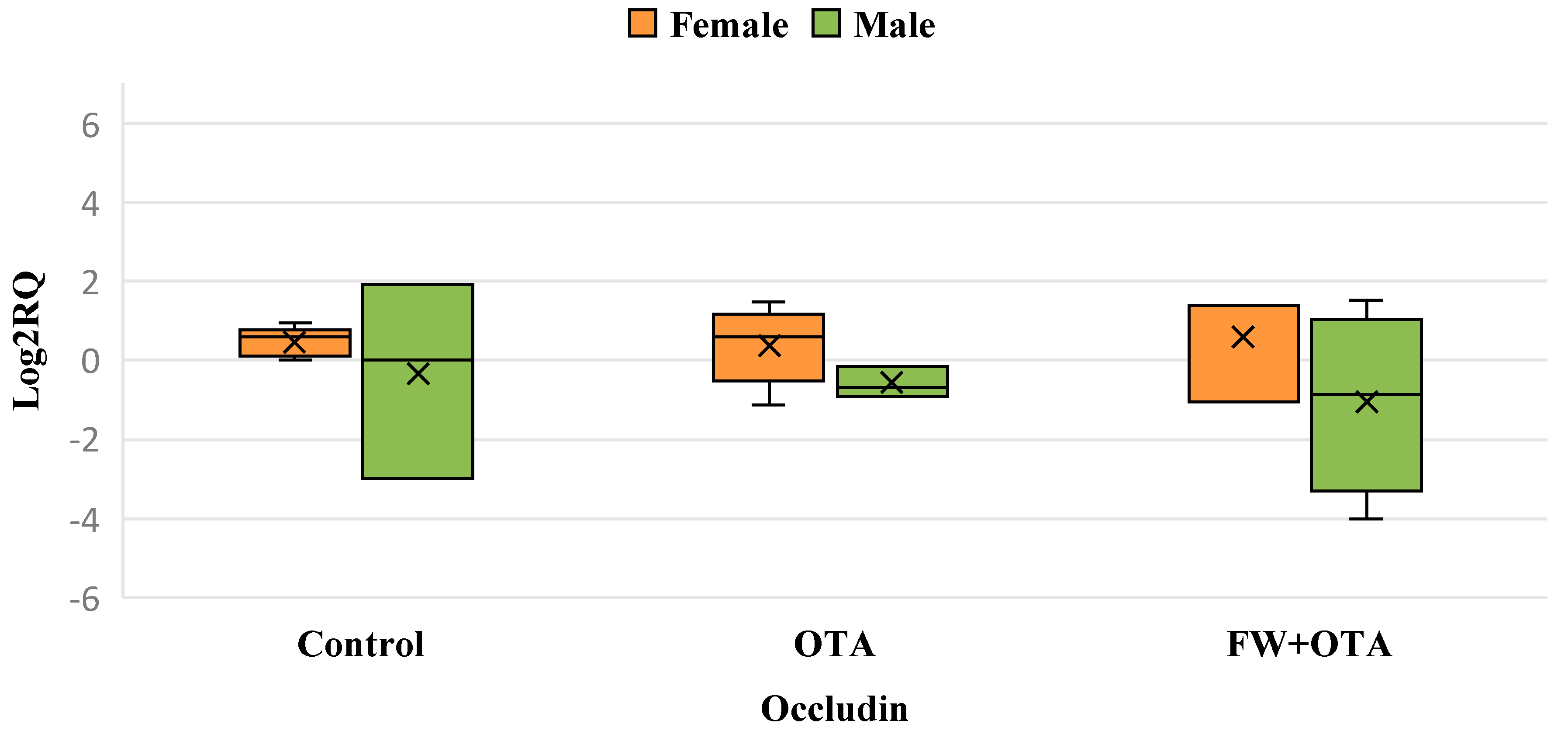
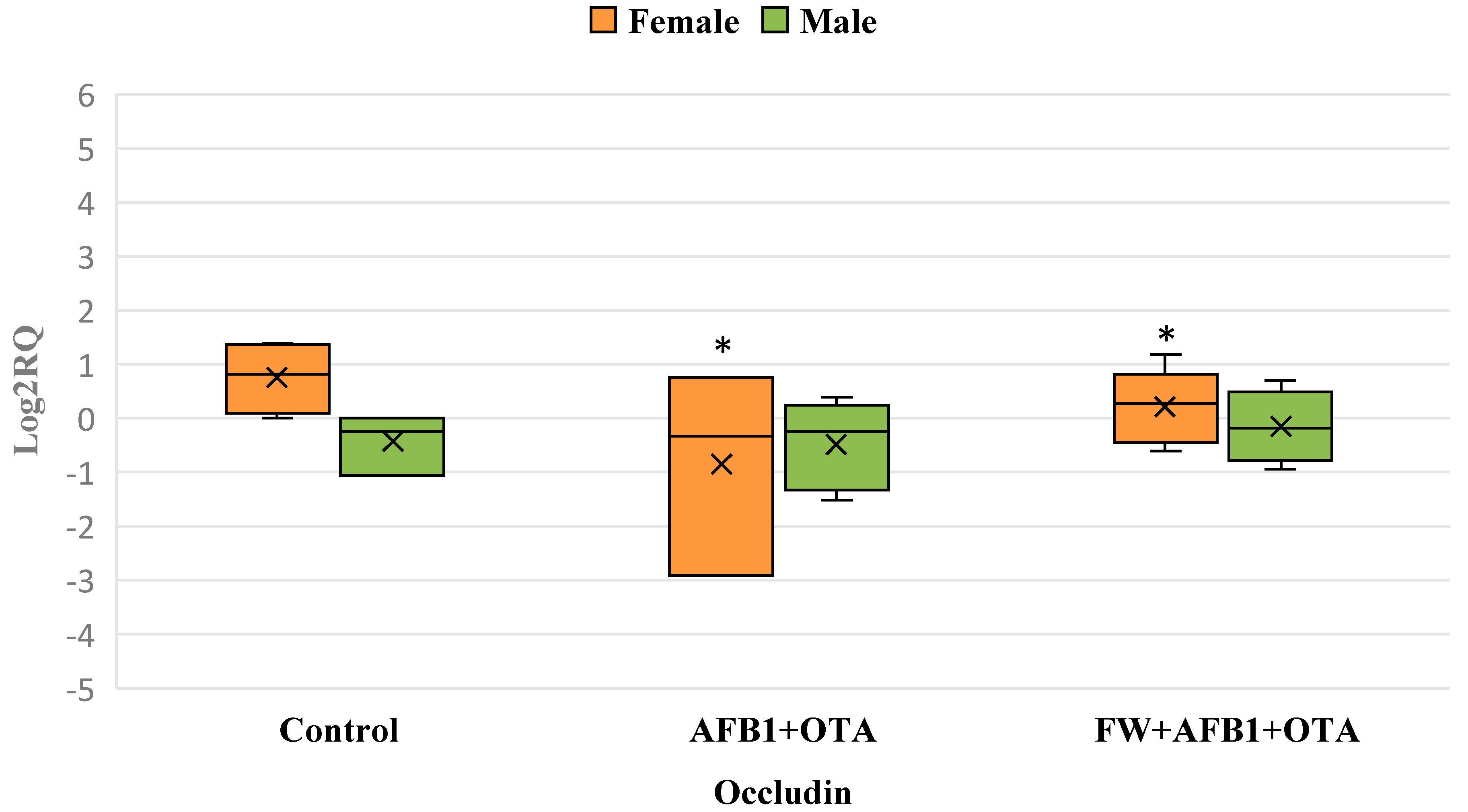
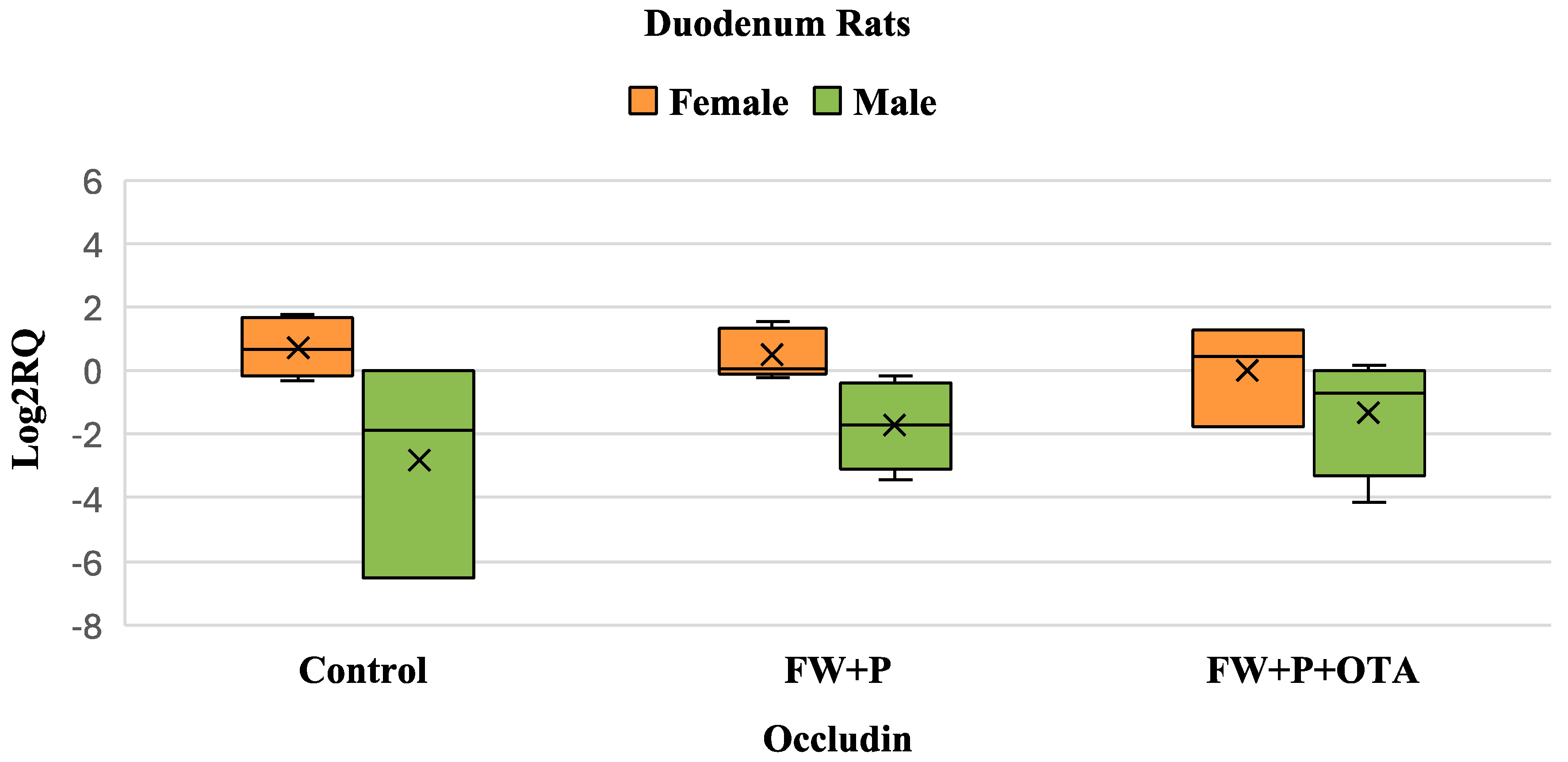
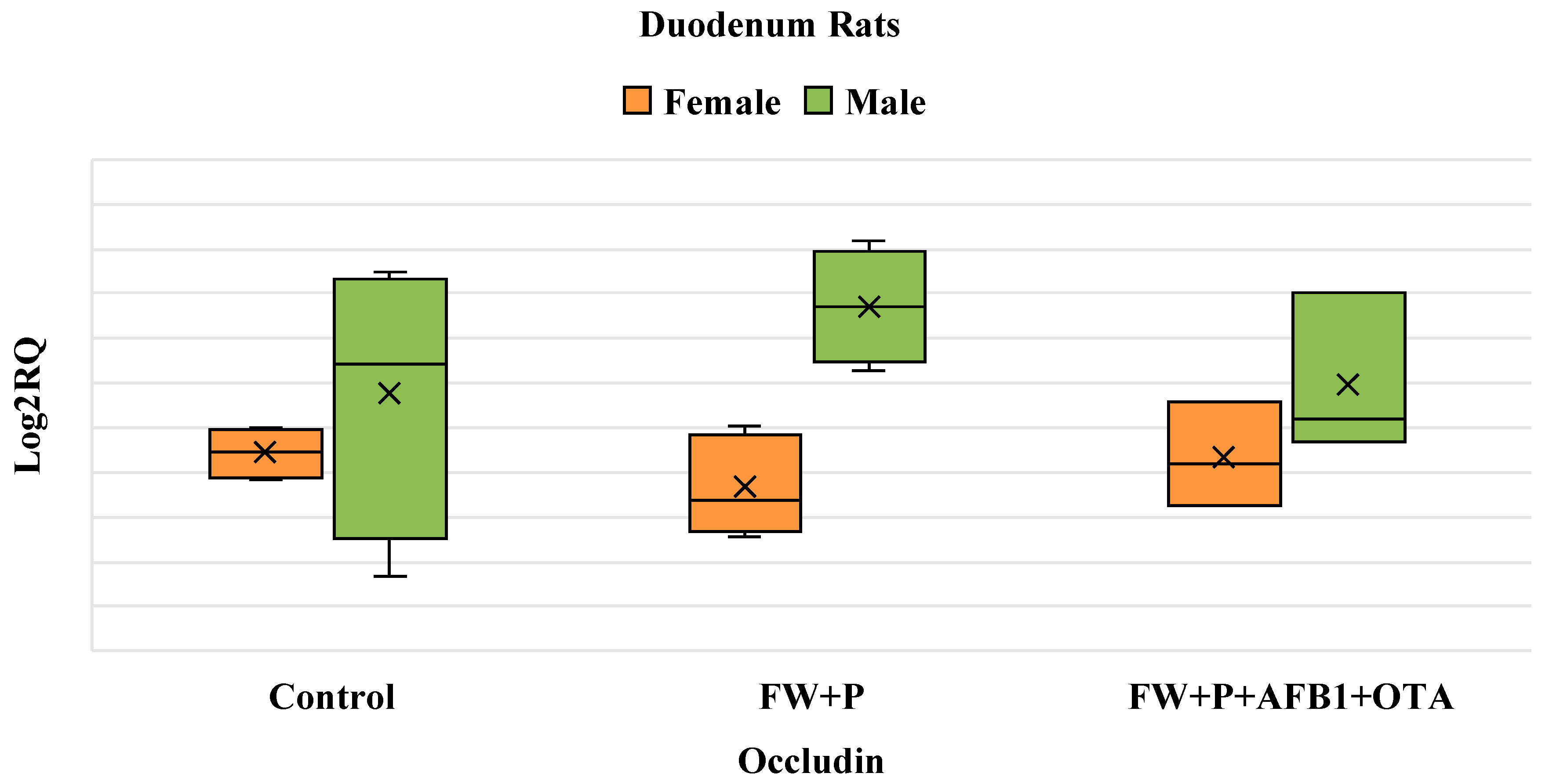
3.5. Differential Gene Expression of Occludin Gene Resulting from Exposure to Mycotoxins and Bioactive Ingredients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFB1 | Aflatoxin B1; |
| AFBO | Aflatoxin B1 exo-8,9-epoxide; |
| Bax | BCL2-Associated X, Apoptosis Regulator; |
| Bcl-2 | B-cell leukemia/lymphoma 2; |
| cDNA | Complementary DNA; |
| DNA | Deoxyribonucleic acid; |
| DON | Deoxynivalenol; |
| ENs | Enniatins; |
| FW | Fermented whey; |
| Hmox1 | Heme oxygenase 1; |
| IARC | International Agency for Research on Cancer; |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells; |
| OTA | Ochratoxin A; |
| P | Pumpkin; |
| p53 | Tumor protein p53; |
| RNA | Ribonucleic acid; |
| ROS | Reactive oxygen species; |
| RQ | Relative quantification; |
| RT-qPCR | Quantitative real-time PCR |
References
- Manyes, L.; Font, G. Mycotoxins: Toxicity, Occurrence, Risk Assessment and Prevention. In Encyclopedia of Human Nutrition; Elsevier: Amsterdam, The Netherlands, 2023; pp. 492–500. ISBN 978-0-323-90816-0. [Google Scholar]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Prandini, A.; Tansini, G.; Sigolo, S.; Filippi, L.; Laporta, M.; Piva, G. On the Occurrence of Aflatoxin M1 in Milk and Dairy Products. Food Chem. Toxicol. 2009, 47, 984–991. [Google Scholar] [CrossRef]
- Fouad, A.M.; Ruan, D.; El-Senousey, H.K.; Chen, W.; Jiang, S.; Zheng, C. Harmful Effects and Control Strategies of Aflatoxin B1 Produced by Aspergillus Flavus and Aspergillus Parasiticus Strains on Poultry: Review. Toxins 2019, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.A.; Khalil, F.; Hassan, H.A.; Amer, I.M.; Kamal, Z. Mycotoxicity and Embryonic Development: I- Aflatoxin B1 Reduces Quality and Birth Rate during Mice Embryonic Development. SVU-Int. J. Vet. Sci. 2023, 6, 1–20. [Google Scholar] [CrossRef]
- Ferreira, R.G.; Cardoso, M.V.; De Souza Furtado, K.M.; Espíndola, K.M.M.; Amorim, R.P.; Monteiro, M.C. Epigenetic Alterations Caused by Aflatoxin B1: A Public Health Risk in the Induction of Hepatocellular Carcinoma. Transl. Res. 2019, 204, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Gao, Y.; Wang, J.; Zheng, N. Integrated Metabolomics and Lipidomics Analysis Reveals Lipid Metabolic Disorder in NCM460 Cells Caused by Aflatoxin B1 and Aflatoxin M1 Alone and in Combination. Toxins 2023, 15, 255. [Google Scholar] [CrossRef]
- El Khoury, A.; Atoui, A. Ochratoxin A: General Overview and Actual Molecular Status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef]
- Klingelhöfer, D.; Braun, M.; Schöffel, N.; Oremek, G.M.; Brüggmann, D.; Groneberg, D.A. Ochratoxin—Characteristics, Influences and Challenges of Global Research. Food Control 2020, 114, 107230. [Google Scholar] [CrossRef]
- Longobardi, C.; Damiano, S.; Andretta, E.; Prisco, F.; Russo, V.; Pagnini, F.; Florio, S.; Ciarcia, R. Curcumin Modulates Nitrosative Stress, Inflammation, and DNA Damage and Protects against Ochratoxin A-Induced Hepatotoxicity and Nephrotoxicity in Rats. Antioxidants 2021, 10, 1239. [Google Scholar] [CrossRef]
- Gan, F.; Zhou, X.; Zhou, Y.; Hou, L.; Chen, X.; Pan, C.; Huang, K. Nephrotoxicity Instead of Immunotoxicity of OTA Is Induced through DNMT1-Dependent Activation of JAK2/STAT3 Signaling Pathway by Targeting SOCS3. Arch. Toxicol. 2019, 93, 1067–1082. [Google Scholar] [CrossRef]
- Wang, H.; Zhai, N.; Chen, Y.; Fu, C.; Huang, K. OTA Induces Intestinal Epithelial Barrier Dysfunction and Tight Junction Disruption in IPEC-J2 Cells through ROS/Ca2+-Mediated MLCK Activation. Environ. Pollut. 2018, 242, 106–112. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, Oxidative Stress and Metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Ülger, T.G.; Uçar, A.; Çakıroğlu, F.P.; Yilmaz, S. Genotoxic Effects of Mycotoxins. Toxicon 2020, 185, 104–113. [Google Scholar] [CrossRef]
- Barajas-Ramírez, J.A.; Moncada-Abaunza, D.A.; Gómez-Espinoza, M.G. Mycotoxins in Foods, from the Field to the Plate: A Review. Int. Food Res. J. 2021, 28, 230–247. [Google Scholar] [CrossRef]
- Sharma, V.; Patial, V. Food Mycotoxins: Dietary Interventions Implicated in the Prevention of Mycotoxicosis. ACS Food Sci. Technol. 2021, 1, 1717–1739. [Google Scholar] [CrossRef]
- Escrivá, L.; Manyes, L.; Vila-Donat, P.; Font, G.; Meca, G.; Lozano, M. Bioaccessibility and Bioavailability of Bioactive Compounds from Yellow Mustard Flour and Milk Whey Fermented with Lactic Acid Bacteria. Food Funct. 2021, 12, 11250–11261. [Google Scholar] [CrossRef]
- Luz, C.; Rodriguez, L.; Romano, R.; Mañes, J.; Meca, G. A Natural Strategy to Improve the Shelf Life of the Loaf Bread against Toxigenic Fungi: The Employment of Fermented Whey Powder. Int. J. Dairy. Tech. 2020, 73, 88–97. [Google Scholar] [CrossRef]
- Fiorbelli, E.; Lapris, M.; Errico, M.; Della Badia, A.; Riahi, I.; Rocchetti, G.; Gallo, A. Mycotoxin Challenge in Dairy Cows: Assessment of the Efficacy of an Anti-Mycotoxin Agent by Adopting an In Vitro Rumen Simulation Method. Toxins 2024, 16, 490. [Google Scholar] [CrossRef] [PubMed]
- Zavistanaviciute, P.; Ruzauskas, M.; Antanaitis, R.; Televicius, M.; Lele, V.; Santini, A.; Bartkiene, E. Antimicrobial and Mycotoxin Reducing Properties of Lactic Acid Bacteria and Their Influence on Blood and Feces Parameters of Newborn Calves. Animals 2023, 13, 3345. [Google Scholar] [CrossRef]
- Bergantin, C.; Maietti, A.; Tedeschi, P.; Font, G.; Manyes, L.; Marchetti, N. HPLC-UV/Vis-APCI-MS/MS Determination of Major Carotenoids and Their Bioaccessibility from “Delica” (Cucurbita Maxima) and “Violina” (Cucurbita Moschata) Pumpkins as Food Traceability Markers. Molecules 2018, 23, 2791. [Google Scholar] [CrossRef]
- Frangiamone, M.; Lozano, M.; Cimbalo, A.; Lazaro, A.; Font, G.; Manyes, L. The Protective Effect of Pumpkin and Fermented Whey Mixture against AFB1 and OTA Immune Toxicity In Vitro. A Transcriptomic Approach. Mol. Nutr. Food Res. 2023, 67, 2200902. [Google Scholar] [CrossRef] [PubMed]
- Vila-Donat, P.; Sánchez, D.; Cimbalo, A.; Mañes, J.; Manyes, L. Effect of Bioactive Ingredients on Urinary Excretion of Aflatoxin B1 and Ochratoxin A in Rats, as Measured by Liquid Chromatography with Fluorescence Detection. Toxins 2024, 16, 363. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization of the United Nations and Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants: Ninety-First Report of the Joint FAO/WHO Expert Committee on Food Additives, 1st ed.; Technical Report Series—World Health Organization Series; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-005458-5. [Google Scholar]
- Rotimi, O.A.; Rotimi, S.O.; Goodrich, J.M.; Adelani, I.B.; Agbonihale, E.; Talabi, G. Time-Course Effects of Acute Aflatoxin B1 Exposure on Hepatic Mitochondrial Lipids and Oxidative Stress in Rats. Front. Pharmacol. 2019, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Pastor, L.; Vettorazzi, A.; Enciso, J.M.; González-Peñas, E.; García-Jalón, J.A.; Monreal, J.I.; López De Cerain, A. Sex Differences in Ochratoxin a Toxicity in F344 Rats after 7 and 21 Days of Daily Oral Administration. Food Chem. Toxicol. 2018, 111, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, N.Z.; Somboro, A.M.; Amoako, D.G.; Arhin, I.; Khumalo, H.M.; Khan, R.B. Toxicogenicity and Mechanistic Pathways of Aflatoxin B1 Induced Renal Injury. Environ. Toxicol. 2021, 36, 1857–1872. [Google Scholar] [CrossRef]
- Kortei, N.K.; Oman Ayiku, P.; Nsor-Atindana, J.; Owusu Ansah, L.; Wiafe-Kwagyan, M.; Kyei-Baffour, V.; Kottoh, I.D.; Odamtten, G.T. Toxicogenic Fungal Profile, Ochratoxin A Exposure and Cancer Risk Characterization through Maize (Zea Mays) Consumed by Different Age Populations in the Volta Region of Ghana. Toxicon 2023, 226, 107085. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Gonçalves, T.; Martins, Z.E.; Bäuerl, C.; Cortés-Macías, E.; Collado, M.C.; Ferreira, I.M.P.L.V.O. Mycotoxin Interactions along the Gastrointestinal Tract: In Vitro Semi-Dynamic Digestion and Static Colonic Fermentation of a Contaminated Meal. Toxins 2022, 14, 28. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.-Y.; You, S.; Song, G.; Lim, W. Neurotoxic Effects of Aflatoxin B1 on Human Astrocytes in Vitro and on Glial Cell Development in Zebrafish in Vivo. J. Hazard. Mater. 2020, 386, 121639. [Google Scholar] [CrossRef]
- Xiao, S.; Wu, Y.; Gao, S.; Zhou, M.; Liu, Z.; Xiong, Q.; Jiang, L.; Yuan, G.; Li, L.; Yang, L. Deciphering the Hazardous Effects of AFB1 and T-2 Toxins: Unveiling Toxicity and Oxidative Stress Mechanisms in PK15 Cells and Mouse Kidneys. Toxins 2023, 15, 503. [Google Scholar] [CrossRef]
- Frangiamone, M.; Lázaro, Á.; Cimbalo, A.; Font, G.; Manyes, L. In Vitro and in Vivo Assessment of AFB1 and OTA Toxic Effects and the Beneficial Role of Bioactive Compounds. A Systematic Review. Food Chem. 2024, 447, 138909. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How Does P53 Induce Apoptosis and How Does This Relate to P53-Mediated Tumour Suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef]
- Kalkavan, H.; Green, D.R. MOMP, Cell Suicide as a BCL-2 Family Business. Cell Death Differ. 2018, 25, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, B.; Li, X.; Nawaz, M.Y.; Saleemi, M.K.; Li, G.; Jin, B.; Wang, L.; Xu, Y. Toxicodynamic of Combined Mycotoxins: MicroRNAs and Acute-phase Proteins as Diagnostic Biomarkers. Comp. Rev. Food Sci. Food Safe 2024, 23, e13338. [Google Scholar] [CrossRef]
- Cimbalo, A.; Alonso-Garrido, M.; Font, G.; Frangiamone, M.; Manyes, L. Transcriptional Changes after Enniatins A, A1, B and B1 Ingestion in Rat Stomach, Liver, Kidney and Lower Intestine. Foods 2021, 10, 1630. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.; Singh, A. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Serasanambati, M.; Chilakapati, S.R. Function of Nuclear Factor Kappa B (NF-kB) in Human Diseases-A Review. S. Indian J. Biol. Sci. 2016, 2, 368. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB Signaling in Inflammation and Cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef]
- Yoon, J.W.; Lee, S.I. Gene Expression Profiling after Ochratoxin A Treatment in Small Intestinal Epithelial Cells from Pigs. J. Anim. Sci. Technol. 2022, 64, 842–853. [Google Scholar] [CrossRef]
- Sheu, M.-L.; Shen, C.-C.; Chen, Y.-S.; Chiang, C.-K. Ochratoxin A Induces ER Stress and Apoptosis in Mesangial Cells via a NADPH Oxidase-Derived Reactive Oxygen Species-Mediated Calpain Activation Pathway. Oncotarget 2017, 8, 19376–19388. [Google Scholar] [CrossRef]
- Wu, J.; He, C.; Bu, J.; Luo, Y.; Yang, S.; Ye, C.; Yu, S.; He, B.; Yin, Y.; Yang, X. Betaine Attenuates LPS-Induced Downregulation of Occludin and Claudin-1 and Restores Intestinal Barrier Function. BMC Vet. Res. 2020, 16, 75. [Google Scholar] [CrossRef]
- Escrivá, L.; Agahi, F.; Vila-Donat, P.; Mañes, J.; Meca, G.; Manyes, L. Bioaccessibility Study of Aflatoxin B1 and Ochratoxin A in Bread Enriched with Fermented Milk Whey and/or Pumpkin. Toxins 2021, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Garrido, M.; Pallarés, N.; Font, G.; Tedeschi, P.; Manyes, L.; Lozano, M. The Role of Pumpkin Pulp Extract Carotenoids against Mycotoxin Damage in the Blood Brain Barrier In Vitro. Arch. Ind. Hyg. Toxicol. 2021, 72, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Yadavalli, R.; Valluru, P.; Raj, R.; Reddy, C.N.; Mishra, B. Biological Detoxification of Mycotoxins: Emphasizing the Role of Algae. Algal Res. 2023, 71, 103039. [Google Scholar] [CrossRef]
- Pauletto, M.; Giantin, M.; Tolosi, R.; Bassan, I.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Discovering the Protective Effects of Resveratrol on Aflatoxin B1-Induced Toxicity: A Whole Transcriptomic Study in a Bovine Hepatocyte Cell Line. Antioxidants 2021, 10, 1225. [Google Scholar] [CrossRef]

| Gene | NCBI Accession Number | Sequence | Annealing (°C) | Efficiency (%) | Linearity (R2) |
|---|---|---|---|---|---|
| 18S rRNA | NM_213557.1 | F: GAGCGTGTGATCACCATCAT R: TCCTTCACGTCCTTCTGTCT | 60 | 111 | 0.993 |
| p53 | NM_030989.4 | F: GTTCCGAGAGCTGAATGAGG R: TTTTATGGCGGGACGTAGAC | 60 | 125 | 0.989 |
| NF-κB | NM_017059.2 | F: CTTCTCGGAGTCCCTCACTG R: CCAATAGCAGCTGGAAAAGC | 60 | 108 | 0.981 |
| Bax | NM_001276711.2 | F: AAGAAGCTGAGCGAGTGTCT R: CAAAGATGGTCACTGTCTGC | 58 | 104 | 0.991 |
| Hmox1 | NM_012580.2 | F: GACGCATATACCCGCTACCT R: AAGGCGGTCTTAGCCTCTTC | 60 | 99 | 0.990 |
| Occludin | NM_031329.3 | F: AGTACATGGCTGCTGCTGAG R: CCCACCATCCTCTTGATGTT | 60 | 101 | 0.993 |
| Group | p53 | Bax | NF-κB | Hmox-1 | |
|---|---|---|---|---|---|
| Male | Control | (−0.81 ± 2.07) | (−1.18 ± 2.04) | (0.30 ± 0.91) | (0.07 ± 0.61) |
| OTA | (−0.04 ± 1.82) | (0.77 ± 1.52) * | (3.72 ± 0.52) ** | (1.69 ± 1.25) ** | |
| FW + OTA | (1.28 ± 2.12) * | (−0.95 ± 2.36) | (2.13 ± 1.49) ** | (0.99 ± 0.84) | |
| Female | Control | (−0.15 ± 0.73) | (−1.80 ± 1.57) | (1.82 ± 1.55) | (−0.19 ± 0.31) |
| OTA | (4.62 ± 0.27) *** | (−2.34 ± 0.24) | (3.92 ± 0.99) *** | (2.06 ± 0.72) *** | |
| FW + OTA | (3.91 ± 0.88) *** | (−1.75 ± 0.71) | (3.61 ± 1.89) * | (0.80 ± 1.01) |
| Group | p53 | Bax | NF-κB | Hmox-1 | |
|---|---|---|---|---|---|
| Male | Control | (−1.18 ± 2.13) | (−0.95 ± 2.58) | (−0.40 ± 1.92) | (1.68 ± 1.19) |
| AFB1 + OTA | (−0.37 ± 1.26) | (−2.81 ± 2.23) * | (0.99 ± 1.63) | (1.19 ± 2.39) | |
| FW + AFB1 + OTA | (0.36 ± 2.07) | (−2.03 ± 2.63) | (2.19 ± 1.68) ** | (2.01 ± 1.42) | |
| Female | Control | (1.68 ± 1.30) | (−1.36 ± 1.76) | (0.70 ± 0.92) | (1.39 ± 1.38) |
| AFB1 + OTA | (2.75 ± 1.46) | (−0.80 ± 0.83) | (0.70 ± 0.70) | (1.60 ± 1.01) | |
| FW + AFB1 + OTA | (1.73 ± 2.01) | (0.15 ± 2.19) | (0.96 ± 1.91) | (0.59 ± 1.44) * |
| Group | p53 | Bax | NF-κB | Hmox-1 | |
|---|---|---|---|---|---|
| Male | Control | (−0.05 ± 0.37) | (−1.09 ± 1.37) | (−2.22 ± 1.92) | (1.15 ± 1.26) |
| FW + P | (−2.14 ± 2.16) *** | (−0.76 ± 0.64) | (−0.53 ± 0.64) ** | (1.64 ± 1.37) | |
| FW + P + AFB1 | (−1.28 ± 1.99) *** | (−2.95 ± 1.52) | (−2.95 ± 1.47) | (0.57 ± 1.52) | |
| Female | Control | (1.47 ± 1.19) | (−0.54 ± 0.47) | (1.27 ± 0.98) | (2.27 ± 2.09) |
| FW + P | (1.87 ± 2.48) | (−0.23 ± 1.33) | (1.48 ± 2.12) | (0.97 ± 1.48) | |
| FW + P + AFB1 | (0.81 ± 0.86) | (2.31 ± 2.30) | (2.31 ± 1.37) | (3.36 ± 1.51) |
| Group | p53 | Bax | NF-κB | Hmox-1 | |
|---|---|---|---|---|---|
| Male | FW + P | (−2.24 ± 1.89) | (−0.80 ± 0.84) | (−2.95 ± 2.86) | (−2.21 ± 1.53) |
| FW + P + OTA | (−2.86 ± 2.19) | (−2.69 ± 0.62) ** | (−3.24 ± 1.15) | (−2.90 ± 1.31) | |
| FW + P + AFB1 + OTA | (−1.76 ± 2.17) | (−1.21 ± 0.65) | (−2.55 ± 1.26) | (−2.29 ± 1.47) | |
| Female | FW + P | (0.35 ± 0.79) | (1.56 ± 1.64) | (0.34 ± 0.90) | (0.64 ± 2.23) |
| FW + P + OTA | (−0.52 ± 0.84) * | (2.76 ± 1.57) *** | (4.47 ± 1.46) | (5.15 ± 1.63) *** | |
| FW + P + AFB1 + OTA | (0.57 ± 0.94) | (4.16 ± 2.53) *** | (4.16 ± 2.53) | (3.92 ± 2.62) *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafai, S.; Cimbalo, A.; Manyes, L. Evaluation of Dietary Bioactive Agents Against Aflatoxin B1 and Ochratoxin A-Induced Duodenal Toxicity in Rats. Foods 2025, 14, 1793. https://doi.org/10.3390/foods14101793
Rafai S, Cimbalo A, Manyes L. Evaluation of Dietary Bioactive Agents Against Aflatoxin B1 and Ochratoxin A-Induced Duodenal Toxicity in Rats. Foods. 2025; 14(10):1793. https://doi.org/10.3390/foods14101793
Chicago/Turabian StyleRafai, Sarra, Alessandra Cimbalo, and Lara Manyes. 2025. "Evaluation of Dietary Bioactive Agents Against Aflatoxin B1 and Ochratoxin A-Induced Duodenal Toxicity in Rats" Foods 14, no. 10: 1793. https://doi.org/10.3390/foods14101793
APA StyleRafai, S., Cimbalo, A., & Manyes, L. (2025). Evaluation of Dietary Bioactive Agents Against Aflatoxin B1 and Ochratoxin A-Induced Duodenal Toxicity in Rats. Foods, 14(10), 1793. https://doi.org/10.3390/foods14101793








