Proteomic Analysis of Bifidobacterium animalis AR668 and AR668-R1 Under Aerobic Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain, Media, and Culture Condition
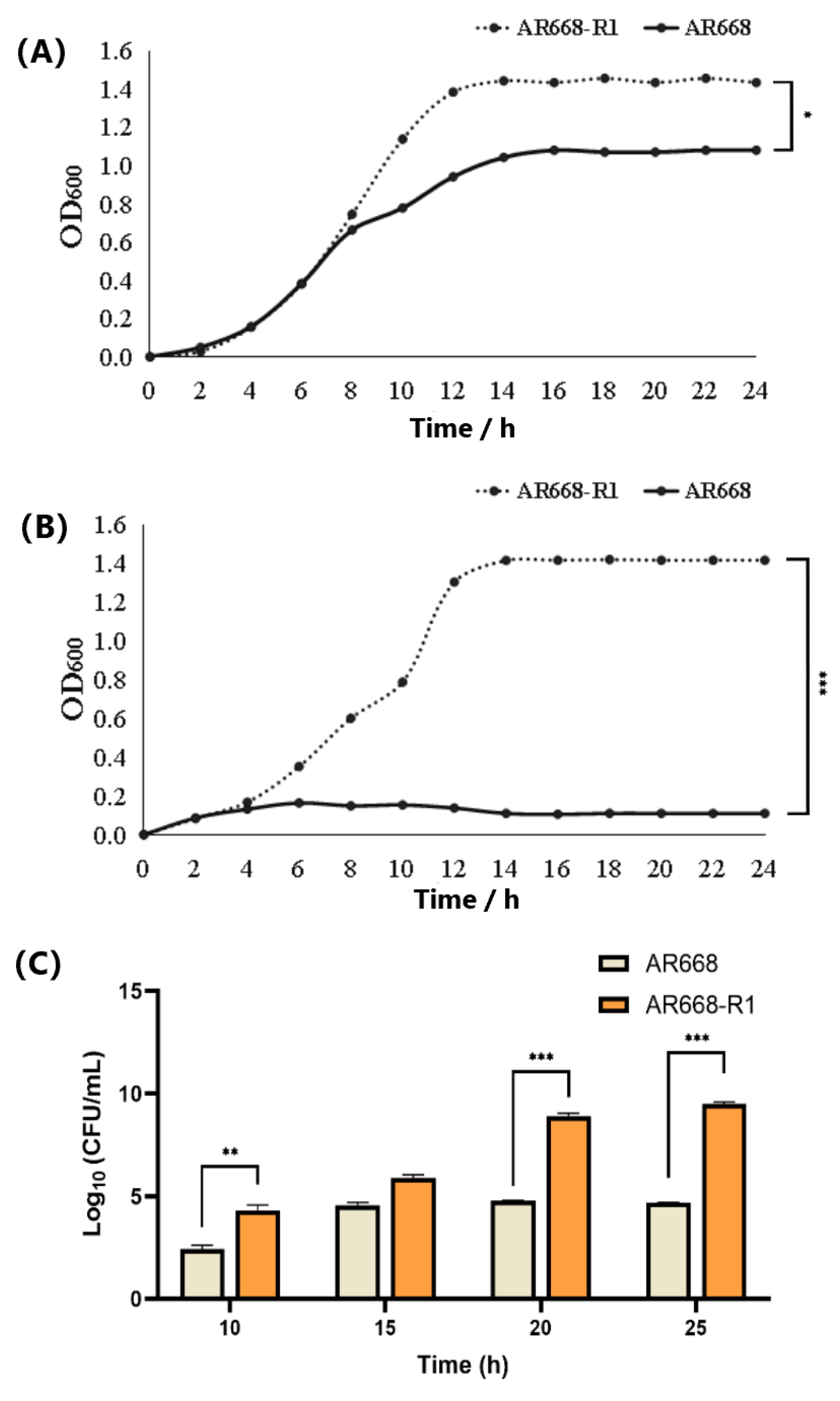
2.2. Hydrogen Peroxide Tolerance and Cell Growth of AR668 and AR668-R1
2.3. Protein Sample Preparation, Sequencing, and Enrichment Analysis
2.4. RNA Preparations and Gene Expression Analysis by Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.5. Plasmid Construction
2.6. Statistical Analysis
3. Results and Discussion
3.1. Hydrogen Peroxide Tolerance and Cell Growth of AR668-R1 and AR668
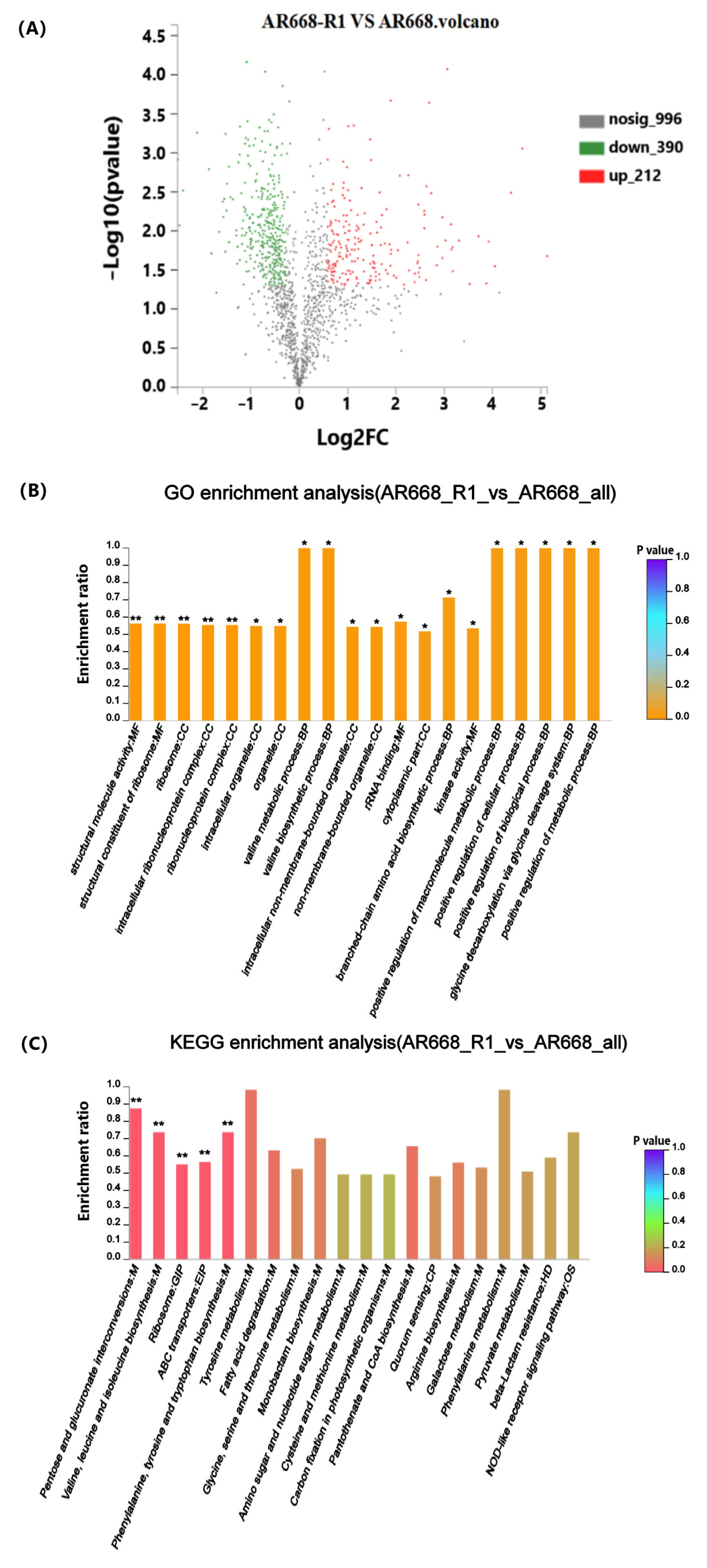
3.2. Functional Enrichment Significance Analysis
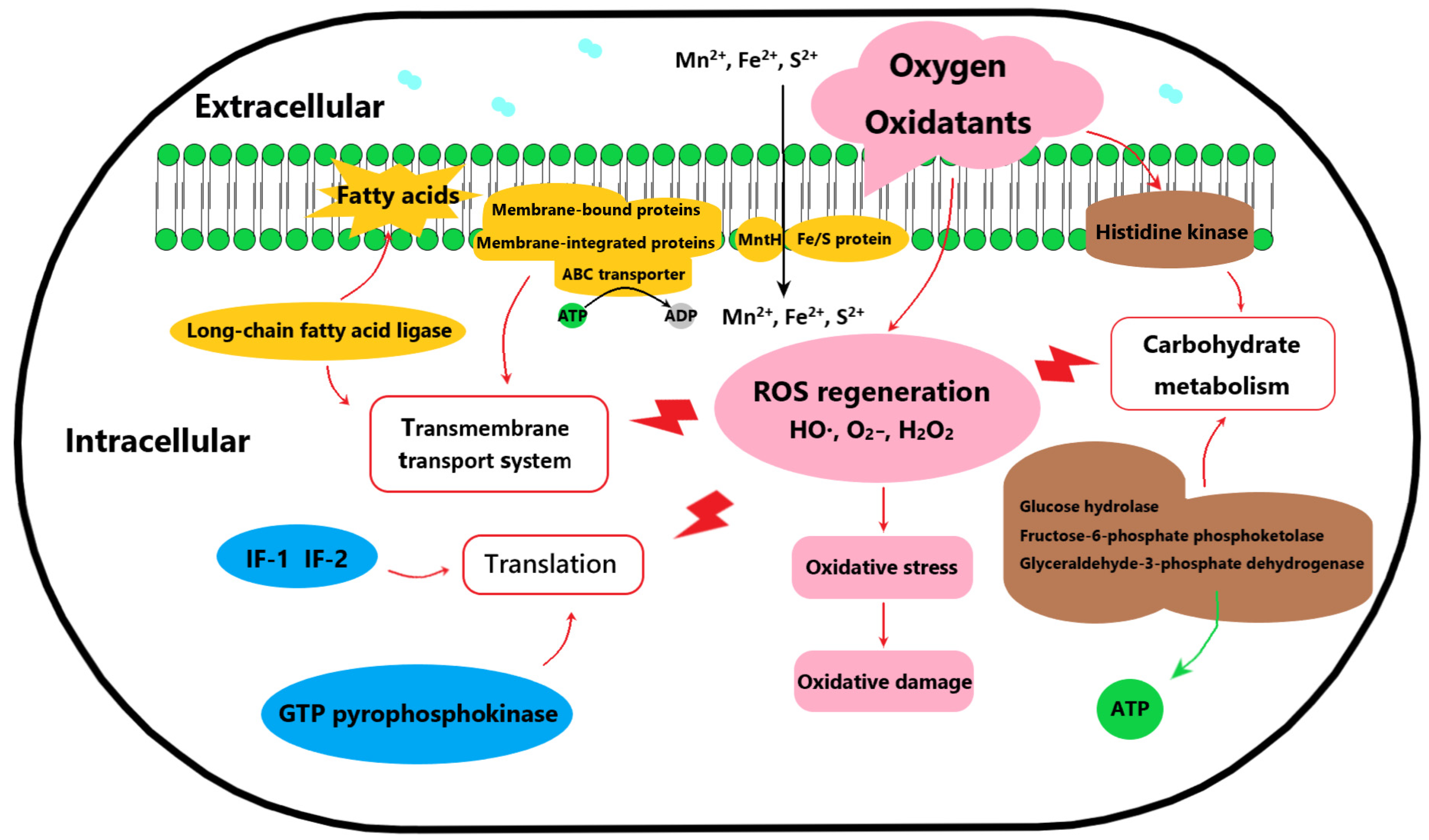
3.3. Analysis of Differential Expressed Proteins
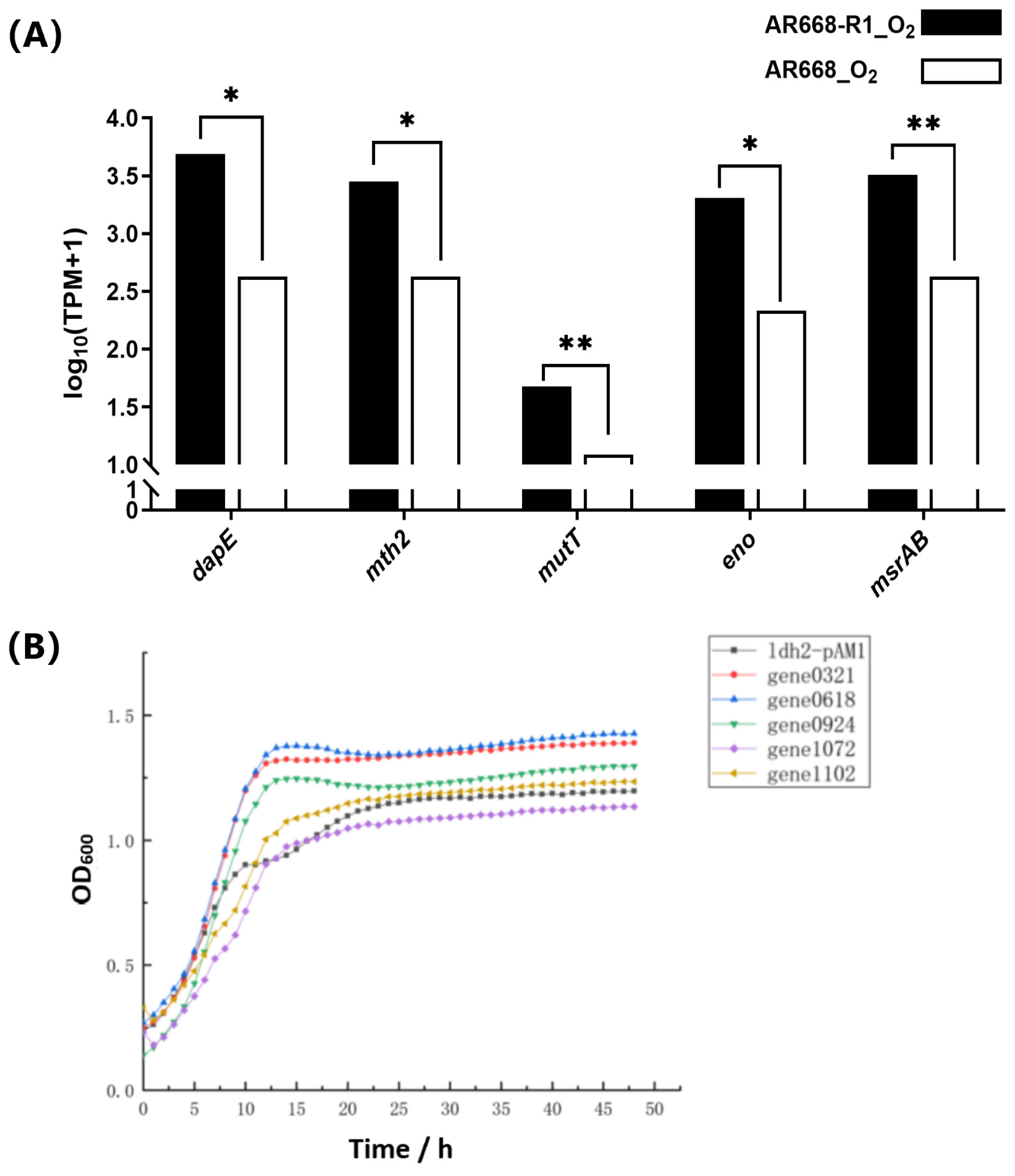
3.4. Validation of Potential Oxygen-Tolerance Proteins of B. animalis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evdokimova, S.A.; Karetkin, B.A.; Guseva, E.V.; Gordienko, M.G.; Khabibulina, N.V.; Panfilov, V.I.; Menshutina, N.V.; Gradova, N.B. A study and modeling of Bifidobacterium and Bacillus coculture continuous fermentation under distal intestine simulated conditions. Microorganisms 2022, 10, 929. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Lin, W.; Liu, S.J.; Jiao, Z.; Li, X. Probiotics, prebiotics, and postbiotics in health and disease. MedComm 2023, 4, e420. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, S.; Kwok, L.Y.; Sun, Z.; Wang, J.; Zhang, H. Probiotic Bifidobacterium animalis ssp. lactis Probio-M8 improves the fermentation and probiotic properties of fermented milk. J. Dairy Sci. 2024, 107, 6643–6657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hou, Y.; Zhang, S.; Jing, N.; Zhang, H.; Xie, Y.; Liu, H.; Yan, J.; Ren, J.; Jin, J. Bifidobacterium animalis A12, a probiotic strain that promotes glucose and lipid metabolism, improved the texture and aroma of the fermented sausage. Foods 2023, 12, 336. [Google Scholar] [CrossRef]
- Pimentel, T.C.; de Assis, B.B.T.; dos Santos Rocha, C.; Marcolino, V.A.; Rosset, M.; Magnani, M. Prebiotics in non-dairy products: Technological and physiological functionality, challenges, and perspectives. Food Biosci. 2022, 46, 101585. [Google Scholar] [CrossRef]
- Baussier, C.; Oriol, C.; Durand, S.; Py, B.; Mandin, P. Small RNA OxyS induces resistance to aminoglycosides during oxidative stress by controlling Fe–S cluster biogenesis in Escherichia coli. Proc. Natl. Acad. Sci. USA 2024, 121, e2317858121. [Google Scholar] [CrossRef]
- Cronin, M.; Zomer, A.; Fitzgerald, G.; van Sinderen, D. Identification of iron-regulated genes of Bifidobacterium breve UCC2003 as a basis for controlled gene expression. Bioengineered 2012, 3, 159–169. [Google Scholar] [CrossRef]
- Schöpping, M.; Vesth, T.; Jensen, K.; Franzén, C.J.; Zeidan, A.A. Genome-wide assessment of stress-associated genes in bifidobacteria. Appl. Environ. Microbiol. 2022, 88, e02251-21. [Google Scholar] [CrossRef]
- Zomer, A.; van Sinderen, D. Intertwinement of stress response regulons in Bifidobacterium breve UCC2003. Gut Microbes 2010, 1, 100–102. [Google Scholar] [CrossRef]
- Satoh, T.; Todoroki, M.; Kobayashi, K.; Niimura, Y.; Kawasaki, S. Purified thioredoxin reductase from O2-sensitive Bifidobacterium bifidum degrades H2O2 by interacting with alkyl hydroperoxide reductase. Anaerobe 2019, 57, 45–54. [Google Scholar] [CrossRef]
- Schöpping, M.; Zeidan, A.A.; Franzén, C.J. Stress response in bifidobacteria. Microbiol. Mol. Biol. Rev. 2022, 86, e00170-21. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Pan, H.; Zhu, Z.; Li, Q. The complete genome sequence of Bifidobacterium longum LTBL16, a potential probiotic strain from healthy centenarians with strong antioxidant activity. Genomics 2020, 112, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Yaghoobi, M.; Zhang, S.; Li, P.; Li, Q.; Dogan, B.; Ahnrud, G.P.; Flock, G.; Marek, P.; Simpson, K.W.; et al. Adaptive Laboratory Evolution of Probiotics toward Oxidative Stress Using a Microfluidic-Based Platform. Small 2024, 20, 2306974. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhong, W.; Feng, S.; Tang, Z.; Zhang, Y.; Ai, L.; Xiong, Z. Identification of new reference genes for colony counting by reverse-transcription quantitative PCR in Bifidobacterium animalis. J. Dairy Sci. 2023, 106, 7477–7485. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, M.; Song, X.; Xia, Y.; Ai, L.; Xiong, Z. Oxygen tolerance mechanism of Bifidobacterium animalis AR668-R1 based on genomic and phenotypic analyses. LWT 2025, 215, 117207. [Google Scholar] [CrossRef]
- Sahoo, S.; Mahapatra, S.R.; Misra, N.; Suar, M. Application of genomics, transcriptomics, and proteomics in probiotic research. In Probiotic Beverages; Panda, S.K., Kellershohn, J., Russell, I., Eds.; Academic Press: Newton, MA, USA, 2021; pp. 235–256. [Google Scholar] [CrossRef]
- Li, J.; Xiong, Z.; Yang, M.; Song, X.; Wang, G.; Xia, Y.; Ai, L. Improvement of electroporation-mediated transformation efficiency for Bifidobacterium animalis AR668. Food Biosci. 2024, 58, 103638. [Google Scholar] [CrossRef]
- Villa-Rodríguez, E.; Ibarra-Gámez, C.; de Los Santos-Villalobos, S. Extraction of high-quality RNA from Bacillus subtilis with a lysozyme pre-treatment followed by the Trizol method. J. Microbiol. Methods 2018, 147, 14–16. [Google Scholar] [CrossRef]
- Kocaefe-Özşen, N.; Yilmaz, B.; Alkım, C.; Arslan, M.; Topaloğlu, A.; Kısakesen, H.L.; Gülsev, E.; Çakar, Z.P. Physiological and molecular characterization of an oxidative stress-resistant Saccharomyces cerevisiae strain obtained by evolutionary engineering. Front. Microbiol. 2022, 13, 822864. [Google Scholar] [CrossRef]
- Starosta, A.L.; Lassak, J.; Jung, K.; Wilson, D.N. The bacterial translation stress response. FEMS Microbiol. Rev. 2014, 38, 1172–1201. [Google Scholar] [CrossRef]
- Gao, X.; Kong, J.; Zhu, H.; Mao, B.; Cui, S.; Zhao, J. Lactobacillus, Bifidobacterium and Lactococcus response to environmental stress: Mechanisms and application of cross-protection to improve resistance against freeze-drying. J. Appl. Microbiol. 2022, 132, 802–821. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H., Jr. Comparative analyses of the transport proteins encoded within the genomes of nine Bifidobacterium species. Microb. Physiol. 2022, 32, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Duboux, S.; Muller, J.A.; De Franceschi, F.; Mercenier, A.; Kleerebezem, M. Using fluorescent promoter-reporters to study sugar utilization control in Bifidobacterium longum NCC 2705. Sci. Rep. 2022, 12, 10477. [Google Scholar] [CrossRef] [PubMed]
- Schöpping, M.; Goel, A.; Jensen, K.; Faria, R.A.; Franzén, C.J.; Zeidan, A.A. Novel Insights into the molecular mechanisms underlying robustness and stability in probiotic bifidobacteria. Appl. Environ. Microbiol. 2023, 89, e00082-23. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Yu, R.; Xiao, M.; Khaskheli, G.; Sun, X.; Ma, H.; Ren, F.; Zhang, B.; Chen, S. Transcriptomic analysis of Bifidobacterium longum subsp. longum BBMN68 in response to oxidative shock. Sci. Rep. 2018, 8, 17085. [Google Scholar] [CrossRef]
- Zhu, M.; Dai, X. Maintenance of translational elongation rate underlies the survival of Escherichia coli during oxidative stress. Nucleic Acids Res. 2019, 47, 7592–7604. [Google Scholar] [CrossRef]
- Ha, K.P.; Edwards, A.M. DNA repair in Staphylococcus aureus. Microbiol. Mol. Biol. Rev. 2021, 85, e00091-21. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, J.; Sun, M.; Yu, P.; Ma, Y.; Xie, L.; Chen, L. Comparative secretomic and proteomic analysis reveal multiple defensive strategies developed by Vibrio cholerae against the heavy metal (Cd2+, Ni2+, Pb2+, and Zn2+) stresses. Front. Microbiol. 2023, 14, 1294177. [Google Scholar] [CrossRef]
- Scheible, M.; Nguyen, C.T.; Luong, T.T.; Lee, J.H.; Chen, Y.W.; Chang, C.; Wittchen, M.; Camacho, M.I.; Tiner, B.L.; Wu, C.; et al. The fused methionine sulfoxide reductase MsrAB promotes oxidative stress defense and bacterial virulence in Fusobacterium nucleatum. MBio 2022, 13, e03022-21. [Google Scholar] [CrossRef]
| Strains/Plasmids | Descriptions | References |
|---|---|---|
| Strains | ||
| Escherichia coli Top10 | Host for cloning | Our laboratory |
| B. animalis AR668 | Wild-type, isolated from infant feces | Our laboratory |
| B. animalis AR668-R1 | High oxygen-tolerant, obtained through ALE using AR668 as the parental strain | Our laboratory |
| Plasmids | ||
| pAM1-ldh2 | Emr (erythromycin resistance), Ampr (ampicillin resistance); pAM1 derivative for promoter Pldh2 replacement | [17] |
| pLYP0321 | Emr, Ampr; pAM1-ldh2 derivative containing the gene0321 | This work |
| pLYP0618 | Emr, Ampr; pAM1-ldh2 derivative containing the gene0618 | This work |
| pLYP0924 | Emr, Ampr; pAM1-ldh2 derivative containing the gene0924 | This work |
| pLYP1072 | Emr, Ampr; pAM1-ldh2 derivative containing the gene1072 | This work |
| pLYP1102 | Emr, Ampr; pAM1-ldh2 derivative containing the gene1102 | This work |
| The ID in Database | Symbol | Function | Differential Regulation | FC (AR668-R1/AR668) |
|---|---|---|---|---|
| A0A2M9HL62 | rsmG | Ribosomal RNA small subunit methyltransferase G | up | 24.48 |
| A0A0L7CYV1 | cspA | Cold-shock protein | up | 20.85 |
| D3R4Y3 | msrAB | peptide methionine sulfoxide reductase MsrA/MsrB | up | 8.82 |
| A0A087BER2 | rpsF | 30S ribosomal protein S6 | up | 8.34 |
| A0A315RYH0 | TMEM175 | DUF1211 domain-containing protein | up | 7.78 |
| D3R6P3 | - | Capsular polysaccharide synthesis protein | up | 6.63 |
| D3R6Y8 | eno | enolase 1/2/3 | up | 6.45 |
| A0A087B4R0 | tktAB | Transketolase | up | 6.19 |
| A0A0L0LVN5 | gatC | Aspartyl/glutamyl-tRNA (Asn/Gln) amidotransferase subunit C | up | 6.04 |
| A0A068Z383 | MutT | DNA mismatch repair protein | up | 6.01 |
| A0A1C7FV15 | gadC | Amino acid permease | up | 6.01 |
| A0A087AF24 | IF2 | Translation initiation factor IF-2 | up | 5.52 |
| D3R5K0 | dapE | Conserved membrane protein (succinyl-diaminopimelate desuccinylase) | up | 4.77 |
| K4ILB4 | talB | Transaldolase | up | 4.23 |
| A0A086ZT91 | - | Gp18 | up | 3.71 |
| A0A315RUP5 | MTH2 | 8-oxo-dGTP diphosphatase | up | 3.50 |
| D3R6Z0 | - | Collagen adhesion protein | up | 3.28 |
| A0A087CFX6 | rplX | 50 S ribosomal protein L24 | up | 3.23 |
| A0A0L7BVT2 | rpmG | 50 S ribosomal protein L33 | up | 3.17 |
| D3R5P9 | livM | ABC transporter permease protein | up | 3.03 |
| A0A2M9H761 | gapA | Type I glyceraldehyde-3-phosphate dehydrogenase | up | 2.80 |
| A0A315S119 | nrdH | NrdH-redoxin | up | 2.77 |
| A0A0L7BLN2 | - | DeoR family transcriptional regulator | up | 2.55 |
| A0A0L7BTZ0 | - | Uncharacterized protein | up | 2.51 |
| A0A087DR62 | infA | Translation initiation factor IF-1 | up | 2.44 |
| A0A0L7BN57 | priA | Phosphoribosylanthranilate isomerase PriA | up | 2.34 |
| A0A261G534 | fadD | AMP-binding protein | up | 2.33 |
| A0A0L7BLU9 | efp | Elongation factor P | up | 2.31 |
| B8DWS1 | atpC | ATP synthase epsilon chain | up | 2.28 |
| A0A315RWG9 | ctpA | Copper-translocating P-type ATPase | up | 2.26 |
| A0A2N3QGE9 | ffh | Signal recognition particle protein | up | 2.18 |
| A0A2N3QFA6 | rplR | 50 S ribosomal protein L18 | up | 2.10 |
| B8DV28 | - | Zn-ribbon protein | up | 2.07 |
| A0A2N5J2G0 | - | Argininosuccinate synthase | up | 2.05 |
| A0A315S096 | fadD | Long-chain fatty acid-CoA ligase | up | 2.03 |
| D3R3Q3 | - | Hypothetical transporter (Sulfate transport family) | up | 2.02 |
| A0A315S6K7 | purC | Phosphoribosylaminoimidazole-succinocarboxamide synthase | up | 2.01 |
| A0A086ZTE6 | talA | Transaldolase | down | 0.50 |
| A0A315RY65 | sufC | Fe-S cluster assembly ATPase SufC | down | 0.49 |
| D3R515 | yajC | YajC | down | 0.49 |
| A0A1C7FUB9 | ftsH | ATP-dependent zinc metalloprotease FtsH | down | 0.49 |
| D3R4Q0 | malL | oligo-1,6-glucosidase | down | 0.49 |
| D3R3A3 | ppk1 | Polyphosphate kinase | down | 0.49 |
| A0A1V8PLA3 | ahpC | Alkyl hydroperoxide reductase | down | 0.49 |
| D3R6J3 | MalT | MalT regulatory protein | down | 0.49 |
| D3R4I6 | - | DedA family protein | down | 0.48 |
| B8DSM1 | musE | Possible solute binding protein of ABC transporter | down | 0.48 |
| A0A261G6F6 | ACSL | AMP-binding protein | down | 0.48 |
| A0A0L7BT82 | - | ABC transporter permease | down | 0.47 |
| A0A0L7CYV9 | hupB | Integration host factor | down | 0.46 |
| A0A386K0D5 | musE | ABC transporter substrate-binding protein | down | 0.46 |
| A0A133L2E1 | xfp | D-xylulose 5-phosphate/D-fructose 6-phosphate phosphoketolase | down | 0.46 |
| D3R7L4 | srtA | Putative integral membrane protein | down | 0.46 |
| B8DW33 | secY | Protein translocase subunit SecY | down | 0.45 |
| A0A0L7BMA7 | aqpZ | Major intrinsic protein | down | 0.45 |
| A0A315T437 | K06890 | BAX inhibitor (BI)-1/YccA family protein | down | 0.44 |
| A0A087DDX0 | srtA | Sortase | down | 0.44 |
| D3R4S6 | butAC | (S,S)-butane-2,3-diol dehydrogenase | down | 0.43 |
| D3R7N6 | - | Transporter | down | 0.43 |
| A0A0L7BSH9 | - | ABC transporter substrate-binding protein | down | 0.42 |
| A0A087CM59 | rpmA | 50 S ribosomal protein L27 | down | 0.41 |
| D3R7X1 | htsT | Membrane-bound protein | down | 0.39 |
| D3R462 | xynB | Beta-xylosidase | down | 0.37 |
| A0A087D495 | mrp | Iron-sulfur cluster carrier protein | down | 0.36 |
| D3R5S2 | pflACE | Pyruvate formate-lyase activating enzyme | down | 0.36 |
| A0A133L2D5 | pflD | Formate C-acetyltransferase | down | 0.35 |
| A0A0A0UBM4 | trxA | Thioredoxin | down | 0.35 |
| A0A261FCR3 | ftsH | ATP-dependent zinc metalloprotease FtsH | down | 0.34 |
| A0A2T3G7W9 | livK | ABC transporter substrate-binding protein | down | 0.34 |
| Q564C5 | galR | LacI family DNA-binding transcriptional regulator | down | 0.33 |
| D3R5W5 | amdH | Dihydrodipicolinate reductase | down | 0.27 |
| D3R5W3 | - | GTP pyrophosphokinase | down | 0.23 |
| A0A315T5L1 | mntH | Divalent metal cation transporter MntH | down | 0.19 |
| A0A174A5I1 | pflD | Formate acetyltransferase | down | 0.18 |
| A0A126SV27 | hupB | Integration host factor | down | 0.17 |
| The ID in Database | Gene ID | Gene | Predict Functions |
|---|---|---|---|
| D3R5K0 | gene0321 | dapE | membrane protein |
| A0A315RUP5 | gene0618 | mth2 | 7,8-dihydro-8-oxoguanine-triphosphatase |
| A0A068Z383 | gene0924 | mutT | DNA mismatch repair protein MutT |
| D3R6Y8 | gene1072 | eno | enolase |
| D3R4Y3 | gene1102 | msrAB | peptide methionine sulfoxide reductase MsrA/MsrB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhao, X.; Yang, M.; Song, X.; Wang, G.; Xia, Y.; Zhao, L.; Xiong, Z.; Ai, L. Proteomic Analysis of Bifidobacterium animalis AR668 and AR668-R1 Under Aerobic Culture. Foods 2025, 14, 1766. https://doi.org/10.3390/foods14101766
Liu Y, Zhao X, Yang M, Song X, Wang G, Xia Y, Zhao L, Xiong Z, Ai L. Proteomic Analysis of Bifidobacterium animalis AR668 and AR668-R1 Under Aerobic Culture. Foods. 2025; 14(10):1766. https://doi.org/10.3390/foods14101766
Chicago/Turabian StyleLiu, Yaping, Xiaoxiao Zhao, Miao Yang, Xin Song, Guangqiang Wang, Yongjun Xia, Liang Zhao, Zhiqiang Xiong, and Lianzhong Ai. 2025. "Proteomic Analysis of Bifidobacterium animalis AR668 and AR668-R1 Under Aerobic Culture" Foods 14, no. 10: 1766. https://doi.org/10.3390/foods14101766
APA StyleLiu, Y., Zhao, X., Yang, M., Song, X., Wang, G., Xia, Y., Zhao, L., Xiong, Z., & Ai, L. (2025). Proteomic Analysis of Bifidobacterium animalis AR668 and AR668-R1 Under Aerobic Culture. Foods, 14(10), 1766. https://doi.org/10.3390/foods14101766





