The Regulation of the NF-κB p65 and Nrf2/HO-1 Signaling Pathways by Fucoxanthin in Human THP-1 Monocyte Macrophages Under a Lipopolysaccharide-Induced Inflammation Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Apparatus
2.2. Cell Culture of THP
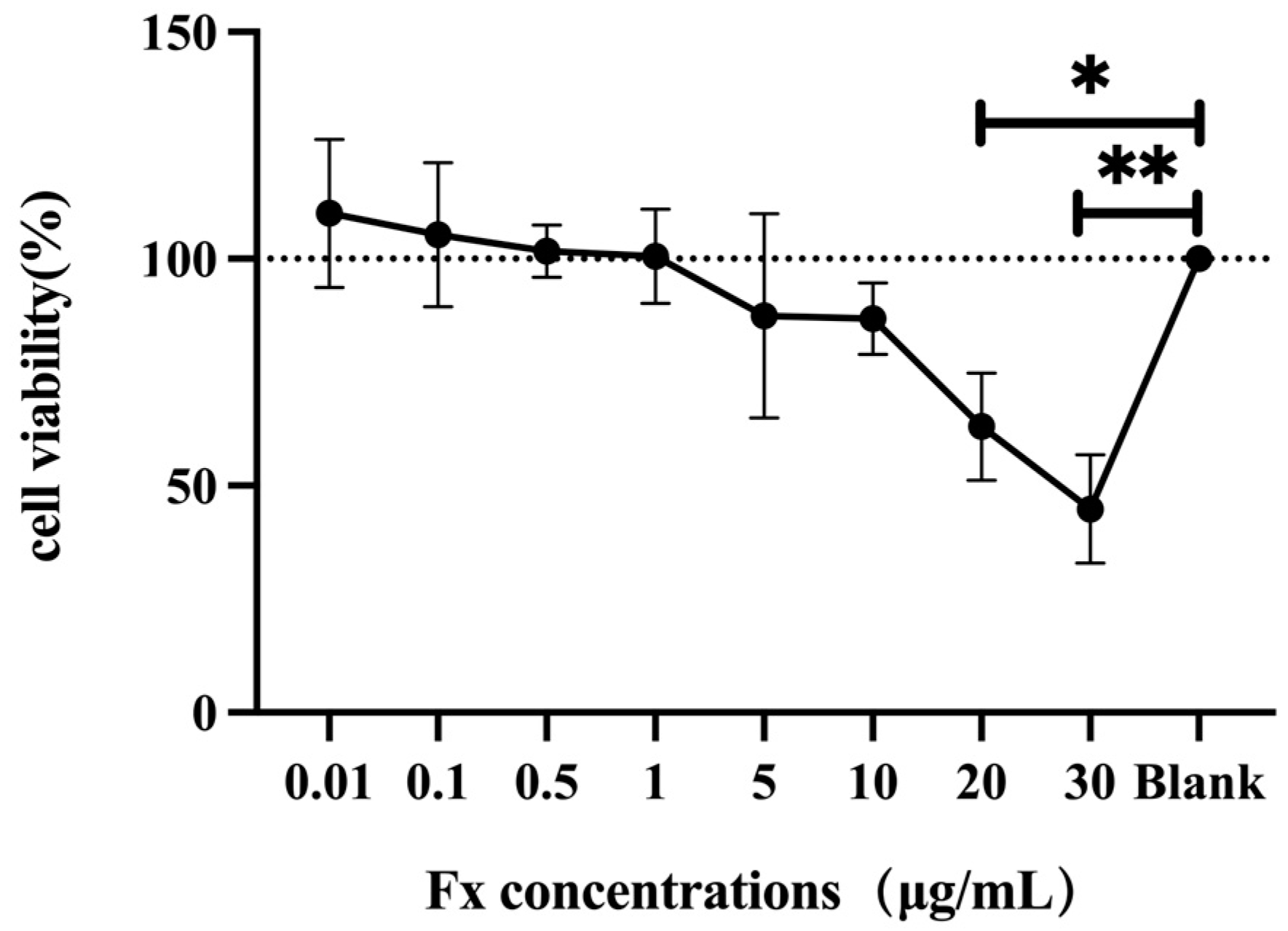
2.3. MTT Assay Method
2.4. CCK-8 Assay Method
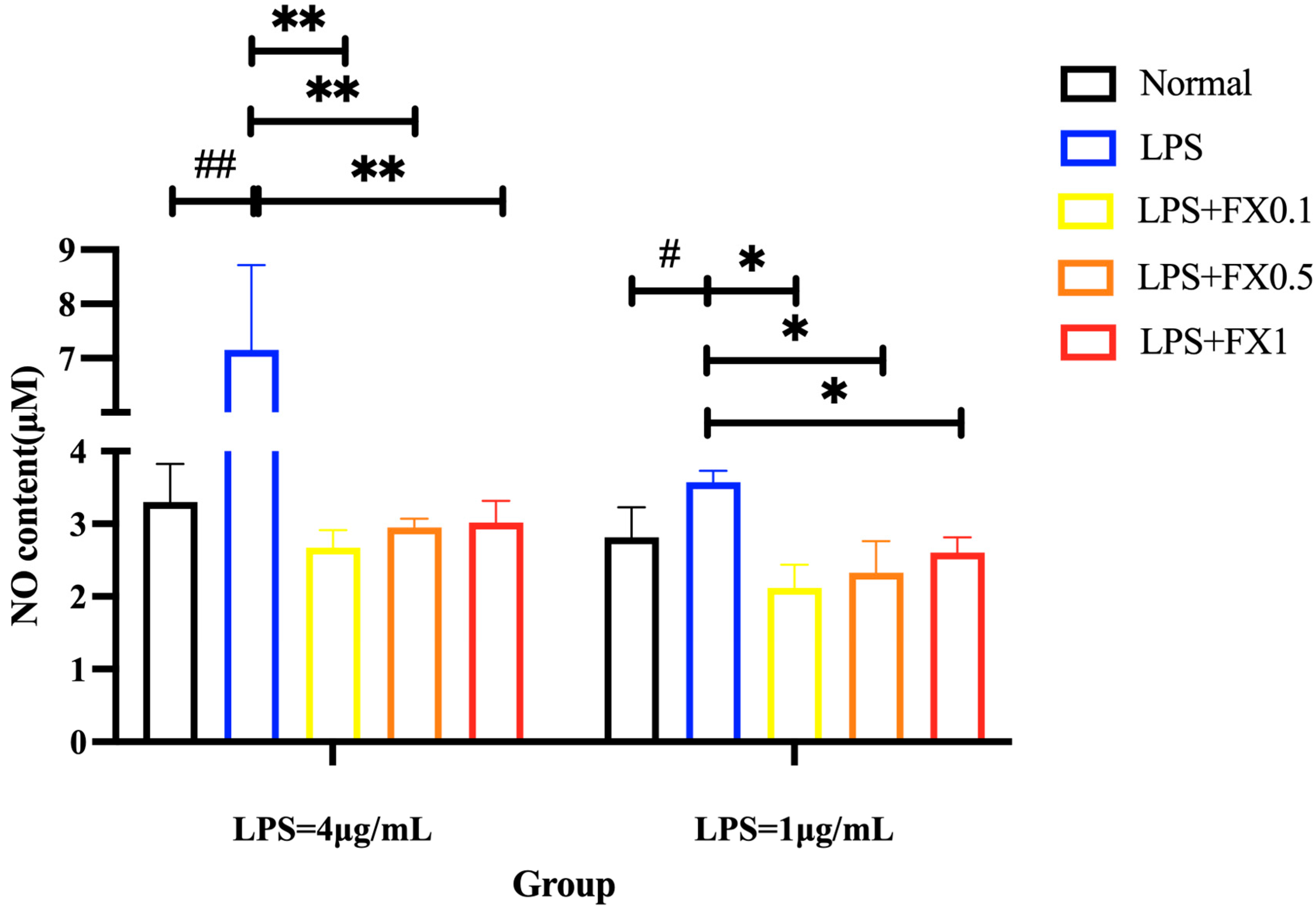
2.5. Griess Method for Measuring NO Content
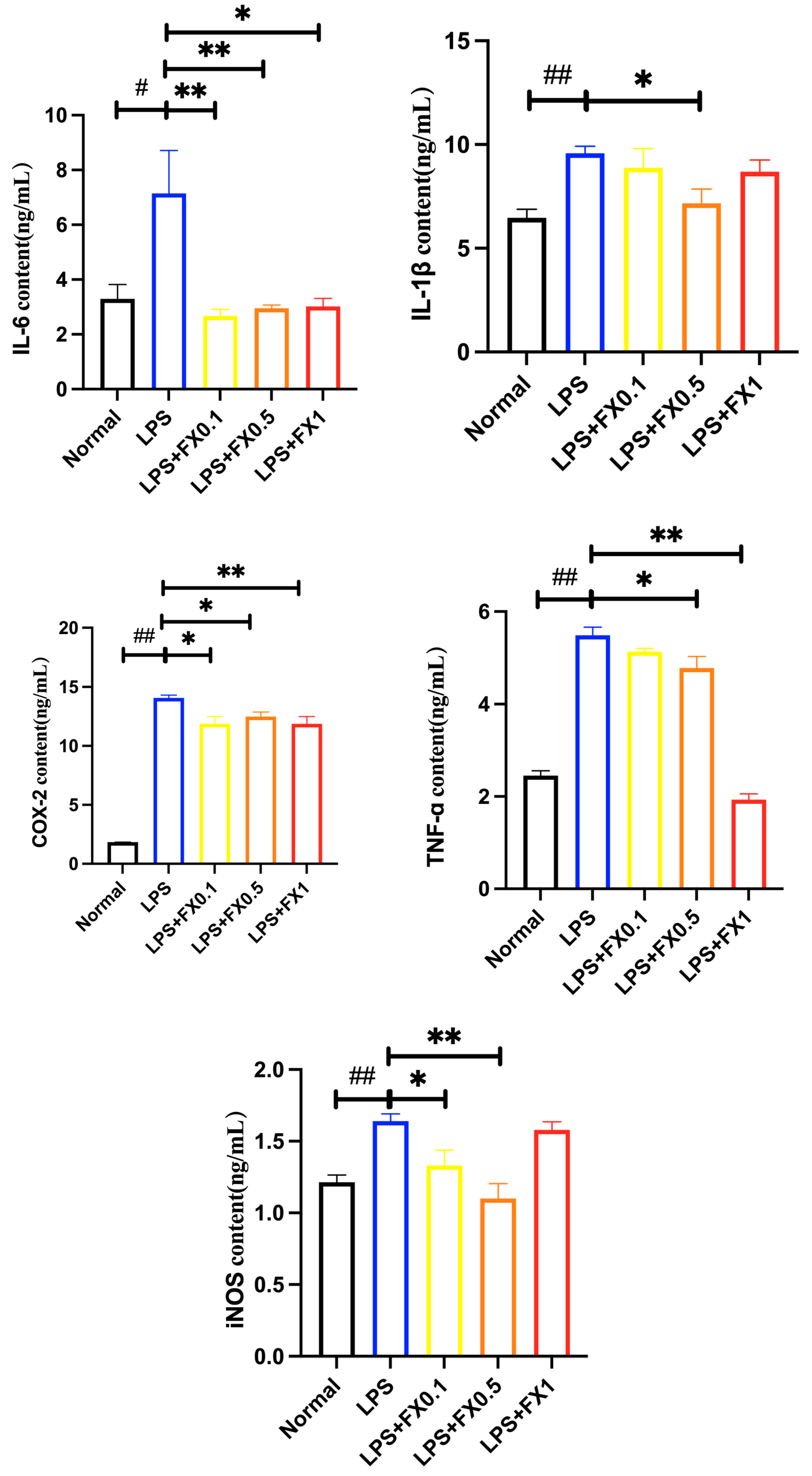
2.6. Measurement of Inflammatory Factor Levels
2.7. Measurement of Oxidase Activity and Oxidative Damage
2.8. Intracellular Reactive Oxygen Species (ROS) Assay
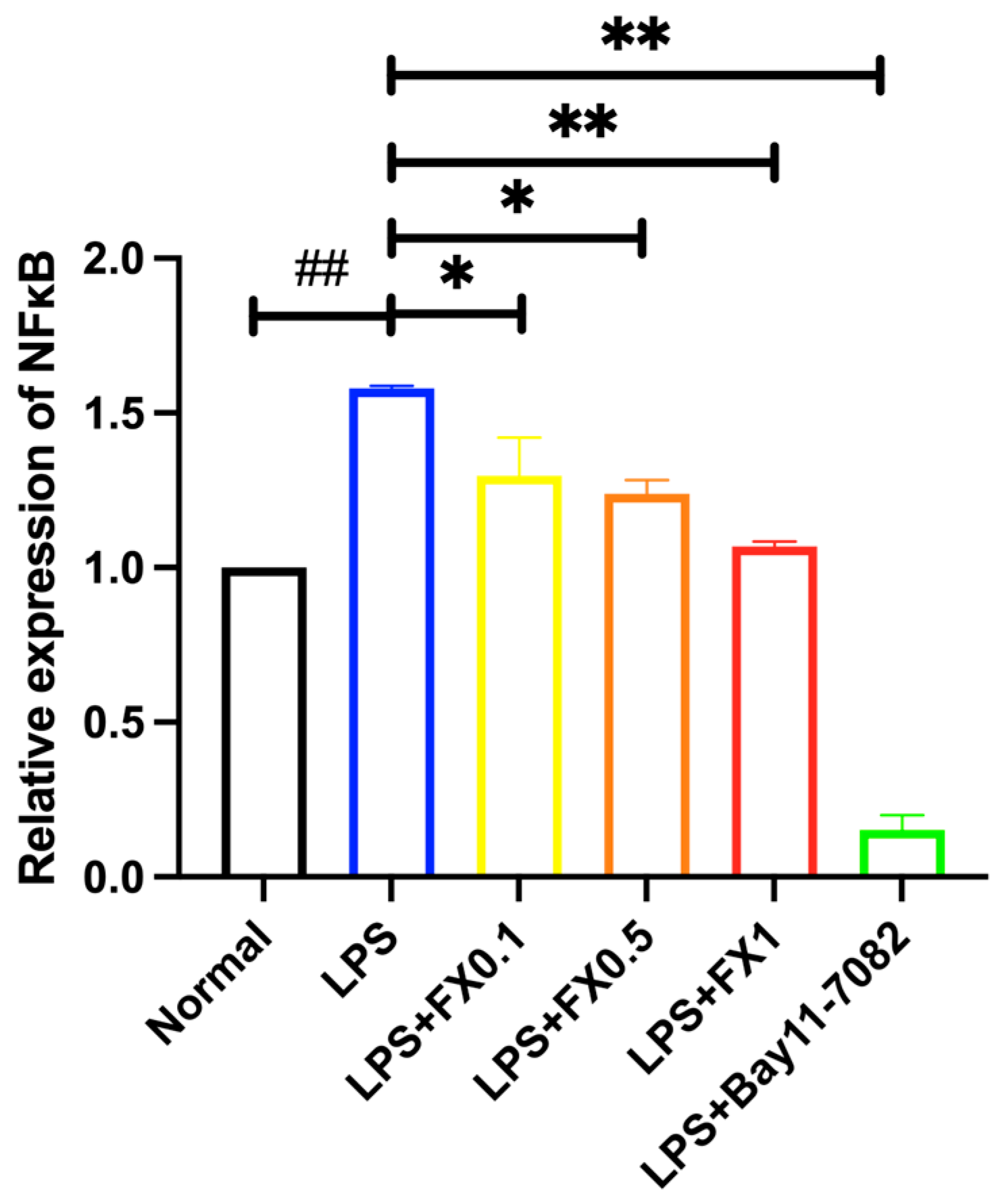
2.9. Gene Expression Measurement of NF-κB p65, Nrf2, and Their Associated Factors
2.10. Determination of Protein Expression of NF-κB, Nrf2, and Their Related Factors
2.11. Statistical Analysis
3. Results and Discussion
3.1. Toxic Effect of Fucoxanthin on Human THP-1 Cells
3.2. The Anti-Inflammatory Effect of Fucoxanthin on LPS-Induced Human THP-1 Cells
3.2.1. Fucoxanthin Reduced LPS-Induced NO Production in THP-1 Cells
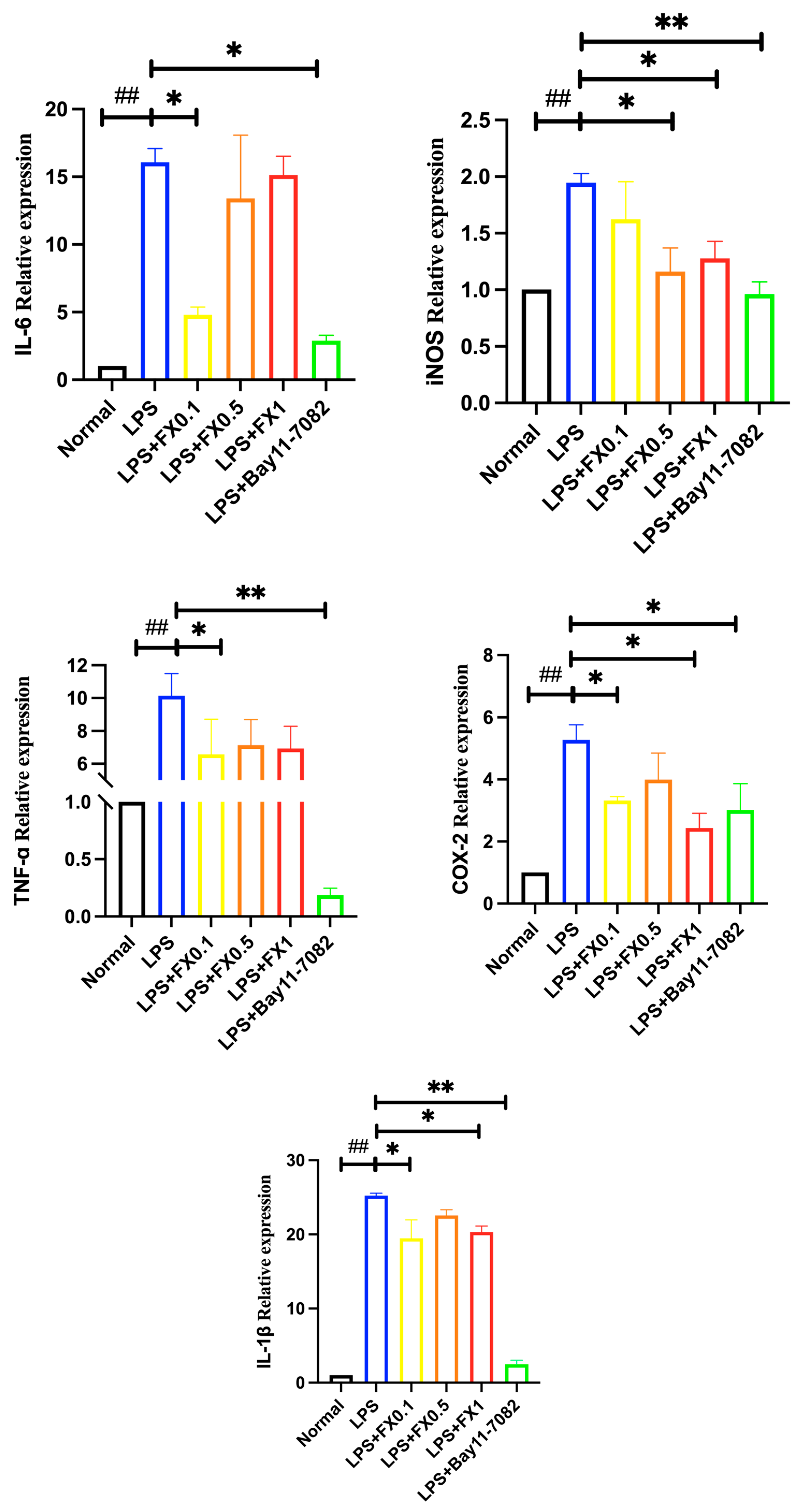
3.2.2. Fucoxanthin Decreased the Production of Inflammatory Factors Induced by LPS in THP-1 Cells
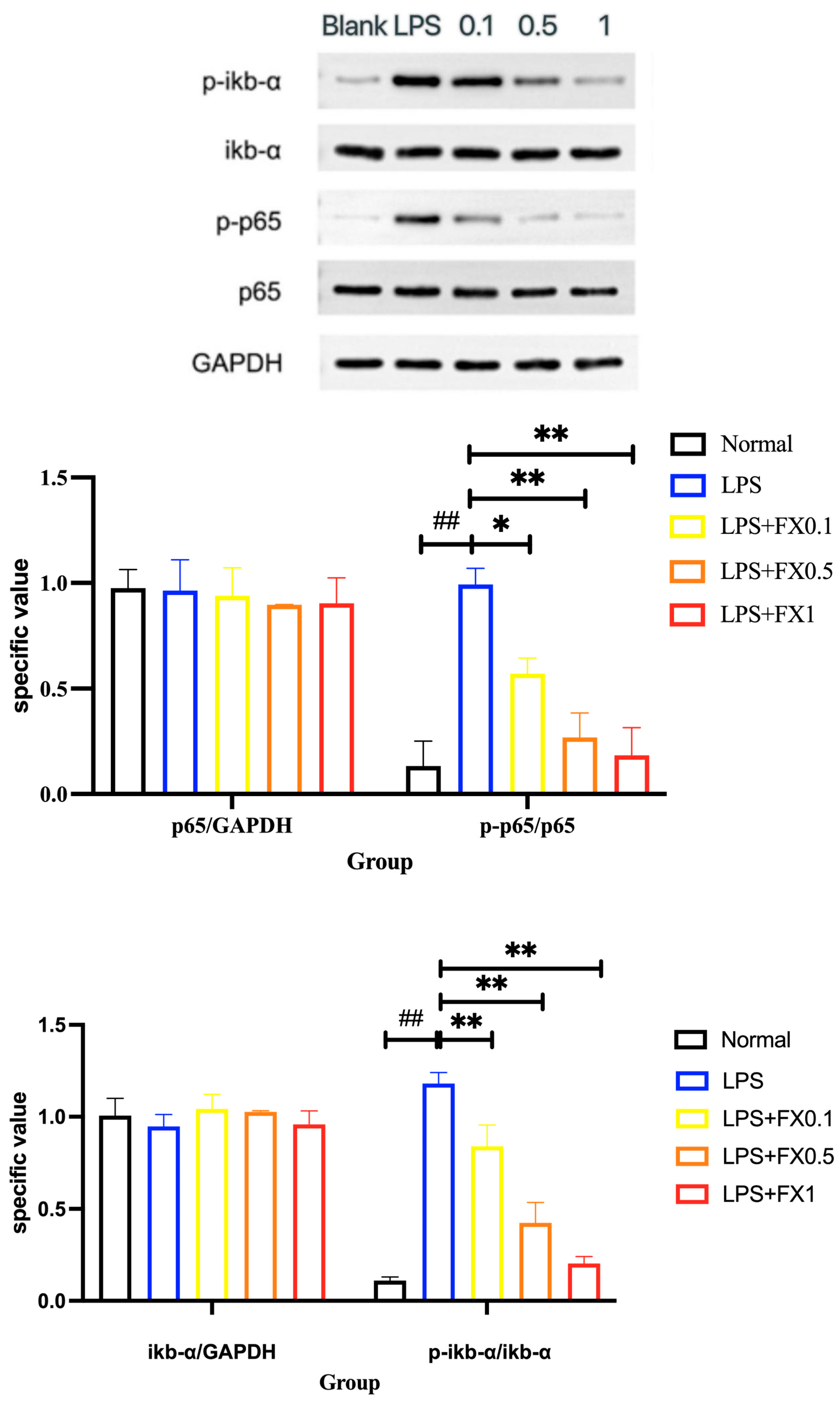
3.2.3. Regulation of Fucoxanthin on NF-κB Signaling Pathway and Its Upstream and Downstream Genes in THP-1 Cell Inflammatory Model
3.2.4. Inflammatory Pathway Protein Expression Was Inhibited by Fucoxanthin in THP-1 Cellular Inflammation Model
3.3. Protective Effect of Fucoxanthin on LPS-Induced Oxidative Reactions Human THP-1 Cells
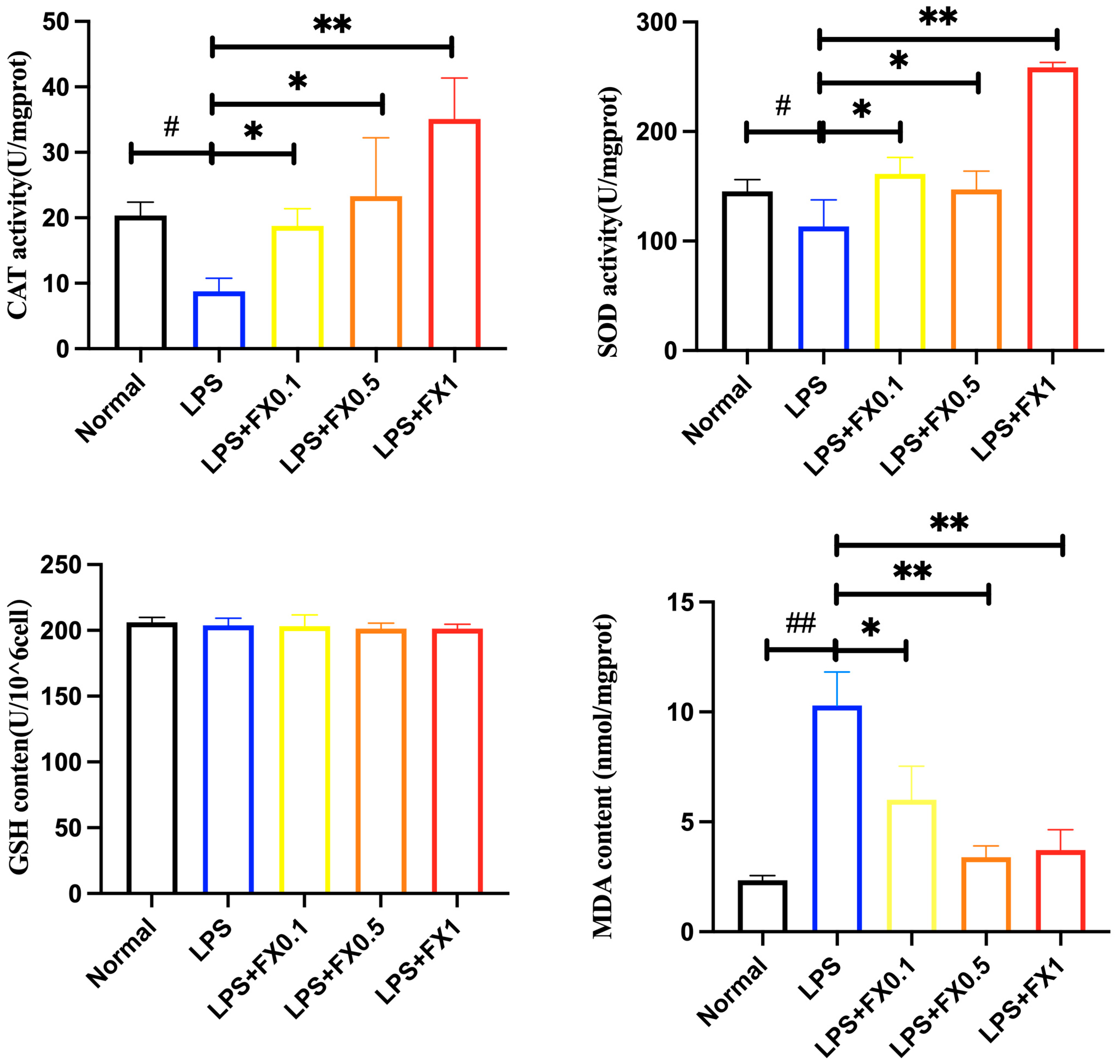
3.3.1. Effect of Fucoxanthin on Intracellular Antioxidant Enzyme Activity and GSH and MDA Production in THP-1 Cell Inflammatory Model
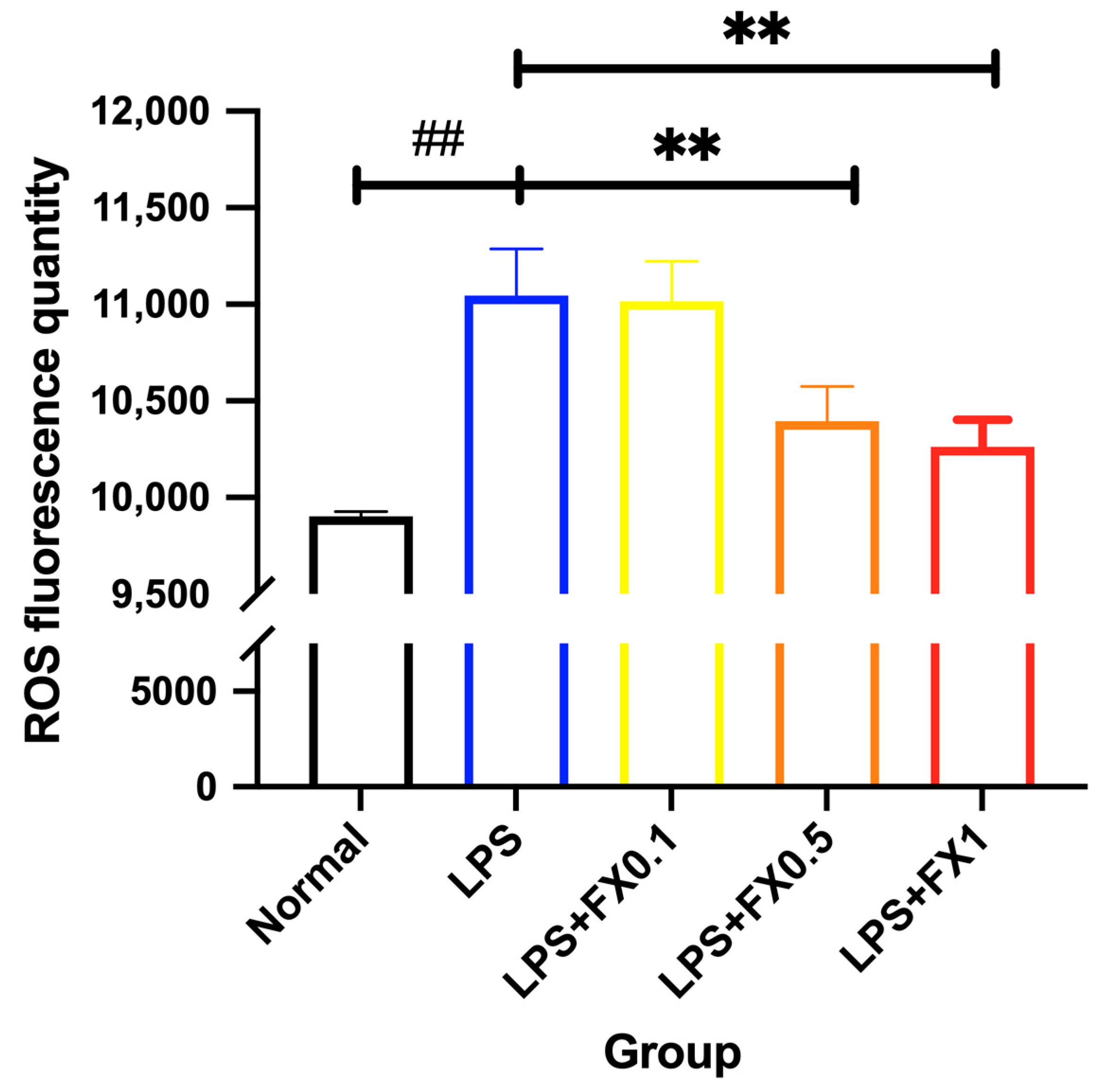
3.3.2. Inhibition of Intracellular ROS Generation by Fucoxanthin in THP-1 Cell Inflammation Model
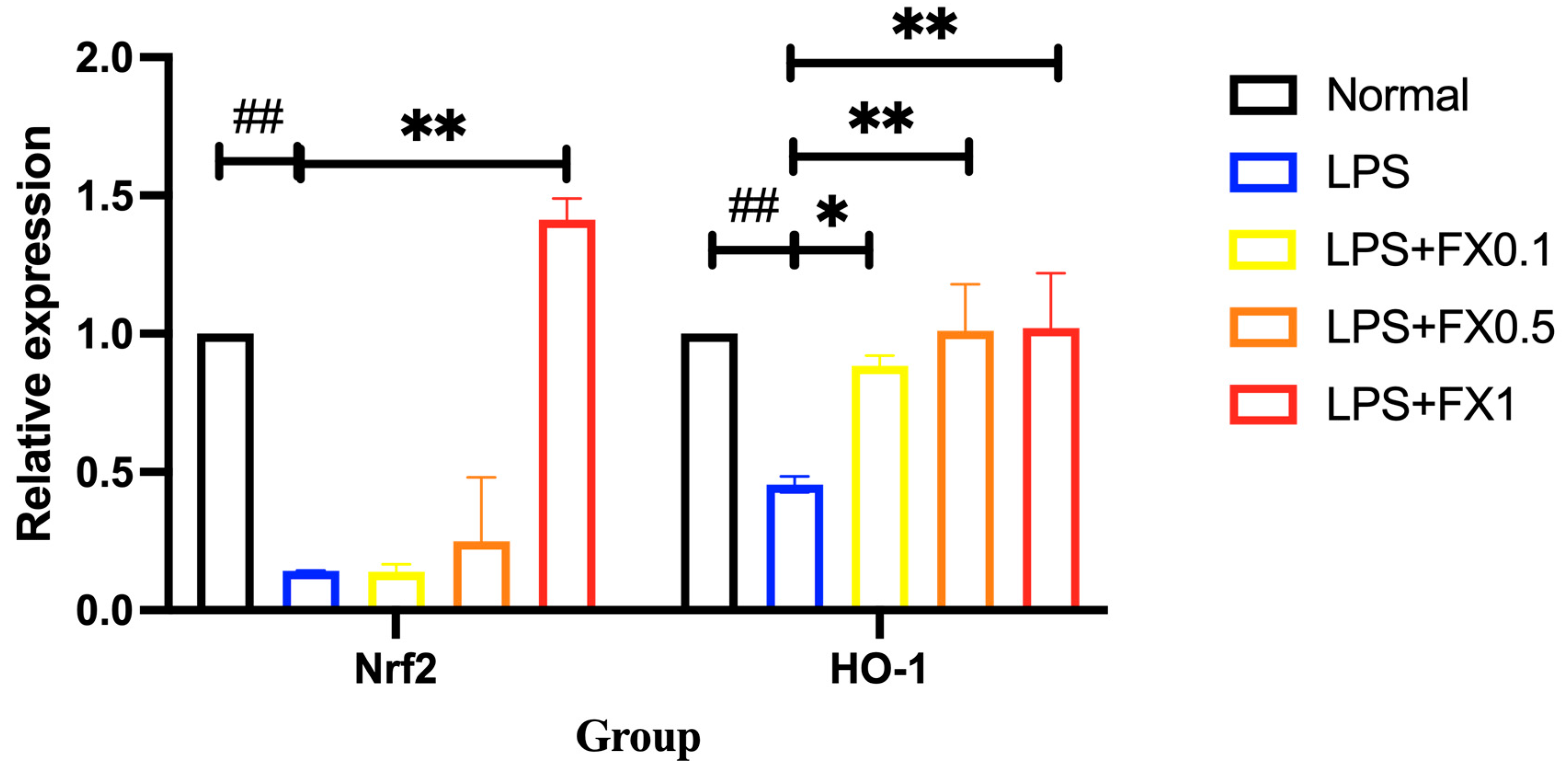
3.3.3. Fucoxanthin Activates the Nrf2 Signaling Pathway and Its Downstream Genes in a Model of THP-1 Cellular Inflammation
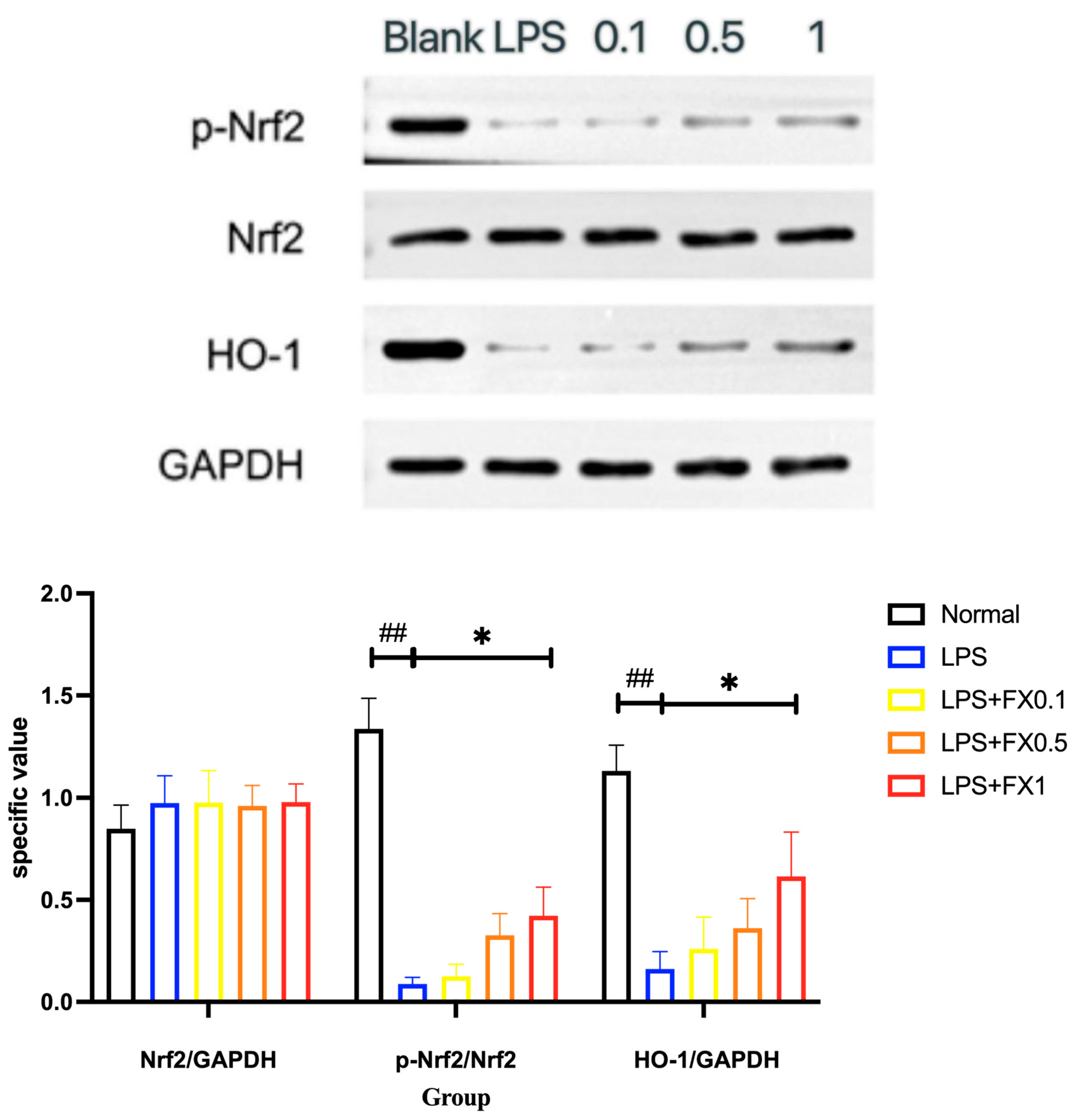
3.3.4. Fucoxanthin Inhibits the Expression of the Nrf2 Signaling Pathway and Its Downstream Proteins in a THP-1 Cellular Inflammation Model
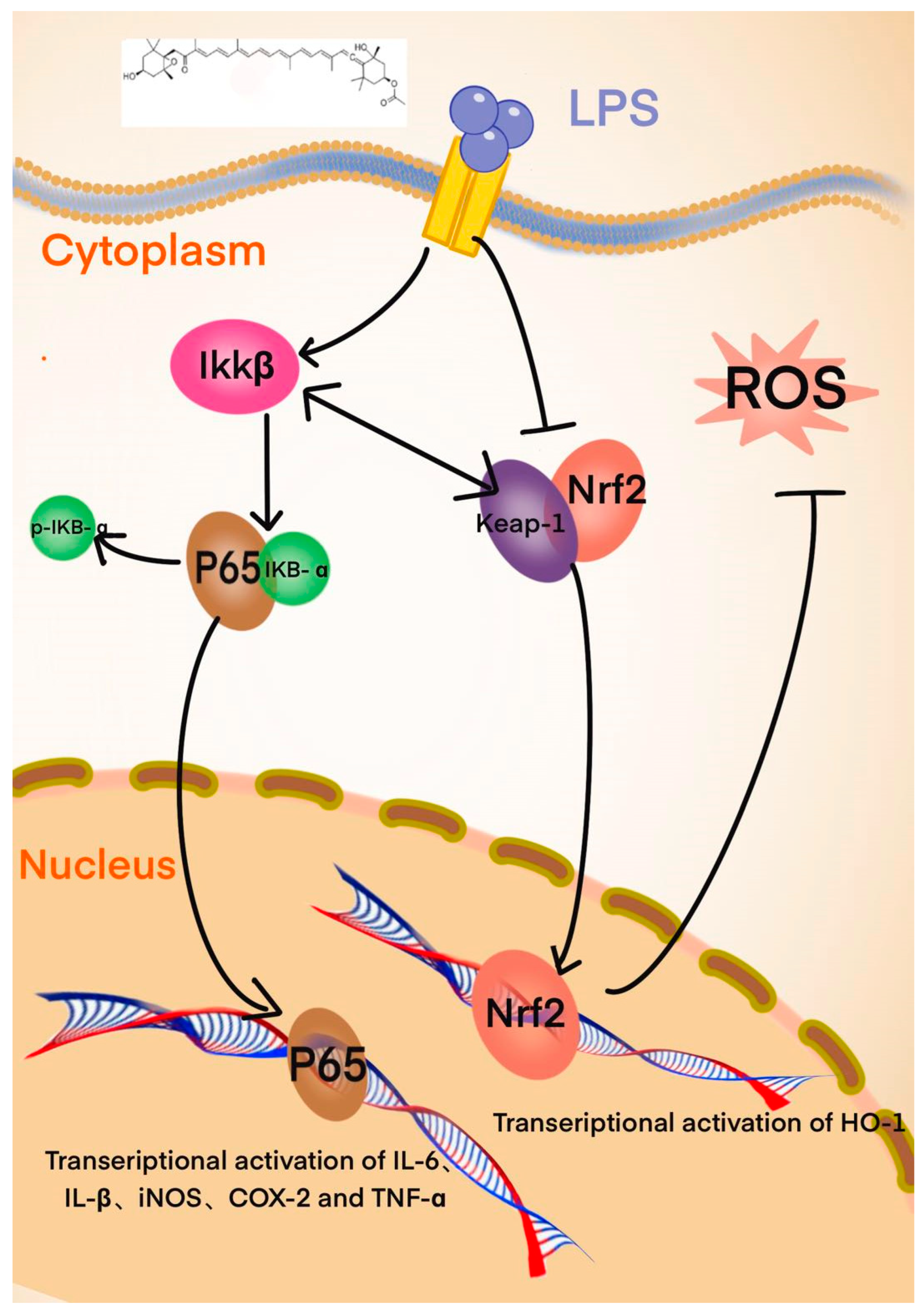
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhu, Y.Y.; Luan, Q.; Zen, F.Z.; Wang, X.N.; Yuan, Y.J.; Cai, L.J. Research progress on anti-inflammatory, anti-cancer activity and mechanism of fucoxanthin. Food Ind. Sci. Technol. 2024, 45, 341–350. [Google Scholar]
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Xu, L.; He, D. Research progress on therapeutic effect and mechanism of tripterygium wilfordii and its derivatives on rheumatoid arthritis. Shizhen Tradit. Chin. Med. 2022, 7, 33. [Google Scholar] [CrossRef]
- Chen, C.Y.; Peng, W.H.; Tsai, K.D.; Hsu, S.L. Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007, 81, 1602–1614. [Google Scholar] [CrossRef]
- Li, H. Anti-Inflammatory Activity and Anti-Inflammatory/Antioxidant Cotransport Mechanism of Flavonoids Based on the Regulation of NF-κB Signalling Pathway. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2019. [Google Scholar]
- Wei, X.; Hou, L. Research status of NF-κB-IKKβ pathway as drug development target. Mod. Med. Health 2018, 34, 4. [Google Scholar]
- Boma, F.; Eddie-Amadi, B.F.; Ezejiofor, A.N.; Orish, C.N.; Cirovic, A.; Cirovic, A.; Orisakwe, O.E. Banana peel ameliorated hepato-renal damage and exerted anti-inflammatory and anti-apoptotic effects in metal mixture mediated hepatic nephropathy by activation of Nrf2/Hmox-1 and inhibition of Nfkb pathway. SAGE Open Med. 2023, 11, 23978473231167422. [Google Scholar]
- Nomura, T.; Kikuchi, M.; Kubodera, A.; Kawaakami, Y. Proton-donative antioxidant activity of fucoxanthin with 1,1-Diphenyl-2-Picrylhydrazyl (DPPH). Biochem. Mol. Biol. Int. 2010, 42, 361–370. [Google Scholar]
- Zhang, W.Y.; Gao, B.Y.; Lei, X.Q.; Li, A.F.; Wu, J.; Zhang, C.W. Research progress on physicochemical and biological properties, preparation technology and its physiological activity of fucoxanthin. Chin. Mar. Drugs 2015, 34, 15. [Google Scholar]
- Xia, X.F.; Dong, S.B.; Li, Z.Y.; Dao, J.W.; Yang, Q. Study on the regulation of oxidative damage by polysaccharides based on Keap1-Nrf2/ARE signalling pathway. China Sci. Technol. Inf. 2021, 9, 2. [Google Scholar]
- Xiao, H.F.; Fu, J.J.; Wang, Y.L.; Li, F.Y.; Song, Y.D. Effect of chicoric acid on reactive oxygen species-induced oxidative damage to lipids and DNA. Food Sci. 2018, 39, 235–240. [Google Scholar]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef]
- Sui, J.X.; Wang, H.; Liu, T.Z. Research progress on the properties of diatom rock phycocyanin and its biosynthesis. Mar. Sci. 2019, 43, 130–138. [Google Scholar]
- Zeng, J.; Deng, C.H.; Fu, H.J.; Liu, C.; Gu, Y.; Zou, Y.X.; Chang, H. Activation of Nrf2/HO-1 pathway by theaflavins protects vascular endothelial cells from oxidative stress injury. Tea Sci. 2020, 40, 632–640. [Google Scholar]
- Wang, R.; Shen, L. Effects of shengcao phenol on Nrf2/HO-1 pathway in mice with experimental colitis. Chin. J. Immunol. 2020, 36, 928–932. [Google Scholar]
- Zhang, H.N.; Zhou, Y.F.; Liu, J.B.; Pang, Q.H. Exploring the hepatoprotective efficacy of quercetin and its mechanism of action based on NF-κB and Nrf2 signalling pathways. Northwest J. Agric. 2020, 29, 143–149. [Google Scholar]
- Gu, F.; Fu, X.; Sun, L.Q.; Wang, H.L.; Chen, Y.X.; Cai, Y.Z. Resveratrol attenuates inflammation and oxidative stress in coxsackievirus B3-infected cardiomyocytes of lactating mice through the Nrf2/ARE pathway. J. Virol. 2020, 36, 63–69. [Google Scholar]
- Chen, C.; Yin, Y.; Wu, X.F.; Wu, R.R.; Xu, Y.Y. Progress in activation of Nrf2 by reactive oxygen species through MAPKs and PI3K/AKT pathway. China Public Health 2016, 32, 870–873. [Google Scholar]
- Li, H.; Xu, Y.Y.; Gao, J.Y.; Yu, Y.Y.; Gong, A.D. Anti-inflammatory effect of dandelion root polysaccharide in vitro study. J. Anim. Med. 2019, 40, 5. [Google Scholar]
- Zhao, D.R.; Jiang, Y.S.; Sun, J.Y.; Li, H.H.; Sun, X.T.; Zhao, M.M. Amelioration of 4-methylguaiacol on LPS-induced inflammation in THP-1 cells through NF-κB/IκBα/AP-1 and Nrf2/HO-1 signaling pathway. J. Funct. Foods 2019, 55, 95–103. [Google Scholar] [CrossRef]
- Liu, Y.X.; Liu, J.L.; Guo, K.; Huang, M.; Huang, L.Q.; Peng, J.L.; Chen, Y.Q.; Su, J.Q. Inhibition of LPS-induced cellular inflammatory response by fucoidan through NF-κB signalling pathway. J. Fujian Norm. Univ. Nat. Sci. Ed. 2019, 2, 8. [Google Scholar]
- Yuan, B.; Mo, J. Research progress on the correlation between NF-κB/COX-2 signalling pathway and venous thrombosis. J. Gannan Med. Coll. 2018, 38, 295–300. [Google Scholar]
- Hu, Y.Y.L.; Mai, W.H.; Chen, L.H.; Cao, K.L.; Zhang, B.; Zhang, Z.L.; Liu, Y.J.; Lou, H.F.; Duan, S.M.; Gao, Z.H. mTOR-mediated metabolic reprogramming shapes distinct microglia functions in response to lipopolysaccharide and ATP. Glia 2020, 68, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wu, P.A.; Li, Y.; Li, Y.F.; Liu, D.L.; Liu, X.F. Research progress of small molecule activators in Keap1-Nrf2-ARE signaling pathway. Chin. Pharmacol. Bull. 2019, 35, 1342–1345. [Google Scholar]
- Pei, C.X.; Liu, J.L.; Jia, N.; He, Y.C.; Wang, Z.X.; Wang, F. Based on Nrf2/HO-1/GPX4 signals to explore the protective effect of Notoginseng saponin R1 on high altitude pulmonary edema induced by hypoxia. World Sci. Technol.—Mod. Tradit. Chin. Med. 2024, 26, 3209–3217. [Google Scholar]
- Cao, Q.; Wang, P.L.; Jiang, Y.Q.; Li, N.; Ni, Z. Effect of Puerarin on Lipopolysaccharide-Induced Inflammatory Response in Human Gingival Fibroblasts by Regulating Nrf2/HO-1 Signaling Pathway. Prog. Mod. Biomed. 2024, 24, 3422–3428. [Google Scholar]
- Tan, W.; Fu, X.Y.; Yang, R.Y.; Ma, L.; Ding, H.; Liu, X.D.; Zhang, W. Astragaloside regulates Nrf2/HO-1 signaling pathway on vascular endothelial cells Effects of oxidative damage. J. Hunan Univ. Chin. Med. 2024, 44, 1592–1600. [Google Scholar]
- Zhang, Q.Y.; Zhang, S.J.; Cui, C.C.; Dong, X.Y.; Du, X.C.; Bao, Y.Z.; Shi, W.Y. The Effects of Fuzi Lizhong Oral Liquid on Growth Performance and Renal Function in Chicks. Chin. J. Vet. Sci. 2025, 2. Available online: https://www.cnki.com.cn/Article/CJFDTotal-ZGSY202502017.htm (accessed on 22 April 2025).
- Wang, B.; Yuan, N.; Zhang, Y.H.; Zeng, L. Scutellarein Reduces LPS-induced Acute Lung Injury via Regulation of PI3K/Nrf2/HO-1 in Endotoxemic Rats. Zhongnan J. Med. Sci. 2016, 44, 409–413. [Google Scholar]
- Chiang, Y.F.; Chen, H.Y.; Chang, Y.J.; Shih, Y.H.; Shieh, T.M.; Wang, K.L.; Hsia, S.M. Protective effects of fucoxanthin on high glucose and 4-hydroxynonenal (4- HNE)-induced injury in human retinal pigment epithelial cells. Antioxidants 2020, 9, 1176. [Google Scholar] [CrossRef]
- Takatani, N.; Kono, Y.; Beppu, F.; Okamatsu-Ogura, Y.; Hosokawa, M. Fucoxanthin inhibits hepatic oxidative stress, inflammation, and fibrosis in diet-induced nonalcoholic steatohepatitis model mice. Biochem. Biophys. Res. Commun. 2020, 528, 305–310. [Google Scholar] [CrossRef]
- Sun, J.; Qin, E.; Tang, J.X.; Cao, M.Y.; Shen, J.X. Doxycycline regulates lipopolysaccharide-induced inflammatory factor levels in THP-1 cells through upregulation of autophagy. Chin. J. Pathophysiol. 2019, 35, 1489–1494. [Google Scholar]
- Liu, Y.; Zhi, L.C.; Wang, H.R.; Zhao, L.; Ren, D.D.; He, Y.H.; Wang, Q.K. Inhibition of fucoxanthin from copper algae on α-glucosidase activity and its hypoglycemic effect. J. Dalian Ocean Univ. 2023, 38, 120–128. [Google Scholar]
- Hang, H.; Sun, Y. Study on the mechanism of TNF-α expression in glial cells induced by Aβ_(1-42) oligomer through PI3K-AKT signaling pathway. Res. Dev. Nat. Prod. 2018, 30, 385–389+468. [Google Scholar]
- Huang, F.L.; Yang, C.H.; Zhu, C.C.; Zhang, S.F. To investigate the intervention mechanismof Jiawei Shengjiangsan on renal injury in Diabetic kidney diseasemicebased on PI3K/Akt/NF-κB signaling pathway. Chin. J. Tradit. Chin. Med. 2024, 52, 20–26. [Google Scholar]
- Lin, H.W.; Liu, C.W.; Yang, D.J.; Chen, C.C.; Chen, S.Y.; Tseng, J.K.; Chang, T.J.; Chang, Y.Y. Dunaliella salina alga extract inhibits the production of interleukin-6, nitric oxide, and reactive oxygen species by regulating nuclear factor-κB/Janus kinase/signal transducer and activator of transcription in virus-infected RAW264.7cells. J. Food Drug Anal. 2017, 25, 908–918. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.E.; Chung, D.K. Manganese superoxide dismutase induced by lipoteichoic acid isolated from Staphylococcus aureus regulates cytokine production in THP-1 cells. J. Microbiol. Immunol. Infect. 2022, 55, 36–43. [Google Scholar] [CrossRef]
- Rubio, V.; García-Pérez, A.I.; Herráez, A.; Diez, J.C. Different roles of Nrf2 and NFKB in the antioxidant imbalance produced by esculetin or quercetin on NB4 leukemia cells. Chem. Biol. Interact. 2018, 294, 158–166. [Google Scholar] [CrossRef]
- Grossmann, M.; Metcalf, D.; Merryfull, J.; Beg, A.; Baltimore, D.; Gerondakis, S. The combined absence of the transcription factors Rel and RelA leads to multiple hemopoietic cell defects. Proc. Natl. Acad. Sci. USA 1999, 96, 11848–11853. [Google Scholar] [CrossRef]
- Ramyaa, P.; Krishnaswamy, R.; Padma, V.V. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells—Up regulation of Nrf2 expression and down regulation of NF-κB and COX-2. Biochim. Biophys. Acta 2014, 1840, 681–692. [Google Scholar] [CrossRef]
- Frelin, C.; Imbert, V.; Griessinger, E.; Peyron, A.C.; Rochet, N.; Philip, P.; Dageville, C.; Srvent, A.; Hummelsberger, M.; Bérard, E. Targeting NF-κB activation via pharmacologic inhibition of IKK2-induced apoptosis of human acute myeloid leukemia cells. Blood 2005, 105, 804–811. [Google Scholar] [CrossRef]
- Cuadrado, A.; Moreno-Murciano, P.; Pedraza-Chaverri, J. The transcription factor Nrf2 as a new therapeutic target in Parkinson’s disease. Expert Opin. Ther. Targets 2009, 13, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.C. The Nrf2-ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef]
- Tang, Q.; Feng, Z.H.; Tong, M.J.; Xu, J.X.; Zheng, G.; Shen, L.Y.; Shang, P.; Zhang, Y.; Liu, H.X. Piceatannol inhibits the IL-1β-induced inflammatory response in human osteoarthritic chondrocytes and ameliorates osteoarthritis in mice by activating Nrf2. Food Funct. 2017, 8, 3926–3937. [Google Scholar] [CrossRef]
- Keutgens, A.; Robert, I.; Viatour, P.; Chariot, A. Deregulated NF-kappaB activity in haematological malignancies. Biochem. Pharmacol. 2006, 72, 1069–1080. [Google Scholar] [CrossRef]
- Fredriksson, L.; Herpers, B.; Benedetti, G.; Matadin, Q.; Puigvert, J.C.; Bont, H.D.; Dragovic, S.; Vermeulen, N.P.E.; Commandeur, J.N.M.; Danen, E. Diclofenac inhibits tumor necrosis factor-α-induced nuclear factor-κB activation causing synergistic hepatocyte apoptosis. Hepatology 2011, 53, 2027–2041. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, L.; Wink, S.; Herpers, B.; Benedetti, G.; Hadi, M.; de Bont, H.; Groothuis, G.; Luijten, M.; Danen, E.; de Graauw, M.; et al. Drug-induced endoplasmic reticulum and oxidative stress responses independently sensitize toward TNFα-mediated hepatotoxicity. Toxicol. Sci. 2014, 140, 144–159. [Google Scholar] [CrossRef]
- Herpers, B.; Wink, S.; Fredriksson, L.; Di, Z.; Hendriks, G.; Vrieling, H.; De Bont, H.; Bob, V.D.W. Activation of the Nrf2 response by intrinsic hepatotoxic drugs correlates with suppression of NF-κB activation and sensitizes toward TNFα-induced cytotoxicity. Arch. Toxicol. 2016, 90, 1163–1179. [Google Scholar] [CrossRef]
- Rushworth, S.A.; Zaitseva, L.; Murray, M.Y.; Shah, N.M.; Bowles, K.M.; MacEwan, D.J. The high Nrf2 expression in human acute myeloid leukemia is driven by NF-κB and underlies its chemo-resistance. Blood 2012, 120, 5188–5198. [Google Scholar] [CrossRef] [PubMed]
- Asha, A.; Ila, D.; Des, C.; Tapas, S. Redox Regulation in Cancer: A Double-edged Sword with Therapeutic Potential. Oxid. Med. Cell. Longev. 2010, 3, 23–34. [Google Scholar]
- Lee, W.J.; Hsiao, M.; Chang, J.L.; Yang, S.F.; Tseng, T.H.; Cheng, C.W.; Chow, J.M.; Lin, K.H.; Lin, Y.W.; Liu, C.C.; et al. Quercetin induces mitochondrial-derived apoptosis via reactive oxygen species-mediated ERK activation in HL-60 leukemia cells and xenograft. Arch. Toxicol. 2015, 89, 1103–1117. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Lee, H.; Rangasamy, T.; Reddy, S.P.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Investig. 2006, 116, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Lee, H.K.; Shin, J.H.; Lee, J.K. Up-down regulation of HO-1 and iNOS gene expressions by ethyl pyruvate via recruiting p300 to Nrf2 and depriving it from p65. Free. Radic. Biol. Med. 2013, 65, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.F.; Kuo, H.P.; Liu, M.; Chou, C.K.; Hung, M.C. Keap1 e3 ligase-mediated downregulation of nf-kappab signaling by targeting ikkbeta. Mol. Cell 2009, 36, 131–140. [Google Scholar] [CrossRef]
- Braak, B.T.; Wink, S.; Hiemstra, S.; Middleton, A.; White, A.; van de Water, B. Mapping the dynamics of Nrf2 antioxidant and NF-κB inflammatory responses by soft electrophilic chemicals in human liver cells defines the transition from adaptive to adverse responses. Toxicol. In Vitro 2022, 84, 105419. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Sequence Content (5′ to 3′) |
|---|---|
| GAPDH-F | GCACCGTCAAGGCTGAGAAC |
| GAPDH-R | TGGTGAAGACGCCAGTGGA |
| IL-6-F | AAGCCAGAGCTGTGCAGATGAGTA |
| IL-6-R | TGTCCTGCAGCCACTGGTTC |
| TNF-α-F | TAAGAGGGAGAGAAGCAACTA |
| TNF-α-R | TCAGTATGTGAGAGGAAGAGA |
| COX-2-F | CCAGCACTTCACGCATCAG |
| COX-2-R | GCTGTCTAGCCAGAGTTTCACC |
| iNOS-F | CAGCAAGCAGCAGAATGAGTCC |
| iNOS-R | TGCATCCAGCTTGACCAGAGA |
| IL-1β-F | CCAGGGACAGGATATGGAGCA |
| IL-1β-R | TTCAACACGCAGGACAGGTACAG |
| iκb-α-F | CACTCCATCCTGAAGGCTACCA |
| iκb-α-R | AAGGGCAGTCCGGCCATTA |
| NF-kB p65-F | GACGCATTGCTGTGCCTTC |
| NF-kB p65-R | TTGATGGTGCTCAGGGATGAC |
| Nrf2-F | TGGGCCCATTGATGTTTCTG |
| Nrf2-R | TGCCACACTGGGACTTGTGTTTA |
| HO-1-F | TCTCTCTGGAAAGGAGGAAGGA |
| HO-1-R | AGGAACTGAGGATGCTGAAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, T.; Liu, J.; Sun, J.; Niu, J.; Ren, D.; Ma, Y.; He, Y.; Liu, S.; Wang, Q. The Regulation of the NF-κB p65 and Nrf2/HO-1 Signaling Pathways by Fucoxanthin in Human THP-1 Monocyte Macrophages Under a Lipopolysaccharide-Induced Inflammation Model. Foods 2025, 14, 1746. https://doi.org/10.3390/foods14101746
Zhang L, Li T, Liu J, Sun J, Niu J, Ren D, Ma Y, He Y, Liu S, Wang Q. The Regulation of the NF-κB p65 and Nrf2/HO-1 Signaling Pathways by Fucoxanthin in Human THP-1 Monocyte Macrophages Under a Lipopolysaccharide-Induced Inflammation Model. Foods. 2025; 14(10):1746. https://doi.org/10.3390/foods14101746
Chicago/Turabian StyleZhang, Linyi, Tong Li, Jingyi Liu, Jiyan Sun, Jinkun Niu, Dandan Ren, Yichao Ma, Yunhai He, Shu Liu, and Qiukuan Wang. 2025. "The Regulation of the NF-κB p65 and Nrf2/HO-1 Signaling Pathways by Fucoxanthin in Human THP-1 Monocyte Macrophages Under a Lipopolysaccharide-Induced Inflammation Model" Foods 14, no. 10: 1746. https://doi.org/10.3390/foods14101746
APA StyleZhang, L., Li, T., Liu, J., Sun, J., Niu, J., Ren, D., Ma, Y., He, Y., Liu, S., & Wang, Q. (2025). The Regulation of the NF-κB p65 and Nrf2/HO-1 Signaling Pathways by Fucoxanthin in Human THP-1 Monocyte Macrophages Under a Lipopolysaccharide-Induced Inflammation Model. Foods, 14(10), 1746. https://doi.org/10.3390/foods14101746





