Diversity of Lactiplantibacillus plantarum in Wild Fermented Food Niches
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Bacterial Isolation and Cultivation
2.3. Genetic Identification of Lactic Acid Bacteria via 16S rRNA Analysis
2.4. Whole-Genome Sequencing of Lactiplantibacillus plantarum Strains
2.4.1. Data Generation from Whole-Genome Sequencing
2.4.2. Genome Assembly and Quality Control
2.4.3. Genome Prediction and Functional Annotation
2.5. Phylogenetic Analysis
2.6. Statistical Analysis
3. Results and Discussion
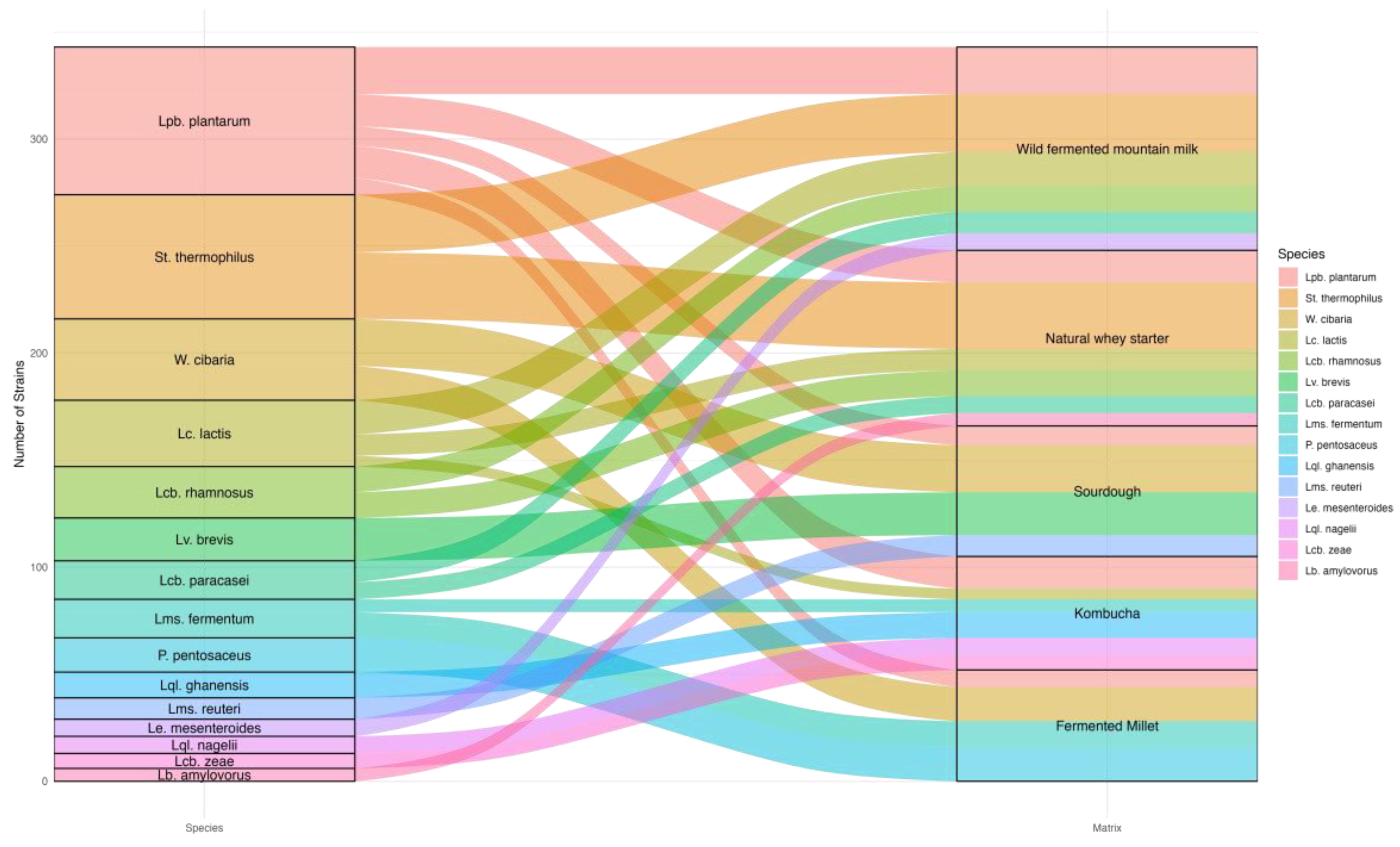
3.1. Distribution of Lactic Acid Bacteria
3.2. Inter-Specie Diversity
3.3. Phylogenomic Structure of Lactiplantibacillus plantarum Strains
3.4. Diversity of Carbohydrate-Active Enzymes in Lactiplantibacillus plantarum Strains: Insights from WGS Analysis
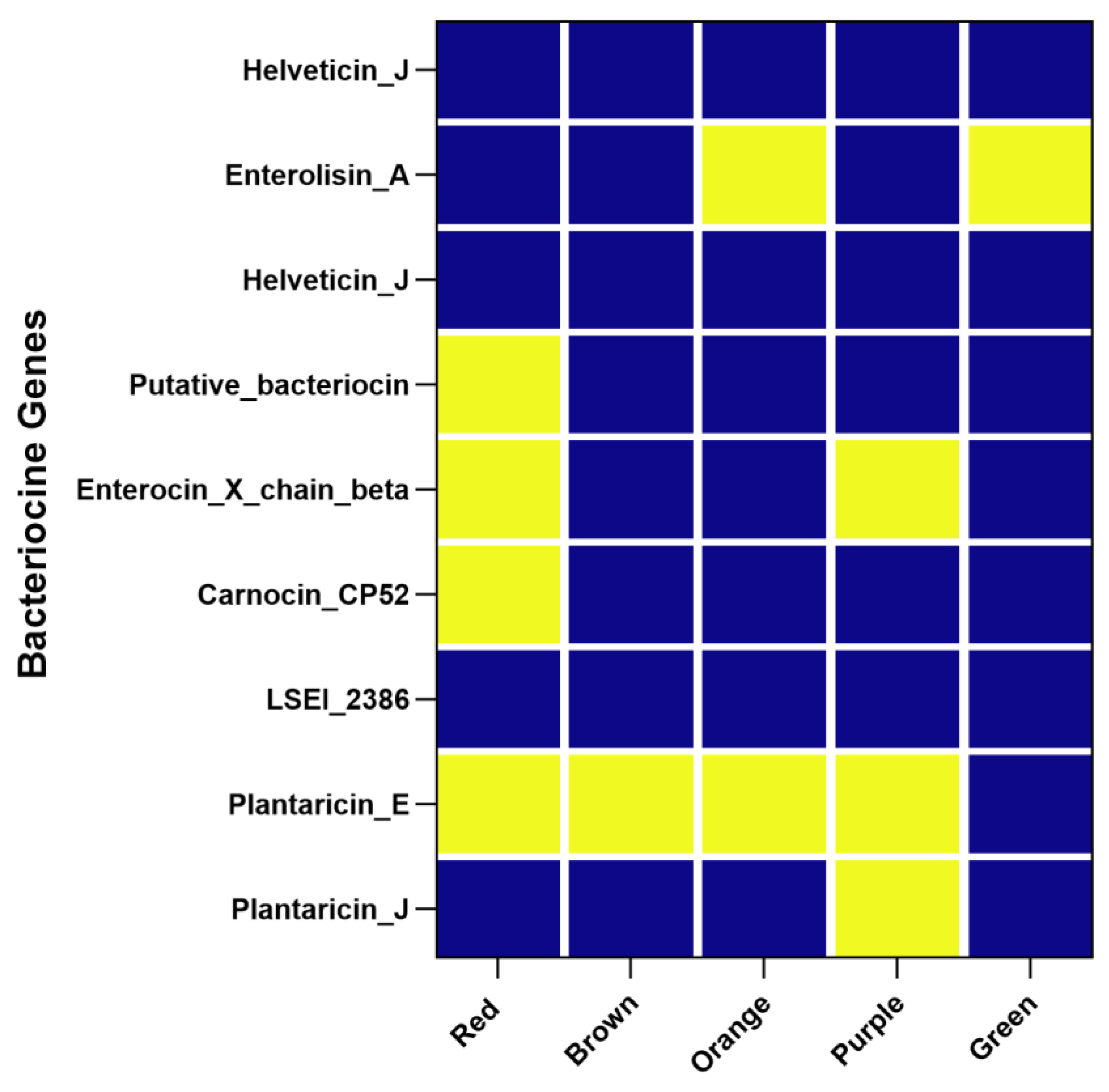
3.5. Diversity of Antimicrobial Compounds in Lactiplantibacillus plantarum Strains: Insights from WGS Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrocino, I.; Rantsiou, K.; Cocolin, L. Microbiome and -omics application in food industry. Int. J. Food Microbiol. 2022, 377, 109781. [Google Scholar] [CrossRef]
- Parlindungan, E.; Jones, O.A. Diversity of Lactiplantibacillus plantarum in Wild Fermentations with Low Technological Intervention. Metabolomics 2023, 19, 99. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, N.; Battista, N.; Prete, R.; Corsetti, A. Health-promoting role of Lactiplantibacillus plantarum isolated from fermented foods. Microorganisms 2021, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Tufariello, M.; De Simone, N.; Fragasso, M.; Grieco, F. Biodiversity of oenological lactic acid bacteria: Species-and strain-dependent plus/minus effects on wine quality and safety. Fermentation 2021, 7, 24. [Google Scholar] [CrossRef]
- Marcelli, V.; Osimani, A.; Aquilanti, L. Research progress on the use of lactic acid bacteria as natural bio-preservatives against Pseudomonas spp. in meat and meat products: A review. Food Res. Int. 2024, 196, 115129. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Raymundo, A.; Moreira, J.B.; Prista, C. Exploring the Potential of Lactic Acid Bacteria Fermentation as a Clean Label Alternative for Use in Yogurt Production. Appl. Sci. 2025, 15, 2686. [Google Scholar] [CrossRef]
- Colautti, A.; Camprini, L.; Ginaldi, F.; Comi, G.; Reale, A.; Coppola, F.; Iacumin, L. Safety traits, genetic and technological characterization of Lacticaseibacillus rhamnosus strains. LWT 2024, 207, 116578. [Google Scholar] [CrossRef]
- Testa, B.; Coppola, F.; Iorizzo, M.; Di Renzo, M.; Coppola, R.; Succi, M. Preliminary Characterisation of Metschnikowia pulcherrima to Be Used as a Starter Culture in Red Winemaking. Beverages 2024, 10, 88. [Google Scholar] [CrossRef]
- Nazzaro, F.; Ombra, M.N.; Coppola, F.; De Giulio, B.; d’Acierno, A.; Coppola, R.; Fratianni, F. Antibacterial Activity and Prebiotic Properties of Six Types of Lamiaceae Honey. Antibiotics 2024, 13, 868. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, N.; Yadav, A.; Bhandari, K. Harnessing the Power of Bacteriocins: A Comprehensive Review on Sources, Mechanisms, and Applications in Food Preservation and Safety. Curr. Microbiol. 2025, 82, 174. [Google Scholar] [CrossRef]
- Gephart, G.J.; Abdelhamid, A.G.; Yousef, A.E. Comparative genomics and phenotypic assessment of lactic acid bacteria isolated from artisanal cheese as potential starter cultures. LWT 2024, 210, 116849. [Google Scholar] [CrossRef]
- Xie, Z.; McAuliffe, O.; Jin, Y.-S.; Miller, M.J. Invited review: Genomic Modifications of Lactic Acid Bacteria and Their Applications in Dairy Fermentation. J. Dairy Sci. 2024, 107, 8749–8764. [Google Scholar] [CrossRef]
- He, X.; Cui, Y.; Jia, Q.; Zhuang, Y.; Gu, Y.; Fan, X.; Ding, Y. Response mechanisms of lactic acid bacteria under environmental stress and their application in the food industry. Food Biosci. 2025, 64, 105938. [Google Scholar] [CrossRef]
- Tremonte, P.; Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Ibanez, E.; Coppola, R. Antimicrobial Effect of Malpighia punicifolia and Extension of Water Buffalo Steak Shelf-Life. J. Food Sci. 2016, 81, M97–M105. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, S.J.; Pannella, G.; Coppola, F.; Vergalito, F.; Maiuro, L.; Succi, M.; Sorrentino, E.; Tremonte, P.; Coppola, R. Plant-Based Ingredients Utilized as Fat Replacers and Natural Antimicrobial Agents in Beef Burgers. Foods 2024, 13, 3229. [Google Scholar] [CrossRef] [PubMed]
- Coppola, F.; Lombardi, S.J.; Tremonte, P. Edible Insect Meals as Bioactive Ingredients in Sustainable Snack Bars. Foods 2025, 14, 702. [Google Scholar] [CrossRef]
- Khiabani, A.; Xiao, H.; Wätjen, A.P.; Tovar, M.; Poulsen, V.K.; Hansen, E.B.; Bang-Berthelsen, C.H. Exploring the Diversity and Potential Use of Flower-Derived Lactic Acid Bacteria in Plant-Based Fermentation: Insights into Exo-Cellular Polysaccharide Production. Foods 2024, 13, 2907. [Google Scholar] [CrossRef]
- Siezen, R.J.; van Hylckama Vlieg, J.E. Genomic diversity and versatility of Lactobacillus plantarum, a natural metabolic engineer. Microb. Cell Factories 2011, 10, S3. [Google Scholar] [CrossRef]
- Sorrentino, E.; Reale, A.; Tremonte, P.; Maiuro, L.; Succi, M.; Tipaldi, L.; Di Renzo, T.; Pannella, G.; Coppola, R. Lactobacillus plantarum 29 inhibits Penicillium spp. involved in the spoilage of black truffles (Tuber aestivum). J. Food Sci. 2013, 78, M1188–M1194. [Google Scholar] [CrossRef]
- Iorizzo, M.; Albanese, G.; Letizia, F.; Testa, B.; Tremonte, P.; Vergalito, F.; Lombardi, S.J.; Succi, M.; Coppola, R.; Sorrentino, E. Probiotic potentiality from versatile Lactiplantibacillus plantarum strains as resource to enhance freshwater fish health. Microorganisms 2022, 10, 463. [Google Scholar] [CrossRef]
- Divyashree, S.; Ramu, R.; Sreenivasa, M.Y. Evaluation of new candidate probiotic lactobacillus strains isolated from a traditional fermented food-multigrain-millet dosa batter. Food Biosci. 2024, 57, 103450. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, Z.; Qiao, J.; Liang, Y.; Yang, P.; Zhao, S.; Ren, G.; Zhang, L. Impact of Lactobacillus plantarum fermentation on the structural, physicochemical, emulsification, and digestibility properties of foxtail millet protein. Food Chem. 2025, 482, 144141. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Wijesekara, T.; Xu, B. New insights into recent development, health benefits, emerging technologies, and future trends of cereal-based fermented products. Process Biochem. 2024, 147, 433–439. [Google Scholar] [CrossRef]
- Mohanan, M.M.; Shetty, R.; Bhat, P.S.; Deepashree, V.S.; Thimulappa, R.K.; Bang-Berthelsen, C.H.; Mudnakudu-Nagaraju, K.K. Isolation and characterization of biological traits of millet-derived lactic acid bacteria. Int. J. Food Sci. Technol. 2025, 60, vvaf074. [Google Scholar] [CrossRef]
- Kitwetcharoen, H.; Chamnipa, N.; Thanonkeo, S.; Klanrit, P.; Tippayawat, P.; Klanrit, P.; Yamada, M.; Thanonkeo, P. Enhancing kombucha functionality: Utilizing dried pineapple peels and cores as an alternative ingredient for improved antioxidant and antimicrobial properties. LWT 2025, 216, 117358. [Google Scholar] [CrossRef]
- Mutukumira, A.N.; Todorov, S.D. Probiotic Potential of Isolated Cultures from Spontaneously or Naturally Fermented Food Products. Foods 2024, 13, 1817. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Nguyen, Q.D. Evolution of Kombucha Tea from Isolated Acetic Acid Bacteria, Lactic Acid Bacteria and Yeast in Single-and Mixed-Cultures: Characteristics, Bioactivities, Fermentation Performance and Kinetics. Food Biotechnol. 2024, 38, 86–117. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of sourdough fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef]
- Paramithiotis, S. Lactiplantibacillus plantarum, the Integral Member of Vegetable Fermentations. Appl. Biosci. 2025, 4, 7. [Google Scholar] [CrossRef]
- Yılmaz, B.; Ningrum, A.; Pateiro, M.; Lorenzo, J.M. Functional and health-promoting properties of lactic acid bacteria from sourdough-based and other agro-food products. In Handbook of Sourdough Microbiota and Fermentation; Academic Press: Cambridge, MA, USA, 2025; pp. 147–160. [Google Scholar] [CrossRef]
- Hassan, A.A.A.; Jin, Y.H.; Mah, J.H. Effects of Lactic Acid Bacteria on Reducing the Formation of Biogenic Amines and Improving the Formation of Antioxidant Compounds in Traditional African Sourdough Flatbread Fermentation. Antioxidants 2024, 13, 844. [Google Scholar] [CrossRef]
- Ayed, L.; M’hir, S.; Nuzzolese, D.; Di Cagno, R.; Filannino, P. Harnessing the health and techno-functional potential of lactic acid bacteria: A comprehensive review. Foods 2024, 13, 1538. [Google Scholar] [CrossRef]
- Cuvas-Limon, R.B.; Nobre, C.; Cruz, M.; Rodriguez-Jasso, R.M.; Ruíz, H.A.; Loredo-Treviño, A.; Texeira, J.A.; Belmares, R. Spontaneously fermented traditional beverages as a source of bioactive compounds: An overview. Crit. Rev. Food Sci. Nutr. 2021, 61, 2984–3006. [Google Scholar] [CrossRef]
- Bintsis, T.; Papademas, P. The evolution of fermented milks, from artisanal to industrial products: A critical review. Fermentation 2022, 8, 679. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Hojjati, M.; Goodarzi Shamsabadi, B. Isolation and identification of Lactiplantibacillus plantarum MOHA1 strain from local yogurt and evaluation of its probiotic, antibacterial and safety properties. Food Res. J. 2024, 34, 29–52. [Google Scholar] [CrossRef]
- Coelho, M.C.; Malcata, F.X.; Silva, C.C. Lactic acid bacteria in raw-milk cheeses: From starter cultures to probiotic functions. Foods 2022, 11, 2276. [Google Scholar] [CrossRef] [PubMed]
- Marasco, R.; Gazzillo, M.; Campolattano, N.; Sacco, M.; Muscariello, L. Isolation and identification of lactic acid bacteria from natural whey cultures of buffalo and cow milk. Foods 2022, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Spano, G.; Capozzi, V. Safety evaluation of starter cultures. In Starter Cultures in Food Production; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 101–128. [Google Scholar] [CrossRef]
- Vesković, S. Natural Antimicrobial Compounds in Food Safety. In Natural Food Preservation: Controlling Loss, Advancing Safety; Springer Nature: Cham, Switzerland, 2025; pp. 133–192. [Google Scholar]
- Criscuolo, A. A fast alignment-free bioinformatics procedure to infer accurate distance-based phylogenetic trees from genome assemblies. Res. Ideas Outcomes 2019, 5, e36178. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- RC Team. Writing R Extensions; R Foundation for Statistical Computing: Vienna, Austria, 1999. [Google Scholar]
- Kondrotiene, K.; Zavistanaviciute, P.; Aksomaitiene, J.; Novoslavskij, A.; Malakauskas, M. Lactococcus lactis in dairy fermentation—Health-promoting and probiotic properties. Fermentation 2023, 10, 16. [Google Scholar] [CrossRef]
- Neviani, E.; Levante, A.; Gatti, M. The Microbial Community of Natural Whey Starter: Why Is It a Driver for the Production of the Most Famous Italian Long-Ripened Cheeses? Fermentation 2024, 10, 186. [Google Scholar] [CrossRef]
- Sudheer, A.; Dastidar, D.G.; Ghosh, G.; Taj, Z.; Nidhin, I.K.; Chattopadhyay, I. Comprehensive genomics, probiotic, and antibiofilm potential analysis of Streptococcus thermophilus strains isolated from homemade and commercial dahi. Sci. Rep. 2025, 15, 7089. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N. Fermentation’s pivotal role in shaping the future of plant-based foods: An integrative review of fermentation processes and their impact on sensory and health benefits. Appl. Food Res. 2024, 4, 100468. [Google Scholar] [CrossRef]
- Liu, S.; Hu, J.; Zhong, Y.; Hu, X.; Yin, J.; Xiong, T.; Nie, S.; Xie, M. A review: Effects of microbial fermentation on the structure and bioactivity of polysaccharides in plant-based foods. Food Chem. 2024, 440, 137453. [Google Scholar] [CrossRef]
- Fatima, S.; Khan, F.; Raza, M.A.; Qarni, M.M.; Khan, S.; Sarwar, R.; Ahmad, N.; Mahmood, W.; Ahmad, S.; Sattar, Z. Plant-based fermented foods: A review of microbiological, biochemical, and sensory properties. Res. Med. Sci. Rev. 2025, 3, 147–165. [Google Scholar]
- Graham, A.E.; Ledesma-Amaro, R. The microbial food revolution. Nat. Commun. 2023, 14, 2231. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Velázquez, R.; Flörl, L.; Lavrinienko, A.; Sebechlebská, Z.; Merk, L.; Greppi, A.; Bokulich, N.A. The future is fermented: Microbial biodiversity of fermented foods is a critical resource for food innovation and human health. Trends Food Sci. Technol. 2024, 150, 104569. [Google Scholar] [CrossRef]
- Dreier, M.; Bettera, L.; Berthoud, H.; Fuchsmann, P.; Tintrop, L.K.; Bachmann, H.P.; Guggisberg, D.; Schmidt, R.S. How raw milk-based adjunct cultures influence microbial diversity in cheese. Int. Dairy J. 2025, 166, 106249. [Google Scholar] [CrossRef]
- Mangia, N.P.; Cottu, M.; Mura, M.E.; Murgia, M.A.; Blaiotta, G. Technological parameters, anti-listeria activity, biogenic amines formation and degradation ability of L. plantarum strains isolated from sheep-fermented sausage. Microorganisms 2021, 9, 1895. [Google Scholar] [CrossRef]
- Plessas, S. Advancements in the use of fermented fruit juices by lactic acid bacteria as functional foods: Prospects and challenges of Lactiplantibacillus (Lpb.) plantarum subsp. plantarum application. Fermentation 2021, 8, 6. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, N.; Bottacini, F.; Van Sinderen, D.; Gahan, C.G.; Corsetti, A. Comparative genomics of Lactiplantibacillus plantarum: Insights into probiotic markers in strains isolated from the human gastrointestinal tract and fermented foods. Front. Microbiol. 2022, 13, 854266. [Google Scholar] [CrossRef]
- Pannella, G.; Lombardi, S.J.; Coppola, F.; Vergalito, F.; Iorizzo, M.; Succi, M.; Tremonte, P.; Iannini, C.; Sorrentino, E.; Coppola, R. Effect of biofilm formation by Lactobacillus plantarum on the malolactic fermentation in model wine. Foods 2020, 9, 797. [Google Scholar] [CrossRef]
- Shahrampour, D.; Khomeiri, M. Comparison of probiotic potential, antimicrobial, antioxidant and technological characteristics of various Lactiplantibacillus plantarum strains isolated from fermented foods. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Yap, M.; Ercolini, D.; Álvarez-Ordóñez, A.; O’Toole, P.W.; O’Sullivan, O.; Cotter, P.D. Next-generation food research: Use of meta-omic approaches for characterizing microbial communities along the food chain. Annu. Rev. Food Sci. Technol. 2022, 13, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Zotta, T.; Giavalisco, M.; Parente, E.; Picariello, G.; Siano, F.; Ricciardi, A. Selection of Lactiplantibacillus strains for the production of fermented table olives. Microorganisms 2022, 10, 625. [Google Scholar] [CrossRef]
- Carlino, N.; Blanco-Míguez, A.; Punčochář, M.; Mengoni, C.; Pinto, F.; Tatti, A.; Manghi, P.; Armanini, F.; Avagliano, M.; Barcenilla, C.; et al. Unexplored microbial diversity from 2,500 food metagenomes and links with the human microbiome. Cell 2024, 187, 5775–5795. [Google Scholar] [CrossRef] [PubMed]
- Gustaw, K.; Niedźwiedź, I.; Rachwał, K.; Polak-Berecka, M. New insight into bacterial interaction with the matrix of plant-based fermented foods. Foods 2021, 10, 1603. [Google Scholar] [CrossRef]
- McCullough, H.C.; Song, H.S.; Auchtung, J.M. Diversity in chemical subunits and linkages: A key molecular determinant of microbial richness, microbiota interactions, and substrate utilization. Microbiol. Spectr. 2025, 13, e02618-24. [Google Scholar] [CrossRef]
- Banicod, R.J.S.; Ntege, W.; Njiru, M.N.; Abubakar, W.H.; Kanthenga, H.T.; Javaid, A.; Khan, F. Production and transformation of biogenic amines in different food products by the metabolic activity of the lactic acid bacteria. Int. J. Food Microbiol. 2024, 428, 110996. [Google Scholar] [CrossRef]
- Han, Z.; Shi, S.; Yao, B.; Shinali, T.S.; Shang, N.; Wang, R. Recent Insights in Lactobacillus-Fermented Fruit and Vegetable Juice: Compositional Analysis, Quality Evaluation, and Functional Properties. Food Rev. Int. 2025, 1–35. [Google Scholar] [CrossRef]
- Katz, S.E. Fermentation as a co-evolutionary force. In Cured, Smoked, and Fermented: Proceedings of the Oxford Symposium on Food and Cookery; Oxford Symposium: Oxford, UK, 2010. [Google Scholar]
- Sun, Z.; Harris, H.M.B.; McCann, A.; Guo, C.; Argimón, S.; Zhang, W.; Yang, X.; Jeffery, I.B.; Cooney, J.C.; Kagawa, T.F.; et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Commun. 2022, 13, 8322. [Google Scholar] [CrossRef]
- Bae, D.Y.; Moon, S.H.; Lee, T.G.; Ko, Y.S.; Cho, Y.C.; Kang, H.; Park, C.-S.; Kang, J.-S.; Oh, Y.; Cho, H.S. Consequences of Domestication on Gut Microbiome: A Comparative Analysis Between Wild Boars and Domestic Pigs. Animals 2025, 15, 747. [Google Scholar] [CrossRef]
- Li, D.D.; Zhang, Z.; Zhang, P.; Wang, J.N.; Liu, Y.; Li, Y.Z. Deterministic assembly processes shaping habitat-specific glycoside hydrolase composition. Glob. Ecol. Biogeogr. 2024, 33, 189–202. [Google Scholar] [CrossRef]
- Borowska, M.; Buttimer, C.; Bottacini, F.; Arendt, E.K.; Coffey, A. Comparative genomic analysis of selected lactic acid bacteria and phenotypic association of the key genes involved in fructan and ribose utilisation. Discov. Bact. 2025, 2, 5. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Trinh, H.P.; Park, H.D. Genome-based analysis reveals niche differentiation among Firmicutes in full-scale anaerobic digestion systems. Bioresour. Technol. 2025, 418, 131993. [Google Scholar] [CrossRef] [PubMed]
- Zhadyra, S.; Tao, F.; Xu, P. Exploring the Microbiome and Functional Metabolism of Fermented Camel Milk (Shubat) Using Metagenomics. Foods 2025, 14, 1102. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S. Structural and Catalytical Features of Different Amylases and their Potential Applications. Jordan J. Biol. Sci. 2022, 15, 311–337. [Google Scholar] [CrossRef]
- Paventi, G.; Di Martino, C.; Coppola, F.; Iorizzo, M. β-Glucosidase Activity of Lactiplantibacillus plantarum: A Key Player in Food Fermentation and Human Health. Foods 2025, 14, 1451. [Google Scholar] [CrossRef]
- Liang, T.; Jiang, T.; Liang, Z.; Zhang, N.; Dong, B.; Wu, Q.; Gu, B. Carbohydrate-active enzyme profiles of Lactiplantibacillus plantarum strain 84-3 contribute to flavor formation in fermented dairy and vegetable products. Food Chem. X 2023, 20, 101036. [Google Scholar] [CrossRef]
- Lin, M.; Jama, S.M.; Cheng, Z.; Zong, Y.; Su, Y.; Liu, W.; Liu, L. Exploring CAZymes Differences in Pediococcus acidilactici Strain OM681363 and Lacticaseibacillus paracasei Strain ON606241 Based on Whole-Genome Sequencing. Fermentation 2025, 11, 64. [Google Scholar] [CrossRef]
- King, M.L.; Xing, X.; Reintjes, G.; Klassen, L.; Low, K.E.; Alexander, T.W.; Waldner, M.; Patel, T.R.; Wade Abbott, D. In vitro and ex vivo metabolism of chemically diverse fructans by bovine rumen Bifidobacterium and Lactobacillus species. Anim. Microbiome 2024, 6, 50. [Google Scholar] [CrossRef]
- Mkadem, W.; Belguith, K.; Indio, V.; Oussaief, O.; Guluzade, G.; ElHatmi, H.; Boudhrioua, N. Assessment of the Anti-Listeria Effect of Citrus limon Peel Extract In Silico, In Vitro, and in Fermented Cow Milk During Cold Storage. Foods 2025, 14, 661. [Google Scholar] [CrossRef] [PubMed]
- Sornsenee, P.; Kooltheat, N.; Wongprot, D.; Suksabay, P.; Nam, T.G.; Permpoon, U.; Romyasamit, C. Antibacterial, Antioxidant, and Anti-inflammatory Activities of Lacticaseibacillus paracasei Lysates Isolated from Fermented Palm Sap. Probiotics Antimicrob. Proteins 2025, 17, 1–13. [Google Scholar] [CrossRef]
- Leisner, J.J.; Laursen, B.G.; Prévost, H.; Drider, D.; Dalgaard, P. Carnobacterium: Positive and negative effects in the environment and in foods. FEMS Microbiol. Rev. 2007, 31, 592–613. [Google Scholar] [CrossRef]
- Dishan, A.; Gönülalan, Z. Lacticaseibacillus paracasei AD22 Stress Response in Brined White Cheese Matrix: In Vitro Probiotic Profiles and Molecular Characterization. Probiotics Antimicrob. Proteins 2024, 16, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Ennahar, S.; Deschamps, N. Anti-Listeria effect of enterocin A, produced by cheese-isolated Enterococcus faecium EFM01, relative to other bacteriocins from lactic acid bacteria. J. Appl. Microbiol. 2000, 88, 449–457. [Google Scholar] [CrossRef] [PubMed]
- de Souza, N.A.A.; de Carvalho, L.; Nogueira, M.H.; Furlaneto, M.C.; Maia, L.F. Potential of Enterocin from Enterococcus durans MF5 in Controlling Listeria Species. J. Dairy Res. 2025, 92, 1–9. [Google Scholar] [CrossRef]
- Smaoui, S.; Echegaray, N.; Kumar, M.; Chaari, M.; D’Amore, T.; Shariati, M.A.; Rebezov, M.; Lorenzo, J.M. Beyond conventional meat preservation: Saddling the control of bacteriocin and lactic acid bacteria for clean label and functional meat products. Appl. Biochem. Biotechnol. 2024, 196, 3604–3635. [Google Scholar] [CrossRef]
- Jadhav, S.P.; Shah, U.B.; Shelke, K. Current Facts about Clean Label Food Products. In Food Intolerances; CRC Press: Boca Raton, FL, USA, 2024; pp. 162–200. [Google Scholar] [CrossRef]


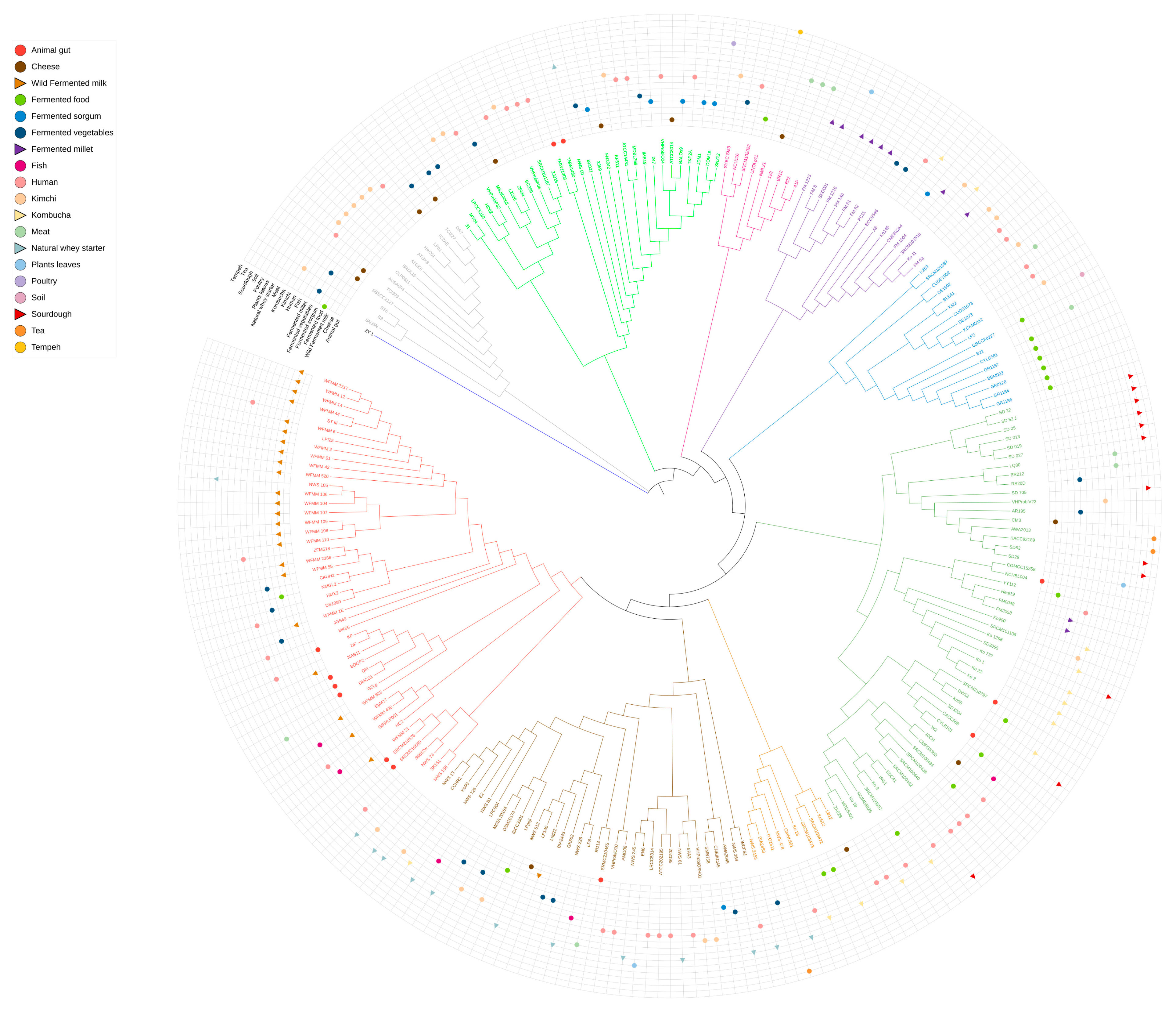
 red (dominant in milk and partly in whey),
red (dominant in milk and partly in whey),  brown (natural whey starter),
brown (natural whey starter),  orange (whey and kombucha),
orange (whey and kombucha),  purple (mainly millet, also kombucha), and
purple (mainly millet, also kombucha), and  green (sourdough, millet, kombucha), illustrating a narrower phylogenetic distribution in animal-derived matrices and higher diversity in plant-based sources.
green (sourdough, millet, kombucha), illustrating a narrower phylogenetic distribution in animal-derived matrices and higher diversity in plant-based sources.
 red (dominant in milk and partly in whey),
red (dominant in milk and partly in whey),  brown (natural whey starter),
brown (natural whey starter),  orange (whey and kombucha),
orange (whey and kombucha),  purple (mainly millet, also kombucha), and
purple (mainly millet, also kombucha), and  green (sourdough, millet, kombucha), illustrating a narrower phylogenetic distribution in animal-derived matrices and higher diversity in plant-based sources.
green (sourdough, millet, kombucha), illustrating a narrower phylogenetic distribution in animal-derived matrices and higher diversity in plant-based sources.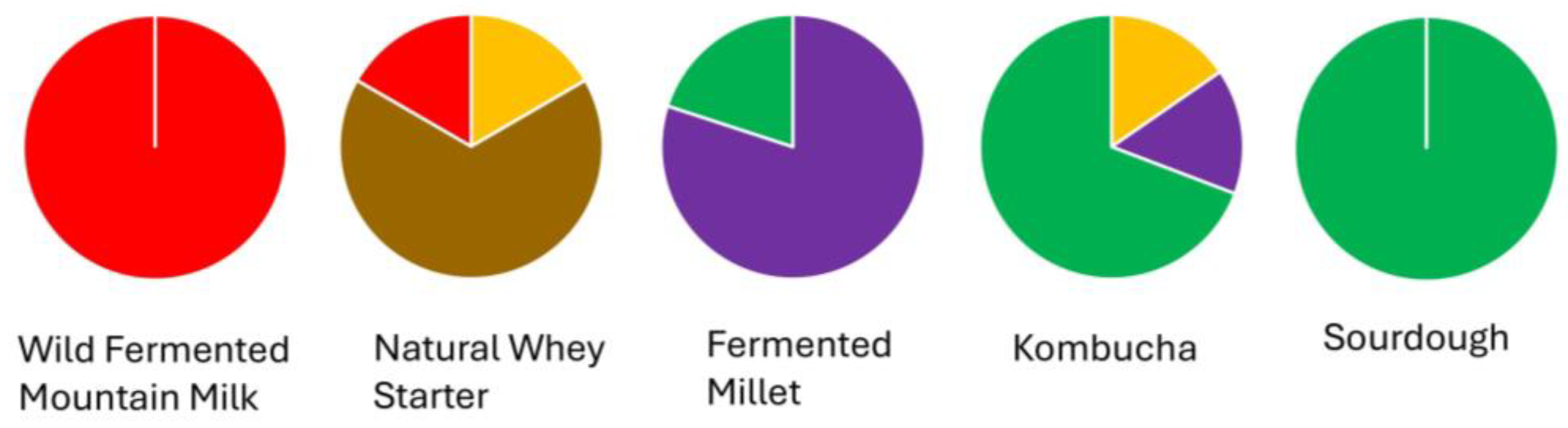

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iarusso, I.; Mahony, J.; Pannella, G.; Lombardi, S.J.; Gagliardi, R.; Coppola, F.; Pellegrini, M.; Succi, M.; Tremonte, P. Diversity of Lactiplantibacillus plantarum in Wild Fermented Food Niches. Foods 2025, 14, 1765. https://doi.org/10.3390/foods14101765
Iarusso I, Mahony J, Pannella G, Lombardi SJ, Gagliardi R, Coppola F, Pellegrini M, Succi M, Tremonte P. Diversity of Lactiplantibacillus plantarum in Wild Fermented Food Niches. Foods. 2025; 14(10):1765. https://doi.org/10.3390/foods14101765
Chicago/Turabian StyleIarusso, Ilenia, Jennifer Mahony, Gianfranco Pannella, Silvia Jane Lombardi, Roberto Gagliardi, Francesca Coppola, Michela Pellegrini, Mariantonietta Succi, and Patrizio Tremonte. 2025. "Diversity of Lactiplantibacillus plantarum in Wild Fermented Food Niches" Foods 14, no. 10: 1765. https://doi.org/10.3390/foods14101765
APA StyleIarusso, I., Mahony, J., Pannella, G., Lombardi, S. J., Gagliardi, R., Coppola, F., Pellegrini, M., Succi, M., & Tremonte, P. (2025). Diversity of Lactiplantibacillus plantarum in Wild Fermented Food Niches. Foods, 14(10), 1765. https://doi.org/10.3390/foods14101765








