Antioxidant/Anti-Inflammatory Potential and Sensory Acceptance of Granola Bars Developed with Sorghum Sprout Flour Irradiated with UV-A LED Light
Abstract
1. Introduction
2. Materials and Methods
2.1. Sorghum Sprouting
2.2. Granola Bar Preparation
2.3. Obtaining Free and Bound Compounds
2.4. Determination of the Total Phenol Content
2.5. Antioxidant Capacity
2.5.1. 1,1-Diphenyl-2-Picrylhydrazyl (DPPH)
2.5.2. Trolox Equivalent Antioxidant Capacity (TEAC)
2.6. Quantification of Phenols via Ultra-High-Performance Liquid Chromatography Diode Array Detection (UHPLC-DAD)
2.7. In Vitro Digestion
2.8. Anti-Inflammatory Capacity
2.8.1. Cell Culture
2.8.2. Effect of Extracts on Cell Viability
2.8.3. Determination of Nitric Oxide (NO) Production
2.9. Sensory Evaluation
Just-About-Right (JAR) Test
2.10. Statistical Analysis
3. Results and Discussion
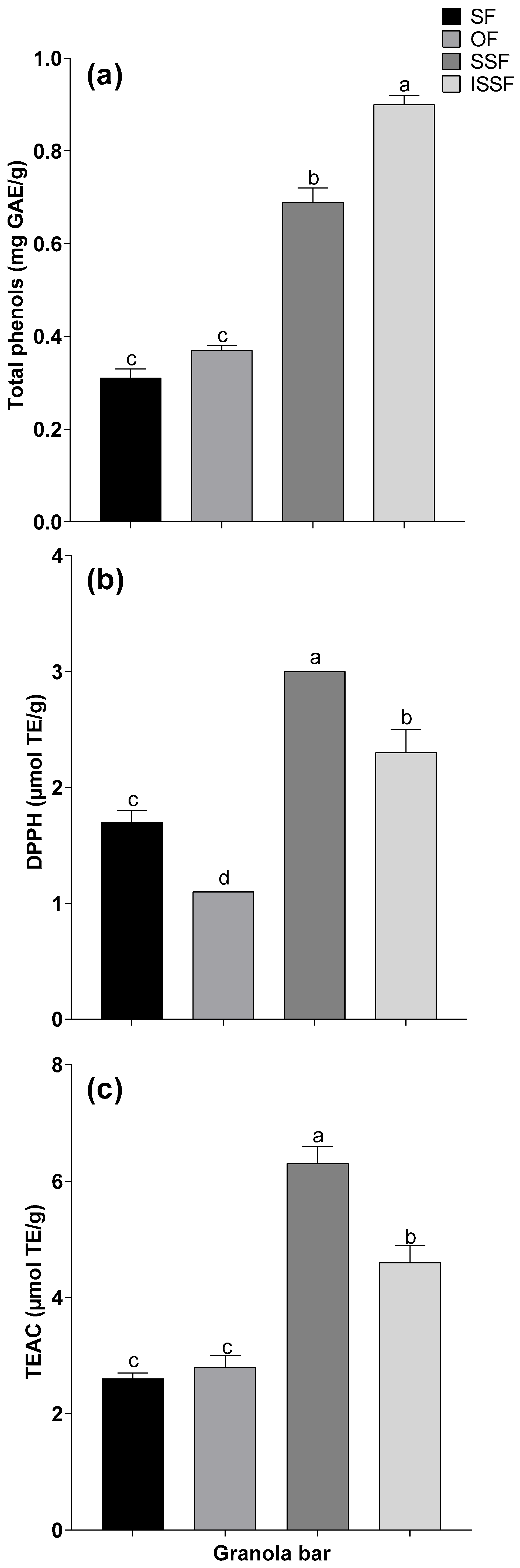
3.1. Total Phenol Contents and Antioxidant Activities in the Granola Bars
3.2. Phenolic Acids and Flavonoids
3.3. Bioaccessibility and Antioxidant Recovery of Phenolic Compounds
3.4. Phenolic Acids and Flavonoids Found in the Intestinal Fraction
3.5. Anti-Inflammatory Effects
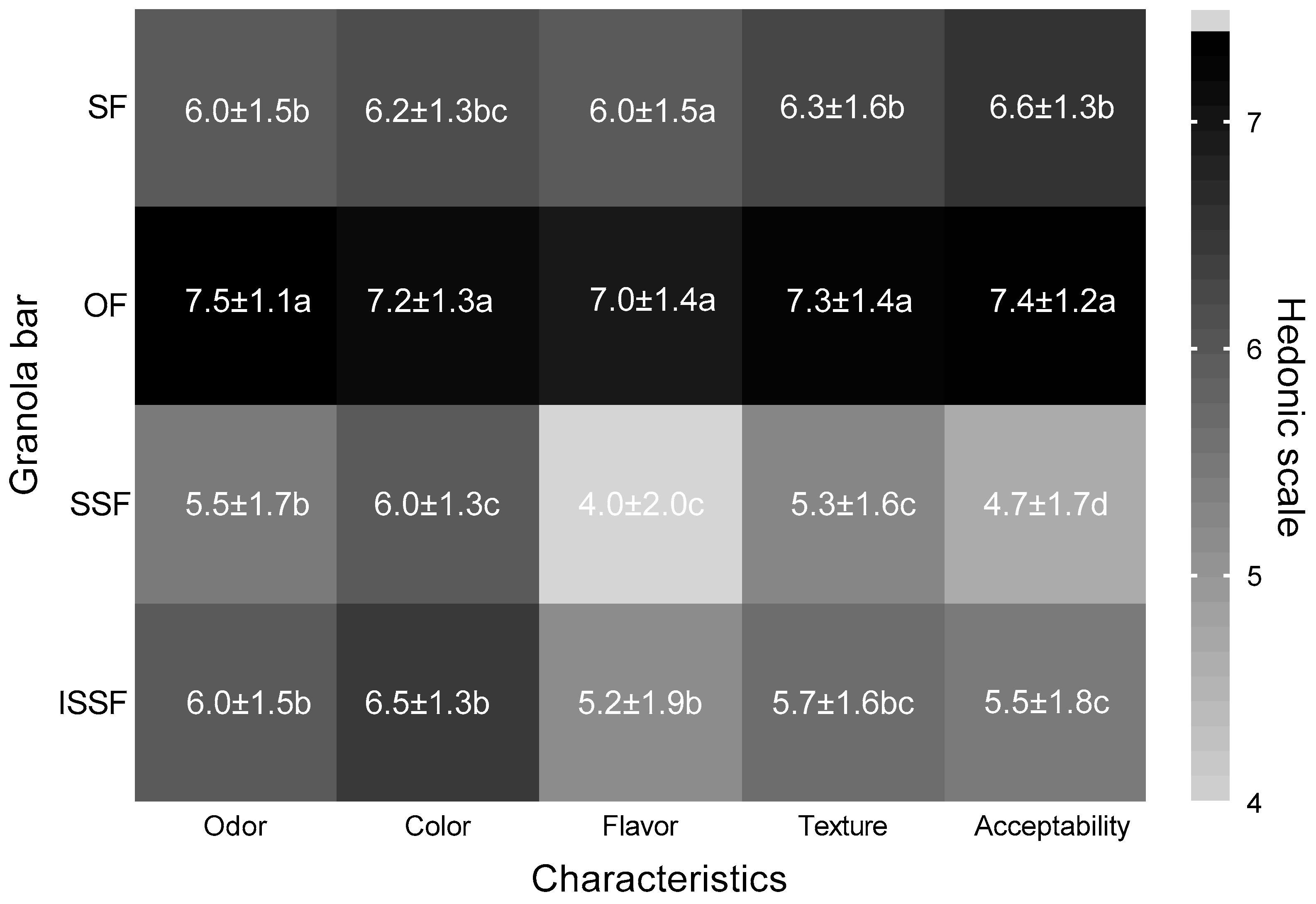
3.6. Sensory Evaluation, Penalty Analysis, and Consumption Intention
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| ATCC | American Type Culture Collection |
| CO2 | Carbon dioxide |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl sulfoxide |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| HCl | Hydrochloric acid |
| iNOS | Inducible nitric oxide synthase |
| JAR | Just about right |
| KCl | Potassium chloride |
| LPS | Lipopolysaccharide |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NaOH | Sodium hydroxide |
| ROS | Reactive oxygen species |
| TEAC | Trolox equivalent antioxidant capacity |
| TNF-α | Tumor necrosis factor α |
| UHPLC-DAD | Ultra-high-performance liquid chromatography diode array detection |
| UV-A | Ultraviolet light A |
References
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.; Brown, W.A.; Mingrone, G. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025, 13, 221–262. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.; Guedes AM, M.; dos Santos KM, O.; Deliza, R. Healthy food innovation in sustainable food system 4.0: Integration of entrepreneurship, research, and education. Curr. Opin. Food Sci. 2021, 42, 215–223. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, G.; Li, Y.; Shi, G.; Li, M. Potential application of plant-based functional foods in the development of immune boosters. Front. Pharmacol. 2021, 12, 637782. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Rai, S.; Joshi, A. Role of functional foods and nutraceuticals for depression, post-traumatic stress disorder, and suicidal behaviors. In Nutraceutical Fruits and Foods for Neurodegenerative Disorders; Academic Press: Cambridge, MA, USA, 2024; pp. 101–122. [Google Scholar]
- Villarruel-López, A.; Ascencio, F.; Nuño, K. Microalgae, a potential natural functional food source–a review. Pol. J. Food Nutr. Sci. 2017, 67, 251–263. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Vasconcelos, M.H.; Morales, P.; Ferreira, I.C. Functional foods based on extracts or compounds derived from mushrooms. Trends Food Sci. Technol. 2017, 66, 48–62. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, D.H.; Jo, S.; Cho, M.J.; Cho, Y.R.; Lee, Y.J.; Byun, S. Immunomodulatory functional foods and their molecular mechanisms. Exp. Mol. Med. 2022, 54, 1–11. [Google Scholar] [CrossRef]
- Zahid, M.K.; Ahmad, D.; Amin, R.; Bao, J. Sorghum starch: Composition, structure, functionality, and strategies for its improvement. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70101. [Google Scholar] [CrossRef] [PubMed]
- Proietti, I.; Frazzoli, C.; Mantovani, A. Exploiting nutritional value of staple foods in the world’s semi-arid areas: Risks, benefits, challenges and opportunities of sorghum. Healthcare 2015, 3, 172–193. [Google Scholar] [CrossRef]
- de Morais Cardoso, L.; Pinheiro, S.S.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Sorghum (Sorghum bicolor L.): Nutrients, bioactive compounds, and potential impact on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 372–390. [Google Scholar] [CrossRef]
- Chávez, D.; Ascheri, J.; Martins, A.; Carvalho, C.; Bernardo, C.; Teles, A. Sorghum, an alternative cereal for gluten-free product. Rev. Chil. Nutr. 2018, 45, 169–177. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Elahi, F.; Daliri, E.B.M.; Tyagi, A.; Chen, X.Q.; Chelliah, R.; Oh, D.H. UHPLC-ESI-QTOF-MS/MS characterization, antioxidant and antidiabetic properties of sorghum grains. Food Chem. 2021, 337, 127788. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Yun, D.; Baek, Y.; Kim, H.J.; Lee, H.G. Antihypertensive effects of the combined extract of Sorghum bicolor, Vigna angularis, and Eleusine coracana in spontaneously hypertensive rats. Sci. Rep. 2024, 14, 803. [Google Scholar] [CrossRef]
- Francis, N.; Rao, S.; Blanchard, C.; Santhakumar, A. Black sorghum phenolic extract regulates expression of genes associated with oxidative stress and inflammation in human endothelial cells. Molecules 2019, 24, 3321. [Google Scholar] [CrossRef]
- Chen, X.; Shen, J.; Xu, J.; Herald, T.; Smolensky, D.; Perumal, R.; Wang, W. Sorghum phenolic compounds are associated with cell growth inhibition through cell cycle arrest and apoptosis in human hepatocarcinoma and colorectal adenocarcinoma cells. Foods 2021, 10, 993. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Arvizu, G.; Castro-Jácome, T.P.; Tovar-Pérez, E.; Alcántara-Quintana, E. Antiproliferative, apoptotic, and antimigratory activities of kafirins on cervical cancer-derived cell lines. Rev. Med. Inst. Mex. Seguro Soc. 2023, 61 (Suppl 1), S4–S11. [Google Scholar]
- Rezaee, N.; Fernando, W.B.; Hone, E.; Sohrabi, H.R.; Johnson, S.K.; Gunzburg, S.; Martins, R.N. Potential of Sorghum polyphenols to prevent and treat Alzheimer’s disease: A review article. Front. Aging Neurosci. 2021, 13, 729949. [Google Scholar] [CrossRef] [PubMed]
- Arbex, P.M.; de Castro Moreira, M.E.; Toledo RC, L.; de Morais Cardoso, L.; Pinheiro-Sant’ana, H.M.; dos Anjos Benjamin, L.; Licursi, L.; Piler Carvalho, C.W.; Vieira Queiroz, V.A.; Martino HS, D. Extruded sorghum flour (Sorghum bicolor L.) modulate adiposity and inflammation in high fat diet-induced obese rats. J. Funct. Foods. 2018, 42, 346–355. [Google Scholar] [CrossRef]
- Jeon, Y.; Lee, H.; Kim, H.J.; Heo, H.; Hong, S.; Kim, Y.; Lee, J. Changes in the Antioxidant, Anti-Diabetic, and Anti-Hypertensive Activities of Sorghum (Sorghum bicolor L.) Due to Microwave Treatment. J. Korean Soc. Food Sci. Nutr. 2024, 53, 787–795. [Google Scholar] [CrossRef]
- Reyna-Reyna, L.Y.; Montaño-Leyva, B.; Valencia, D.; Cinco-Moroyoqui, F.J.; González-Vega, R.I.; Bernal-Mercado, A.T.; Ballesteros-Monreal, M.G.; Mendez-Encinas, M.A.; Del-Toro-Sánchez, C.L. Antioxidant, antibacterial, anti-inflammatory, and antiproliferative activity of sorghum lignin (Sorghum bicolor) treated with ultrasonic pulses. Metabolites 2023, 13, 394. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted grains: A comprehensive review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef]
- Hassan, S.; Imran, M.; Ahmad, M.H.; Khan, M.I.; Xu, C.; Khan, M.K.; Muhammad, N. Phyto-chemical characterization of ultrasound-processed sorghum sprouts for the use in functional foods. Int. J. Food Prop. 2020, 23, 853–863. [Google Scholar] [CrossRef]
- Deore, A.; Athmaselvi, K.A.; Venkatachalapathy, N. Effect of ultrasound and microwave pre-treatment on sprouting, GABA, bioactive compounds, and other physicochemical properties of sorghum. Grain Oil Sci. Technol. 2023, 6, 91–99. [Google Scholar] [CrossRef]
- Deng, M.; Qian, H.; Chen, L.; Sun, B.; Chang, J.; Miao, H.; Caim, C.; Wang, Q. Influence of pre-harvest red light irradiation on main phytochemicals and antioxidant activity of Chinese kale sprouts. Food Chem. 2017, 222, 1–5. [Google Scholar] [CrossRef]
- Sun, K.; Peng, Y.; Wang, M.; Li, W.; Li, Y.; Chen, J. Effect of red and blue light on the growth and antioxidant activity of alfalfa sprouts. Horticulturae 2024, 10, 76. [Google Scholar] [CrossRef]
- del Socorro Sánchez Correa, M.; el Rocío Reyero Saavedra, M.; Parra, E.A.; Ontiveros, E.N.; del Carmen Benítez Flores, J.; Montiel, J.G.; Contreras, J.E.; Urrutia, E.L.; Acevedo, J.G.; Nopala, G.E.; et al. Ultraviolet radiation and its effects on plants. In Abiotic Stress in Plants-Adaptations to Climate Change; Olvieira, M., Fernandes Silva, A.A., Eds.; IntechOpen: London, UK, 2023. [Google Scholar]
- Pulli, T.; Dönsberg, T.; Poikonen, T.; Manoocheri, F.; Kärhä, P.; Ikonen, E. Advantages of white LED lamps and new detector technology in photometry. Light Sci. Appl. 2015, 4, e332. [Google Scholar] [CrossRef]
- Rai, N.; Morales, L.O.; Aphalo, P.J. Perception of solar UV radiation by plants: Photoreceptors and mechanisms. Plant Physiol. 2021, 186, 1382–1396. [Google Scholar] [CrossRef]
- Lim, Y.J.; Lyu, J.I.; Kwon, S.J.; Eom, S.H. Effects of UV-A radiation on organ-specific accumulation and gene expression of isoflavones and flavonols in soybean sprout. Food Chem. 2021, 339, 128080. [Google Scholar] [CrossRef] [PubMed]
- Camara, F.; Coulibaly, W.H.; Mian TM, A.S.; Ahoussi KJ, B.; Coulibaly, S.; Varadarajan, V.; Matei, F. Household Production of Cookies from Sorghum (Sorghum bicolor) with a Low Glycaemic Index in Prevention and Management of Type 2 Diabetes in Côte d’Ivoire. Curr. Nutr. Food Sci. 2024, 21, 341–349. [Google Scholar] [CrossRef]
- Indrianingsih, A.W.; Khasanah, Y.; Darsih, C.; Hastuti, H.P.; Suryani, A.E.; Hastuti, M.; Wiyono, T. Sorghum cookies fortified with Garcinia mangostana peel extract: Formulation, characterization, and evaluation of antioxidant and antidiabetic activity. Bioact. Carbohydr. 2025, 33, 100467. [Google Scholar] [CrossRef]
- Ruiz Hernández, A.A.; Rouzaud Sández, O.; Frías, J.; Ayala Zavala, F.; Astiazarán García, H.; Robles Sánchez, M. Optimization of the duration and intensity of UV-A radiation to obtain the highest free phenol content and antioxidant activity in sprouted sorghum (Sorghum bicolor L. Moench). Plant Foods Hum. Nutr. 2022, 77, 317–318. [Google Scholar] [CrossRef]
- Zamora-Gasga, V.M.; Bello-Pérez, L.A.; Ortíz-Basurto, R.I.; Tovar, J.; Sáyago-Ayerdi, S.G. Granola bars prepared with Agave tequilana ingredients: Chemical composition and in vitro starch hydrolysis. LWT-Food Sci. Technol. 2014, 56, 309–314. [Google Scholar] [CrossRef]
- Salazar Lopez, N.J.; Loarca-Piña, G.; Campos-Vega, R.; Gaytán Martínez, M.; Morales Sánchez, E.; Esquerra-Brauer, J.M.; Robles Sánchez, M. The extrusion process as an alternative for improving the biological potential of sorghum bran: Phenolic compounds and antiradical and anti-inflammatory capacity. Evid-Based Complement. Alternat. Med. 2016, 2016, 8387975. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Beta, T. Phenolic acid composition and antioxidant potential of insoluble and soluble dietary fibre extracts derived from select whole-grain cereals. Food Res. Int. 2013, 51, 518–525. [Google Scholar] [CrossRef]
- López-Bascón, M.A.; De Castro, M.L. Soxhlet extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 327–354. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; González-Aguilar, G.A.; Loarca-Piña, G.; Cinco-Moroyoqui, F.J.; Rouzaud-Sández, O.; Domínguez-Avila, J.A.; Robles-Sánchez, M. Contribution and interactions of hydroxycinnamic acids found in bran and wholegrain sorghum (Sorghum bicolor L. Moench): Effects on the antioxidant capacity and inhibition of human erythrocyte hemolysis. Oxid. Med. Cell. Longev. 2017, 2017, 8219023. [Google Scholar] [CrossRef]
- Blancas-Benitez, F.J.; Mercado-Mercado, G.; Quirós-Sauceda, A.E.; Montalvo-González, E.; González-Aguilar, G.A.; Sáyago-Ayerdi, S.G. Bioaccessibility of polyphenols associated with dietary fiber and in vitro kinetics release of polyphenols in Mexican ‘Ataulfo’ mango (Mangifera indica L.) by-products. Food Funct. 2015, 6, 859–868. [Google Scholar] [CrossRef]
- Lee, K.M.; Kalyani, D.; Tiwari, M.K.; Kim, T.S.; Dhiman, S.S.; Lee, J.K.; Kim, I.W. Enhanced enzymatic hydrolysis of rice straw by removal of phenolic compounds using a novel laccase from yeast Yarrowia lipolytica. Bioresour. Technol. 2012, 123, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Salazar-López, N.J.; González-Aguilar, G.A.; Rouzaud-Sández, O.; Robles-Sánchez, M. Bioaccessibility of hydroxycinnamic acids and antioxidant capacity from sorghum bran thermally processed during simulated in vitro gastrointestinal digestion. J. Food Sci. Technol. 2018, 55, 2021–2030. [Google Scholar] [CrossRef]
- Kemp, S.E.; Hollowood, T.; Hort, J. Sensory Evaluation: A Practical Handbook; Wiley Publishers: Chichester, UK, 2011. [Google Scholar]
- Lin, F.; Li, X.; Jia, N.; Feng, F.; Huang, H.; Huang, J.; Fan, S.; Ciais, P.; Song, X.P. The impact of Russia-Ukraine conflict on global food security. Glob. Food Sec. 2023, 36, 100661. [Google Scholar] [CrossRef]
- Pol, K.; de Graaf, C.; Meyer, D.; Mars, M. The efficacy of daily snack replacement with oligofructose-enriched granola bars in overweight and obese adults: A 12-week randomised controlled trial. Br. J. Nutr. 2018, 119, 1076–1086. [Google Scholar] [CrossRef]
- Kumar, S.; Velraja, S.; Arambakkam, H.J. Antioxidant-Rich Peridialytic Granola Bar for Hemodialytic Subjects. Biomed. Bio-technol. Res. J. 2022, 6, 105–108. [Google Scholar]
- Hitayezu, R.; Baakdah, M.M.; Kinnin, J.; Henderson, K.; Tsopmo, A. Antioxidant activity, avenanthramide and phenolic acid contents of oat milling fractions. J. Cereal Sci. 2015, 63, 35–40. [Google Scholar] [CrossRef]
- Arivalagan, M.; Roy, T.K.; Yasmeen, A.M.; Pavithra, K.C.; Jwala, P.N.; Shivasankara, K.S.; Kanade, S.R. Extraction of phenolic compounds with antioxidant potential from coconut (Cocos nucifera L.) testa and identification of phenolic acids and flavonoids using UPLC coupled with TQD-MS/MS. LWT-Food Sci. Technol. 2018, 92, 116–126. [Google Scholar] [CrossRef]
- Velázquez Ríos, I.O.; González-García, G.; Mellado-Mojica, E.; Veloz García, R.A.; Dzul Cauich, J.G.; López, M.G.; García-Vieyra, M.I. Phytochemical profiles and classification of Agave syrups using 1H-NMR and chemometrics. Food Sci. Nutr. 2019, 7, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Uslu, N.; Özcan, M.M. Effect of microwave heating on phenolic compounds and fatty acid composition of cashew (Anacardium occidentale) nut and oil. J. Saudi Soc. Agric. Sci. 2019, 18, 344–347. [Google Scholar] [CrossRef]
- Ruiz-Hernández, A.A.; Cárdenas-López, J.L.; Cortez-Rocha, M.O.; González-Aguilar, G.A.; Robles-Sánchez, R.M. Optimization of germination of white sorghum by response surface methodology for preparing porridges with biological potential. CyTA J. Food. 2021, 19, 49–55. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Insoluble-bound phenolics in food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Wu, W.; Mustafa, G.; Yang, Y.; Yang, P. Molecular mechanisms of rice seed germination. New Crops 2025, 2, 100051. [Google Scholar] [CrossRef]
- Barnes, W.J.; Anderson, C.T. Release, recycle, rebuild: Cell-wall remodeling, autodegradation, and sugar salvage for new wall biosynthesis during plant development. Mol. Plant 2018, 11, 31–46. [Google Scholar] [CrossRef]
- Gomes, M.P.; Garcia, Q.S. Reactive oxygen species and seed germination. Biologia 2013, 68, 351–357. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Samadi, M.; Kazemeini, S.A.; Ozturk, M.; Ludwiczak, A.; Piernik, A. ROS homeostasis and antioxidants in the halophytic plants and seeds. Plants 2023, 12, 3023. [Google Scholar] [CrossRef] [PubMed]
- Begum, K.; Hasan, N.; Shammi, M. Selective Biotic Stressors’ Action on Seed Germination: A Review. Plant Sci. 2024, 346, 112156. [Google Scholar] [CrossRef]
- Khan, K.A.; Saleem, M.H.; Afzal, S.; Hussain, I.; Ameen, F.; Fahad, S. Ferulic acid: Therapeutic potential due to its antioxidant properties, role in plant growth, and stress tolerance. Plant Growth Regul. 2024, 104, 1329–1353. [Google Scholar] [CrossRef]
- Ahmed, A.; Tariq, A.; Habib, S. Interactive biology of auxins and phenolics in plant environment. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A., Eds.; Springer: Singapore, 2020; Volume 1, pp. 117–133. [Google Scholar]
- Ali, A.S.; Elozeiri, A.A. Metabolic processes during seed germination. Adv. Seed Biol. 2017, 141–166. [Google Scholar]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Dhal, S.; Pal, H. Regulation of glycolysis and Krebs cycle during biotic and abiotic stresses. In Photosynthesis and Respiratory Cycles during Environmental Stress Response in Plants, 1st ed.; Somali, D., Harshata, P., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2022; pp. 263–308. [Google Scholar]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic acid pathway in biosynthesis of phenolic compounds. In Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, M., García-Mateos, R., Palma-Tenango, M., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination, 2nd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Rosental, L.; Nonogaki, H.; Fait, A. Activation and regulation of primary metabolism during seed germination. Seed Sci. Res. 2014, 24, 1–15. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin−Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar]
- Foti, M.C. Use and Abuse of the DPPH• Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef]
- Nićiforović, N.; Abramovič, H. Sinapic acid and its derivatives: Natural sources and bioactivity. Comp. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Arts, M.J.; Dallinga, J.S.; Voss, H.P.; Haenen, G.R.; Bast, A. A new approach to assess the total antioxidant capacity using the TEAC assay. Food Chem. 2004, 88, 567–570. [Google Scholar] [CrossRef]
- Xiao, J. Recent advances on the stability of dietary polyphenols. Efood 2022, 3, e21. [Google Scholar] [CrossRef]
- Šterna, V.; Segliņa, D.; Krasnova, I.; Ķince, T.; Jansone, Z.; Bleidere, M. Sprouted hulless barley grains and their application possibilities for the functional sweet snacks development. Proc. Latv. Acad. Sci. Sect. B 2022, 76, 116–123. [Google Scholar] [CrossRef]
- Bazhay-Zhezherun, S.; Antoniuk, M.; Smulska, J. Biologically activated wheat grain as a functional component of glazed bars. Ukr. Food J. 2015, 4, 310. [Google Scholar]
- Finnie, S.; Brovelli, V.; Nelson, D. Sprouted grains as a food ingredient. In Sprouted Grains; AACC International Press: Duxford, UK, 2019; pp. 113–142. [Google Scholar]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Bishayee, A. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran J. Basic Med. Sci. 2019, 22, 225. [Google Scholar]
- Ruiz-Hernández, A.A.; Rouzaud-Sández, O.; Frias, J.; Ayala-Zavala, F.; Astiazarán-García, H.; Salazar–López, N.J.; Sánchez, M.R. Antioxidant and anti-inflammatory potential of a food produced from irradiated (UV-A LED) sorghum sprouts subjected to in vitro gastrointestinal simulation. J. Funct. Foods 2023, 110, 105857. [Google Scholar] [CrossRef]
- Seneviratne, K.N.; HapuarachchI, C.D.; Ekanayake, S. Comparison of the phenolic-dependent antioxidant properties of coconut oil extracted under cold and hot conditions. Food Chem. 2009, 114, 1444–1449. [Google Scholar] [CrossRef]
- Mahayothee, B.; Koomyart, I.; Khuwijitjaru, P.; Siriwongwilaichat, P.; Nagle, M.; Müller, J. Phenolic compounds, antioxidant activity, and medium chain fatty acids profiles of coconut water and meat at different maturity stages. Int. J. Food Prop. 2016, 19, 2041–2051. [Google Scholar] [CrossRef]
- Moreno Gracia, B.; Laya Reig, D.; Rubio-Cabetas, M.J.; Sanz García, M.Á. Study of phenolic compounds and antioxidant capacity of spanish almonds. Foods 2021, 10, 2334. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wu, M.; Zhang, Y.; Zhao, X.; Yu, J.; Zheng, X. The structure-dependent self-association of five phenolic acids in aqueous solution. Magn. Reson. Chem. 2014, 52, 460–466. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Tranchant, C.C.; Alhamad, M.N.; Rababah, T.; Gammoh, S.; Almajwal, A.; Alli, I. Phenolic and protein contents of differently prepared protein co-precipitates from flaxseed and soybean and antioxidant activity and angiotensin inhibitory activity of their phenolic fractions. NFS J. 2020, 21, 65–72. [Google Scholar] [CrossRef]
- Rawel, H.M.; Rohn, S. Nature of hydroxycinnamate-protein interactions. Phytochem. Rev. 2010, 9, 93–109. [Google Scholar] [CrossRef]
- Shahidi, F.; Dissanayaka, C.S. Phenolic-protein interactions: Insight from in-silico analyses–a review. Food Prod. Prosec. Nutr. 2023, 5, 2. [Google Scholar] [CrossRef]
- Costa, T.D.S.; Rogez, H.; Pena, R.D.S. Adsorption capacity of phenolic compounds onto cellulose and xylan. Food Sci. Technol. Int. 2015, 35, 314–320. [Google Scholar] [CrossRef]
- Xue, H.; Du, X.; Fang, S.; Gao, H.; Xie, K.; Wang, Y.; Tan, J. The interaction of polyphenols-polysaccharides and their applications: A review. Int. J. Biol. Macromol. 2024, 278, 134594. [Google Scholar] [CrossRef]
- Coello, K.E.; Frias, J.; Martínez-Villaluenga, C.; Cartea, M.E.; Velasco, P.; Peñas, E. Manufacture of healthy snack bars supplemented with moringa sprout powder. LWT-Food Sci. Technol. 2022, 154, 112828. [Google Scholar] [CrossRef]
- Rajagukguk, Y.V.; Arnold, M.; Sidor, A.; Kulczyński, B.; Brzozowska, A.; Schmidt, M.; Gramza-Michałowska, A. Antioxidant activity, probiotic survivability, and sensory properties of a phenolic-rich pulse snack bar enriched with Lactiplantibacillus plantarum. Foods 2022, 11, 309. [Google Scholar] [CrossRef]
- Yang, F. Nutritional Evaluation of Germinated Wheat and Its Use in a Nutritional Bar; National Library of Canada (Bibliothèque Nationale du Canada): Ottawa, ON, Canada, 2002. [Google Scholar]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef]
- Cabrera-Ramírez, A.H.; Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Morales-Sánchez, E.; Campos-Vega, R.; Gaytán-Martínez, M. Effect of the nixtamalization process on the protein bioaccessibility of white and red sorghum flours during in vitro gastrointestinal digestion. Food Res. Int. 2020, 134, 109234. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Alminger, M.; Aura, A.M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, G.J.; Requena, T.; Santos, C.N. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Chen, J.; McClements, D.J.; Hu, P.; Ye, X.; Liu, C.; Li, T. Protein–polyphenol interactions enhance the antioxidant capacity of phenolics: Analysis of rice glutelin–procyanidin dimer interactions. Food Funct. 2019, 10, 765–774. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods. 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Bhattarai, R.R.; Jayasree Joshi, T.; Sruthi, N.U.; Rao, P.S.; Johnson, S. Effects of extrusion cooking on nutritional and health attributes of sorghum and millets: Special reference to protein and starch digestibility. Int. J. Food Sci. Technol. 2025, 60, vvae093. [Google Scholar] [CrossRef]
- Li, C.X.; Wang, F.R.; Zhang, B.; Deng, Z.Y.; Li, H.Y. Stability and antioxidant activity of phenolic compounds during in vitro digestion. J. Food Sci. 2023, 88, 696–716. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Vargas-Requena, C.L.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Polyphenolic compounds and digestive enzymes: In vitro non-covalent interactions. Molecules 2017, 22, 669. [Google Scholar] [CrossRef]
- Szawara-Nowak, D.; Bączek, N.; Zieliński, H. Antioxidant capacity and bioaccessibility of buckwheat-enhanced wheat bread phenolics. J. Food Sci. Technol. 2016, 53, 621–630. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Che, G.; Chen, M.; Li, X.; Xiao, J.; Liu, L.; Guo, L. Effect of UV-A irradiation on bioactive com-pounds accumulation and hypoglycemia-related enzymes activities of broccoli and radish sprouts. Plants 2024, 13, 450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xing, B.; Sun, M.; Zhou, B.; Ren, G.; Qin, P. Changes in bio-accessibility, polyphenol profile and antioxidants of quinoa and djulis sprouts during in vitro simulated gastrointestinal digestion. Food Sci. Nutr. 2020, 8, 4232–4241. [Google Scholar] [CrossRef] [PubMed]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, L.V.; Gorshkov, O.V.; Mokshina, N.E.; Gorshkova, T.A. Differential expression of α-l-arabinofuranosidases during maize (Zea mays L.) root elongation. Planta 2015, 241, 1159–1172. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Mota, T.R.; Salatta, F.V.; de Almeida, G.H.; Olher, V.G.; Oliveira, M.A.; Dos Santos, W.D. Feruloyl esterase activity and its role in regulating the feruloylation of maize cell walls. Plant Physiol. Biochem. 2020, 156, 49–54. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Zajac, N. Digestion and absorption of phenolic compounds assessed by in vitro simulation methods. A review. Rocz. Państw. Zakł. Higi. 2013, 64, 79–84. [Google Scholar]
- Milinčić, D.D.; Stanisavljević, N.S.; Pešić, M.M.; Kostić, A.Ž.; Stanojević, S.P.; Pešić, M.B. The Bioaccessibility of Grape-Derived Phenolic Compounds: An Overview. Foods 2025, 14, 607. [Google Scholar] [CrossRef]
- Maldonado-Valderrama, J.; Wilde, P.; Macierzanka, A.; Mackie, A. The role of bile salts in digestion. Adv. Colloid Interface Sci. 2011, 165, 36–46. [Google Scholar] [CrossRef]
- Bellesi, F.A.; Pilosof, A.M. Potential implications of food proteins-bile salts interactions. Food Hydrocoll. 2021, 118, 106766. [Google Scholar] [CrossRef]
- Mikkelsen, M.S.; Cornali, S.B.; Jensen, M.G.; Nilsson, M.; Beeren, S.R.; Meier, S. Probing interactions between β-glucan and bile salts at atomic detail by 1H–13C NMR assays. J. Agric. Food Chem. 2014, 62, 11472–11478. [Google Scholar] [CrossRef]
- Naumann, S.; Haller, D.; Eisner, P.; Schweiggert-Weisz, U. Mechanisms of interactions between bile acids and plant com-pounds—A review. Int. J. Mol. Sci. 2020, 21, 6495. [Google Scholar] [CrossRef] [PubMed]
- Goodman, B.E. Insights into digestion and absorption of major nutrients in humans. Adv. Physiol. Educ. 2010, 34, 44–53. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Fu, L. Dietary protein-phenolic interactions: Characterization, biochemical-physiological consequences, and potential food applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 3589–3615. [Google Scholar] [CrossRef]
- Özdal, T.; Yalçınkaya, İ.E.; Toydemir, G.; Çapanoglu, E. Polyphenol-Protein Interactions and Changes in Functional Properties and Digestibility. In Encyclopedia of Food Chemistry, 1st ed.; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 566–577. [Google Scholar]
- Sták, M.; Clark, M.; Suur, B.E.; Börgeson, E. Inflammation and resolution in obesity. Nat. Rev. Endocrinol. 2025, 21, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive oxygen species signaling and oxidative stress: Transcriptional regulation and evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Kobayashi, J. Nitric oxide and insulin resistance. Immunoendocrinology 2015, 2, 10-14800. [Google Scholar]
- Conforti, F.; Menichini, F. Phenolic compounds from plants as nitric oxide production inhibitors. Curr. Med. Chem. 2011, 18, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, M.J. Cell viability assays: Introduction. In Mammalian Cell Viability; Methods in Molecular Biology; Stoddart, M., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 1–6. [Google Scholar]
- Lee, G.B.; Kim, Y.; Lee, K.E.; Vinayagam, R.; Singh, M.; Kang, S.G. Anti-inflammatory effects of quercetin, rutin, and troxerutin result from the inhibition of NO production and the reduction of COX-2 levels in RAW 264.7 cells treated with LPS. Appl. Biochem. Biotechnol. 2024, 196, 1–22. [Google Scholar] [CrossRef]
- Mu, K.; Kitts, D.D. Gallic acid mitigates intestinal inflammation and loss of tight junction protein expression using a 2D-Caco-2 and RAW 264.7 co-culture model. Arch. Biochem. Biophys. 2024, 756, 109978. [Google Scholar] [CrossRef]
- Hwang, D.; Kang, M.J.; Kang CWKim, G.D. Kaempferol-3-O-β-rutinoside suppresses the inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells via the NF-κB and MAPK pathways. Int. J. Mol. Med. 2019, 44, 2321–2328. [Google Scholar] [CrossRef]
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Tian, C.; Guo, Y.; Chang, Y.; Zhao, J.; Cui, C.; Liu, M. Dose-effect relationship on anti-inflammatory activity on LPS induced RAW 264.7 cells and antioxidant activity of rutin in vitro. Acta Pol. Pharm. 2019, 76, 511–522. [Google Scholar] [CrossRef]
- Elseweidy, M.M.; Amin, R.S.; Atteia, H.H.; El-Zeiky, R.R.; Al-Gabri, N.A. New insight on a combination of policosanol and 10-dehydrogingerdione phytochemicals as inhibitors for platelet activation biomarkers and atherogenicity risk in dyslipidemic rabbits: Role of CETP and PCSK9 inhibition. Appl. Biochem. Biotechnol. 2018, 186, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Satora, P.; Michalik, J. Transformations of phenolic compounds in an in vitro model simulating the human alimentary tract. Food Technol. Biotechnol. 2009, 47, 456–463. [Google Scholar]
- Scholl, C.; Lepper, A.; Lehr, T.; Hanke, N.; Schneider, K.L.; Brockmöller, J.; Stingl, J.C. Population nutrikinetics of green tea extract. PLoS ONE 2018, 13, e0193074. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Aigster, A.; Duncan, S.E.; Conforti, F.D.; Barbeau, W.E. Physicochemical properties and sensory attributes of resistant starch-supplemented granola bars and cereals. LWT-Food Sci. Technol. 2011, 44, 2159–2165. [Google Scholar] [CrossRef]
- Setyaningtiyas, T.; Fitriyanti, A.R.; Latrobdiba, Z.M.; Sya’di, Y.K. Sugar content, crude fiber content, antioxidant activity, and sensory characteristics of sorghum (Sorghum bicolor (L.) Moench) snack bar with addition of klutuk banana (Musa balbisiana colla) flour. Nat. Nutr. J./MGI 2024, 19, 95–106. [Google Scholar] [CrossRef]
- Xu, L.; Ye, Q.; Cao, Q.; Liu, Y.; Li, X.; Liu, Z.; Gong, Y.; Zhang, S.; Yin, J.; Xu, Y. Effects of the Taste Substances and Metal Cations in Green Tea Infusion on the Turbidity of EGCG–Mucin Mixtures. Foods 2024, 13, 1172. [Google Scholar] [CrossRef]
- Fan, X.; Jiao, X.; Liu, J.; Jia, M.; Blanchard, C.; Zhou, Z. Characterizing the volatile compounds of different sorghum cultivars by both GC-MS and HS-GC-IMS. Food Res. Int. 2021, 140, 109975. [Google Scholar] [CrossRef]
- Momanyi, D.; Owino, W.; Makokha, A. Formulation, nutritional and sensory evaluation of baobab based ready-to-eat sorghum and cowpea blend snack bars. Sci. Afr. 2020, 7, e00215. [Google Scholar] [CrossRef]


| Ingredient | Experimental Granola Bars | |||
|---|---|---|---|---|
| SF | OF | SSF | ISSF | |
| Oat flakes(g) | 50 | 50 | 50 | 50 |
| Coconut oil (g) | 4.3 | 4.3 | 4.3 | 4.3 |
| Agave honey (g) | 27 | 27 | 27 | 27 |
| Cashews (g) | 12.5 | 12.5 | 12.5 | 12.5 |
| Almonds (g) | 12.5 | 12.5 | 12.5 | 12.5 |
| Coconut (g) | 12.5 | 12.5 | 12.5 | 12.5 |
| SF (g) | 12.5 | -- | -- | -- |
| OF (g) | -- | 12.5 | -- | -- |
| SSF(g) | -- | -- | 12.5 | -- |
| ISSF(g) | -- | -- | -- | 12.5 |
| Phenols (µg/g) | SF | OF | SSF | ISSF |
|---|---|---|---|---|
| Gallic acid | 627.71 ± 68.47 b | 345.50 ± 6.33 c | 678.70 ± 8.83 b | 1228.55 ± 1.22 a |
| Protocatechuic acid | 25.29 ± 0.47 | - | - | - |
| p-Coumaric acid | 104.38 ± 0.38 a | 16.17 ± 0.45 b | - | - |
| Ferulic acid | - | - | - | - |
| Sinapic acid | - | - | - | - |
| Catechin | 25.43 ± 0.15 d | 27.81 ± 0.23 c | 1202.28 ± 2.02 b | 1223.41 ± 2.14 a |
| Assay | Bars | |||
|---|---|---|---|---|
| SF | OF | SSF | ISSF | |
| Total phenols | 34.1 | 51.4 | 87.9 | 119.9 |
| DPPH | 95.5 | 87.4 | 59.2 | 201.0 |
| TEAC | 41.7 | 60.9 | 43.9 | 225.8 |
| Phenols (µg/g) | SF | OF | SSF | ISSF |
|---|---|---|---|---|
| Gallic acid | - | - | 658.82 ± 15.05 b | 1856.89 ± 7.81 a |
| Protocatechuic acid | 487.01 ± 10.88 c | 180.78 ± 7.30 d | 2834.01 ± 35.12 a | 977.93 ± 6.73 b |
| p-Coumaric acid | 53.19 ± 0.63 a | 18.23 ± 0.85 d | 35.52 ± 3.66 c | 40.29 ± 0.89 b |
| Ferulic acid | 50.73 ± 0.34 a | 7.65 ± 0.87 d | 36.26 ± 0.49 b | 7.38 ± 0.76 c |
| Sinapic acid | - | 64.57 ± 2.188 c | 89.32 ± 0.91 a | 77.79 ± 0.24 b |
| Catechin | 23.11 ± 0.19 d | 48.87 ± 0.29 c | 100.83 ± 0.11 b | 1672.59 ± 4.43 a |
| Sample | Concentration (µg/mL) | Cell Viability (%) | Nitric Oxide (NO) (nM) | ||
|---|---|---|---|---|---|
| Granola Bar | Intestinal | Granola Bar | Intestinal | ||
| LPS+ | - | 100.1 | 129.4 a | 129.4 a | |
| LPS- | - | 108.2 | 3.6 c | 3.6 j | |
| SF | 25 | 98.7 | 103.6 | 111.6 ± 2.5 bA | 102.8 ± 0.5 eB |
| 12.5 | 104.6 | 102.2 | 109.9 ± 2.3 bA | 105.5 ± 0.7 dB | |
| 6.25 | 96.8 | 102.1 | 108.6 ± 1.5 bB | 112.4 ± 0.2 bA | |
| OF | 25 | 100.6 | 96.9 | 102.5 ± 1.0 bA | 74.7 ± 1.6 hB |
| 12.5 | 105.4 | 103.6 | 109.7 ± 1.0 bA | 96.8 ± 4.4 fB | |
| 6.25 | 100.5 | 102.4 | 112.1 ± 2.1 bA | 110.4 ± 0.3 cA | |
| SSF | 25 | 102.5 | 93.9 | 107.0 ± 0.4 bA | 71.1 ± 3.8 hiB |
| 12.5 | 105.4 | 92.2 | 111.8 ± 1.5 bA | 84.2 ± 5.3 gB | |
| 6.25 | 100.9 | 93.5 | 112.5 ± 0.4 bA | 96.7 ± 4.9 fB | |
| ISSF | 25 | 105.1 | 94.8 | 109.3 ± 1.1 bA | 68.3 ± 1.7 iB |
| 12.5 | 105.8 | 91.9 | 109.5 ± 2.5 bA | 69.9 ± 3.5 iB | |
| 6.25 | 101.5 | 107.6 | 112.5 ± 1.3 bA | 74.4 ± 3.9 hiB | |
| Variable | Level | OF | SF | SSF | ISSF |
|---|---|---|---|---|---|
| Sweetness | Little sweet | −0.085 | 1.017 | 0.929 | 1.336 |
| JAR | |||||
| Very sweet | 0.008 | 0.817 | 0.633 | 1.434 | |
| Herbal | Little herbal | 0.212 | 0.495 | 0.575 | 0.400 |
| JAR | |||||
| Very herbal | 0.198 | 0.572 | 1.300 * | 1.600 | |
| Firmness | Unsteady | 0.421 | 0.096 | 0.329 | −0.204 |
| JAR | |||||
| Very firm | 0.856 | −0.245 | 0.126 | −0.206 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Hernández, A.A.; Rouzaud-Sández, O.; Valenzuela-González, M.; Domínguez-Avila, J.A.; González-Aguilar, G.A.; Robles-Sánchez, M. Antioxidant/Anti-Inflammatory Potential and Sensory Acceptance of Granola Bars Developed with Sorghum Sprout Flour Irradiated with UV-A LED Light. Foods 2025, 14, 1787. https://doi.org/10.3390/foods14101787
Ruiz-Hernández AA, Rouzaud-Sández O, Valenzuela-González M, Domínguez-Avila JA, González-Aguilar GA, Robles-Sánchez M. Antioxidant/Anti-Inflammatory Potential and Sensory Acceptance of Granola Bars Developed with Sorghum Sprout Flour Irradiated with UV-A LED Light. Foods. 2025; 14(10):1787. https://doi.org/10.3390/foods14101787
Chicago/Turabian StyleRuiz-Hernández, Alan A., Ofelia Rouzaud-Sández, Maribel Valenzuela-González, J. Abraham Domínguez-Avila, Gustavo A. González-Aguilar, and Maribel Robles-Sánchez. 2025. "Antioxidant/Anti-Inflammatory Potential and Sensory Acceptance of Granola Bars Developed with Sorghum Sprout Flour Irradiated with UV-A LED Light" Foods 14, no. 10: 1787. https://doi.org/10.3390/foods14101787
APA StyleRuiz-Hernández, A. A., Rouzaud-Sández, O., Valenzuela-González, M., Domínguez-Avila, J. A., González-Aguilar, G. A., & Robles-Sánchez, M. (2025). Antioxidant/Anti-Inflammatory Potential and Sensory Acceptance of Granola Bars Developed with Sorghum Sprout Flour Irradiated with UV-A LED Light. Foods, 14(10), 1787. https://doi.org/10.3390/foods14101787








