Ginseng Soluble Dietary Fiber Reverses Obesity via the PPAR/AMPK Signaling Pathway and Improves Intestinal Flora in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GSDF
2.3. Animal Experimental Design
2.4. Determination of Basic Indicators
2.5. Serum Biochemical Index and ELISA Assay
2.6. Organizational Analysis
2.7. Western Blotting
2.8. Microbiome Analysis
2.9. Statistical Analysis
3. Results
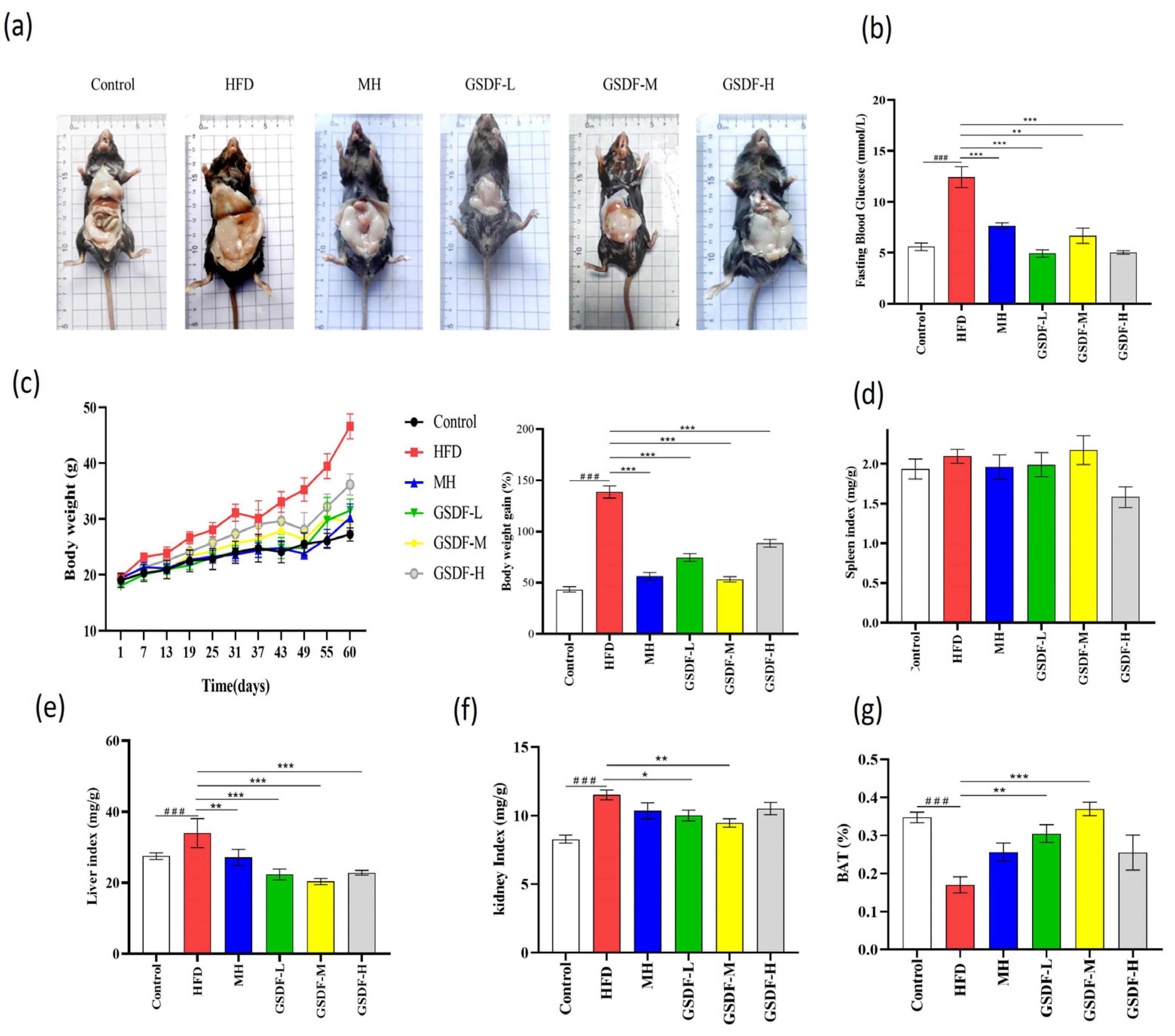
3.1. Effect of GSDF on Basic Indicators in HFD-Induced Obese Mice
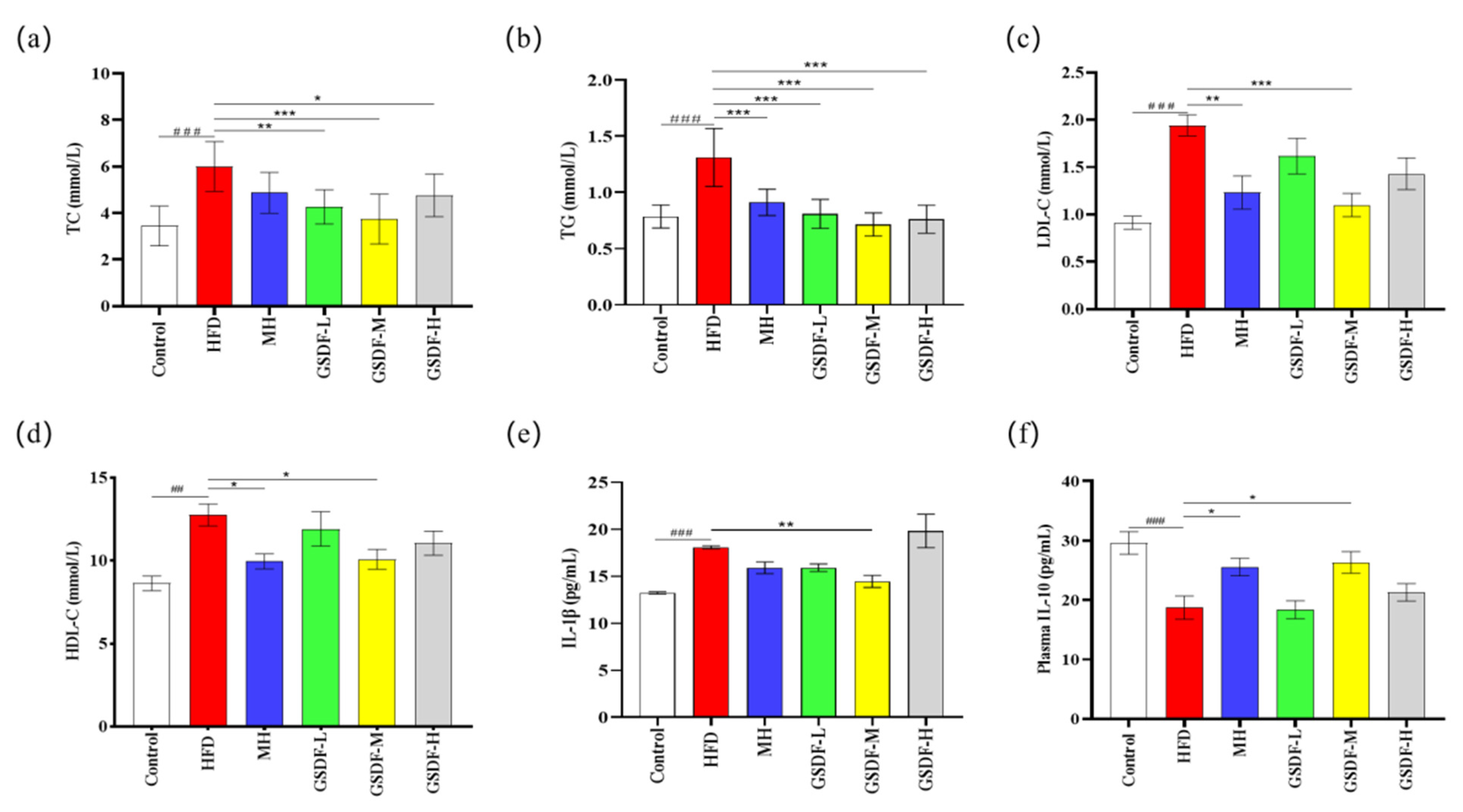
3.2. Effect of GSDF on Blood Lipids and Inflammatory Factors
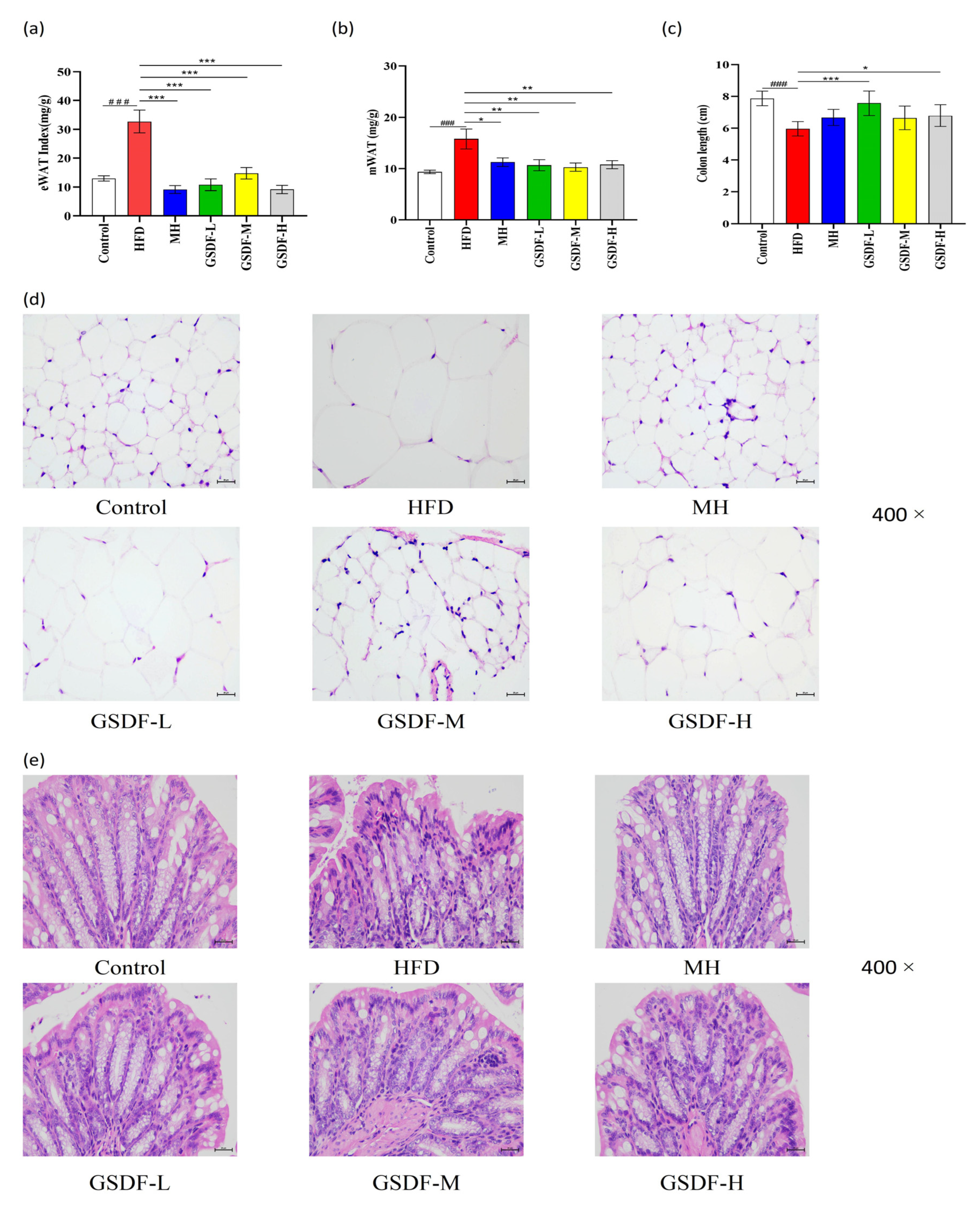
3.3. Effects of GSDF on Histopathology in HFD-Induced Obese Mice
3.4. Effect of GSDF on the PPAR/AMPK Pathway
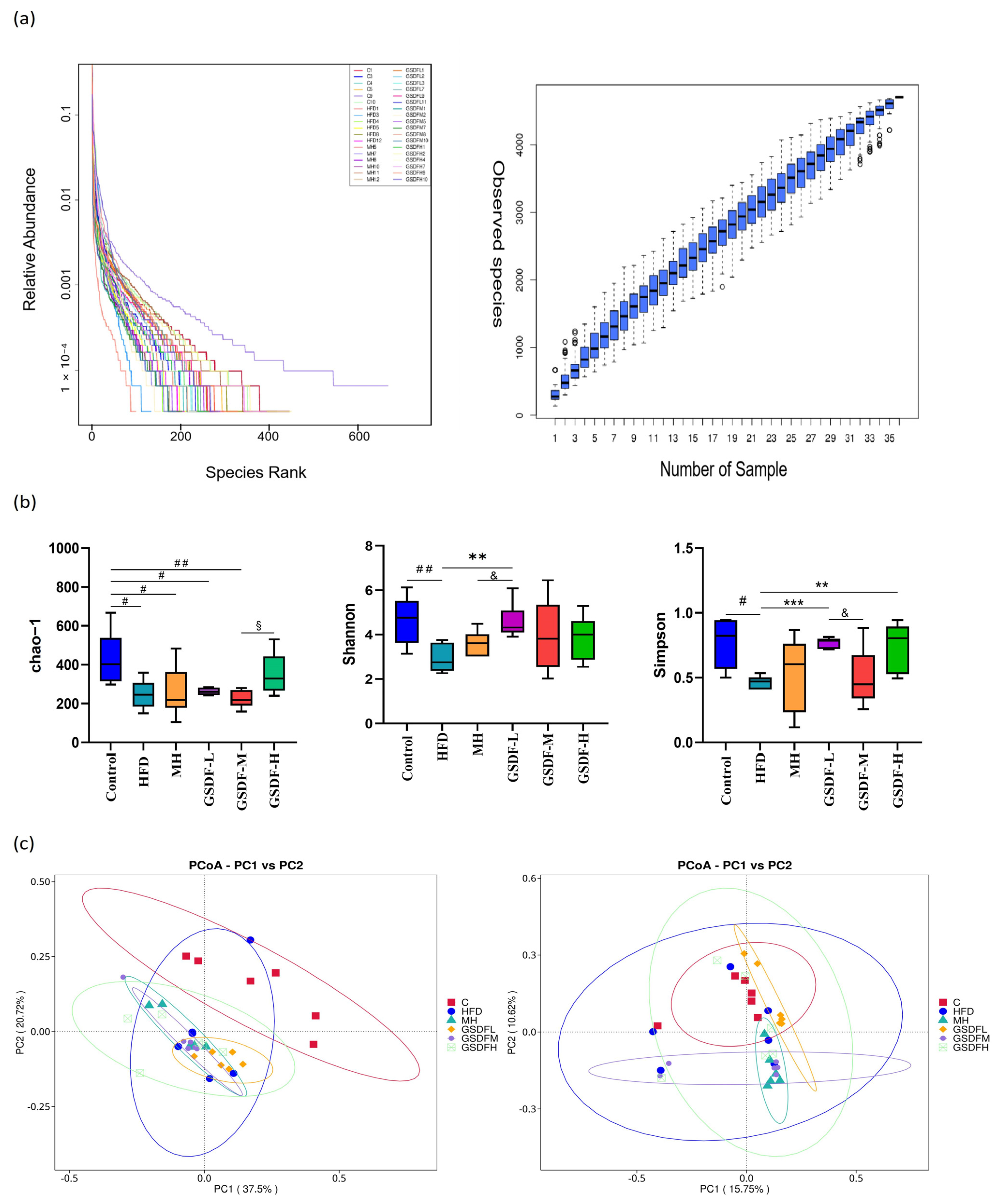
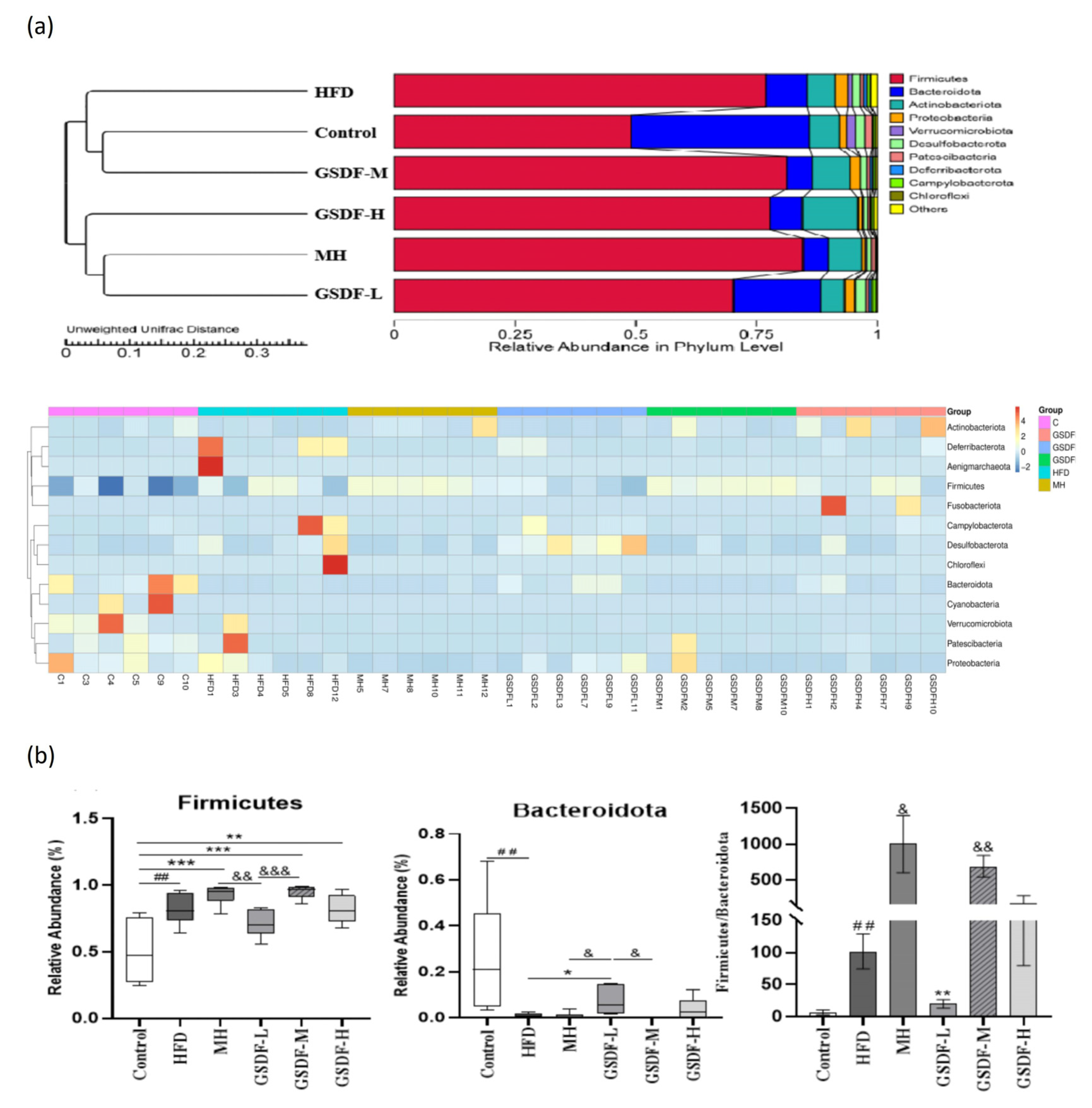
3.5. Composition and Diversity of Mouse Intestinal Microflora
3.6. Analysis of GSDF in Gut Microbiota Composition
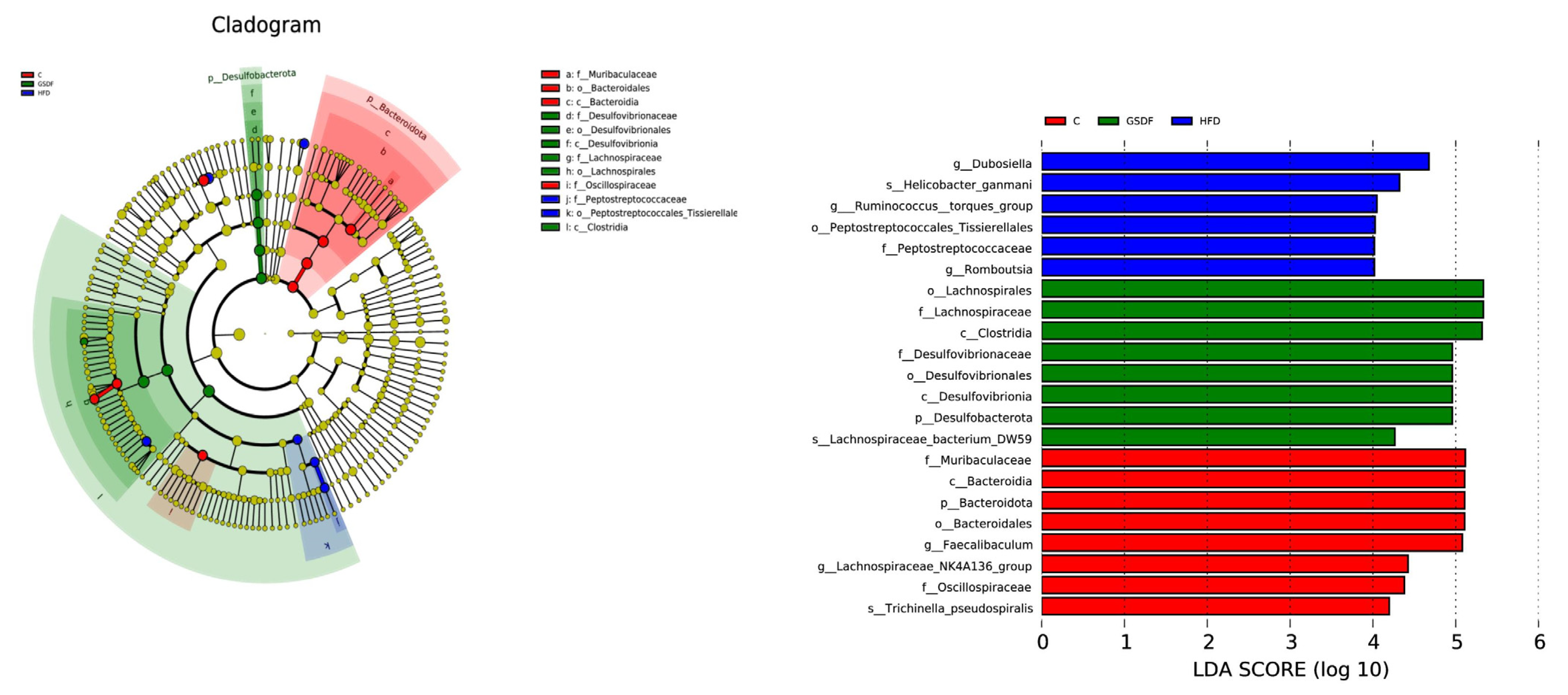
3.7. LEfSe Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, L.P.; Andrade, G.M.; Mayoral, E.P.; Huerta, T.H.; Canseco, S.P.; Rodal Canales, F.J.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Pérez Santiago, A.D.; Alpuche, J.J.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11–21. [Google Scholar] [PubMed]
- Pafili, K.; Roden, M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol. Metab. 2021, 50, 101–122. [Google Scholar] [CrossRef]
- Feng, K.; Zhu, X.; Chen, T.; Peng, B.; Lu, M.; Zheng, H.; Huang, Q.; Ho, C.T.; Chen, Y.; Cao, Y. Prevention of Obesity and Hyperlipidemia by Heptamethoxyflavone in High-fat Diet-induced Rats. J. Agric. Food Chem. 2019, 67, 2476–2489. [Google Scholar] [CrossRef]
- Kumar, S.; Behl, T.; Sachdeva, M.; Sehgal, A.; Kumari, S.; Kumar, A.; Kaur, G.; Yadav, H.N.; Bungau, S. Implicating the effect of ketogenic diet as a preventive measure to obesity and diabetes mellitus. Life Sci. 2021, 264, 118–132. [Google Scholar] [CrossRef]
- Cote, A.T.; Harris, K.C.; Panagiotopoulos, C.; Sandor, G.G.; Devlin, A.M. Childhood obesity and cardiovascular dysfunction. J. Am. Coll. Cardiol. 2013, 62, 1309–1319. [Google Scholar] [CrossRef]
- Peng, J.; Wang, A.J.; Li, Z.Y.; Lin, H.E. Based on 16S rRNA gene sequencing, the effect of gin-xiao granules on the intestinal microbiota of patients with blood-heat-dampness-stagnation and toxin-type psoriasis was investigated. Inf. Chin. Med. 2024, 41, 48–56. [Google Scholar]
- Asadi, A.; Shadab Mehr, N.; Mohamadi, M.H.; Shokri, F.; Heidary, M.; Sadeghifard, N.; Khoshnood, S. Obesity and gut-microbiota-brain axis: A narrative review. J. Clin. Lab. Anal. 2022, 36, e24420. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Makki, K.; Brolin, H.; Petersen, N.; Henricsson, M.; Christensen, D.P.; Khan, M.T.; Wahlström, A.; Bergh, P.O.; Tremaroli, V.; Schoonjans, K.; et al. 6α-hydroxylated bile acids mediate TGR5 signalling to improve glucose metabolism upon dietary fiber supplementation in mice. Gut 2023, 72, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, T.; Liu, R.; Zhang, M.; Wang, R. Soluble Dietary Fiber Reduces Trimethylamine Metabolism via Gut Microbiota and Co-Regulates Host AMPK Pathways. Mol. Nutr. Food Res. 2017, 61, 473–484. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, W.; Chen, Y.; Lei, L.; Li, F.; Zhao, J.; Zeng, K.; Ming, J. Dietary fiber of Tartary buckwheat bran modified by steam explosion alleviates hyperglycemia and modulates gut microbiota in db/db mice. Food Res. Int. 2022, 157, 111–122. [Google Scholar] [CrossRef]
- Li, Y.; Xia, D.; Chen, J.; Zhang, X.; Wang, H.; Huang, L.; Shen, J.; Wang, S.; Feng, Y.; He, D.; et al. Dietary fibers with different viscosity regulate lipid metabolism via ampk pathway: Roles of gut microbiota and short-chain fatty acid. Poult. Sci. 2022, 101, 101–115. [Google Scholar] [CrossRef]
- Lin, S.C.; Hardie, D.G. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.E.; Xu, H.E.; Melcher, K. Structure and Physiological Regulation of AMPK. Int. J. Mol. Sci. 2018, 19, 3534. [Google Scholar] [CrossRef] [PubMed]
- Abbott, B.D. Review of the expression of peroxisome proliferator-activated receptors alpha (PPAR alpha), beta (PPAR beta), and gamma (PPAR gamma) in rodent and human development. Reprod. Toxicol. 2009, 27, 246–257. [Google Scholar] [CrossRef]
- Scott, R.E.; Florine, D.L.; Wille, J.J.; Yun, K. Coupling of growth arrest and differentiation at a distinct state in the G1 phase of the cell cycle: GD. Proc. Natl. Acad. Sci. USA 1982, 79, 845–849. [Google Scholar] [CrossRef]
- Liu, H.; Lu, X.; Hu, Y.; Fan, X. Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy. Pharmacol. Res. 2020, 161, 105–118. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, H.Y. Ginseng-derived compounds as potential anticancer agents targeting cancer stem cells. J. Ginseng Res. 2024, 48, 266–275. [Google Scholar] [CrossRef]
- Hao, M.; Ding, C.; Peng, X.; Chen, H.; Dong, L.; Zhang, Y.; Chen, X.; Liu, W.; Luo, Y. Ginseng under forest exerts stronger anti-aging effects compared to garden ginseng probably via regulating PI3K/AKT/mTOR pathway, SIRT1/NF-κB pathway and intestinal flora. Phytomedicine 2022, 105, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.B.; Li, M.J.; Huo, X.H.; Gong, T.J.; Han, X.; Liu, J.H.; Liu, S.; Sun, Y.S. Ginsenoside Rg5 Improves Sleep by Regulating Energy Metabolism in Sleep-Deprived Rats. Am. J. Chin. Med. 2023, 51, 1845–1864. [Google Scholar] [CrossRef]
- Huang, J.; Liu, D.; Wang, Y.; Liu, L.; Li, J.; Yuan, J.; Jiang, Z.; Jiang, Z.; Hsiao, W.W.; Liu, H.; et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 2022, 71, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Li, S.S.; Qu, D.; Chen, J.B.; Li, Z.M.; Sun, Y.S. Study on the nutritional composition, polysaccharide structure and thermal stability of ginseng dietary fiber. Spec. Res. 2020, 02, 20–26. [Google Scholar]
- Hua, M.; Sun, Y.; Shao, Z.; Lu, J.; Lu, Y.; Liu, Z. Functional soluble dietary fiber from ginseng residue: Polysaccharide characterization, structure, antioxidant, and enzyme inhibitory activity. J. Food Biochem. 2020, 44, e13524. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Bai, C.; Li, Z.M.; Huo, X.H.; Li, W.; Sun, Y.S.; Sha, J.Y. Ginseng Soluble Dietary Fiber Enhances Spermatogenic Potential in Obese Mice via the MAPK Signaling Pathway. J. Food Biochem. 2024, 2024, 623–635. [Google Scholar] [CrossRef]
- Yang, Y.; Pang, F.; Zhou, M.; Guo, X.; Yang, Y.; Qiu, W.; Liao, C.; Chen, Y.; Tang, C. Electroacupuncture Reduces Inflammatory Bowel Disease in Obese Mice by Activating the Nrf2/HO-1 Signaling Pathways and Repairing the Intestinal Barrier. Diabetes Metab. Syndr. Obes. 2024, 17, 435–452. [Google Scholar] [CrossRef]
- Deol, P.; Ruegger, P.; Logan, G.D.; Shawki, A.; Li, J.; Mitchell, J.D.; Yu, J.; Piamthai, V.; Radi, S.H.; Hasnain, S.; et al. Diet high in linoleic acid dysregulates the intestinal endocannabinoid system and increases susceptibility to colitis in Mice. Gut Microbes 2023, 15, 2229–2257. [Google Scholar] [CrossRef]
- Chen, H.; Ye, C.; Wu, C.; Zhang, J.; Xu, L.; Wang, X.; Xu, C.; Zhang, J.; Guo, Y.; Yao, Q. Berberine inhibits high fat diet-associated colorectal cancer through modulation of the gut microbiota-mediated lysophosphatidylcholine. Int. J. Biol. Sci. 2023, 19, 2097–2113. [Google Scholar] [CrossRef]
- Fenzl, A.; Kiefer, F.W. Brown adipose tissue and thermogenesis. Horm. Mol. Biol. Clin. Investig. 2014, 19, 25–37. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Song, Y.; Xie, H.; Dong, M. An update on brown adipose tissue and obesity intervention: Function, regulation and therapeutic implications. Front. Endocrinol. 2023, 13, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Zheng, Z.; Gao, W.; Zhu, Z.; Li, S.; Chen, X.; Cravotto, G.; Sui, Y.; Zhou, L. Complexes of Soluble Dietary Fiber and Polyphenols from Lotus Root Regulate High-Fat Diet-Induced Hyperlipidemia in Mice. Antioxidants 2024, 13, 466. [Google Scholar] [CrossRef]
- Luo, J.; Qi, J.; Wang, W.; Luo, Z.; Liu, L.; Zhang, G.; Zhou, Q.; Liu, J.; Peng, X. Antiobesity Effect of Flaxseed Polysaccharide via Inducing Satiety due to Leptin Resistance Removal and Promoting Lipid Metabolism through the AMP-Activated Protein Kinase (AMPK) Signaling Pathway. J. Agric. Food Chem. 2019, 67, 7040–7049. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yang, B.; Shi, Y.; Du, Y.; Lv, Y.; Liu, J.; Liu, E.; Xu, H.; Deng, L.; Chen, X.Y. Adlay (Coix lacryma-jobi L.) Polyphenol Improves Hepatic Glucose and Lipid Homeostasis through Regulating Intestinal Flora via AMPK Pathway. Mol. Nutr. Food Res. 2022, 66, e2200447. [Google Scholar] [CrossRef]
- Jayachandran, M.; Christudas, S.; Zheng, X.; Xu, B. Dietary fiber konjac glucomannan exerts an antidiabetic effect via inhibiting lipid absorption and regulation of PPAR-γ and gut microbiome. Food Chem. 2023, 403, 134–144. [Google Scholar] [CrossRef]
- Peng, Z.; Ban, K.; Wawrose, R.A.; Gover, A.G.; Kozar, R.A. Protection by enteral glutamine is mediated by intestinal epithelial cell peroxisome proliferator-activated receptor-γ during intestinal ischemia/reperfusion. Shock 2015, 43, 327–333. [Google Scholar] [CrossRef]
- Victor, N.A.; Wanderi, E.W.; Gamboa, J.; Zhao, X.; Aronowski, J.; Deininger, K.; Lust, W.D.; Landreth, G.E.; Sundararajan, S. Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur. J. Neurosci. 2006, 24, 1653–1663. [Google Scholar] [CrossRef]
- Gong, M.; Yuan, Y.; Shi, X.; Huang, H.; Liu, J.; Zhao, J.; Xu, Q. Compound oolong tea ameliorates lipid accumulation through AMPK-PPAR pathway of hepatic lipid metabolism and modulates gut microbiota in HFD induced mice. Food Res. Int. 2024, 196, 115–127. [Google Scholar] [CrossRef]
- Mota de Sá, P.; Richard, A.J.; Hang, H.; Stephens, J.M. Transcriptional Regulation of Adipogenesis. Compr. Physiol. 2017, 7, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Almeida, A.; Mitchell, A.L.; Boland, M.; Forster, S.C.; Gloor, G.B.; Tarkowska, A.; Lawley, T.D.; Finn, R.D. A new genomic blueprint of the human gut microbiota. Nature 2019, 568, 499–504. [Google Scholar] [CrossRef]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Itav, S.; Rothschild, D.; Meijer, M.T.; Levy, M.; Moresi, C.; Dohnalová, L.; Braverman, S.; Rozin, S.; Malitsky, S.; et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 2016, 540, 544–551. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.T.; Cheng, P.C.; Pan, T.M. Anti-obesity effects of gut microbiota are associated with lactic acid bacteria. Appl. Microbiol. Biotechnol. 2014, 98, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, Y.; Wei, J.; Wang, X.; Yang, H.; Wang, S. Stabilization of hypoxia-inducible factor 1α and regulation of specific gut microbes by EGCG contribute to the alleviation of ileal barrier disorder and obesity. Food Funct. 2024, 15, 9983–9994. [Google Scholar] [CrossRef]
- Petersen, C.; Bell, R.; Klag, K.A.; Lee, S.H.; Soto, R.; Ghazaryan, A.; Buhrke, K.; Ekiz, H.A.; Ost, K.S.; Boudina, S.; et al. T cell-mediated regulation of the microbiota protects against obesity. Science 2019, 365, eaat9351. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Bai, C.; Sha, J.; Huo, X.; Qu, D.; Chen, J. Ginseng Soluble Dietary Fiber Reverses Obesity via the PPAR/AMPK Signaling Pathway and Improves Intestinal Flora in Mice. Foods 2025, 14, 1716. https://doi.org/10.3390/foods14101716
Zhang Y, Bai C, Sha J, Huo X, Qu D, Chen J. Ginseng Soluble Dietary Fiber Reverses Obesity via the PPAR/AMPK Signaling Pathway and Improves Intestinal Flora in Mice. Foods. 2025; 14(10):1716. https://doi.org/10.3390/foods14101716
Chicago/Turabian StyleZhang, Yue, Chen Bai, Jiyue Sha, Xiaohui Huo, Di Qu, and Jianbo Chen. 2025. "Ginseng Soluble Dietary Fiber Reverses Obesity via the PPAR/AMPK Signaling Pathway and Improves Intestinal Flora in Mice" Foods 14, no. 10: 1716. https://doi.org/10.3390/foods14101716
APA StyleZhang, Y., Bai, C., Sha, J., Huo, X., Qu, D., & Chen, J. (2025). Ginseng Soluble Dietary Fiber Reverses Obesity via the PPAR/AMPK Signaling Pathway and Improves Intestinal Flora in Mice. Foods, 14(10), 1716. https://doi.org/10.3390/foods14101716




