Integrated Analysis of the Transcriptome and Metabolome Reveals the Network Regulating Fruit Taste in Sponge Gourd (Luffa cylindrica)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of Amino Acids Content
2.3. Determination of Soluble Solids, Ascorbic Acid, and Crude Protein Content
2.4. Transcriptome Sequencing and Analysis
2.5. Metabolome Determination and Analysis
2.6. DEGs Validation via qRT-PCR
2.7. Statistical Analysis
3. Results
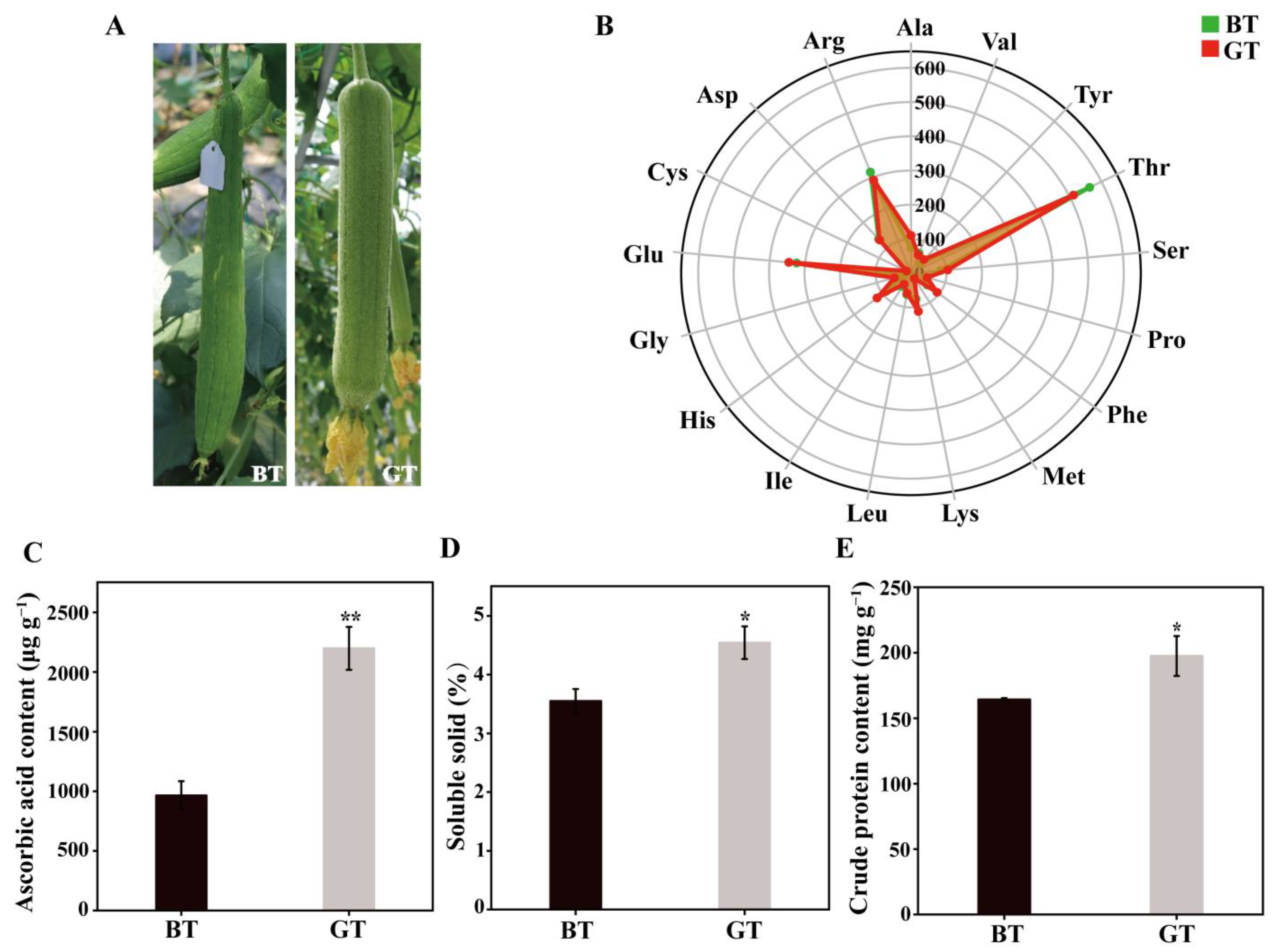
3.1. Differences in Morphology and Compounds Between BT and GT
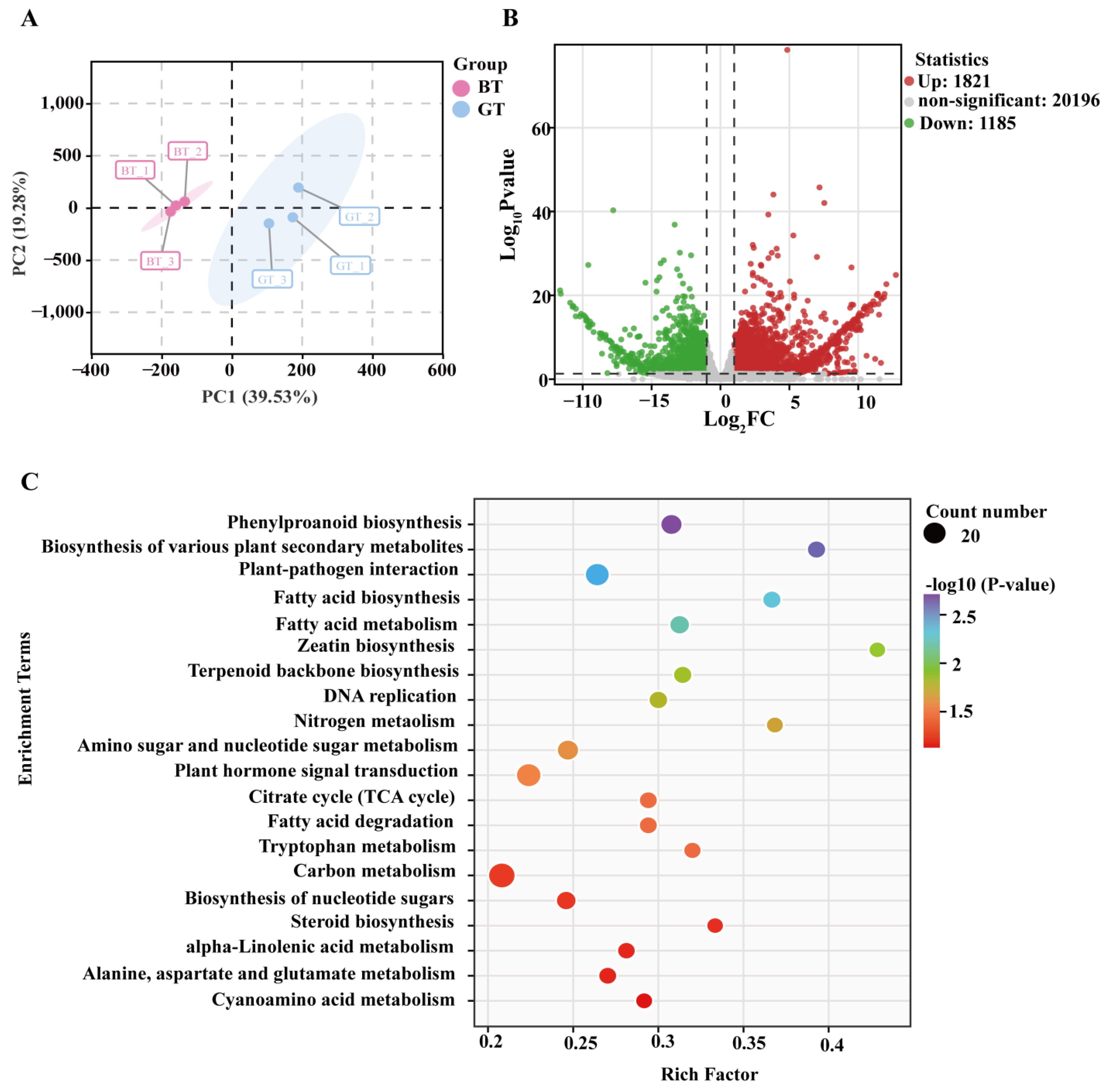
3.2. Analysis of Transcriptome Data
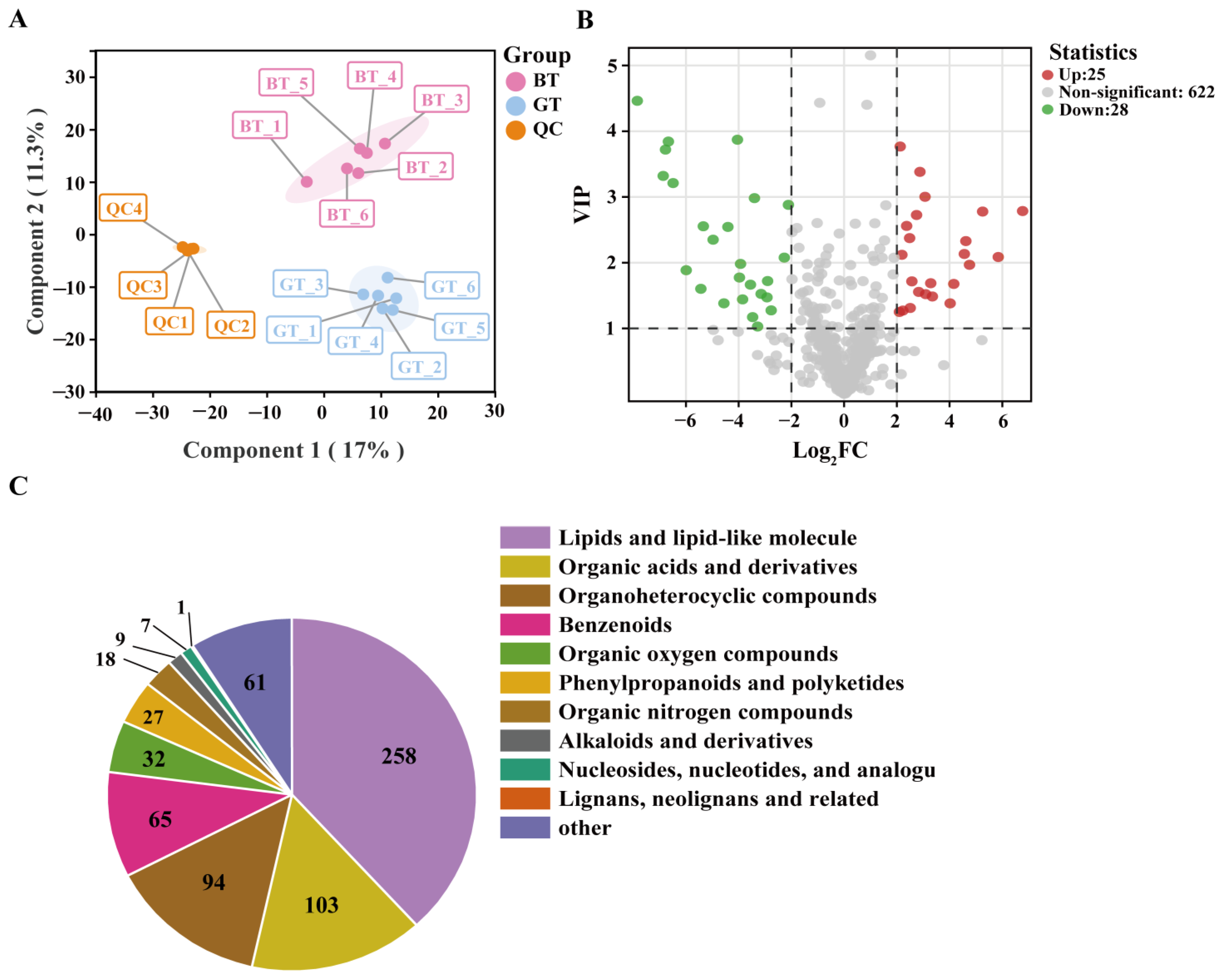
3.3. Analysis of Metabolome Data
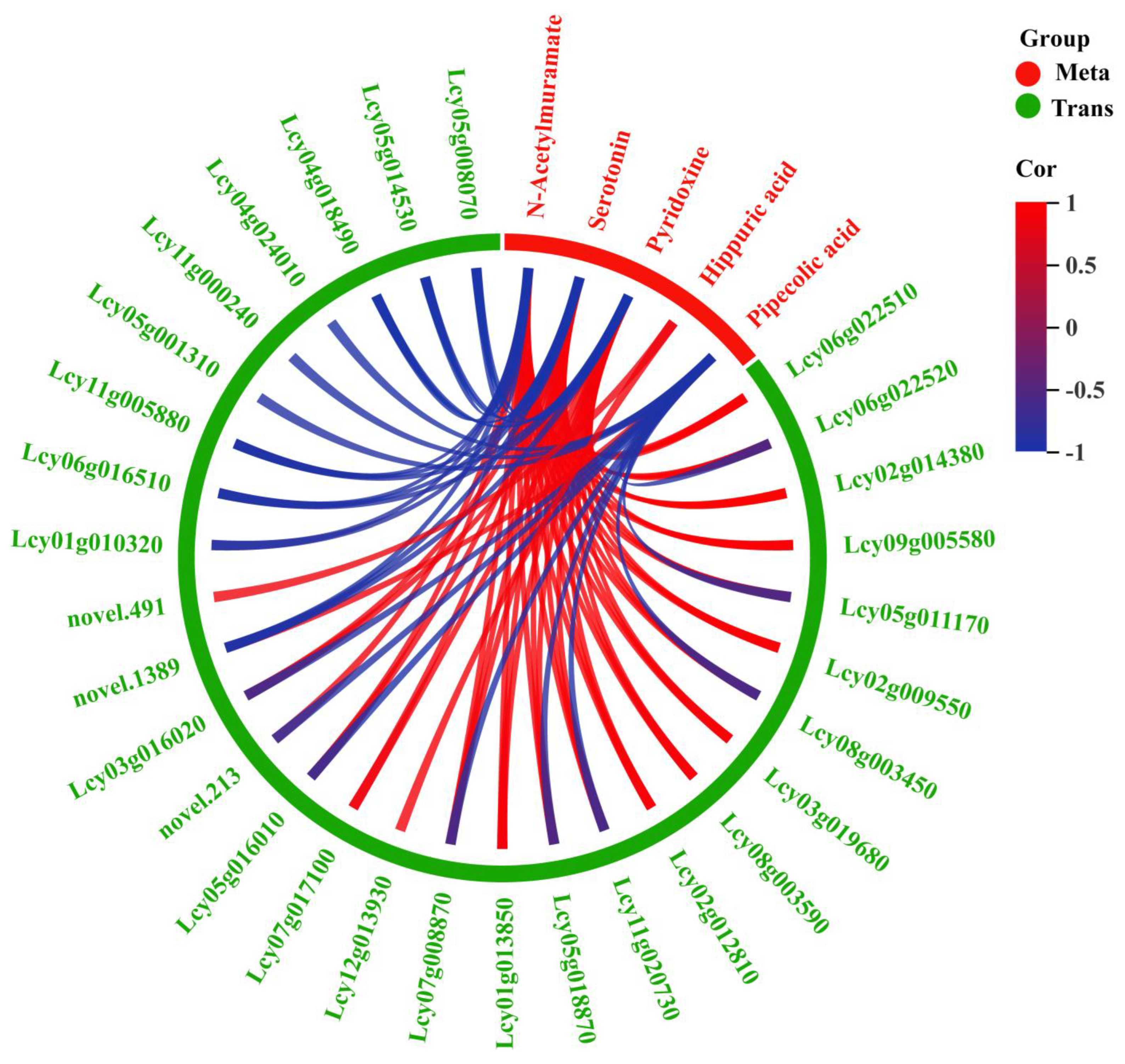
3.4. Integrated Analysis of Transcriptome and Metabolome Results
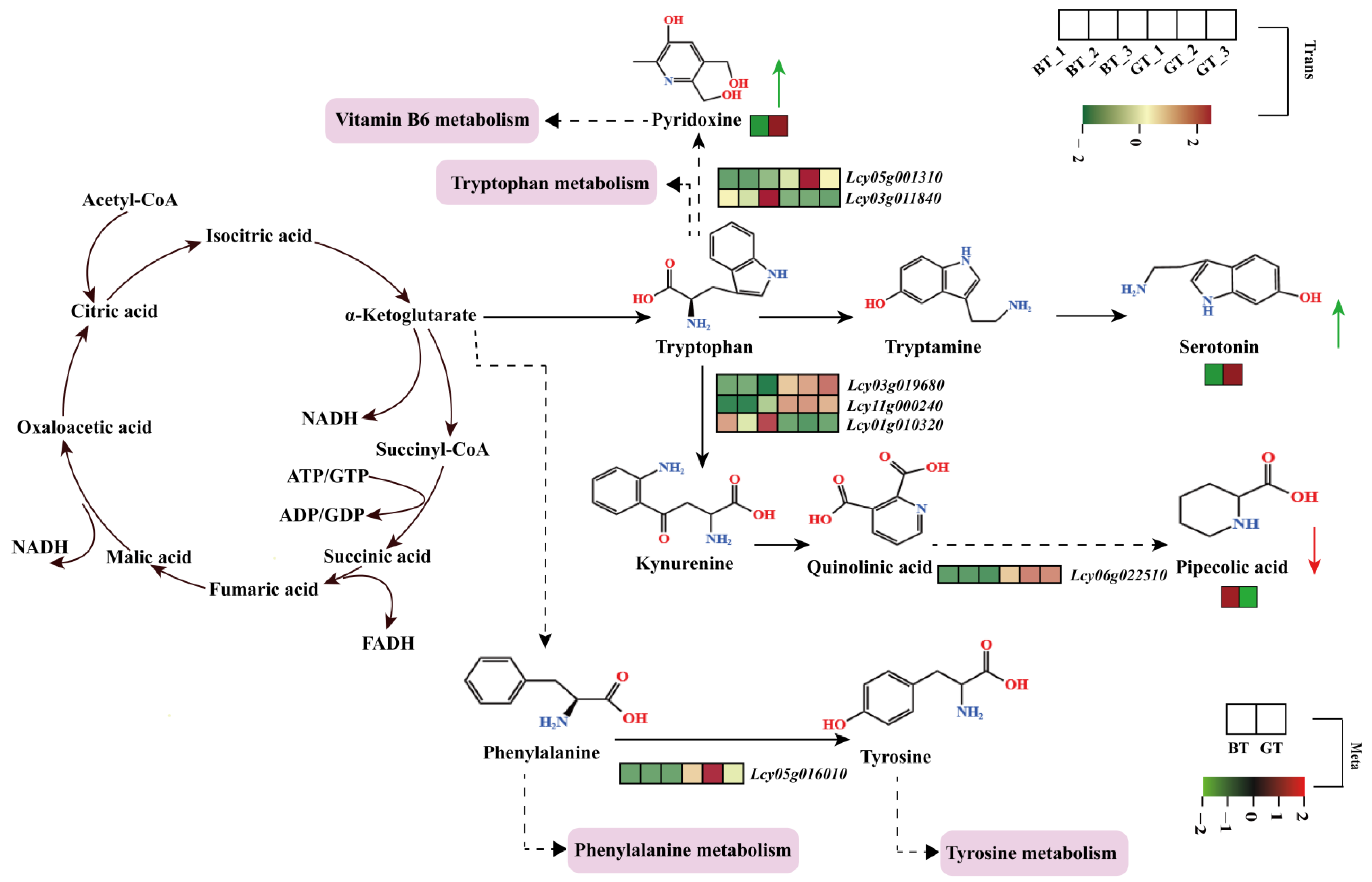
3.5. Potential Mechanism Model Underlying the Differences in Fruit Taste
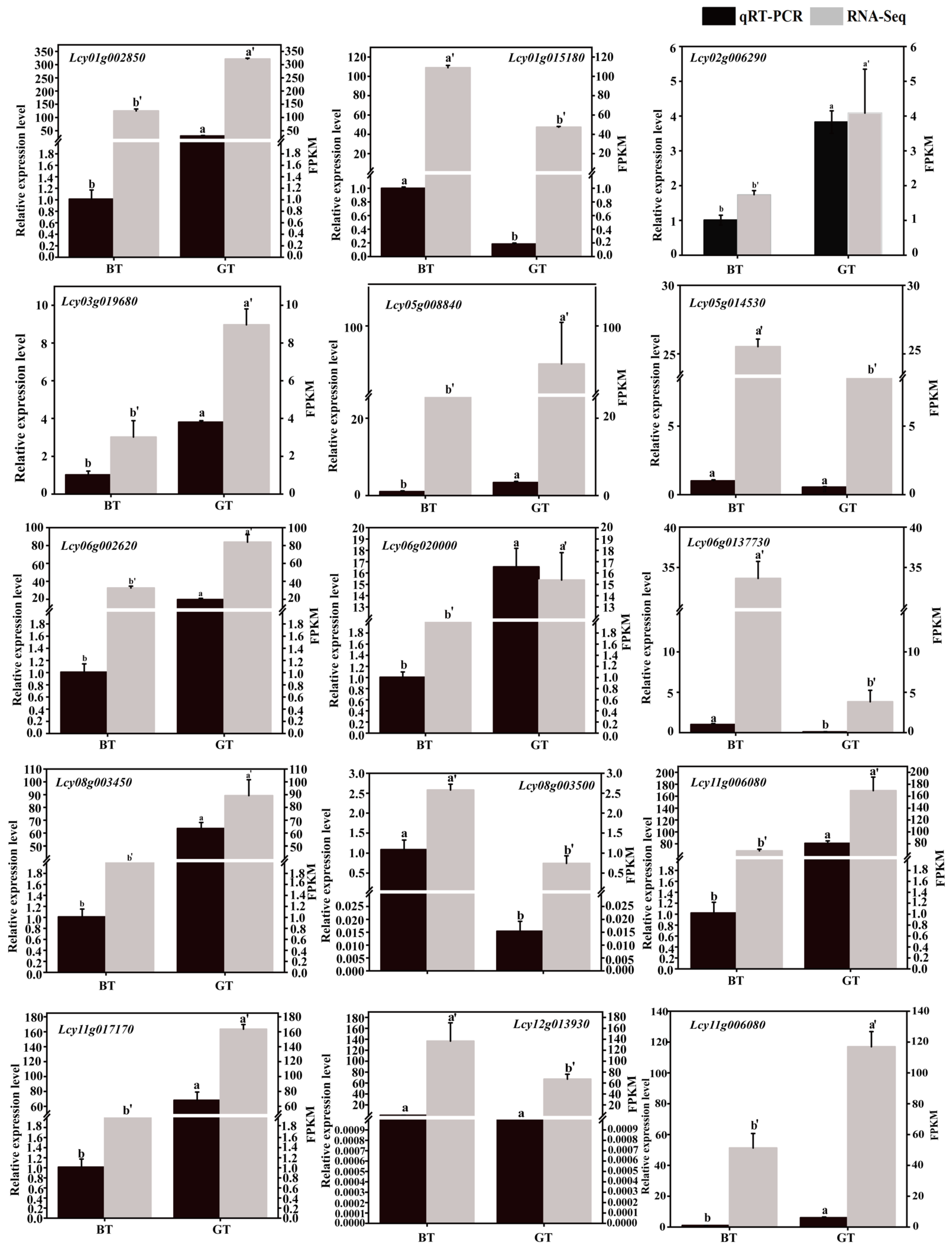
3.6. Validation of DEGs via qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, H.; Zhao, G.; Gong, H.; Li, J.; Luo, C.; He, X.; Luo, S.; Zheng, X.; Liu, X.; Guo, J.; et al. A high-quality sponge gourd (Luffa cylindrica) genome. Hortic. Res. 2020, 7, 128. [Google Scholar] [CrossRef]
- Partap, S.; Kumar, A.; Sharma, N.K.; Jha, K.K. Luffa cylindrica: An important medicinal plant. J. Nat. Prod. Plant Resour. 2012, 2, 127–134. [Google Scholar]
- Tyagi, R.; Sharma, V.; Sureja, A.K.; Das Munshi, A.; Arya, L.; Saha, D.; Verma, M. Genetic diversity and population structure detection in sponge gourd (Luffa cylindrica) using ISSR, SCoT and morphological markers. Physiol. Mol. Biol. Plants 2020, 26, 119–131. [Google Scholar] [CrossRef]
- Shendge, P.N.; Belemkar, S. Therapeutic potential of Luffa acutangula: A review on its traditional uses, phytochemistry, pharmacology and toxicological aspects. Front. Pharmacol. 2018, 9, 1177. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, X.; Yan, W.; Lou, L.; Xu, X.; Chen, X. Characterization of differences in the composition and content of volatile compounds in cucumber fruit. Foods 2022, 11, 1101. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, S.; Lu, X.; He, N.; Zhu, H.; Dou, J.; Liu, W. Comparative transcriptome analysis reveals key genes potentially related to soluble sugar and organic acid accumulation in watermelon. PLoS ONE 2018, 13, e0190096. [Google Scholar] [CrossRef]
- Umer, M.J.; Bin Safdar, L.; Gebremeskel, H.; Zhao, S.; Yuan, P.; Zhu, H.; Kaseb, M.O.; Anees, M.; Lu, X.; He, N.; et al. Identification of key gene networks controlling organic acid and sugar metabolism during watermelon fruit development by integrating metabolic phenotypes and gene expression profiles. Hortic. Res. 2020, 7, 193. [Google Scholar] [CrossRef]
- Kaleem, M.M.; Nawaz, M.A.; Ding, X.; Wen, S.; Shireen, F.; Cheng, J.; Bie, Z. Comparative analysis of pumpkin rootstocks mediated impact on melon sensory fruit quality through integration of non-targeted metabolomics and sensory evaluation. Plant Physiol. Biochem. 2022, 192, 320–330. [Google Scholar] [CrossRef]
- Feng, X.; Ma, J.; Liu, Z.; Li, X.; Wu, Y.; Hou, L.; Li, M. Analysis of glucosinolate content and metabolism related genes in different parts of Chinese flowering cabbage. Front. Plant Sci. 2022, 12, 767898. [Google Scholar] [CrossRef]
- Sajjad, M.; Xue, S.; Zhou, M.; Li, G.; Xu, Y.; Liu, L.; Zhu, J.; Meng, Q.; Jin, Q.; Du, H.; et al. Decoding comparative taste and nutrition regulation in Chinese cabbage via integrated metabolome and transcriptome analysis. Food Res. Int. 2024, 195, 114943. [Google Scholar] [CrossRef]
- Miao, L.; Di, Q.; Sun, T.; Li, Y.; Duan, Y.; Wang, J.; Yan, Y.; He, C.; Wang, C.; Yu, X. Integrated metabolome and transcriptome analysis provide insights into the effects of grafting on fruit flavor of cucumber with different rootstocks. Int. J. Mol. Sci. 2019, 20, 3592. [Google Scholar] [CrossRef]
- Kemp, T.R.; Knavel, D.E.; Stoltz, L.P.; Lundin, R.E. 3,6-Nonadien-1-ol from Citrullus vulgaris and Cucumis melo. Phytochemistry 1974, 13, 1167–1170. [Google Scholar] [CrossRef]
- Mayobre, C.; Garcia-Mas, J.; Pujol, M. A matter of smell: The complex regulation of aroma production in melon. Food Chem. 2024, 460, 140640. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Otunola, G.A.; Afolayan, A.J. Phytochemical screening and in vitro evaluation of antioxidant and antimicrobial activities of Kedrostis africana (L.) Cogn. Asian Pac. J. Trop. Biomed. 2017, 7, 901–908. [Google Scholar] [CrossRef]
- Manchali, S.; Chidambara Murthy, K.N.; Vishnuvardana; Patil, B.S. Nutritional composition and health benefits of various botanical types of melon (Cucumis melo L.). Plants 2021, 10, 1755. [Google Scholar] [CrossRef]
- Jaskani, M.J.; Kwon, S.W.; Kim, D.H. Comparative study on vegetative, reproductive and qualitative traits of seven diploid and tetraploid watermelon lines. Euphytica 2005, 145, 259–268. [Google Scholar] [CrossRef]
- da Silva, S.S.; de Lima, G.S.; de Lima, V.L.A.; Gheyi, H.R.; dos Anjos Soares, L.A.; Oliveira, J.P.M.; de Araújo, A.C.; Gomes, J.P. Production and quality of watermelon fruits under salinity management strategies and nitrogen fertilization. Semin. Ciências Agrárias 2020, 41, 2923–2936. [Google Scholar] [CrossRef]
- Salo-väänänen, P.P.; Koivistoinen, P.E. Determination of protein in foods: Comparison of net protein and crude protein (N × 6.25) values. Food Chem. 1996, 57, 27–31. [Google Scholar] [CrossRef]
- Parkhomchuk, D.; Borodina, T.; Amstislavskiy, V.; Banaru, M.; Hallen, L.; Kylvia, S.; Lehrach, H.; Soldatov, A. Transcriptome analysis by strand-specific sequencing of complementary DNA. Nucleic Acids Res. 2009, 37, e123. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; World, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An efficient general-purpose program for assigning sequence reads to genomicfeatures. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Ai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, L.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J. Global metabolic profiling of animal andhuman tissues via UPLC-MS. Nat. Protoc. 2012, 8, 17–32. [Google Scholar] [CrossRef]
- Gasic, K.; Hernandez, A.; Korban, S.S. RNA extraction from different apple tissues rich in polyphenols and polysaccharides for cDNA library construction. Plant Mol. Biol. Rep. 2004, 22, 437–438. [Google Scholar] [CrossRef]
- Li, F.; Min, D.; Ren, C.; Dong, L.; Shu, P.; Cui, X.; Zhang, X. Ethylene altered fruit cuticular wax, the expression of cuticular wax synthesis-related genes and fruit quality during cold storage of apple (Malus domestica Borkh. c.v. Starkrimson) fruit. Postharvest Biol. Technol. 2019, 149, 58–65. [Google Scholar] [CrossRef]
- Zhang, X.; Min, D.; Li, F.; Ji, N.; Meng, D.; Li, L. Synergistic effects of L-arginine and methyl salicylate on alleviating postharvest disease caused by Botrysis cinerea in tomato fruit. J. Agric. Food Chem. 2017, 65, 4890–4896. [Google Scholar] [CrossRef]
- Morrison, R.I. The isolation of L-pipecolinic acid from Trifolium repens. Biochem. J. 1953, 53, 474. [Google Scholar] [CrossRef]
- Chen, Y.C.; Holmes, E.C.; Rajniak, J.; Kim, J.G.; Tang, S.; Fischer, C.R.; Sattely, E.S. N-hydroxy-pipecolic acid is a mobile metabolite that induces systemic disease resistance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E4920–E4929. [Google Scholar] [CrossRef]
- Holmes, E.C.; Chen, Y.C.; Mudgett, M.B.; Sattely, E.S. Arabidopsis UGT76B1 glycosylates N-hydroxy-pipecolic acid and inactivates systemic acquired resistance in tomato. Plant Cell 2021, 33, 750–765. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liang, X.; Fang, H.; Wang, J.; Liu, X.; Li, Y.; Shi, K. Transcriptomic and genetic approaches reveal that the pipecolate biosynthesis pathway simultaneously regulates tomato fruit ripening and quality. Plant Physiol. Biochem. 2023, 201, 107920. [Google Scholar] [CrossRef]
- Zhu, C.; Lu, Q.; Zhou, X.; Li, J.; Yue, J.; Wang, Z.; Pan, S. Metabolic variations of organic acids, amino acids, fatty acids and aroma compounds in the pulp of different pummelo varieties. LWT-Food Sci. Technol. 2020, 130, 109445. [Google Scholar] [CrossRef]
- Angonese, M.; Motta, G.E.; de Farias, N.S.; Molognoni, L.; Daguer, H.; Brugnerotto, P.; de Oliveira Costa, A.C.; Müller, C.M.O. Organic dragon fruits (Hylocereus undatus and Hylocereus polyrhizus) grown at the same edaphoclimatic conditions: Comparison of phenolic and organic acids profiles and antioxidant activities. LWT-Food Sci. Technol. 2021, 149, 111924. [Google Scholar] [CrossRef]
- Hajeb, P.; Jinap, S. Umami taste components and their sources in Asian foods. Crit. Rev. Food Sci. Nutr. 2015, 55, 778–791. [Google Scholar] [CrossRef]
- McCormick, D.B. Two interconnected B vitamins: Riboflavin and pyridoxine. Physiol. Rev. 1989, 69, 1170–1198. [Google Scholar] [CrossRef]
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, global distribution, and nutritional importance of citrus fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Zhang, P.; Tang, F.; Cai, W.; Zhao, X.; Shan, C. Evaluating the effect of lactic acid bacteria fermentation on quality, aroma, and metabolites of chickpea milk. Front. Nutr. 2022, 9, 1069714. [Google Scholar] [CrossRef]
- Lo’Ay, A.A.; El-Khateeb, A.Y. Impact of exogenous pyridoxin treatments of ‘Williams’ banana on the activities of starch degrading enzymes. Sci. Hortic. 2019, 243, 48–54. [Google Scholar] [CrossRef]
- Guo, J.; Dong, L.; Kandel, S.L.; Jiao, Y.; Shi, L.; Yang, Y.; Shi, A.; Mou, B. Transcriptomic and metabolomic analysis provides insights into the fruit quality and yield improvement in tomato under soilless substrate-based cultivation. Agronomy 2022, 12, 923. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, C.; Wang, S.; Zhang, M.; Li, Y.; Xue, Q.; Guo, Q.; Lai, H. Widely targeted metabolomic, transcriptomic, and metagenomic profiling reveal microbe–plant–metabolic reprogramming patterns mediated by Streptomyces pactum Act12 enhance the fruit quality of Capsicum annuum L. Food Res. Int. 2023, 166, 112587. [Google Scholar] [CrossRef] [PubMed]
- Lioe, H.N.; Kinjo, A.; Yasuda, S.; Kuba-Miyara, M.; Tachibana, S.; Yasuda, M. Taste and chemical characteristics of low molecular weight fractions from Tokuyo–Japanese fermented soybean curd. Food Chem. 2018, 252, 265–270. [Google Scholar] [CrossRef]
- Li, Y.; He, C.; Yu, X.; Zhou, J.; Ran, W.; Chen, Y.; Ni, D. Effects of red-light withering on the taste of black tea as revealed by non-targeted metabolomics and transcriptomics analysis. LWT-Food Sci. Technol. 2021, 147, 111620. [Google Scholar] [CrossRef]
- Liu, R.; Deng, Y.; Liu, Y.; Wang, Z.; Yu, S.; Nie, Y.; Zhu, W.; Zhou, Z.; Diao, J. Combined analysis of transcriptome and metabolome reveals the potential mechanism of the enantioselective effect of chiral penthiopyrad on tomato fruit flavor quality. J. Agric. Food Chem. 2022, 70, 10872–10885. [Google Scholar] [CrossRef]
- Chen, J.; Wu, F.; Wang, H.; Guo, C.; Zhang, W.; Luo, P.; Zhou, J.; Hao, W.; Yang, G.; Huang, J. Identification of key taste components in Baccaurea ramiflora Lour. fruit using non-targeted metabolomics. Food Sci. Hum. Wellness 2023, 12, 94–101. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, Y.; Qiu, W.; Li, Z.; Gao, L.; Dong, W.; Zhang, P.; Zhou, S.; Wang, X.; Zhu, Y.; Sun, Y. Integrated Analysis of the Transcriptome and Metabolome Reveals the Network Regulating Fruit Taste in Sponge Gourd (Luffa cylindrica). Foods 2025, 14, 1753. https://doi.org/10.3390/foods14101753
Chai Y, Qiu W, Li Z, Gao L, Dong W, Zhang P, Zhou S, Wang X, Zhu Y, Sun Y. Integrated Analysis of the Transcriptome and Metabolome Reveals the Network Regulating Fruit Taste in Sponge Gourd (Luffa cylindrica). Foods. 2025; 14(10):1753. https://doi.org/10.3390/foods14101753
Chicago/Turabian StyleChai, Yaqian, Wenjing Qiu, Zhikun Li, Luyao Gao, Wenqi Dong, Peng Zhang, Shengjun Zhou, Xin Wang, Yuqiang Zhu, and Yuyan Sun. 2025. "Integrated Analysis of the Transcriptome and Metabolome Reveals the Network Regulating Fruit Taste in Sponge Gourd (Luffa cylindrica)" Foods 14, no. 10: 1753. https://doi.org/10.3390/foods14101753
APA StyleChai, Y., Qiu, W., Li, Z., Gao, L., Dong, W., Zhang, P., Zhou, S., Wang, X., Zhu, Y., & Sun, Y. (2025). Integrated Analysis of the Transcriptome and Metabolome Reveals the Network Regulating Fruit Taste in Sponge Gourd (Luffa cylindrica). Foods, 14(10), 1753. https://doi.org/10.3390/foods14101753




