Nutritional and Chemical Characterization of Red and Purple Potatoes Peels: A Polyphenol-Rich By-Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Samples
2.3. Macronutrients Determination
2.3.1. Total Carbohydrate Determination
2.3.2. Determination of Total Protein
2.3.3. Total Lipids Determination
2.4. Micronutrients Determination
2.5. Polyphenols Extraction
2.6. HPLC Polyphenols Analysis
2.7. Determination of Total Polyphenols
2.8. DPPH Radical-Scavenging Assay
2.9. FRAP: Ferric-Reducing Antioxidant Power
2.10. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Composition
3.1.1. Macronutrients
3.1.2. Mineral Content
3.2. Functional Composition
3.2.1. Total Polyphenol Content and Antioxidant Potential
3.2.2. Quantitative Analysis of Polyphenols in Red and Purple Potatoes Peel
3.3. PCA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Valorisation of food agro-industrial by-products: From the past to the present and perspectives. J. Environ. Manag. 2021, 299, 113571. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.; Hoang, S.A.; Bradney, L.; Dutta, S.; Xiong, X.; Tsang, D.C.; Ramadass, K.; Vinu, A.; Kirkham, M.; Bolan, N.S. A review on the valorisation of food waste as a nutrient source and soil amendment. Environ. Pollut. 2021, 272, 115985. [Google Scholar] [CrossRef] [PubMed]
- Gebrechristos, H.Y.; Chen, W. Utilization of potato peel as eco-friendly products: A review. Food Sci. Nutr. 2018, 6, 1352–1356. [Google Scholar] [CrossRef]
- Djaman, K.; Koudahe, K.; Koubodana, H.D.; Saibou, A.; Essah, S. Tillage Practices in Potato (Solanum tuberosum L.) Production: A Review. Am. J. Potato Res. 2022, 99, 1–19. [Google Scholar] [CrossRef]
- Navarre, D.A.; Brown, C.R.; Sathuvalli, V.R. Potato Vitamins, Minerals and Phytonutrients from a Plant Biology Perspective. Am. J. Potato Res. 2019, 96, 111–126. [Google Scholar] [CrossRef]
- Joshi, A.; Sethi, S.; Arora, B.; Azizi, A.F.; Thippeswamy, B. Potato Peel Composition and Utilization. Potato Nutr. Food Secur. 2020, 229–245. [Google Scholar] [CrossRef]
- Liang, S.; Mcdonald, A.G. Chemical and Thermal Characterization of Potato Peel Waste and Its Fermentation Residue as Potential Resources for Biofuel and Bioproducts Production. J. Agric. Food Chem. 2014, 62, 8421–8429. [Google Scholar] [CrossRef]
- Rai, D.; Aziz, A.; Mccue, K.; Rockhold, D.; Belknap, W. Ultrasonic extraction of steroidal alkaloids from potato peel waste. Ultrason. Sonochem. 2014, 21, 1470–1476. [Google Scholar]
- Gaudino, E.C.; Colletti, A.; Grillo, G.; Tabasso, S.; Cravotto, G. Emerging processing technologies for the recovery of valuable bioactive compounds from potato peels. Foods 2020, 9, 1598. [Google Scholar] [CrossRef]
- Iriondo-Dehond, M.; Miguel, E.; Del Castillo, M.D. Food byproducts as sustainable ingredients for innovative and healthy dairy foods. Nutrients 2018, 10, 1358. [Google Scholar] [CrossRef]
- Trigo, J.P.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M.E. High value-added compounds from fruit and vegetable by-products–Characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 1388–1416. [Google Scholar] [CrossRef]
- Joly, N.; Souidi, K.; Depraetere, D.; Wils, D.; Martin, P. Potato By-Products as a Source of Natural Chlorogenic Acids and Phenolic Compounds: Extraction, Characterization, and Antioxidant Capacity. Molecules 2021, 26, 177. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Eichhorn, S.; Winterhalter, P. Anthocyanins from pigmented potato (Solanum tuberosum L.) varieties. Food Res. Int. 2005, 38, 943–948. [Google Scholar] [CrossRef]
- Oertel, A.; Matros, A.; Hartmann, A.; Arapitsas, P.; Dehmer, K.J.; Martens, S.; Mock, H.-P. Metabolite profiling of red and blue potatoes revealed cultivar and tissue specific patterns for anthocyanins and other polyphenols. Planta 2017, 246, 281–297. [Google Scholar] [CrossRef]
- Jansen, G.; Flamme, W. Coloured potatoes (Solanum tuberosum L.)—Anthocyanin content and tuber quality. Genet. Resour. Crop Evol. 2006, 53, 1321–1331. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Ž.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The Therapeutic Potential of Anthocyanins: Current Approaches Based on Their Molecular Mechanism of Action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Chen, Y.; Vaidyanathan, S. Simultaneous assay of pigments, carbohydrates, proteins and lipids in microalgae. Anal. Chim. Acta 2013, 776, 31–40. [Google Scholar] [CrossRef]
- Bligh, W.J.; Dyer, E.G. Canadian Journal of Biochemistry and Physiology. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Mishra, S.K.; Suh, W.I.; Farooq, W.; Moon, M.; Shrivastav, A.; Park, M.S.; Yang, J.-W. Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour. Technol. 2014, 155, 330–333. [Google Scholar] [CrossRef]
- D’amelia, V.; Sarais, G.; Fais, G.; Dessì, D.; Giannini, V.; Garramone, R.; Carputo, D.; Melito, S. Biochemical Characterization and Effects of Cooking Methods on Main Phytochemicals of Red and Purple Potato Tubers, a Natural Functional Food. Foods 2022, 11, 384. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Axelrod, D.; Koppel, D.E.; Schlessinger, J.; Elson, E.; Webb, W.W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 1976, 16, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer Acceptance toward Functional Foods: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 1217. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Kaur, S.; Aggarwal, P. Techno and bio functional characterization of industrial potato waste for formulation of phytonutrients rich snack product. Food Biosci. 2022, 49, 101824. [Google Scholar] [CrossRef]
- Martinez-Fernandez, J.S.; Seker, A.; Davaritouchaee, M.; Gu, X.; Chen, S. Recovering Valuable Bioactive Compounds from Potato Peels with Sequential Hydrothermal Extraction. Waste Biomass Valorization 2021, 12, 1465–1481. [Google Scholar] [CrossRef]
- Cozma, A.; Velciov, A.; Popescu, S.; Mihut, C.; Duma Copcea, A.; Lato, A.; Chis, C.; Rada, M. Determination of some nutritional parameters of potato peel-preliminary research. Res. J. Agric. Sci 2024, 56, 28–34. [Google Scholar]
- Sampaio, S.L.; Petropoulos, S.A.; Alexopoulos, A.; Heleno, S.A.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C. Potato peels as sources of functional compounds for the food industry: A review. Trends Food Sci. Technol. 2020, 103, 118–129. [Google Scholar] [CrossRef]
- Kårlund, A.; Gómez-Gallego, C.; Turpeinen, A.M.; Palo-oja, O.-M.; El-Nezami, H.; Kolehmainen, M. Protein Supplements and Their Relation with Nutrition, Microbiota Composition and Health: Is More Protein Always Better for Sportspeople? Nutrients 2019, 11, 829. [Google Scholar] [CrossRef]
- Waglay, A.; Karboune, S.; Alli, I. Potato protein isolates: Recovery and characterization of their properties. Food Chem. 2014, 142, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Pȩksa, A.; Kita, A.; Kulakowska, K.; Aniolowska, M.; Hamouz, K.; Nemś, A. The quality of protein of coloured fleshed potatoes. Food Chem. 2013, 141, 2960–2966. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Pinckaers, P.J.M.; Hendriks, F.K.; Hermans, W.J.; Goessens, J.P.; Senden, J.M.; VAN Kranenburg, J.M.X.; Wodzig, W.K.H.W.; Snijders, T.; VAN Loon, L.J.C. Potato Protein Ingestion Increases Muscle Protein Synthesis Rates at Rest and during Recovery from Exercise in Humans. Med. Sci. Sports Exerc. 2022, 54, 1572–1581. [Google Scholar] [CrossRef]
- Bellumori, M.; Silva, N.A.C.; Vilca, L.; Andrenelli, L.; Cecchi, L.; Innocenti, M.; Balli, D.; Mulinacci, N. A study on the biodiversity of pigmented andean potatoes: Nutritional profile and phenolic composition. Molecules 2020, 25, 3169. [Google Scholar] [CrossRef]
- Vaitkevičienė, N. A comparative study on proximate and mineral composition of coloured potato peel and flesh. J. Sci. Food Agric. 2019, 99, 6227–6233. [Google Scholar] [CrossRef]
- DeMartino, P.; Cockburn, D.W. Resistant starch: Impact on the gut microbiome and health. Curr. Opin. Biotechnol. 2020, 61, 66–71. [Google Scholar] [CrossRef]
- Jardon, K.M.; Canfora, E.E.; Goossens, G.H.; Blaak, E.E. Dietary macronutrients and the gut microbiome: A precision nutrition approach to improve cardiometabolic health. Gut 2022, 71, 1214–1226. [Google Scholar] [CrossRef]
- Streppel, M.T.; Ocké, M.C.; Boshuizen, H.C.; Kok, F.J.; Kromhout, D. Dietary fiber intake in relation to coronary heart disease and all-cause mortality over 40 y: The Zutphen Study. Am. J. Clin. Nutr. 2008, 88, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Han, S.; Zhang, Z.; Zhang, W.; Yang, J.; Wan, Z.; Qin, L. Cereal Fiber Ameliorates High-Fat/Cholesterol-Diet-Induced Atherosclerosis by Modulating the NLRP3 Inflammasome Pathway in ApoE-/- Mice. J. Agric. Food Chem. 2018, 66, 4827–4834. [Google Scholar] [CrossRef]
- Yu, E.Y.W.; Wesselius, A.; Mehrkanoon, S.; Brinkman, M.; Brandt, P.v.D.; White, E.; Weiderpass, E.; Le Calvez-Kelm, F.; Gunter, M.; Huybrechts, I.; et al. Grain and dietary fiber intake and bladder cancer risk: A pooled analysis of prospective cohort studies. Am. J. Clin. Nutr. 2020, 112, 1252–1266. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A. Role of phytochemicals in the management of metabolic syndrome. Phytomedicine 2016, 23, 1134–1144. [Google Scholar] [CrossRef]
- Sanjoaquin, M.A.; Appleby, P.N.; Spencer, E.A.; Key, T.J. Nutrition and lifestyle in relation to bowel movement frequency: A cross-sectional study of 20 630 men and women in EPIC–Oxford. Public Health Nutr. 2004, 7, 77–83. [Google Scholar] [CrossRef]
- Crowe, F.L.; Balkwill, A.; Cairns, B.J.; Appleby, P.N.; Green, J.; Reeves, G.K.; Key, T.J.; Beral, V. Source of dietary fibre and diverticular disease incidence: A prospective study of UK women. Gut 2014, 63, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Post, R.E.; Mainous, A.G.; King, D.E.; Simpson, K.N. Dietary fiber for the treatment of type 2 diabetes mellitus: A meta-analysis. J. Am. Board Fam. Med. 2012, 25, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Jeddou, K.B.; Bouaziz, F.; Zouari-Ellouzi, S.; Chaari, F.; Ellouz-Chaabouni, S.; Ellouz-Ghorbel, R.; Nouri-Ellouz, O. Improvement of texture and sensory properties of cakes by addition of potato peel powder with high level of dietary fiber and protein. Food Chem. 2017, 217, 668–677. [Google Scholar] [CrossRef]
- Sharoba, A.; Farrag, M.; Abd, E.A.M. Utilization of some fruits and vegetables waste as a source of dietary fiber and its effect on the cake making and its quality attributes. J. Agroaliment. 2013, 19, 429–444. [Google Scholar]
- Wszelaki, A.L.; Delwiche, J.F.; Walker, S.D.; Liggett, R.E.; Scheerens, J.C.; Kleinhenz, M.D. Sensory quality and mineral and glycoalkaloid concentrations in organically and conventionally grown redskin potatoes (Solanum tuberosum). J. Sci. Food Agric. 2005, 85, 720–726. [Google Scholar] [CrossRef]
- Weaver, C.M. Potassium and health. Adv. Nutr. 2013, 4, 368S–377S. [Google Scholar] [CrossRef] [PubMed]
- Jing, T.; Li, J.; He, Y.; Shankar, A.; Saxena, A.; Tiwari, A.; Maturi, K.C.; Solanki, M.K.; Singh, V.; Eissa, M.A.; et al. Role of calcium nutrition in plant Physiology: Advances in research and insights into acidic soil conditions—A comprehensive review. Plant Physiol. Biochem. 2024, 210, 108602. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Yang, X.; Liu, Z.; Liu, K.; Huang, Y. Spatial distribution of soil nutrients and evaluation of cultivated land in Xuwen county. PeerJ 2022, 10, 1–19. [Google Scholar] [CrossRef]
- Nunes, J.C.S.; Fontes, P.C.R.; Araújo, E.F.; Sediyama, C. Potato plant growth and macronutrient uptake as affected by soil tillage and irrigation systems. Pesqui. Agropecu. Bras. 2006, 41, 1787–1792. [Google Scholar] [CrossRef]
- Razzaque, M.S. Magnesium: Are we consuming enough? Nutrients 2018, 10, 1863. [Google Scholar] [CrossRef]
- Andre, C.M.; Ghislain, M.; Bertin, P.; Oufir, M.; Herrera, M.D.R.; Hoffmann, L.; Hausman, J.-F.; Larondelle, A.Y.; Evers, D. Andean potato cultivars (Solarium tuberosum L.) as a source of antioxidant and mineral micronutrients. J. Agric. Food Chem. 2007, 55, 366–378. [Google Scholar] [CrossRef]
- Martins, A.C.; Oliveira-Paula, G.H.; Tinkov, A.A.; Skalny, A.V.; Tizabi, Y.; Bowman, A.B.; Aschner, M. Role of manganese in brain health and disease: Focus on oxidative stress. Free Radic. Biol. Med. 2025, 232, 306–318. [Google Scholar] [CrossRef]
- Panel, E.; Nda, A. Scientific Opinion on Dietary Reference Values for manganese. EFSA J. 2013, 11, 3419. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Fu, W.; Kennett, M.; Cox, A.D.; Lee, D.; Vanamala, J.K.P.; Reddivari, L. Role of Gut Microbiota in the Anti-Colitic Effects of Anthocyanin-Containing Potatoes. Mol. Nutr. Food Res. 2021, 65, 2100152. [Google Scholar] [CrossRef]
- Strugała, P.; Urbaniak, A.; Kuryś, P.; Włoch, A.; Kral, T.; Ugorski, M.; Hof, M.; Gabrielska, J. Antitumor and antioxidant activities of purple potato ethanolic extract and its interaction with liposomes, albumin and plasmid DNA. Food Funct. 2021, 12, 1271–1290. [Google Scholar] [CrossRef]
- Charepalli, V.; Reddivari, L.; Radhakrishnan, S.; Vadde, R.; Agarwal, R.; Vanamala, J.K.P. Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells. J. Nutr. Biochem. 2015, 26, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Ombra, M.N.; Fratianni, F.; Granese, T.; Cardinale, F.; Cozzolino, A.; Nazzaro, F. In vitro antioxidant, antimicrobial and anti-proliferative activities of purple potato extracts (Solanum tuberosum cv Vitelotte noire) following simulated gastro-intestinal digestion. Nat. Prod. Res. 2015, 29, 1087–1091. [Google Scholar] [CrossRef]
- Han, K.H.; Sekikawa, M.; Shimada, K.-I.; Hashimoto, M.; Hashimoto, N.; Noda, T.; Tanaka, H.; Fukushima, M. Anthocyanin-rich purple potato flake extract has antioxidant capacity and improves antioxidant potential in rats. Br. J. Nutr. 2006, 96, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, S.L.; Petropoulos, S.A.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Fernandes, Â.; Leme, C.M.; Alexopoulos, A.; Santos-Buelga, C.; Ferreira, I.C.; et al. Phenolic composition and cell-based biological activities of ten coloured potato peels (Solanum tuberosum L.). Food Chem. 2021, 363, 130360. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Huang, V.; Quiambao, Q.; Noritake, S.; Liu, J.; Kwon, O.; Chintalapati, S.; Young, J.; Levin, C.E.; Tam, C.; et al. Potato Peels and Their Bioactive Glycoalkaloids and Phenolic Compounds Inhibit the Growth of Pathogenic Trichomonads. J. Agric. Food Chem. 2018, 66, 7942–7947. [Google Scholar] [CrossRef]
- Akomolafe, S.F.; Ajayi, O.O.; Agboola, O.E.; Adewale, O.O. Comparative evaluation of the antidiabetic potential of three varieties of Ipomoea batatas L. Toxicol. Rep. 2025, 14, 102015. [Google Scholar] [CrossRef]
- Saenjum, C.; Thim-Uam, A.; Khonthun, C.; Oonlao, P.; Nuntaboon, P.; Surh, Y.-J.; Phromnoi, K. Anthocyanins from a new hybrid sweet potato peel cultivated in Northern Thailand mitigate LPS-induced inflammation and RANKL-induced osteoporosis by regulating ROS-mediated pathways. Inflammopharmacology 2025, 33, 381–399. [Google Scholar] [CrossRef]
- de Albuquerque, T.M.R.; Magnani, M.; Lima, M.D.S.; Castellano, L.R.C.; de Souza, E.L. Effects of digested flours from four different sweet potato (Ipomoea batatas L.) root varieties on the composition and metabolic activity of human colonic microbiota in vitro. J. Food Sci. 2021, 86, 3707–3719. [Google Scholar] [CrossRef]
- Yin, L.; Chen, T.; Li, Y.; Fu, S.; Li, L.; Xu, M.; Niu, Y. A comparative study on total anthocyanin content, composition of anthocyanidin, total phenolic content and antioxidant activity of pigmented potato peel and flesh. Food Sci. Technol. Res. 2016, 22, 219–226. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Gebrechristos, H.Y.; Ma, X.; Xiao, F.; He, Y.; Zheng, S.; Oyungerel, G.; Chen, W. Potato peel extracts as an antimicrobial and potential antioxidant in active edible film. Food Sci. Nutr. 2020, 8, 6338–6345. [Google Scholar] [CrossRef] [PubMed]
- Mäder, J.; Rawel, H.; Kroh, L.W. Composition of phenolic compounds and glycoalkaloids α-solanine and α-chaconine during commercial potato processing. J. Agric. Food Chem. 2009, 57, 6292–6297. [Google Scholar] [CrossRef]
- Visvanathan, R.; Jayathilake, C.; Jayawardana, B.C.; Liyanage, R. Health-beneficial properties of potato and compounds of interest. J. Sci. Food Agric. 2016, 96, 4850–4860. [Google Scholar] [CrossRef]
- Riciputi, Y.; Diaz-De-Cerio, E.; Akyol, H.; Capanoglu, E.; Cerretani, L.; Caboni, M.F.; Verardo, V. Establishment of ultrasound-assisted extraction of phenolic compounds from industrial potato by-products using response surface methodology. Food Chem. 2018, 269, 258–263. [Google Scholar] [CrossRef]
- Amado, I.R.; Franco, D.; Sánchez, M.; Zapata, C.; Vázquez, J.A. Optimisation of antioxidant extraction from Solanum tuberosum potato peel waste by surface response methodology. Food Chem. 2014, 165, 290–299. [Google Scholar] [CrossRef]
- Javed, A.; Ahmad, A.; Tahir, A.; Shabbir, U.; Nouman, M.; Hameed, A. Potato peel waste—Its nutraceutical, industrial and biotechnological applacations. AIMS Agric. Food 2019, 4, 807–823. [Google Scholar] [CrossRef]
- Le, Y.-J.; He, L.-Y.; Li, S.; Xiong, C.-J.; Lu, C.-H.; Yang, X.-Y. Chlorogenic acid exerts antibacterial effects by affecting lipid metabolism and scavenging ROS in Streptococcus pyogenes. FEMS Microbiol. Lett. 2022, 369, fnac061. [Google Scholar] [CrossRef]
- Cheng, D.; Li, H.; Zhou, J.; Wang, S. Chlorogenic acid relieves lead-induced cognitive impairments and hepato-renal damage via regulating the dysbiosis of the gut microbiota in mice. Food Funct. 2019, 10, 681–690. [Google Scholar] [CrossRef]
- Lukitasari, M.; Rohman, M.S.; Nugroho, D.A.; Widodo, N.; Nugrahini, N.I.P. Cardiovascular protection effect of chlorogenic acid: Focus on the molecular mechanism. F1000Research 2020, 9, 1462. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Iqbal, M.S.; Srivastava, J.K. Therapeutic promises of chlorogenic acid with special emphasis on its anti-obesity property. Curr. Mol. Pharmacol. 2020, 13, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Cao, Z.; Cao, L.; Ding, G.; Wang, Z.; Xiao, W. Antiviral activity of chlorogenic acid against influenza A (H1N1/H3N2) virus and its inhibition of neuraminidase. Sci. Rep. 2017, 7, 45723. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yamamoto, N.; Jokura, H.; Yamamoto, M.; Fujii, A.; Tokimitsu, I.; Saito, I. Chlorogenic acid attenuates hypertension and improves endothelial function in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 1065–1073. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Pelinson, L.P.; Assmann, C.E.; Palma, T.V.; da Cruz, I.; Pillat, M.M.; Mânica, A.; Stefanello, N.; Weis, G.C.C.; de Oliveira Alves, A.; De Andrade, C.M.; et al. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol. Biol. Rep. 2019, 46, 2085–2092. [Google Scholar] [CrossRef]
- Oršolić, N.; Sirovina, D.; Odeh, D.; Gajski, G.; Balta, V.; Šver, L.; Jembrek, M.J. Efficacy of caffeic acid on diabetes and its complications in the mouse. Molecules 2021, 26, 3262. [Google Scholar] [CrossRef]
- Wang, Y.; Kaur, G.; Kumar, M.; Kushwah, A.S.; Kabra, A.; Kainth, R. Caffeic acid prevents vascular oxidative stress and atherosclerosis against atherosclerogenic diet in rats. Evid.-Based Complement. Altern. Med. 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Q.; Zhang, L.; Wang, Q.; Yang, Z.; Liu, J.; Feng, L. Caffeic acid reduces A53T α-synuclein by activating JNK/Bcl-2-mediated autophagy in vitro and improves behaviour and protects dopaminergic neurons in a mouse model of Parkinson’s disease. Pharmacol. Res. 2019, 150, 104538. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, K.; Zheng, S.; Wang, Y.; Ren, Q.; Li, H.; Ding, L.; Li, W.; Zhang, L. Antibacterial effect of caffeic acid phenethyl ester on cariogenic bacteria and Streptococcus mutans biofilms. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef]
- Wu, Z.-M.; Yu, Z.-J.; Cui, Z.-Q.; Peng, L.-Y.; Li, H.-R.; Zhang, C.-L.; Shen, H.-Q.; Yi, P.-F.; Fu, B.-D. In vitro antiviral efficacy of caffeic acid against canine distemper virus. Microb. Pathog. 2017, 110, 240–244. [Google Scholar] [CrossRef]
- Panchal, S.K.; John, O.D.; Mathai, M.L.; Brown, L. Anthocyanins in Chronic Diseases: The Power of Purple. Nutrients 2022, 14, 1–30. [Google Scholar] [CrossRef]
- Fogelman, E.; Oren-Shamir, M.; Hirschberg, J.; Mandolino, G.; Parisi, B.; Ovadia, R.; Tanami, Z.; Faigenboim, A.; Ginzberg, I. Nutritional value of potato (Solanum tuberosum) in hot climates: Anthocyanins, carotenoids, and steroidal glycoalkaloids. Planta 2019, 249, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Valiñas, M.A.; Lanteri, M.L.; Have, A.T.; Andreu, A.B. Chlorogenic acid, anthocyanin and flavan-3-ol biosynthesis in flesh and skin of Andean potato tubers (Solanum tuberosum subsp. andigena). Food Chem. 2017, 229, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Polit, M.F.; Ayvaz, H.; Tay, D.; Manrique, I. Characterization and quantitation of anthocyanins and other phenolics in native andean potatoes. J. Agric. Food Chem. 2014, 62, 4408–4416. [Google Scholar] [CrossRef] [PubMed]
- Tierno, R.; López, A.; Riga, P.; Arazuri, S.; Jarén, C.; Benedicto, L.; Galarreta, J.I.R.d. Phytochemicals determination and classification in purple and red fleshed potato tubers by analytical methods and near infrared spectroscopy. J. Sci. Food Agric. 2016, 96, 1888–1899. [Google Scholar] [CrossRef]
- de Aguiar Cipriano, P.; Kim, H.; Fang, C.; Venancio, V.P.; Mertens-Talcott, S.U.; Talcott, S.T. In vitro digestion, absorption and biological activities of acylated anthocyanins from purple sweet potatoes (Ipomoea batatas). Food Chem. 2022, 374, 131076. [Google Scholar] [CrossRef]
- Cai, X.Y.; Yang, C.; Shao, L.; Zhu, H.; Wang, Y.; Huang, X.; Wang, S.; Hong, L. Targeting NOX 4 by petunidin improves anoxia/reoxygenation-induced myocardium injury. Eur. J. Pharmacol. 2020, 888, 173414. [Google Scholar] [CrossRef]
- Auger, C.; Chaabi, M.; Anselm, E.; Lobstein, A.; Schini-Kerth, V.B. The red wine extract-induced activation of endothelial nitric oxide synthase is mediated by a great variety of polyphenolic compounds. Mol. Nutr. Food Res. 2010, 54 (Suppl. 2), 171–183. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.B.; Nivetha, R.; Chattopadhyay, I.; Nagini, S. Blueberry and malvidin inhibit cell cycle progression and induce mitochondrial-mediated apoptosis by abrogating the JAK/STAT-3 signalling pathway. Food Chem. Toxicol. 2017, 109, 534–543. [Google Scholar] [CrossRef]
- Huang, W.Y.; Wang, J.; Liu, Y.M.; Zheng, Q.S.; Li, C.Y. Inhibitory effect of Malvidin on TNF-α-induced inflammatory response in endothelial cells. Eur. J. Pharmacol. 2014, 723, 67–72. [Google Scholar] [CrossRef]
- Shih, P.H.; Yeh, C.T.; Yen, G.C. Effects of anthocyanidin on the inhibition of proliferation and induction of apoptosis in human gastric adenocarcinoma cells. Food Chem. Toxicol. 2005, 43, 1557–1566. [Google Scholar] [CrossRef]
- Oliveira, H.; Wu, N.; Zhang, Q.; Wang, J.; Oliveira, J.; de Freitas, V.; Mateus, N.; He, J.; Fernandes, I. Bioavailability studies and anticancer properties of malvidin based anthocyanins, pyranoanthocyanins and non-oxonium derivatives. Food Funct. 2016, 7, 2462–2468. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, J.; Tian, J.; Si, X.; Jiao, X.; Zhang, W.; Gong, E.; Li, B. Blueberry Malvidin-3-galactoside Suppresses Hepatocellular Carcinoma by Regulating Apoptosis, Proliferation, and Metastasis Pathways in Vivo and in Vitro. J. Agric. Food Chem. 2019, 67, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Chen, M.; Zheng, H.; Li, C.; Yang, F.; Niu, Q. Pelargonidin ameliorates MCAO-induced cerebral ischemia/reperfusion injury in rats by the action on the Nrf2/HO-1 pathway. Transl. Neurosci. 2021, 12, 020–031. [Google Scholar] [CrossRef] [PubMed]
- Roghani, M.; Niknam, A.; Jalali-Nadoushan, M.R.; Kiasalari, Z.; Khalili, M.; Baluchnejadmojarad, T. Oral pelargonidin exerts dose-dependent neuroprotection in 6-hydroxydopamine rat model of hemi-parkinsonism. Brain Res. Bull. 2010, 82, 279–283. [Google Scholar] [CrossRef]
- Alisavari, N.; Soleimani-Asl, S.; Zarei, M.; Hashemi-Firouzi, N.; Shahidi, S. Protective effect of chronic administration of pelargonidin on neuronal apoptosis and memory process in amyloid-beta-treated rats. Avicenna J. Phytomed. 2021, 11, 407–416. [Google Scholar] [CrossRef]
- Luna-Vital, D.A.; De Mejia, E.G. Anthocyanins from purple corn activate free fatty acid-receptor 1 and glucokinase enhancing in vitro insulin secretion and hepatic glucose uptake. PLoS ONE 2018, 13, 1–20. [Google Scholar] [CrossRef]
- Matsui, T.; Ebuchi, S.; Kobayashi, M.; Fukui, K.; Sugita, K.; Terahara, N.; Matsumoto, K. Anti-hyperglycemic effect of diacylated anthocyanin derived from Ipomoea batatas cultivar Ayamurasaki can be achieved through the alpha-glucosidase inhibitory action. J. Agric. Food Chem. 2002, 50, 7244–7248. [Google Scholar] [CrossRef] [PubMed]
- André, C.M.; Oufir, M.; Hoffmann, L.; Hausman, J.-F.; Rogez, H.; Larondelle, Y.; Evers, D. Influence of environment and genotype on polyphenol compounds and in vitro antioxidant capacity of native Andean potatoes (Solanum tuberosum L.). J. Food Compos. Anal. 2009, 22, 517–524. [Google Scholar] [CrossRef]
- Hamouz, K.; Lachman, J.; Dvořák, P.; Jůzl, M.; Pivec, V. The effect of site conditions, variety and fertilization on the content of polyphenols in potato tubers. Plant, Soil Environ. 2006, 52, 407–412. [Google Scholar] [CrossRef]
- Ieri, F.; Innocenti, M.; Andrenelli, L.; Vecchio, V.; Mulinacci, N. Rapid HPLC/DAD/MS method to determine phenolic acids, glycoalkaloids and anthocyanins in pigmented potatoes (Solanum tuberosum L.) and correlations with variety and geographical origin. Food Chem. 2011, 125, 750–759. [Google Scholar] [CrossRef]
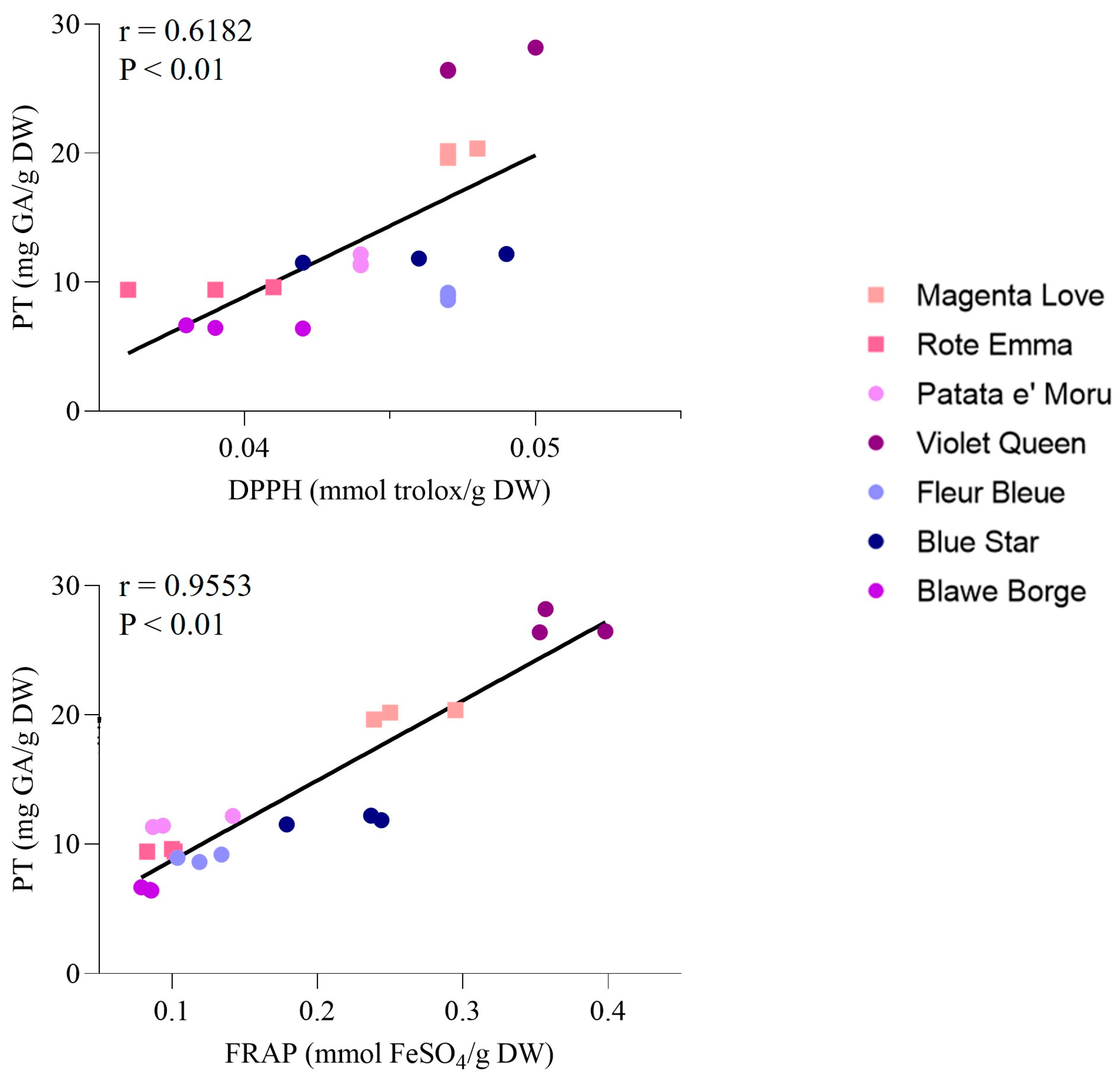
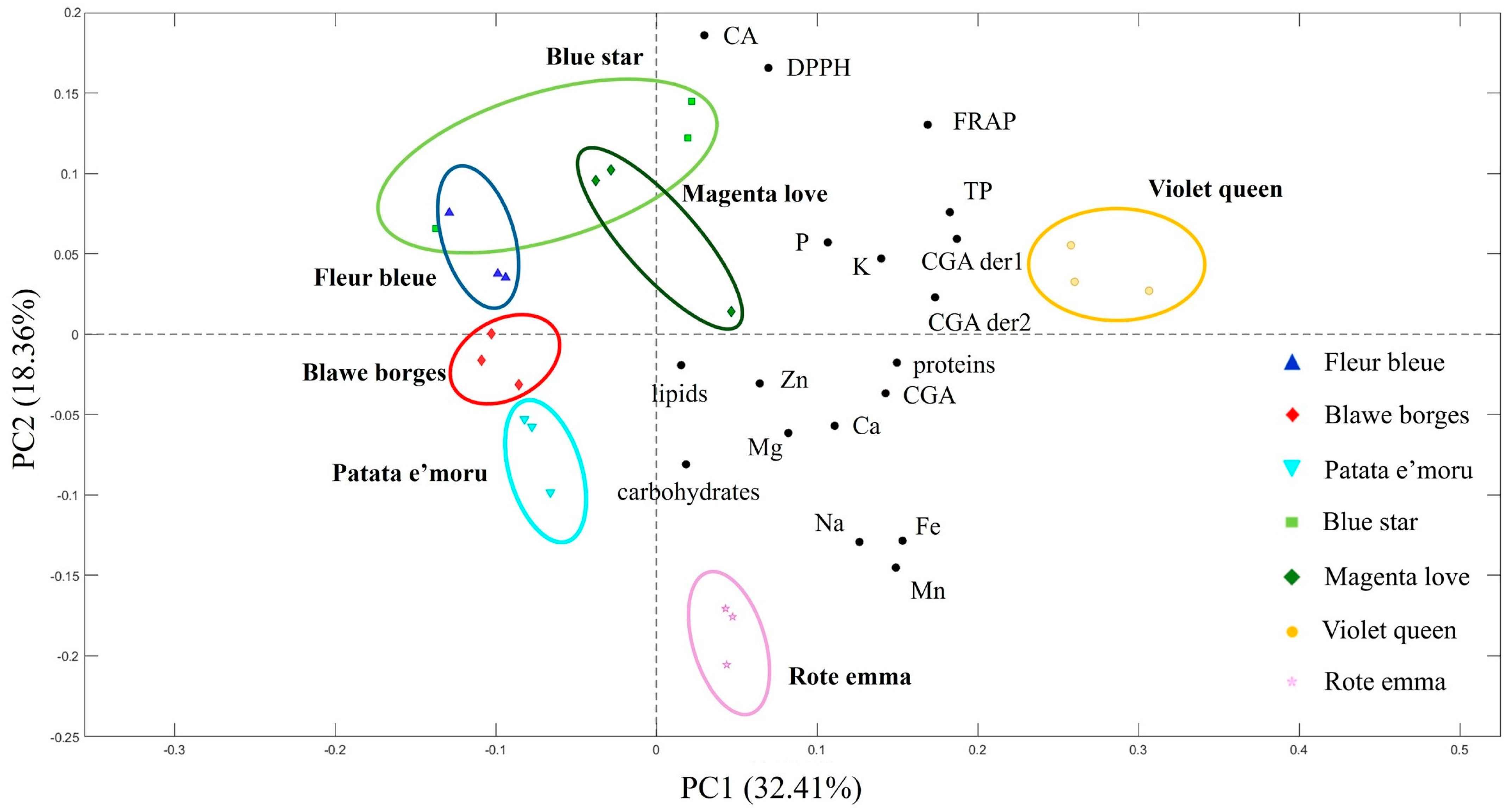
| Patata E’moru | Violet Queen | Fleur Bleue | Blue Star | Blawe Borges | Magenta Love | Rote Emma | |
|---|---|---|---|---|---|---|---|
| Lipids | 0.9 ± 0.0 a | 1.0 ± 0.1 ac | 0.7 ± 0.0 ad | 0.8 ± 0.1 acd | 1.2 ± 0.1 ace | 1.3 ± 0.1 bce | 0.8 ± 0.1 acd |
| Proteins | 24.4 ± 2.6 a | 31.8 ± 3.0 b | 13.9 ± 3.2 cd | 12.1 ± 0.9 c | 12.8 ± 0.5 c | 27.9 ± 2.0 ab | 19.5 ± 0.3 ad |
| Carbohydrates | 87.4 ± 3.0 a | 78.7 ± 7.9 a | 95.1 ± 4.2 a | 77.5 ± 3.0 a | 89.7 ± 7.4 a | 82.0 ± 8.0 a | 92.6 ± 1.2 a |
| Patata E’moru | Violet Queen | Fleur Bleue | Blue Star | Blaw Borges | Magenta Love | Rote Emma | |
|---|---|---|---|---|---|---|---|
| Macro-elements | |||||||
| K | 1399.6 ± 22.4 a | 2375.4 ± 9.4 b | 1475.4 ± 179.6 a | 2135.8 ± 243.6 bd | 1893.4 ± 21.8 cde | 2130.0 ± 24.4 bde | 1649.1 ± 9.7 ae |
| Ca | 621.3 ± 20.2 a | 663.2 ± 35.7 a | 415.1 ± 6.4 bd | 488.5 ± 22.0 be | 370.2 ± 3.3 b | 602.5 ± 109.4 aef | 524.5 ± 11.4 acdf |
| Mg | 141.4 ± 4.5 a | 179.2 ± 4.5 b | 154.5 ± 11.7 a | 148.9 ± 14.0 a | 153.4 ± 4.0 a | 184.5 ± 12.7 b | 178.8 ± 2.8 b |
| Na | 68.6 ± 5.8 a | 89.9 ± 7.1 b | 23.5 ± 2.9 c | 23.6 ± 2.3 c | 25.6 ± 0.9 c | 49.0 ± 4.3 d | 93.0 ± 1.4 b |
| P | 14.2 ± 0.4 a | 20.6 ± 0.1 b | 14.7 ± 1.6 a | 33.4 ± 3.2 c | 13.1 ± 0.1 a | 20.0 ± 0.4 b | 19.2 ± 0.4 b |
| Micro-elements | |||||||
| Fe | 18.7 ± 1.7 a | 51.4 ± 2.2 b | 12.6 ± 1.3 a | 21.9 ± 2.7 ae | 17.2 ± 1.2 a | 29.7 ± 1.1 ce | 71.3 ± 6.1 d |
| Zn | 3.3 ± 0.3 a | 3.7 ± 0.2 a | 1.6 ± 0.0 b | 2.8 ± 0.7 ab | 2.8 ± 0.2 ab | 3.1 ± 0.4 ab | 3.0 ± 0.4 ab |
| Mn | 1.3 ± 0.0 a | 2.1 ± 0.0 b | 1.2 ± 0.1 a | 1.3 ± 0.1 a | 1.2 ± 0.1 a | 1.8 ± 0.2 b | 2.6 ± 0.0 c |
| Cultivar | Total Polyphenols mg GA/g DW | DPPH mmol trolox/g DW | FRAP mmol FeSO4/g DW |
|---|---|---|---|
| Patate e’moru | 11.64 ± 0.46 a | 0.044 ± 0.000 a | 0.090 ± 0.005 a |
| Violet queen | 27.00 ± 1.02 b | 0.048 ± 0.002 a | 0.369 ± 0.030 b |
| Fleur bleue | 8.91 ± 0.29 c | 0.047 ± 0.000 a | 0.119 ± 0.015 a |
| Blue star | 11.85 ± 0.34 a | 0.046 ± 0.003 a | 0.240 ± 0.005 c |
| Blawe borges | 6.51 ± 0.13 d | 0.040 ± 0.002 ab | 0.083 ± 0.004 a |
| Magenta love | 20.06 ± 0.37 e | 0.047 ± 0.000 a | 0.261 ± 0.030 dc |
| Rote emma | 9.48 ± 0.12 c | 0.039 ± 0.003 b | 0.095 ± 0.010 a |
| λ 280 nm | ||||
|---|---|---|---|---|
| Cultivar | Chlorogenic Acid Derivative 1 (£) | Chlorogenic Acid | Chlorogenic Acid Derivative 2 (£) | Caffeic Acid |
| Patate e’moru | traces a | 3.25 ± 0.40 a | 0.24 ± 0.03 a | 0.031 ± 0.01 a |
| Violet queen | 0.88 ± 0.07 b | 10.53 ± 0.95 b | 2.17 ± 0.17 b | 0.15 ± 0.01 b |
| Fleur bleue | 0.11 ± 0.02 c | 1.23 ± 0.13 c | 0.14 ± 0.01 a | 0.13 ± 0.01 bd |
| Blue star | 0.53 ± 0.02 d | 6.45 ± 0.28 d | 1.29 ± 0.07 c | 0.21 ± 0.01 c |
| Blawe borges | 0.28 ± 0.01 e | 1.18 ± 0.04 c | 0.33 ± 0.01 a | 0.13 ± 0.01 be |
| Magenta love | traces a | 2.05 ± 0.04 c | 0.30 ± 0.01 a | 0.12 ± 0.00 cde |
| Rote emma | 0.40 ± 0.02 f | 5.06 ± 0.23 e | 0.60 ± 0.03 d | 0.04 ± 0.00 a |
| λ 520 nm | ||||||
|---|---|---|---|---|---|---|
| Cultivar | Petunidin-3-O-caffeoyl-rutinoside-5-O-glucoside * | Petunidin-3-O-p-coumaryl-rutinoside-5-O-glucoside * | Petunidin-3-O-feruloyl-rutinoside-5-O-glucoside * | Malvidin 3-O-p-coumaroyl-rutinoside-5-O-glucoside * | Pelargonidin-3-O-p-coumaroyl-rutinoside-5-O-glucoside * | Peonidin-3-O-p-coumaroyl-rutinoside-5-glucoside * |
| Patate e’moru | - | 0.11 ± 0.02 a | - a | 1.96 ± 0.22 a | - | - |
| Violet queen | 0.20 ± 0.00 | 7.27 ± 0.73 b | 0.28 ± 0.04 b | 1.19 ± 0.13 b | - | - |
| Fleur bleue | - | 0.36 ± 0.05 a | 0.05 ± 0.01 ad | 0.06 ± 0.01 c | - | - |
| Blue star | - | 1.17± 0.06 c | 0.17 ± 0.01 c | 0.16 ± 0.01 c | - | - |
| Blawe borges | - | 0.32 ± 0.01 a | 0.06 ± 0.00 d | 0.03 ± 0.00 c | - | - |
| Magenta love | - | - | - | - | 0.36 ± 0.01 a | 0.08 ± 0.00 |
| Rote emma | - | - | - | - | 0.99 ± 0.06 b | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dessì, D.; Fais, G.; Sarais, G. Nutritional and Chemical Characterization of Red and Purple Potatoes Peels: A Polyphenol-Rich By-Product. Foods 2025, 14, 1740. https://doi.org/10.3390/foods14101740
Dessì D, Fais G, Sarais G. Nutritional and Chemical Characterization of Red and Purple Potatoes Peels: A Polyphenol-Rich By-Product. Foods. 2025; 14(10):1740. https://doi.org/10.3390/foods14101740
Chicago/Turabian StyleDessì, Debora, Giacomo Fais, and Giorgia Sarais. 2025. "Nutritional and Chemical Characterization of Red and Purple Potatoes Peels: A Polyphenol-Rich By-Product" Foods 14, no. 10: 1740. https://doi.org/10.3390/foods14101740
APA StyleDessì, D., Fais, G., & Sarais, G. (2025). Nutritional and Chemical Characterization of Red and Purple Potatoes Peels: A Polyphenol-Rich By-Product. Foods, 14(10), 1740. https://doi.org/10.3390/foods14101740






