Procyanidins and Anthocyanins in Young and Aged Prokupac Wines: Evaluation of Their Reactivity Toward Salivary Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Wine, Seed and Skin Samples
2.2. Saliva Collection
2.3. Saliva Test
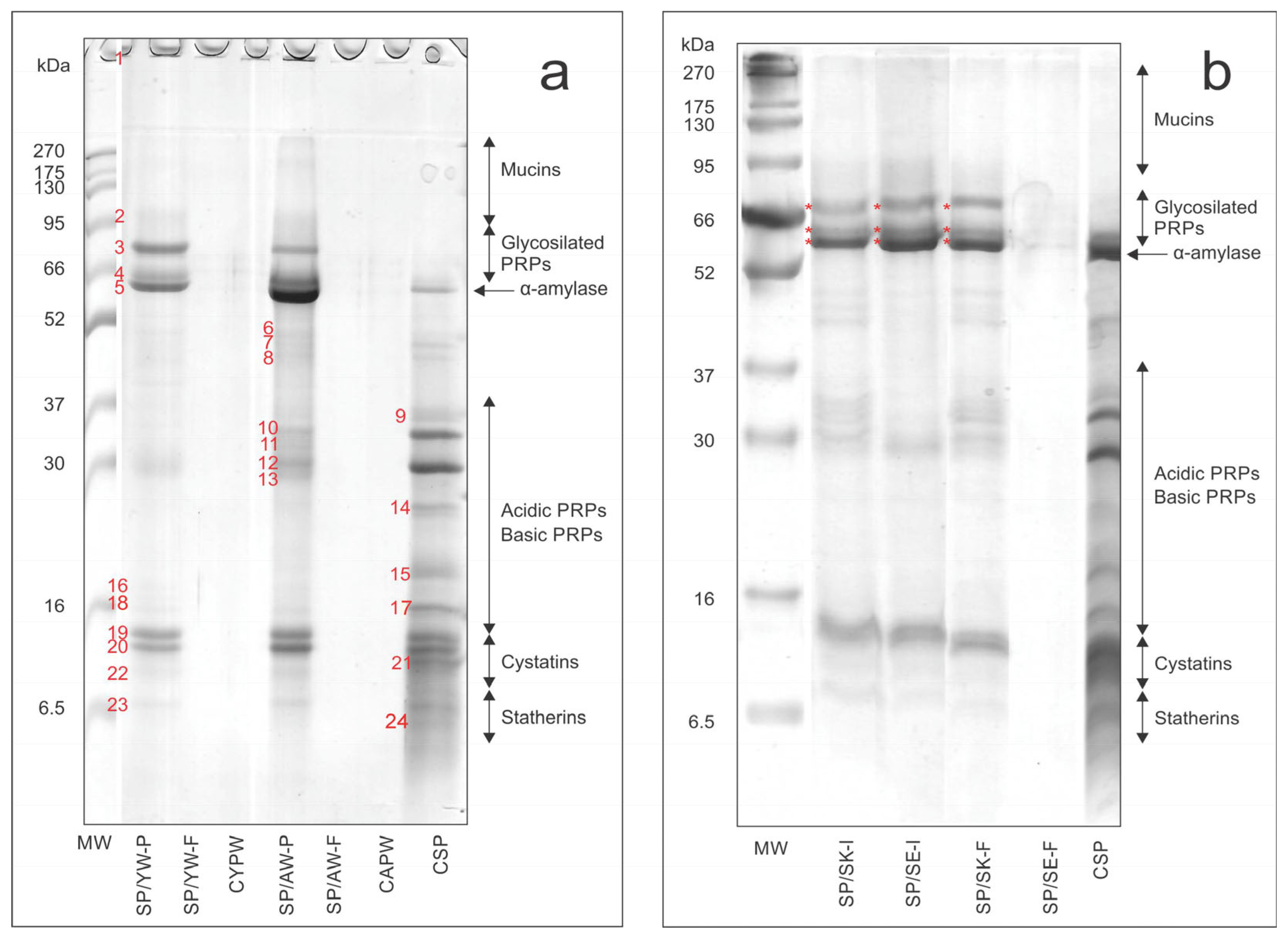
2.4. SDS-PAGE Analysis
- (a)
- Control salivary proteins—CSP;
- (b)
- Salivary protein solution/young wine filtrate (after filtration through 0.22 µm filter)—SP/YW-F;
- (c)
- Salivary protein solution/young wine precipitate (after centrifugation)—SP/YW-P;
- (d)
- Control young Prokupac wine—CYPW;
- (e)
- Salivary protein solution/aged wine filtrate (after filtration through 0.22 µm filter)—SP/AW-F;
- (f)
- Salivary protein solution/aged wine precipitate (after centrifugation)—SP/AW-P;
- (g)
- Control aged Prokupac wine—CAPW.
- (a)
- Salivary protein solution/seed extract after incubation (37 °C, 5 min)—SP/SE-I;
- (b)
- Salivary protein solution/seed extract filtrate (after filtration through 0.22 µm filter)—SP/SE-F;
- (c)
- Salivary protein solution/skin extract after incubation (37 °C, 5 min)—SP/SK-I;
- (d)
- Salivary protein solution/skin extract filtrate (after filtration through 0.22 µm filter)—SP/SK-F;
2.5. Untargeted and Targeted UHPLC-QTOF-MS Analysis
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Untargeted UHPLC-QTOF-MS Profile of Young and Aged Prokupac Wines
3.2. Content of Phenolic Compounds in Young and Aged Prokupac Wines
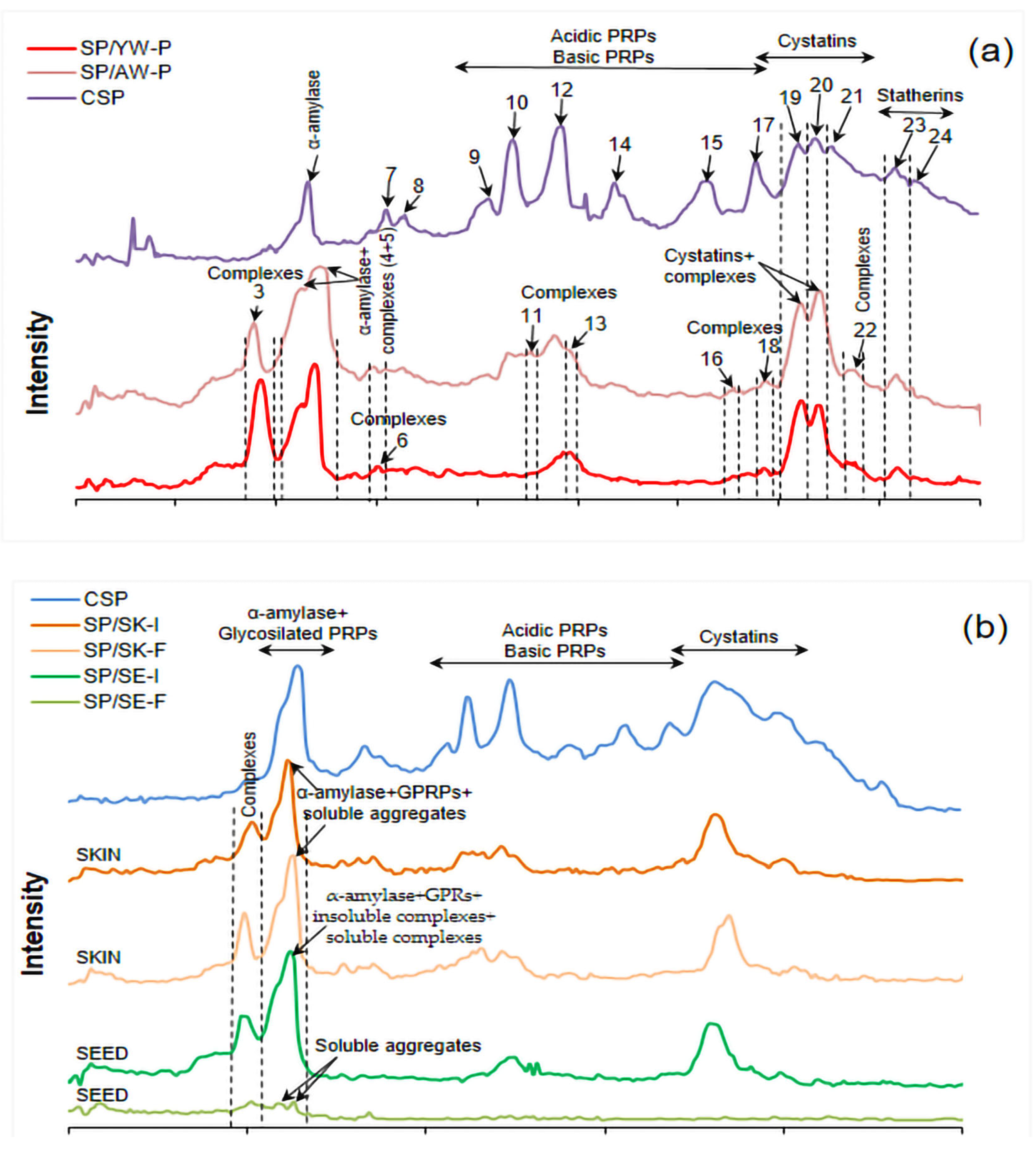
3.3. SDS-PAGE Analysis of Salivary Proteins Before and After Interaction with Wine Samples
3.4. Binding Affinity of Salivary Proteins for Selected Wine Phenolics
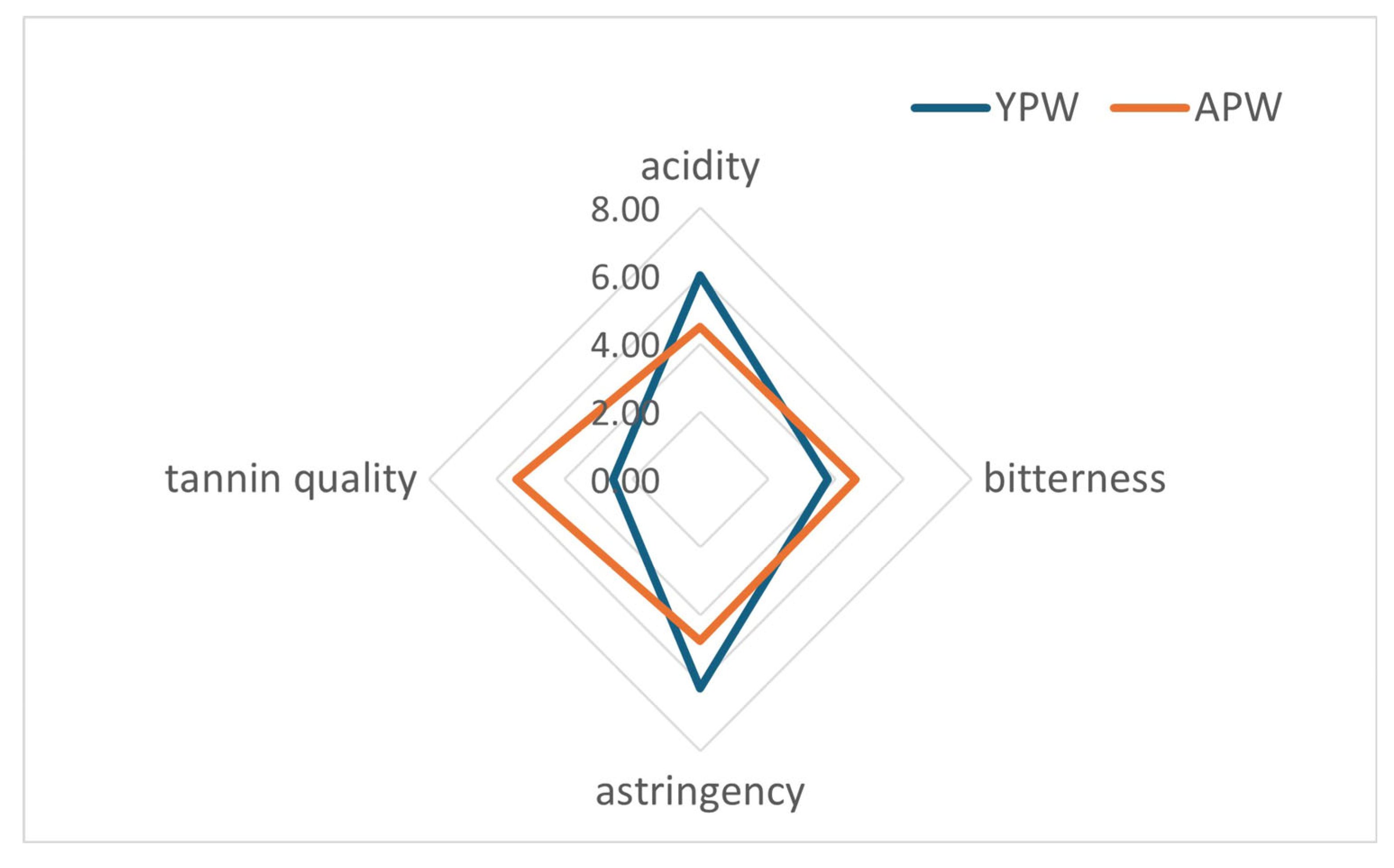
3.5. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- OIV. State of the world vine and wine sector in 2023. Available online: https://www.oiv.int/sites/default/files/2024-04/OIV_STATE_OF_THE_WORLD_VINE_AND_WINE_SECTOR_IN_2023.pdf (accessed on 1 April 2025).
- OIV. World wine production outlook. Available online: https://www.oiv.int/sites/default/files/documents/OIV_2024_World_Wine_Production_Outlook_1.pdf (accessed on 1 April 2025).
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.Ž.; Soković Bajić, S.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Lj Tešić, Ž.; Pešić, M.B. Phenolic compounds and biopotential of grape pomace extracts from Prokupac red grape variety. LWT 2021, 138, 110739. [Google Scholar] [CrossRef]
- Paissoni, M.A.; Waffo-Teguo, P.; Ma, W.; Jourdes, M.; Giacosa, S.; Río Segade, S.; Rolle, L.; Teissedre, P.-L. Sensory assessment of grape polyphenolic fractions: An insight into the effects of anthocyanins on in-mouth perceptions. OENO One 2020, 54, 1059–1075. [Google Scholar] [CrossRef]
- González-Muñoz, B.; Garrido-Vargas, F.; Pavez, C.; Osorio, F.; Chen, J.; Bordeu, E.; O’Brien, J.A.; Brossard, N. Wine astringency: More than just tannin-protein interactions. J. Sci. Food Agric. 2022, 102, 1771–1781. [Google Scholar] [CrossRef]
- Huang, R.; Xu, C. An overview of the perception and mitigation of astringency associated with phenolic compounds. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1036–1074. [Google Scholar] [CrossRef]
- Ma, W.; Waffo-Teguo, P.; Jourdes, M.; Li, H.; Teissedre, P.L. Chemical Affinity between Tannin Size and Salivary Protein Binding Abilities: Implications for Wine Astringency. PLoS ONE 2016, 11, e0161095. [Google Scholar] [CrossRef]
- Freitas, V.; Mateus, N. Protein/Polyphenol Interactions: Past and Present Contributions. Mechanisms of Astringency Perception. Curr. Org. Chem. 2012, 16, 724–746. [Google Scholar] [CrossRef]
- Costa, J.J.; Moreira, F.T.C.; Soares, S.; Brandão, E.; Mateus, N.; De Freitas, V.; Sales, M.G.F. Wine astringent compounds monitored by an electrochemical biosensor. Food Chem. 2022, 395, 133587. [Google Scholar] [CrossRef]
- Greabu, M.; Battino, M.; Mohora, M.; Totan, A.; Didilescu, A.; Spinu, T.; Totan, C.; Miricescu, D.; Radulescu, R. Saliva--a diagnostic window to the body, both in health and in disease. J. Med. Life 2009, 2, 124–132. [Google Scholar]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- McArthur, C.; Sanson, G.D.; Beal, A.M. Salivary proline-rich proteins in mammals: Roles in oral homeostasis and counteracting dietary tannin. J. Chem. Ecol. 1995, 21, 663–691. [Google Scholar] [CrossRef]
- Pascal, C.; Poncet-Legrand, C.; Cabane, B.; Vernhet, A. Aggregation of a Proline-Rich Protein Induced by Epigallocatechin Gallate and Condensed Tannins: Effect of Protein Glycosylation. J. Agric. Food Chem. 2008, 56, 6724–6732. [Google Scholar] [CrossRef]
- Canon, F.; Paté, F.; Cheynier, V.; Sarni-Manchado, P.; Giuliani, A.; Pérez, J.; Durand, D.; Li, J.; Cabane, B. Aggregation of the Salivary Proline-Rich Protein IB5 in the Presence of the Tannin EgCG. Langmuir 2013, 29, 1926–1937. [Google Scholar] [CrossRef] [PubMed]
- Bennick, A. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral Biol. Med. 2002, 13, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Iturmendi, N.; Gambuti, A.; Jourdes, M.; Teissedre, P.L.; Moio, L. Chip electrophoresis as a novel approach to measure the polyphenols reactivity toward human saliva. Electrophoresis 2014, 35, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Pineda, A.M.; Carpenter, G.H.; García-Estévez, I.; Escribano-Bailón, M.T. Influence of Chemical Species on Polyphenol–Protein Interactions Related to Wine Astringency. J. Agric. Food Chem. 2020, 68, 2948–2954. [Google Scholar] [CrossRef]
- Sarni-Manchado, P.; Cheynier, V.; Moutounet, M. Interactions of Grape Seed Tannins with Salivary Proteins. J. Agric. Food Chem. 1999, 47, 42–47. [Google Scholar] [CrossRef]
- Sarni-Manchado, P.; Canals-Bosch, J.-M.; Mazerolles, G.; Cheynier, V. Influence of the Glycosylation of Human Salivary Proline-Rich Proteins on Their Interactions with Condensed Tannins. J. Agric. Food Chem. 2008, 56, 9563–9569. [Google Scholar] [CrossRef]
- Soares, S.; Vitorino, R.; Osório, H.; Fernandes, A.; Venâncio, A.; Mateus, N.; Amado, F.; de Freitas, V. Reactivity of Human Salivary Proteins Families Toward Food Polyphenols. J. Agric. Food Chem. 2011, 59, 5535–5547. [Google Scholar] [CrossRef]
- Soares, S.; García-Estévez, I.; Ferrer-Galego, R.; Brás, N.F.; Brandão, E.; Silva, M.; Teixeira, N.; Fonseca, F.; Sousa, S.F.; Ferreira-da-Silva, F.; et al. Study of human salivary proline-rich proteins interaction with food tannins. Food Chem. 2018, 243, 175–185. [Google Scholar] [CrossRef]
- Hornedo Ortega, R.; Gonzalez-Centeno, M.R.; Chira, K.; Jourdes, M.; Teissedre, P.-L. Phenolic Compounds of Grapes and Wines: Key Compounds and Implications in Sensory Perception. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; IntechOpen: London, UK, 2020. [Google Scholar]
- Paissoni, M.A.; Waffo-Teguo, P.; Ma, W.; Jourdes, M.; Rolle, L.; Teissedre, P. Chemical and sensorial investigation of in-mouth sensory properties of grape anthocyanins. Sci. Rep. 2018, 8, 17098. [Google Scholar] [CrossRef]
- Delić, K.; Milinčić, D.D.; Pešić, M.B.; Lević, S.; Nedović, V.A.; Gancel, A.-L.; Jourdes, M.; Teissedre, P.-L. Grape, wine and pomace anthocyanins: Winemaking biochemical transformations, application and potential benefits. OENO One 2024, 58. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Soares, S.; Mateus, N.; Rivas-Gonzalo, J.; Escribano-Bailón, M.T.; Freitas, V.d. New Anthocyanin–Human Salivary Protein Complexes. Langmuir 2015, 31, 8392–8401. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rochera, B.; Manjón, E.; Escribano-Bailón, M.T.; García-Estévez, I. Role of Anthocyanins in the Interaction between Salivary Mucins and Wine Astringent Compounds. Foods 2023, 12, 3623. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different phenolic compounds activate distinct human bitter taste receptors. J. Agric. Food Chem. 2013, 61, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.; Brandão, E.; Soares, S.; Oliveira, J.; Lopes, P.; Mateus, N.; de Freitas, V. Polyphenolic Characterization of Nebbiolo Red Wines and Their Interaction with Salivary Proteins. Foods 2020, 9, 1867. [Google Scholar] [CrossRef]
- Sun, B.; Sá, M.d.; Leandro, C.; Caldeira, I.; Duarte, F.L.; Spranger, I. Reactivity of Polymeric Proanthocyanidins toward Salivary Proteins and Their Contribution to Young Red Wine Astringency. J. Agric. Food Chem. 2013, 61, 939–946. [Google Scholar] [CrossRef]
- Soares, S.; Brandao, E.; Mateus, N.; Freitas, V. Interaction between red wine procyanidins and salivary proteins: Effect of stomach digestion on the resulting complexes. RSC Adv. 2015, 5, 12664–12670. [Google Scholar] [CrossRef]
- Brandão, E.; Silva, M.S.; García-Estévez, I.; Williams, P.; Mateus, N.; Doco, T.; de Freitas, V.; Soares, S. Inhibition Mechanisms of Wine Polysaccharides on Salivary Protein Precipitation. J. Agric. Food Chem. 2020, 68, 2955–2963. [Google Scholar] [CrossRef]
- Soares, S.; Santos Silva, M.; García-Estévez, I.; Brandão, E.; Fonseca, F.; Ferreira-da-Silva, F.; Teresa Escribano-Bailón, M.; Mateus, N.; de Freitas, V. Effect of malvidin-3-glucoside and epicatechin interaction on their ability to interact with salivary proline-rich proteins. Food Chem. 2019, 276, 33–42. [Google Scholar] [CrossRef]
- de Freitas, V.; Mateus, N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011, 401, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, I.H.n.; Lorenzo, E.S.-P.; Espinosa, A.V. Phenolic composition and magnitude of copigmentation in young and shortly aged red wines made from the cultivars, Cabernet Sauvignon, Cencibel, and Syrah. Food Chem. 2005, 92, 269–283. [Google Scholar] [CrossRef]
- Boulton, R. The Copigmentation of Anthocyanins and Its Role in the Color of Red Wine: A Critical Review. Am. J. Enol. Vitic. 2001, 522, 67–87. [Google Scholar] [CrossRef]
- Manjón, E.; García-Estévez, I.; Escribano-Bailón, M.T. Possible Role of High-Molecular-Weight Salivary Proteins in Astringency Development. Foods 2024, 13, 862. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; García-Estevez, I.; Fonseca, F.; Guerreiro, C.; Ferreira-da-Silva, F.; Mateus, N.; Deffieux, D.; Quideau, S.; de Freitas, V. Interaction between Ellagitannins and Salivary Proline-Rich Proteins. J. Agric. Food Chem. 2019, 67, 9579–9590. [Google Scholar] [CrossRef] [PubMed]
- Zdunić, G.; Gođevac, D.; Šavikin, K.; Krivokuća, D.; Mihailović, M.; Pržić, Z.; Marković, N. Grape Seed Polyphenols and Fatty Acids of Autochthonous Prokupac Vine Variety from Serbia. Chem. Biodivers. 2019, 16, e1900053. [Google Scholar] [CrossRef]
- Pesic, M.B.; Barac, M.B.; Stanojevic, S.P.; Ristic, N.M.; Macej, O.D.; Vrvic, M.M. Heat induced casein–whey protein interactions at natural pH of milk: A comparison between caprine and bovine milk. Small Rumin. Res. 2012, 108, 77–86. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Vidović, B.B.; Gašić, U.M.; Milenković, M.; Kostić, A.Ž.; Stanojević, S.P.; Ilić, T.; Pešić, M.B. A systematic UHPLC Q-ToF MS approach for the characterization of bioactive compounds from freeze-dried red goji berries (L. barbarum L.) grown in Serbia: Phenolic compounds and phenylamides. Food Chem. 2024, 456, 140044. [Google Scholar] [CrossRef]
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. ISO: Geneva, Switzerland, 2007.
- ISO 3591:1977; Sensory Analysis—Apparatus—Wine-Tasting Glass. ISO: Geneva, Switzerland, 1977.
- Kovačević Ganić, K.; Staver, M.; Peršurić, Đ.; Banović, M.; Komes, D.; Gracin, L. Influence of Blending on the Aroma of Malvasia istriana Wine. Food Technol. Biotechnol. 2003, 41, 305–314. [Google Scholar]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic Profiles of Leaves, Grapes and Wine of Grapevine Variety Vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef]
- Jordão, A.; Ricardo-da-Silva, J.; Laureano, O. Extraction of Some Ellagic Tannins and Ellagic Acid from Oak Wood Chips (Quercus pyrenaica L.) in Model Wine Solutions: Effect of Time, pH, Temperature and Alcoholic Content. S. Afr. J. Enol. Vitic. 2005, 26, 86–89. [Google Scholar] [CrossRef][Green Version]
- Matějíček, D.; Mikeš, O.; Klejdus, B.; Štěrbová, D.; Kubáň, V. Changes in contents of phenolic compounds during maturing of barrique red wines. Food Chem. 2005, 90, 791–800. [Google Scholar] [CrossRef]
- Fulcrand, H.; Dueñas, M.; Salas, E.; Cheynier, V. Phenolic Reactions during Winemaking and Aging. Am. J. Enol. Vitic. 2006, 57, 289. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Dabić Zagorac, D.Č.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Ž.L.; Natić, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef]
- Căpruciu, R. Resveratrol in Grapevine Components, Products and By-Products—A Review. Horticulturae 2025, 11, 111. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Changes in the detailed pigment composition of red wine during maturity and ageing: A comprehensive study. Anal. Chim. Acta 2006, 563, 238–254. [Google Scholar] [CrossRef]
- Ramos-Pineda, A.M.; García-Estévez, I.; Soares, S.; de Freitas, V.; Dueñas, M.; Escribano-Bailón, M.T. Synergistic effect of mixture of two proline-rich-protein salivary families (aPRP and bPRP) on the interaction with wine flavanols. Food Chem. 2019, 272, 210–215. [Google Scholar] [CrossRef]
- McRae, J.M.; Falconer, R.J.; Kennedy, J.A. Thermodynamics of Grape and Wine Tannin Interaction with Polyproline: Implications for Red Wine Astringency. J. Agric. Food Chem. 2010, 58, 12510–12518. [Google Scholar] [CrossRef]
- Shahidi, F.; Dissanayaka, C.S. Phenolic-protein interactions: Insight from in-silico analyses—A review. Food Prod. Process. Nutr. 2023, 5, 2. [Google Scholar] [CrossRef]
- Charlton, A.J.; Baxter, N.J.; Khan, M.L.; Moir, A.J.G.; Haslam, E.; Davies, A.P.; Williamson, M.P. Polyphenol/Peptide Binding and Precipitation. J. Agric. Food Chem. 2002, 50, 1593–1601. [Google Scholar] [CrossRef]
- Helmerhorst, E.J.; Zamakhchari, M.; Schuppan, D.; Oppenheim, F.G. Discovery of a Novel and Rich Source of Gluten-Degrading Microbial Enzymes in the Oral Cavity. PLoS ONE 2010, 5, e13264. [Google Scholar] [CrossRef] [PubMed]
- Rashwan, A.K.; Osman, A.I.; Abdelshafy, A.M.; Mo, J.; Chen, W. Plant-based proteins: Advanced extraction technologies, interactions, physicochemical and functional properties, food and related applications, and health benefits. Crit. Rev. Food Sci. Nutr. 2025, 65, 667–694. [Google Scholar] [CrossRef] [PubMed]
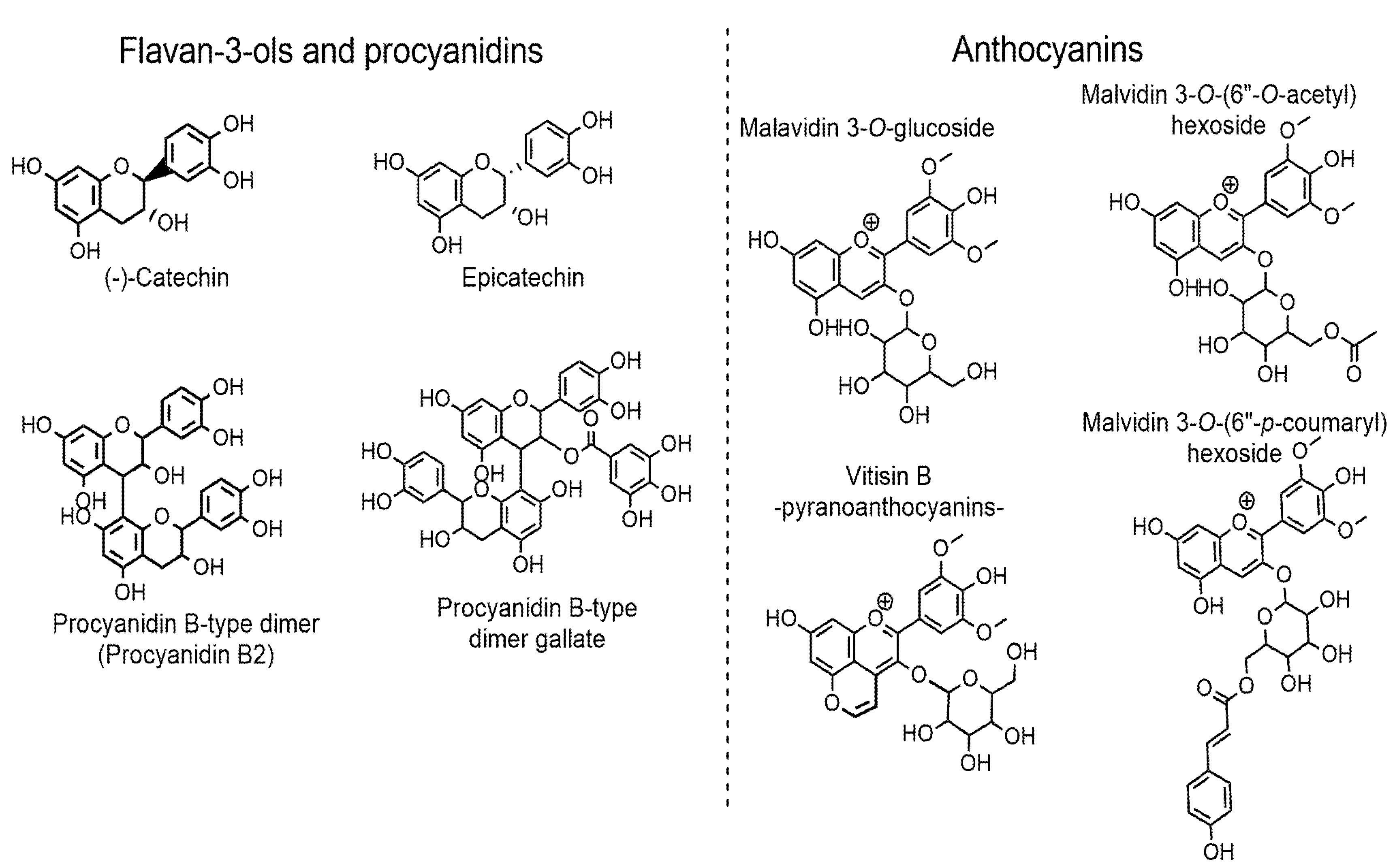

| No. | RT | Compound | Formula | Calculated Mass | m/z Exact Mass | mDa | MS Fragments (Main Fragment) | Samples | |
|---|---|---|---|---|---|---|---|---|---|
| YPW | APW | ||||||||
| Phenolic acid and derivatives | |||||||||
| 1 | 2.80 | Coumaric acid * | C9H7O3− | 163.0395 | 163.0401 | 0.58 | 119.0497(100) | + | − |
| 2 | 7.38 | Vanillic acid * | C8H7O4− | 167.0344 | 167.0356 | 1.17 | 123.0439(100), 107.0133 | + | − |
| 3 | 1.00 | Gallic acid * | C7H5O5− | 169.0137 | 169.0148 | 1.10 | 125.0239(100), 124.0163 | + | + |
| 4 | 4.37 | Caffeic acid * | C9H7O4− | 179.0344 | 179.0356 | 1.17 | 135.0445(100), 134.0371, 107.0499 | + | + |
| 5 | 3.92 | Ferulic acid * | C10H9O4− | 193.0501 | 193.0503 | 0.22 | 134.0365(100), 133.0283, 117.0342, 148.0133, 164.0119 | − | + |
| 6 | 6.59 | Ethyl gallate | C9H9O5− | 197.045 | 197.0465 | 1.50 | 124.0162(100), 125.0227, 169.0144 | + | + |
| 7 | 9.42 | Ethyl caffeic acid | C11H11O4− | 207.0657 | 207.0670 | 1.27 | 133.0292(100), 135.0446, 134.036, 161.0244, 179.0343 | + | + |
| 8 | 3.20 | Coutaric acid | C13H11O8− | 295.0454 | 295.0470 | 1.61 | 119.0501(100), 163.0400 | + | + |
| 9 | 7.52 | Ellagic acid * | C14H5O8− | 300.9984 | 301.0001 | 1.66 | 300.9992(100), 299.9913, 283.9966, 229.016, 201.0202, 151.0033, 245.0144, 185.0251, 173.0229, 257.0103 | + | + |
| 10 | 1.54 | Caftaric acid | C13H11O9− | 311.0403 | 311.0421 | 1.79 | 135.0447(100), 149.0089, 179.0352, 134.0372 | + | + |
| 11 | 4.17 | Fertaric acid | C14H13O9− | 325.056 | 325.0600 | 4.04 | 134.0368(100), 193.0506, 178.027, 149.0089 | + | + |
| 12 | 7.81 | Aesculin | C15H15O9− | 339.0716 | 339.0734 | 1.79 | 161.0241(100), 159.0295, 133.0285, 177.0398, 115.0392 | − | + |
| 13 | 3.84 | Caffeoylquinic acid (like Chlorogenic acid) | C16H17O9− | 353.0873 | 353.0887 | 1.44 | 191.0559(100), 161.0239, 127.0395, 173.0451, 135.0449 | + | − |
| Flavan-3-ols and procyanidins | |||||||||
| 14 | 3.42 | Catechin * | C15H13O6− | 289.0712 | 289.0727 | 1.49 | 123.045(100), 109.0294, 125.0244, 151.0398, 137.0244, 203.0712, 149.025, 221.0821, 187.0402, 245.0813 | + | + |
| 15 | 6.13 | Epicatechin * | C15H13O6− | 289.0712 | 289.0727 | 1.49 | 123.045(100), 109.0294, 125.0244, 151.0399, 137.0243, 203.0713, 149.0253, 221.0819, 187.0403, 245.0820 | + | + |
| 16 | 2.48 | Procyanidin B-type dimer is. I | C30H25O12− | 577.1346 | 577.1365 | 1.90 | 289.0724(100), 407.0780, 125.0243, 245.0805, 161.0248, 137.0242, 273.0408, 425.0884, 451.1036, 255.0339, 229.0511 | + | + |
| 17 | 4.11 | Procyanidin B-type dimer is. II | C30H25O12− | 577.1346 | 577.1365 | 1.90 | 289.0718(100), 407.0776, 125.0241, 245.0798, 161.0249, 137.0239, 273.0404, 425.0885, 451.1047, 255.0377, 229.0512, 205.0485 | + | − |
| 18 | 5.38 | Procyanidin B-type dimer is. III | C30H25O12− | 577.1346 | 577.1365 | 1.90 | 289.0722(100), 407.0778, 125.0242, 245.0803, 161.0250, 137.0242, 273.0407, 425.0882, 451.1031, 229.0512, 205.0476, 109.0291 | + | + |
| 19 | 3.41 | Chalcan-flavan 3-ol dimer is. I (like Gambiriin A1) | C30H27O12− | 579.1503 | 579.1522 | 1.95 | 289.0720(100), 245.0824, 271.0607, 179.0352, 205.0510, 165.0187, 151.0400, 137.0245, 125.0242, 109.0293 | + | − |
| 20 | 6.07 | Chalcan-flavan 3-ol dimer is. II | C30H27O12− | 579.1503 | 579.1522 | 1.95 | 289.0719(100), 245.0824, 271.060719, 179.0352, 205.0510, 165.0188, 151.0397, 137.0241, 125.0241, 109.0293, 221.0825 | + | − |
| 21 | 6.84 | Procyanidin dimer B-type gallate | C37H29O16− | 729.1456 | 729.1481 | 2.54 | 407.0772(100), 289.0716, 125.0239, 451.1023, 169.0141, 577.1319, 271.0612, 287.0567, 441.0825, 161.0246, 245.0591, 203.0206 | + | − |
| Flavonols and glycosides | |||||||||
| 22 | 10.1 | Kaempferol * | C15H9O6− | 285.0399 | 285.0411 | 1.19 | 285.0405(100), 185.0609, 229.0515, 239.035, 159.0447, 211.0396, 143.0497, 151.0038, 227.0347, 255.0301, 268.0370 | + | − |
| 23 | 9.30 | Quercetin | C15H9O7− | 301.0348 | 301.0368 | 1.97 | 151.0036(100), 121.0292, 178.9984, 149.0237, 301.0334, 245.0456, 273.0400, 229.0500, 201.0549 | + | + |
| 24 | 10.3 | Isorhamnetin | C16H11O7− | 315.0505 | 315.0516 | 1.12 | 300.0276(100), 151.0033, 301.031, 107.0133, 271.0251, 283.0259, 255.0293, 227.0344, 243.0301, 179.0001 | + | + |
| 25 | 8.41 | Myricetin * | C15H9O8− | 317.0297 | 317.0315 | 1.76 | 151.0036(100), 137.0241, 107.0137, 178.9987, 165.0191, 227.0349, 243.0311, 271.0247, 317.0306 | + | + |
| 26 | 9.27 | Laricitrin | C16H11O8− | 331.0454 | 331.0473 | 1.91 | 151.0062(100), 316.0231, 178.9995, 271.0243, 317.0257, 287.0179, 259.0252, 243.0300, 107.0135 | - | + |
| 27 | 7.72 | Syringetin | C17H13O8− | 345.061 | 345.0634 | 2.36 | 190.9994(100), 315.0144, 163.0028, 287.0211, 330.0383, 316.019, 271.0243, 259.0244, 243.0282, 345.0607 | + | − |
| 28 | 7.60 | Quercetin 3-O-hexuronide | C21H17O13− | 477.0669 | 477.0687 | 1.78 | 301.0358(100), 151.0034, 178.9984, 283.0251, 273.0403, 255.0301, 245.0451 | + | + |
| 29 | 7.13 | Myricetin 3-O-hexoside | C21H19O13− | 479.0826 | 479.0847 | 2.13 | 316.0229(100), 271.0245, 287.0194, 178.9982, 151.0035, 479.0832 | + | + |
| 30 | 7.05 | Myricetin 3-O-hexuronide | C21H17O14− | 493.0618 | 493.0647 | 2.87 | 317.0304(100), 318.0312, 178.9971, 151.0049, 137.0232, 271.0281, 299.0174 | − | + |
| 31 | 7.65 | Laricitrin 3-O-hexoside | C22H21O13− | 493.0982 | 493.0988 | 0.58 | 330.0382(100), 331.0446, 315.0150, 316.0201, 287.02, 493.1013, 271.0245, 243.0285, 151.0055, 178.9975 | + | - |
| 32 | 8.11 | Syringetin 3-O-hexoside | C23H23O13− | 507.1139 | 507.1156 | 1.73 | 344.0541(100), 345.0591, 507.1147, 273.0405, 301.0369, 316.0588, 329.0321, 258.0160, 151.0034 | + | + |
| Other detected non-anthocyanin flavonoids | |||||||||
| 33 | 9.83 | Naringenin * | C15H11O5− | 271.0606 | 271.0622 | 1.55 | 119.0501(100), 151.0034, 107.0133, 177.0182, 161.0586, 145.0275, 229.0541 | + | + |
| 34 | 7.39 | Taxifolin | C15H11O7− | 303.0505 | 303.0522 | 1.72 | 125.0249(100), 151.0216, 174.0312, 199.0390, 137.0211, 193.0515, 243.0271 | − | + |
| 35 | 5.05 | Dihydromyricetin | C15H11O8− | 319.0454 | 319.0469 | 1.51 | 125.0242(100), 165.019, 151.0038, 167.0346, 137.0241, 175.0040, 193.0137, 205.0501, 233.0457 | + | − |
| 36 | 8.40 | Phloretin 2’-O-hexoside (like Phlorizin) | C21H23O10− | 435.1291 | 435.1316 | 2.48 | 167.0351(100), 273.0778, 125.0238, 274.0802, 179.0348, 123.0452, 168.0388 | + | − |
| Stilbenoids | |||||||||
| 37 | 9.34 | Resveratrol * | C14H11O3− | 227.0708 | 227.0721 | 1.28 | 143.0501(100), 185.0593, 117.0347, 157.0655, 167.0535 | + | + |
| 38 | 8.22 | Resveratrol hexoside (like Piceid) | C20H21O8− | 389.1236 | 389.1253 | 1.66 | 227.0711(100), 185.0605, 143.0499, 159.0811 | + | + |
| Anthocyanins and pyranoanthocyanins | |||||||||
| Malvidin derivatives | |||||||||
| 39 | 6.59 | Malvidin 3-O-glucoside * | C23H25O12+ | 493.1346 | 493.1375 | 2.9 | 331.0831(100), 332.0854, 315.0508, 316.0578, 287.0555 | + | + |
| 40 | 7.13 | Malvidin 3-O-hexoside-acetaldehyde (Vitisin B) | C25H25O12+ | 517.1346 | 517.1367 | 2.1 | 355.0819(100), 356.0854, 317.0662 | + | + |
| 41 | 7.40 | Malvidin 3-O-(6”-acetyl)hexoside | C25H27O13+ | 535.1452 | 535.1475 | 2.33 | 331.0819(100), 332.085, 333.0878, 315.0505 | + | - |
| 42 | 7.45 | 10H-Pyranomalvidin 3-O-(6”-acetyl)hexoside (Malvidin-acetaldehyde adduct I) | C27H27O13+ | 559.1452 | 559.147 | 1.83 | 355.0822(100), 356.0848, 397.0921 | + | − |
| 43 | 7.12 | Malvidin 3-O-hexoside-pyruvate (Vitisin A) | C26H25O14+ | 561.1244 | 561.1266 | 2.17 | 399.0722(100), 400.0754 | + | + |
| 44 | 8.64 | Malvidin 3-O-hexoside-4-vinylphenol | C31H29O13+ | 609.1608 | 609.1626 | 1.78 | 447.1079(100), 448.1112, 431.0755 | - | + |
| 45 | 8.39 | Malvidin 3-O-hexoside-4-vinylcatechol (Pinotin A) | C31H29O14+ | 625.1557 | 625.1577 | 1.97 | 463.1026(100), 464.1059, 447.0745 | − | + |
| 46 | 8.22 | Malvidin 3-O-(6”-p-coumaroyl)hexoside | C32H31O14+ | 639.1714 | 639.1739 | 2.52 | 331.0819(100), 332.085, 333.0876 | + | − |
| 47 | 8.11 | 10H-Pyranomalvidin 3-O-(6”-p-coumaroyl)hexosid (Malvidin-acetaldehyde adduct II) | C34H31O14+ | 663.1714 | 663.1737 | 2.32 | 355.0811(100), 356.0852, 357.087 | + | − |
| Other detected anthocyanins | |||||||||
| 48 | 6.06 | Petunidin 3-O-glucoside | C22H23O12+ | 479.119 | 479.1205 | 1.55 | 317.0657(100), 318.0698, 302.0423 | + | − |
| 49 | 7.59 | Peonidin 3-O-(6”- acetyl)hexoside | C24H25O12+ | 505.1346 | 505.1362 | 1.6 | 301.0704(100), 302.0746, 286.048 | + | − |
| 50 | 8.30 | Peonidin 3-O-(6”-p-coumaroyl)hexoside | C31H29O13+ | 609.1608 | 609.1635 | 2.68 | 301.0708(100), 302.0744, 303.076, 286.0477 | + | − |
| 51 | 8.06 | Petunidin 3-O-(6”-p-coumaroyl)hexoside | C31H29O14+ | 625.1557 | 625.1581 | 2.37 | 317.0661(100), 318.0689, 302.0466 | + | − |
| Compound | Samples | |
|---|---|---|
| YPW | APW | |
| mg/L Wine | ||
| Coumaric acid | 3.07 | – |
| Vanillic acid | 5.95 | – |
| Gallic acid | 24.65 | 19.84 |
| Caffeic acid | 4.72 | 3.39 |
| Ferulic acid | – | 2.09 |
| Ellagic acid | 0.61 | 1.25 |
| Resveratrol | 1.38 | <LOQ |
| Catechin | 16.32 | 11.40 |
| Epicatechin | 6.93 | 2.74 |
| Procyanidin B-type dimer is. I * | 10.33 | 3.63 |
| Procyanidin B-type dimer is. II * | 2.02 | – |
| Procyanidin B-type dimer is. III * | 6.56 | 3.33 |
| Kaempferol | <LOQ | – |
| Myricetin | 2.69 | 2.09 |
| Naringenin | <LOQ | <LOQ |
| Malvidin-3-O-glucoside | 47.20 | 5.80 |
| ∑ | 132.45 | 55.56 |
| No. Polypeptide Band | CSP (%) | SP/AW-P (%) | SP/YW-P (%) | Characteristic of Identified Bands | Band Area Ratio (SP/AW-P)/ (SP/YW-P) |
|---|---|---|---|---|---|
| 2 | − | + | + | Complexes | 1.03 |
| 3 | − | + | + | Complexes | 0.65 |
| 4 + 5 | 100 | 643.4 | 346.9 | α-amylase + GPRPs + complexes | 1.85 |
| 6 | − | + | + | Complexes | 2.09 |
| 7 | 100 | 21.2 | − | - | * |
| 8 | 100 | 60.3 | − | - | * |
| 9 | 100 | − | − | - | - |
| 10 | 100 | 52.3 | − | PRPs | * |
| 11 | − | + | + | Complexes | 4.37 |
| 12 | 100 | 54.2 | 43.0 | PRPs | 1.26 |
| 13 | − | + | + | Complexes | 0.78 |
| 14 | 100 | − | − | PRPs | - |
| 15 | 100 | − | − | PRPs | - |
| 16 | − | + | + | Complexes | 0.93 |
| 17 | 100 | − | − | PRPs | - |
| 18 | − | + | + | Complexes | 1.60 |
| 19 | 100 | 150.4 | 137.0 | Cystatins + complexes | 1.09 |
| 20 | 100 | 120.7 | 75.8 | Cystatins + complexes | 1.59 |
| 21 | 100 | − | − | Cystatins | - |
| 22 | − | + | + | Complexes | 2.86 |
| 23 | 100 | 43.6 | 19.2 | Statherins | 2.27 |
| 24 | 100 | 0 | 0 | Statherins | - |
| Target Compound | m/z Exact Mass | SP/YW | SP/AW |
|---|---|---|---|
| Percentage of Bound Phenolics (%) | |||
| Monomeric flavan-3-ol and procyanidins (ESI−) | |||
| (Epi)catechin | 289.0712 | 4.78 ± 0.95 | 54.00 ± 1.10 |
| Procyanidin dimer (procyanidin B1) | 577.1346 | 28.51 ± 0.85 | 71.25 ± 0.31 |
| Procyanidin trimer (procyanidin C1) | 865.1979 | 29.86 ± 1.94 | 77.87 ± 0.24 |
| Procyanidin tetramer | 1153.2614 | 23.44 ± 1.21 | 74.99 ± 0.95 |
| Procyanidin pentamer | 1441.3248 | 32.16 ± 3.37 | 100 |
| Anthocyanins (malvidin derivatives) and pyranoanthocyanins (ESI+) | |||
| Malavidin 3-O-glucoside | 493.1346 | 3.91 ± 0.35 | 90.01 ± 0.03 |
| Malvidin 3-O-(6″-O-acetyl)hexoside | 535.1452 | / | 100 |
| Malvidin 3-O-(6″-p-coumaroyl)hexoside | 639.1714 | 15.86 ± 0.97 | 100 |
| Malvidin 3-O-hexoside-acetaldehyde (Vitisin B) | 517.1346 | / | 78.31 ± 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delić, K.; Milinčić, D.D.; Petrović, A.V.; Stanojević, S.P.; Gancel, A.-L.; Jourdes, M.; Pešić, M.B.; Teissedre, P.-L. Procyanidins and Anthocyanins in Young and Aged Prokupac Wines: Evaluation of Their Reactivity Toward Salivary Proteins. Foods 2025, 14, 1780. https://doi.org/10.3390/foods14101780
Delić K, Milinčić DD, Petrović AV, Stanojević SP, Gancel A-L, Jourdes M, Pešić MB, Teissedre P-L. Procyanidins and Anthocyanins in Young and Aged Prokupac Wines: Evaluation of Their Reactivity Toward Salivary Proteins. Foods. 2025; 14(10):1780. https://doi.org/10.3390/foods14101780
Chicago/Turabian StyleDelić, Katarina, Danijel D. Milinčić, Aleksandar V. Petrović, Slađana P. Stanojević, Anne-Laure Gancel, Michael Jourdes, Mirjana B. Pešić, and Pierre-Louis Teissedre. 2025. "Procyanidins and Anthocyanins in Young and Aged Prokupac Wines: Evaluation of Their Reactivity Toward Salivary Proteins" Foods 14, no. 10: 1780. https://doi.org/10.3390/foods14101780
APA StyleDelić, K., Milinčić, D. D., Petrović, A. V., Stanojević, S. P., Gancel, A.-L., Jourdes, M., Pešić, M. B., & Teissedre, P.-L. (2025). Procyanidins and Anthocyanins in Young and Aged Prokupac Wines: Evaluation of Their Reactivity Toward Salivary Proteins. Foods, 14(10), 1780. https://doi.org/10.3390/foods14101780







