Unlocking the Functional Food Potential of Capparis spinosa: Optimizing Ultrasound Extraction, Phytochemical Analysis, and Assessing Antioxidative Activities
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Extraction Protocol
2.3.1. Ultrasound-Assisted Extraction (UAE)
2.3.2. Experimental Design
2.4. Spectrophotometric Analysis
2.4.1. Total Phenolic Content
2.4.2. Total Flavonoids Content
2.4.3. Antioxidant Capacity
2.5. Chromatographic Analysis
Phenolic Profile Determination by HPLC
2.6. Cell Culture Experiments Evaluating Extracts of Caper Obtained Under Optimal Conditions
2.6.1. Cell Culture Selection and Culture Conditions
2.6.2. Cytotoxicity
2.6.3. Protective Effect Against Induced Oxidative Stress
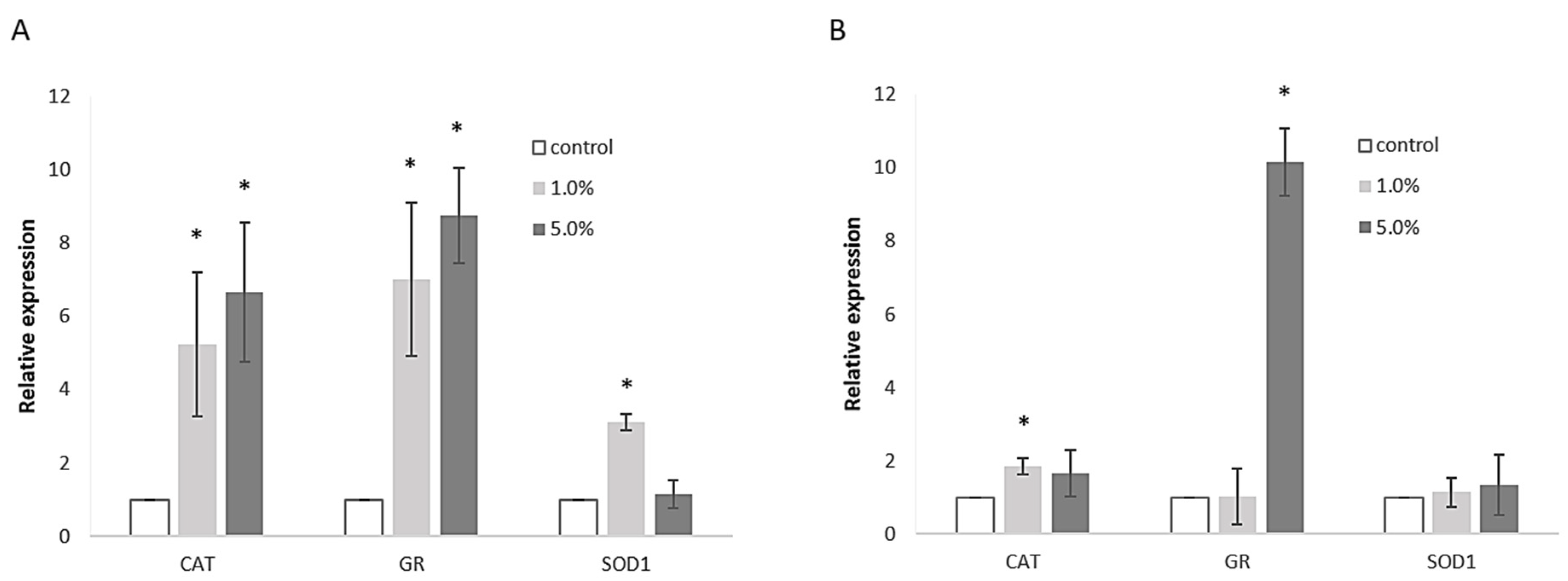
2.6.4. Expression of Genes Related to Oxidative Stress
2.7. Statistical Analysis
3. Results and Discussion
3.1. Impact of Different Parameters on Extraction of Phenolics and Antioxidant Activity of the Extracts
3.2. Parameters Optimization
3.3. HPLC Analysis
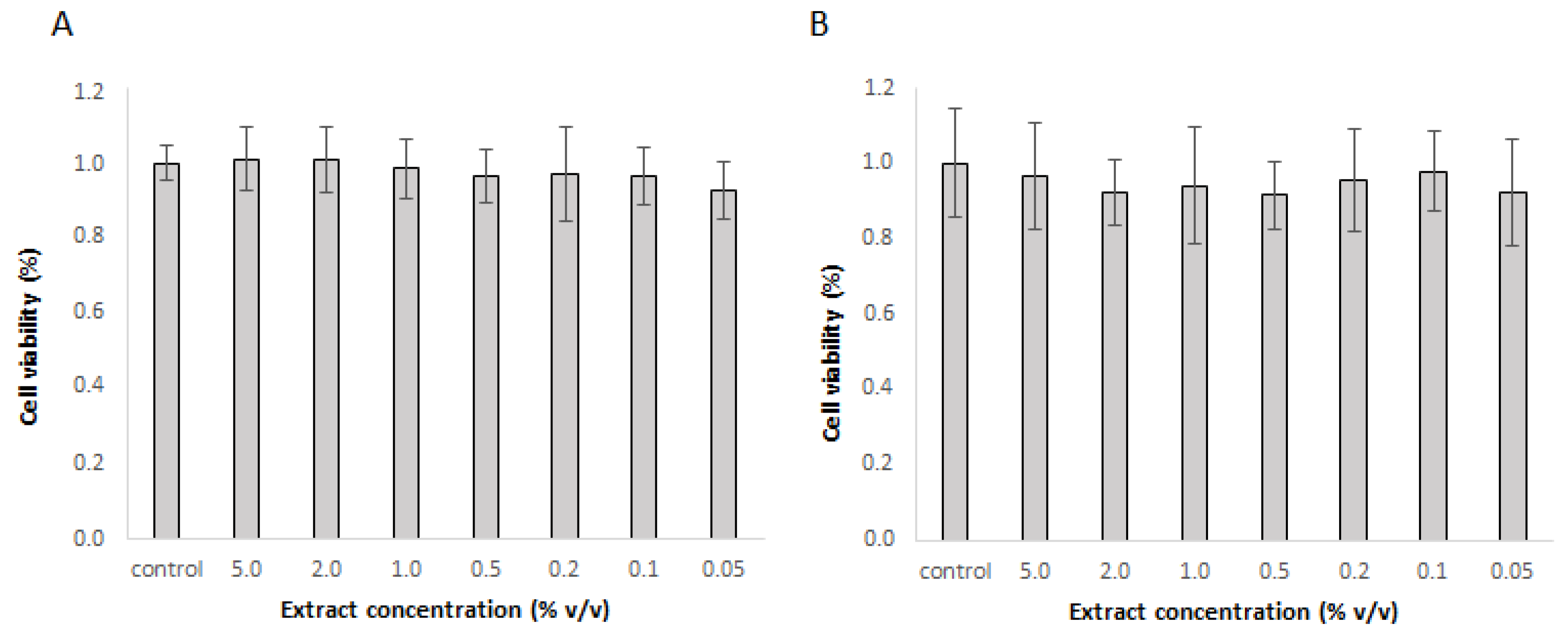
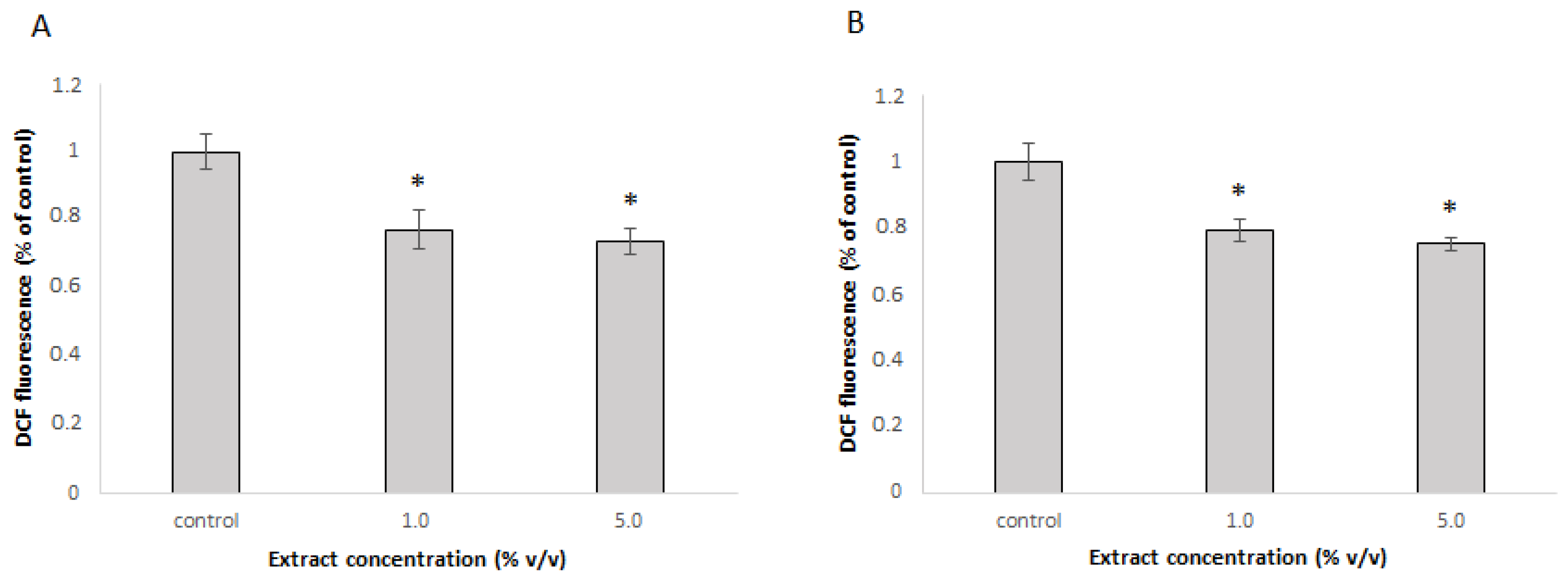
3.4. Cell Culture Response to Extract Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Yang, T.; Wang, C. Capparis spinosa L. as a Potential Source of Nutrition and Its Health Benefits in Foods: A Comprehensive Review of Its Phytochemistry, Bioactivities, Safety, and Application. Food Chem. 2023, 409, 135258. [Google Scholar] [CrossRef]
- Boudries, H.; Nabet, N.; Chougui, N.; Souagui, S.; Loupassaki, S.; Madani, K.; Dimitrov, K. Optimization of Ultrasound-Assisted Extraction of Antioxidant Phenolics from Capparis spinosa Flower Buds and LC–MS Analysis. J. Food Meas. Charact. 2019, 13, 2241–2252. [Google Scholar] [CrossRef]
- Rajhi, I.; Hernandez-Ramos, F.; Abderrabba, M.; Dhia, M.T.B.; Ayadi, S.; Labidi, J. Antioxidant, Antifungal and Phytochemical Investigations of Capparis spinosa L. Agriculture 2021, 11, 1025. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Grimalt, M.; Legua, P.; Almansa, M.S.; Amorós, A.; Carbonell-Barrachina, Á.A.; Hernández, F. Polyphenol Compounds and Biological Activity of Caper (Capparis spinosa L.) Flowers Buds. Plants 2019, 8, 539. [Google Scholar] [CrossRef]
- Grimalt, M.; Hernández, F.; Legua, P.; Amorós, A.; Almansa, M.S. Antioxidant Activity and the Physicochemical Composition of Young Caper Shoots (Capparis spinosa L.) of Different Spanish Cultivars. Sci. Hortic. 2022, 293, 110646. [Google Scholar] [CrossRef]
- Annaz, H.; Sane, Y.; Bitchagno, G.T.M.; Ben Bakrim, W.; Drissi, B.; Mahdi, I.; El Bouhssini, M.; Sobeh, M. Caper (Capparis spinosa L.): An Updated Review on Its Phytochemistry, Nutritional Value, Traditional Uses, and Therapeutic Potential. Front. Pharmacol. 2022, 13, 878749. [Google Scholar] [CrossRef]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant Polyphenols and Their Potential Benefits on Cardiovascular Health: A Review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef]
- Khojasteh Rad, M.; Ghani, A.; Ghani, E. In Vitro Effects of Capparis spinosa L. Extract on Human Sperm Function, DNA Fragmentation, and Oxidative Stress. J. Ethnopharmacol. 2021, 269, 113702. [Google Scholar] [CrossRef]
- Zeng, J.; Dou, Y.; Yan, N.; Li, N.; Zhang, H.; Tan, J.-N. Optimizing Ultrasound-Assisted Deep Eutectic Solvent Extraction of Bioactive Compounds from Chinese Wild Rice. Molecules 2019, 24, 2718. [Google Scholar] [CrossRef]
- Hiranpradith, V.; Therdthai, N.; Soontrunnarudrungsri, A.; Rungsuriyawiboon, O. Optimisation of Ultrasound-Assisted Extraction of Total Phenolics and Flavonoids Content from Centella Asiatica. Foods 2025, 14, 291. [Google Scholar] [CrossRef]
- Jamshaid, S.; Ahmed, D. Optimization of Ultrasound-Assisted Extraction of Valuable Compounds from Fruit of Melia Azedarach with Glycerol-Choline Chloride Deep Eutectic Solvent. Sustain. Chem. Pharm. 2022, 29, 100827. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.J.; Qiu, B.; Zhou, L.; Liu, R.Y.; Huang, J.F.; He, Y.; Xie, Q. Optimization of Ultrasound-Assisted Deep Eutectic Solvents Extraction of Rutin from Ilex Asprella Using Response Surface Methodology. Sci. Rep. 2025, 15, 6205. [Google Scholar] [CrossRef]
- Žlabur, J.Š.; Brajer, M.; Voća, S.; Galić, A.; Radman, S.; Rimac-Brnčić, S.; Xia, Q.; Zhu, Z.; Grimi, N.; Barba, F.J.; et al. Ultrasound as a Promising Tool for the Green Extraction of Specialized Metabolites from Some Culinary Spices. Molecules 2021, 26, 1866. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of Total Flavonoid Content by Aluminum Chloride Assay: A Critical Evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Matić, M.; Stupar, A.; Pezo, L.; Đerić Ilić, N.; Mišan, A.; Teslić, N.; Pojić, M.; Mandić, A. Eco-Friendly Extraction: A Green Approach to Maximizing Bioactive Extraction from Pumpkin (Curcubita moschata L.). Food Chem. X 2024, 22, 101290. [Google Scholar] [CrossRef]
- Mišan, A.Č.; Mimica-Dukić, N.M.; Mandić, A.I.; Sakač, M.B.; Milovanović, I.L.; Sedej, I.J. Development of a Rapid Resolution HPLC Method for the Separation and Determination of 17 Phenolic Compounds in Crude Plant Extracts. Cent. Eur. J. Chem. 2011, 9, 133–142. [Google Scholar] [CrossRef]
- Kramberger, K.; Barlič-Maganja, D.; Pražnikar, Z.J.; Režen, T.; Rozman, D.; Pražnikar, J.; Kenig, S. Whole Transcriptome Expression Array Analysis of Human Colon Fibroblasts Culture Treated with Helichrysum Italicum Supports Its Use in Traditional Medicine. J. Ethnopharmacol. 2022, 296, 115505. [Google Scholar] [CrossRef]
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintas, G.; Burello, E.; Castell, J.V.; Gombau, L. Dichloro-Dihydro-Fluorescein Diacetate (DCFH-DA) Assay: A Quantitative Method for Oxidative Stress Assessment of Nanoparticle-Treated Cells. Toxicol. Vitr. 2013, 27, 954–963. [Google Scholar] [CrossRef]
- Spandidos, A.; Wang, X.; Wang, H.; Seed, B. PrimerBank: A Resource of Human and Mouse PCR Primer Pairs for Gene Expression Detection and Quantification. Nucleic Acids Res. 2010, 38, D792–D799. [Google Scholar] [CrossRef]
- Rani, U.; Kaur, S.; Panesar, P.S.; Chopra, H.K. Effect of Ultrasound on the Extraction of Bioactive Compounds from Jamun (Syzygium cumini L.) Seeds. Biomass Convers. Biorefinery 2025, 1–14. [Google Scholar] [CrossRef]
- Kruszewski, B.; Boselli, E. Blackcurrant Pomace as a Rich Source of Anthocyanins: Ultrasound-Assisted Extraction under Different Parameters. Appl. Sci. 2024, 14, 821. [Google Scholar] [CrossRef]
- Gull, T.; Sultana, B.; Anwar, F.; Nouman, W.; Rosa, E.; Domínguez-Perles, R. HPLC-PDA-ESI-MS n Profiling of Polyphenolics in Different Parts of Capparis spinosa and Capparis Decidua as Function of Harvesting Seasons. J. Appl. Bot. Food Qual. 2019, 92, 73–80. [Google Scholar] [CrossRef]
- Tlili, N.; Feriani, A.; Saadoui, E.; Nasri, N.; Khaldi, A. Capparis spinosa Leaves Extract: Source of Bioantioxidants with Nephroprotective and Hepatoprotective Effects. Biomed. Pharmacother. 2017, 87, 171–179. [Google Scholar] [CrossRef]
- Fattahi, M.; Rahimi, R. Optimization of Extraction Parameters of Phenolic Antioxidants from Leaves of Capparis spinosa Using Response Surface Methodology. Food Anal. Methods 2016, 9, 2321–2334. [Google Scholar] [CrossRef]
- Stefanucci, A.; Zengin, G.; Locatelli, M.; Macedonio, G.; Wang, C.K.; Novellino, E.; Mahomoodally, M.F.; Mollica, A. Impact of Different Geographical Locations on Varying Profile of Bioactives and Associated Functionalities of Caper (Capparis spinosa L.). Food Chem. Toxicol. 2018, 118, 181–189. [Google Scholar] [CrossRef]
- Kianersi, F.; Abdollahi, M.R.; Mirzaie-asl, A.; Dastan, D.; Rasheed, F. Biosynthesis of Rutin Changes in Capparis spinosa Due to Altered Expression of Its Pathway Genes under Elicitors’ Supplementation. Plant Cell. Tissue Organ Cult. 2020, 141, 619–631. [Google Scholar] [CrossRef]
- Hasibuan, P.A.Z.; Simanjuntak, Y.; Hey-Hawkins, E.; Lubis, M.F.; Rohani, A.S.; Park, M.N.; Kim, B.; Syahputra, R.A. Unlocking the Potential of Flavonoids: Natural Solutions in the Fight against Colon Cancer. Biomed. Pharmacother. 2024, 176, 116827. [Google Scholar] [CrossRef]
- Suganya, K.; Poornima, A.; Sumathi, S.; Chigurupati, S.; Alyamani, N.M.; Ghazi Felemban, S.; Bhatia, S.; Al-Harrasi, A.; Sayed Moawad, A. Rutin Induces Endoplasmic Reticulum Stress-Associated Apoptosis in Human Triple-Negative Breast Carcinoma MDA-MB-231 Cells–In Vitro and In Silico Docking Studies. Arab. J. Chem. 2022, 15, 104021. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Qari, H.A.; Oves, M. Rutin (Bioflavonoid) as Cell Signaling Pathway Modulator: Prospects in Treatment and Chemoprevention. Pharmaceuticals 2021, 14, 1069. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-yachi, R.; Aizawa, K.; Shimizu, K.; Akutsu, H.; Nakamura, K. DNMT1/PKR Double Knockdowned HepG2 (HepG2-DP) Cells Have High Hepatic Function and Differentiation Ability. Sci. Rep. 2022, 12, 21173. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, Y.; Zhao, C.; Yang, Y.; Xiong, C.; Zhang, D.; Feng, S.; Wu, J.; Wang, X. Rutin Supplementation Reduces Oxidative Stress, Inflammation and Apoptosis of Mammary Gland in Sheep During the Transition Period. Front. Vet. Sci. 2022, 9, 907299. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, L.; Zhang, X.; Fang, Q.; Xu, Y.; Wang, H. Rutin Alleviates Pb-Induced Oxidative Stress, Inflammation and Cell Death via Activating Nrf2/ARE System in SH-SY5Y Cells. Neurotoxicology 2024, 104, 1–10. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.; Liu, B.; Shi, W.; Shi, J.; Zhang, Z.; Xing, J. Rutin Attenuates H2O2-Induced Oxidation Damage and Apoptosis in Leydig Cells by Activating PI3K/Akt Signal Pathways. Biomed. Pharmacother. 2017, 88, 500–506. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Pressman, P.; Hayes, A.W.; Dhawan, G.; Kapoor, R.; Agathokleous, E.; Calabrese, V. RUTIN, a Widely Consumed Flavonoid, That Commonly Induces Hormetic Effects. Food Chem. Toxicol. 2024, 187, 114626. [Google Scholar] [CrossRef]

| Extraction Temperature (°C) | Ethanol Concentration (%) | Ultrasound Power (%) | TPC (mg GAE/g DW) | TFC (mg GAE/g DW) | DPPH (mg TE/g DW) | Rutin (mg/g DW) |
|---|---|---|---|---|---|---|
| 80 | 30 | 30 | 23.1436 | 90.3909 | 20.6089 | 14.14833 |
| 65 | 50 | 60 | 22.2452 | 91.1791 | 21.1616 | 13.49059 |
| 80 | 30 | 90 | 20.3945 | 94.4752 | 21.1772 | 14.1438 |
| 80 | 50 | 60 | 20.0845 | 94.4752 | 21.3739 | 14.77886 |
| 65 | 50 | 60 | 19.4152 | 88.4563 | 21.1179 | 12.94625 |
| 50 | 50 | 60 | 19.1367 | 90.4482 | 21.3614 | 12.85552 |
| 65 | 70 | 60 | 18.8492 | 97.0834 | 21.3552 | 14.31617 |
| 65 | 50 | 30 | 18.7774 | 84.0997 | 21.0679 | 14.06215 |
| 80 | 70 | 30 | 18.7459 | 101.3826 | 21.3115 | 14.73804 |
| 65 | 30 | 60 | 18.7414 | 90.1759 | 21.2022 | 13.70832 |
| 50 | 30 | 30 | 18.7010 | 91.6520 | 21.0342 | 10.03402 |
| 65 | 50 | 60 | 18.6516 | 132.1364 | 21.8735 | 12.82831 |
| 50 | 30 | 90 | 18.6291 | 89.0152 | 20.9368 | 10.2064 |
| 50 | 70 | 90 | 18.2518 | 101.9988 | 21.4582 | 14.28895 |
| 65 | 50 | 90 | 17.9059 | 90.8638 | 21.3396 | 13.22295 |
| 50 | 70 | 30 | 17.6993 | 99.6486 | 20.0906 | 13.40894 |
| 80 | 70 | 90 | 17.0210 | 107.6595 | 21.3923 | 15.85394 |
| Term | Regression Coefficient | |||
|---|---|---|---|---|
| Empty Cell | TPC | TFC | DPPH | Rutin |
| Intercept | ||||
| β0 | 1369 | 1.23 | 6.39 | 20.23 |
| Linear | ||||
| β1 | 3.15 * | −0.22 * | 3.70 | 7.72 * |
| β2 | 0.56 | −0.8 * | 0.14 | 3.35 * |
| β3 | 3.32 * | 0.67 * | 0.26 | 8.643 × 10−3 |
| β4 | 0.42 | 0.38* | 0.065 | 0.92 |
| Interaction | ||||
| β12 | 1.6 | 0.66 * | 0.29 | 2.59 * |
| β13 | 0.88 | −0.47 | 0.11 | −5.67 * |
| β14 | −0.34 | −0.33 | −0.033 | 1.14 |
| β23 | −1.11 | −0.12 | 0.44 * | 7.86 * |
| β24 | 1.15 | 0.21 | 0.10 | −0.76 |
| β34 | −0.064 | 0.68 * | 0.13 | 2.64 * |
| Quadratic | ||||
| β11 | −2.92 | −0.094 | 0.45 * | −1.75 |
| β22 | 0.48 | 0.54 * | 0.20 | −0.89 |
| β33 | −1.48 | 0.41 | 0.045 | 5.56 * |
| β44 | 0.51 | 0.027 | −0.39 * | −0.36 |
| R2 a | 0.8233 | 0.8393 | 0.9843 | 0.6864 |
| CV b | 17.22 | 35.67 | 6.73 | 8.5588 |
| pm-value c | 0.0034 | 0.0019 | <0.0001 | <0.0001 |
| plf-value d | 0.2013 | 0.057 | 0.0566 | 0.4860 |
| Optimal Parameters | Predicted Values | Obtained Values |
|---|---|---|
| Extraction temperature (°C) | 80 | 80 |
| Ethanol concentration (%) | 62.23 | 62.23 |
| Ultrasound power (%) | 56.05 | 56 |
| TPC (mg GAE/DW) | 19.52 | 19.82 |
| TFC (mg GAE/g DW) | 98.64 | 99.26 |
| DPPH (mg TE/g DW) | 21.4475 | 21.5423 |
| Rutin (mg/g DW) | 15.03 | 15.79 |
| Compound | Concentration (mg/g) |
|---|---|
| Gallic Acid | 0.0408 |
| Catechin equivalent | 2.0675 |
| Caffeic Acid | 0.2495 |
| Syringic Acid | 0.1927 |
| Sinapic Acid | 0.0502 |
| Rutin | 15.5051 |
| Quercetin-3-Glucoside | 4.2687 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naffati, A.; Mišan, A.; Kenig, S.; Kramberger, K.; Jenko Pražnikar, Z.; Petelin, A.; Kljakić, A.C.; Benmerzoug, A.; Elfarrah, N.; Stupar, A. Unlocking the Functional Food Potential of Capparis spinosa: Optimizing Ultrasound Extraction, Phytochemical Analysis, and Assessing Antioxidative Activities. Foods 2025, 14, 1768. https://doi.org/10.3390/foods14101768
Naffati A, Mišan A, Kenig S, Kramberger K, Jenko Pražnikar Z, Petelin A, Kljakić AC, Benmerzoug A, Elfarrah N, Stupar A. Unlocking the Functional Food Potential of Capparis spinosa: Optimizing Ultrasound Extraction, Phytochemical Analysis, and Assessing Antioxidative Activities. Foods. 2025; 14(10):1768. https://doi.org/10.3390/foods14101768
Chicago/Turabian StyleNaffati, Abdulhakim, Aleksandra Mišan, Saša Kenig, Katja Kramberger, Zala Jenko Pražnikar, Ana Petelin, Aleksandra Cvetanović Kljakić, Abdelmoumen Benmerzoug, Nasrien Elfarrah, and Alena Stupar. 2025. "Unlocking the Functional Food Potential of Capparis spinosa: Optimizing Ultrasound Extraction, Phytochemical Analysis, and Assessing Antioxidative Activities" Foods 14, no. 10: 1768. https://doi.org/10.3390/foods14101768
APA StyleNaffati, A., Mišan, A., Kenig, S., Kramberger, K., Jenko Pražnikar, Z., Petelin, A., Kljakić, A. C., Benmerzoug, A., Elfarrah, N., & Stupar, A. (2025). Unlocking the Functional Food Potential of Capparis spinosa: Optimizing Ultrasound Extraction, Phytochemical Analysis, and Assessing Antioxidative Activities. Foods, 14(10), 1768. https://doi.org/10.3390/foods14101768









