Dynamic Change of Aroma Components in Chimonanthus praecox Flower Scented Teas During Absorption and Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Regents
2.2. Collection of Tea and Chimonanthus Flowers Blooming
2.3. Processing of Chimonanthus Tea Samples
2.4. Extraction of Volatile Compounds by the Headspace Solid-Phase Microextraction
2.5. Qualitative and Quantitative Analysis of the Volatiles by GC-MS
2.6. Odor Description
2.7. Statistical Analysis
3. Results
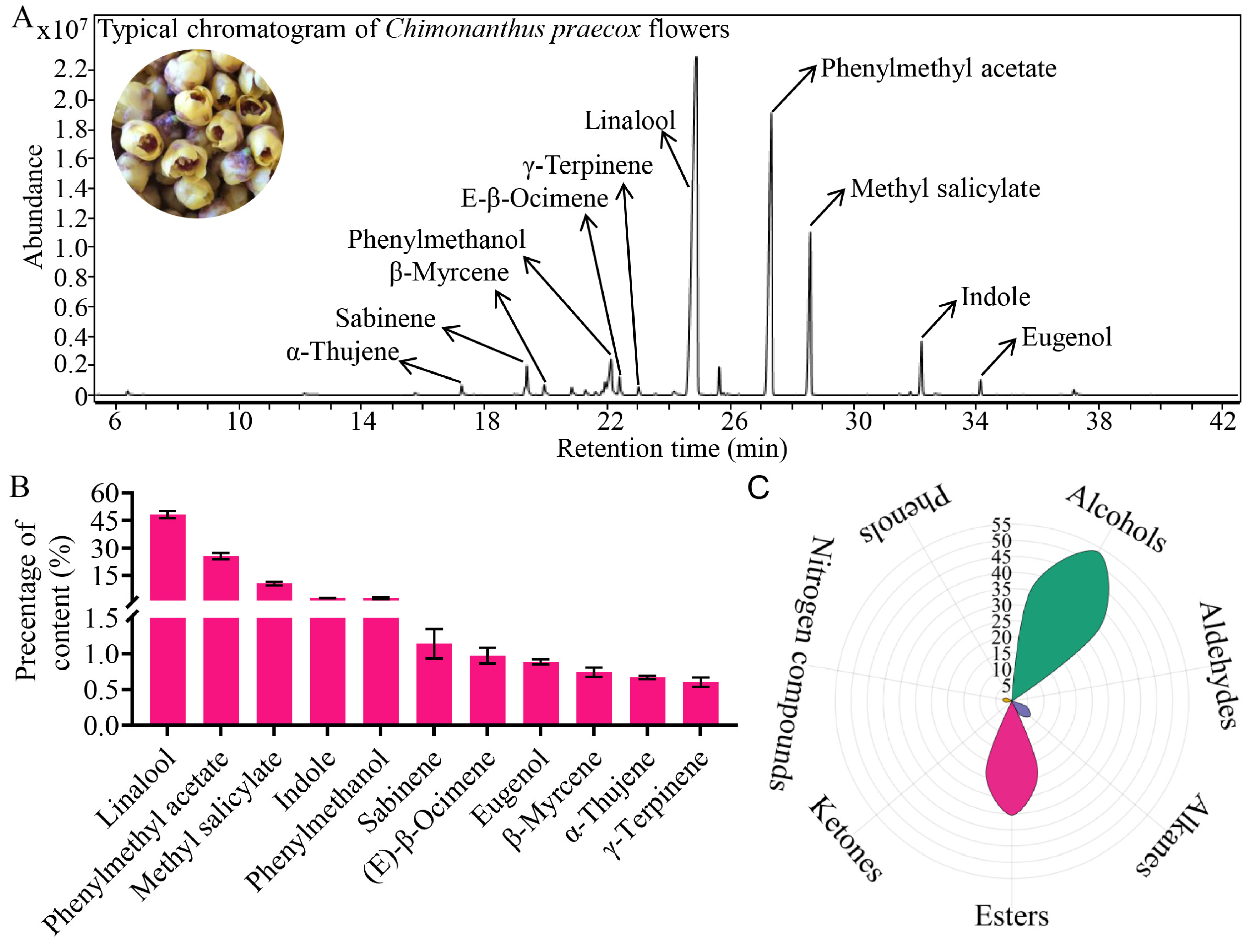
3.1. Characteristic Composition of Volatile in Chimonanthus Flowers
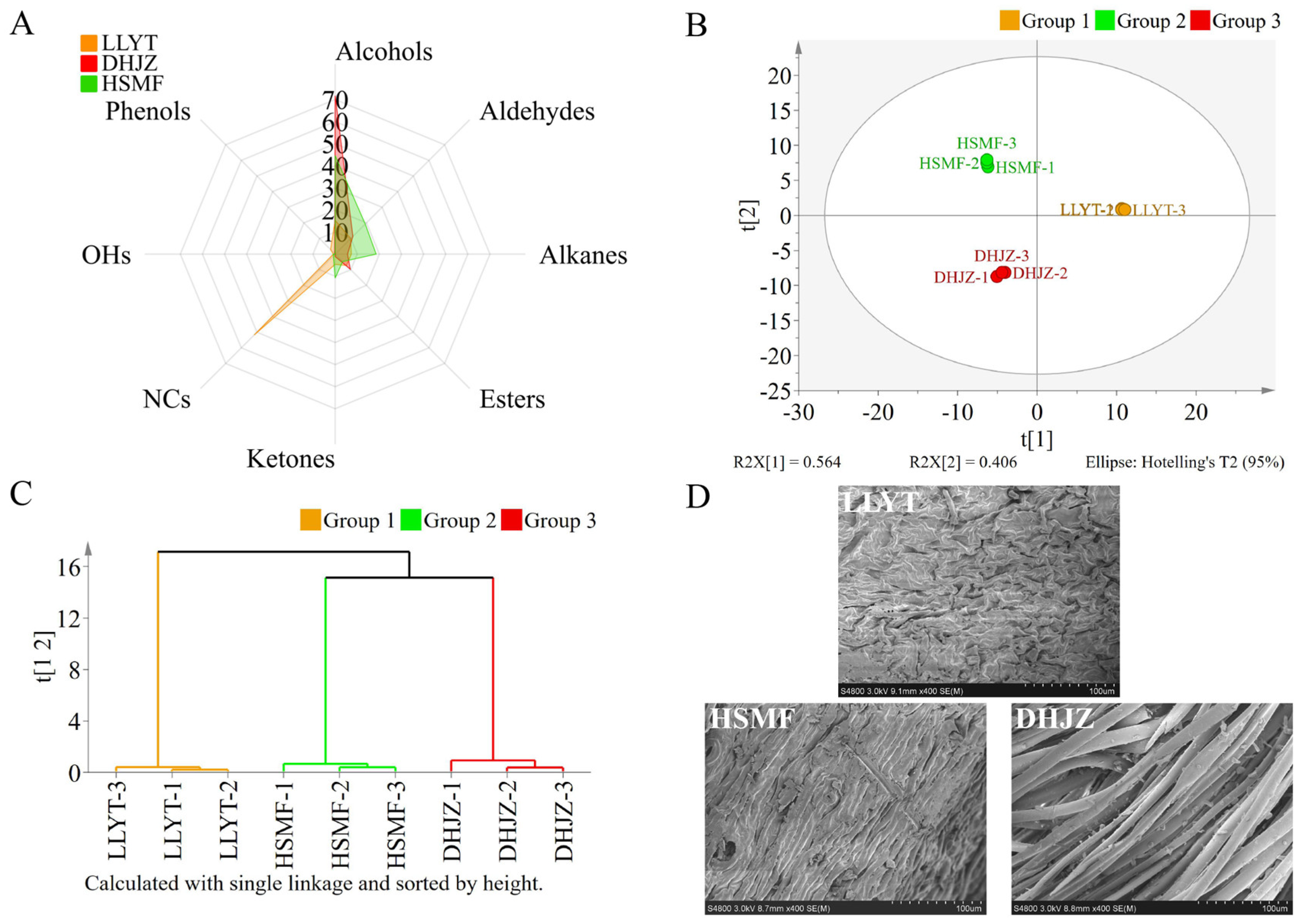
3.2. Aroma Quality of Three Tea Dhools
3.3. Scanning Electron Microscopy (SEM) Examination of Solid Surfaces of Different Tea Dhools
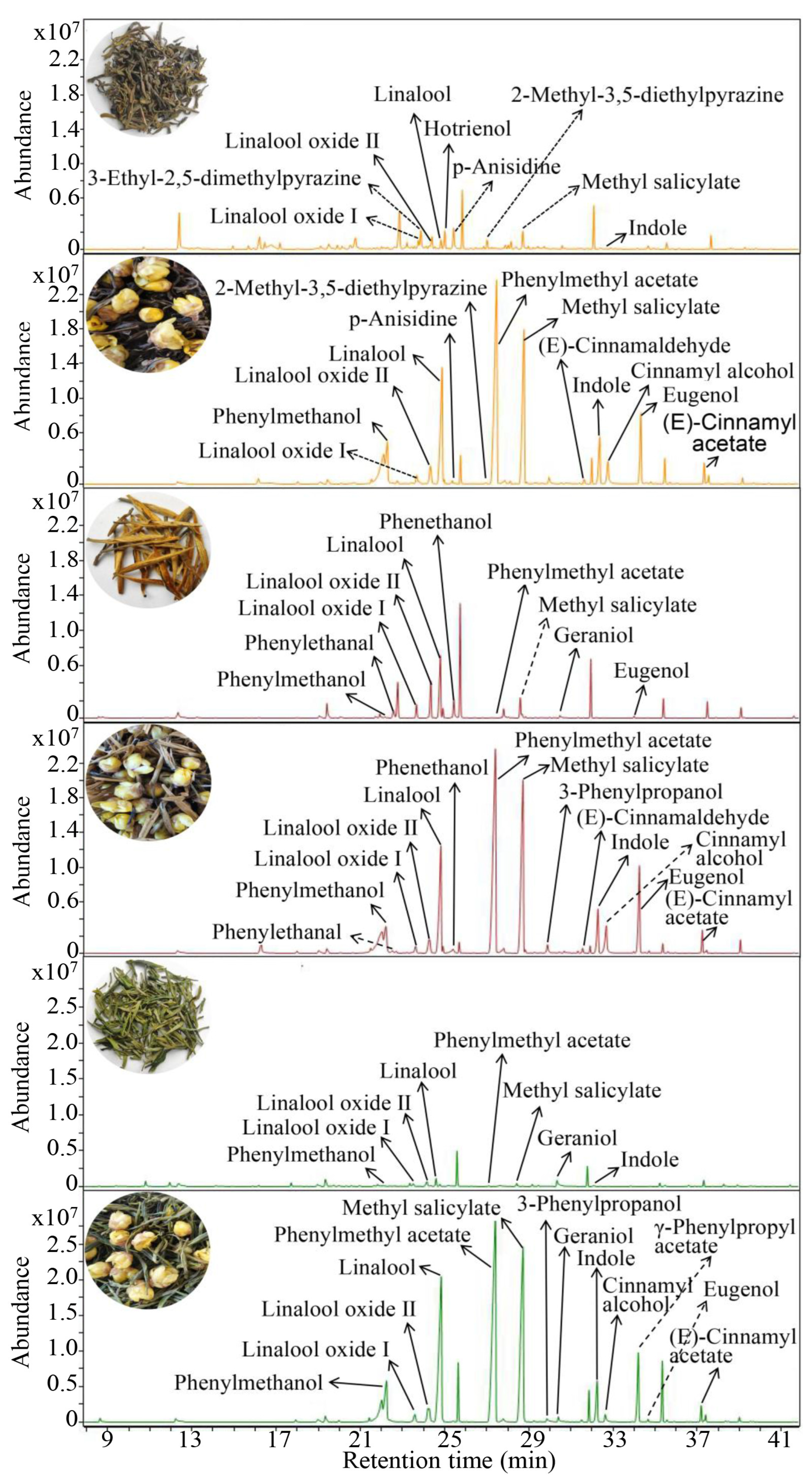
3.4. Contribution of Chimonanthus Flowers Volatiles to Aroma Quality of Three Scented Chimonanthus Teas
3.5. Identification of New Aroma Components After Scenting of Tea Dhool with Chimonanthus Flowers
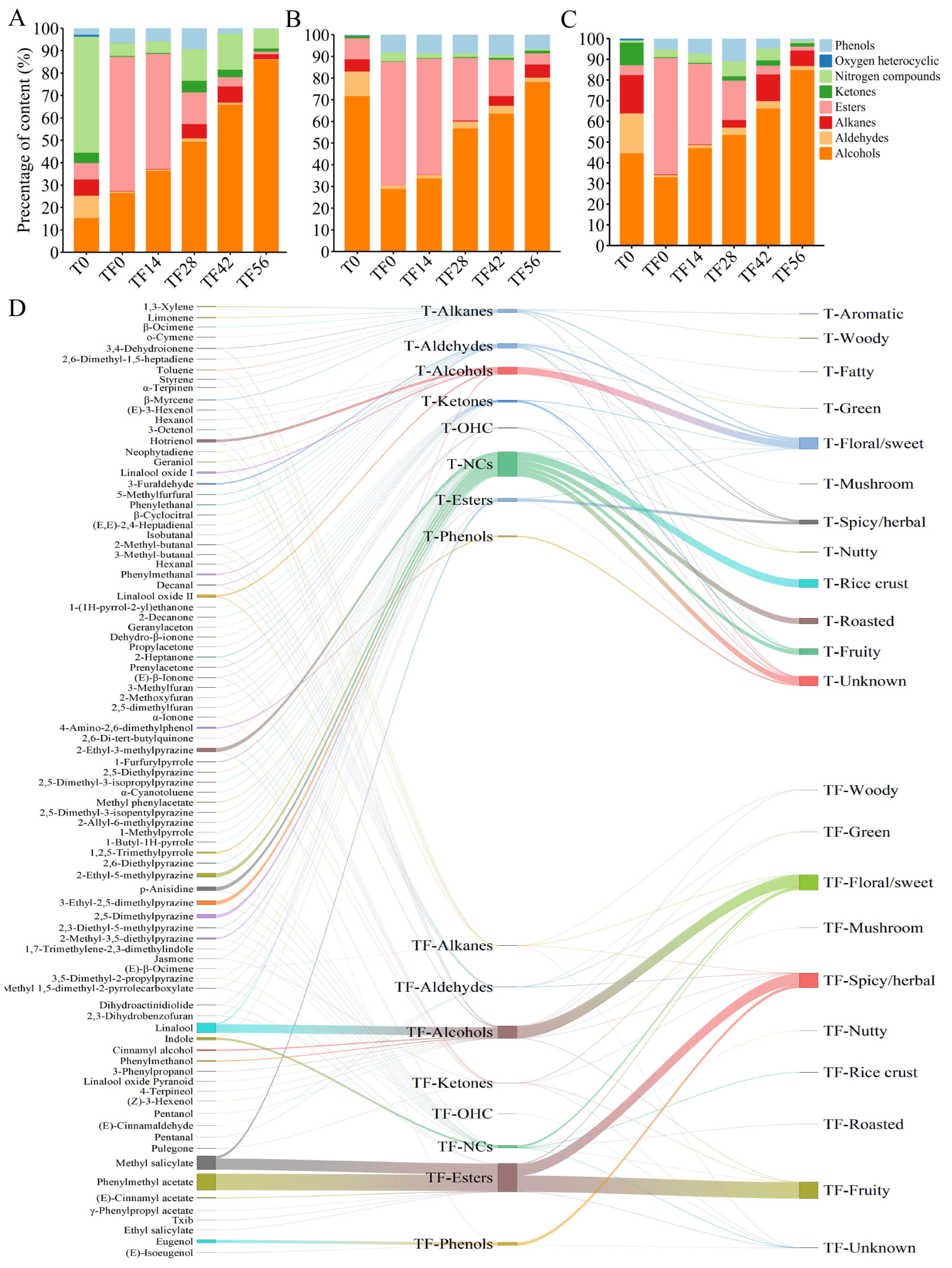
3.6. Changes of Seven Types of Volatile Substances Added After Scenting During Storage
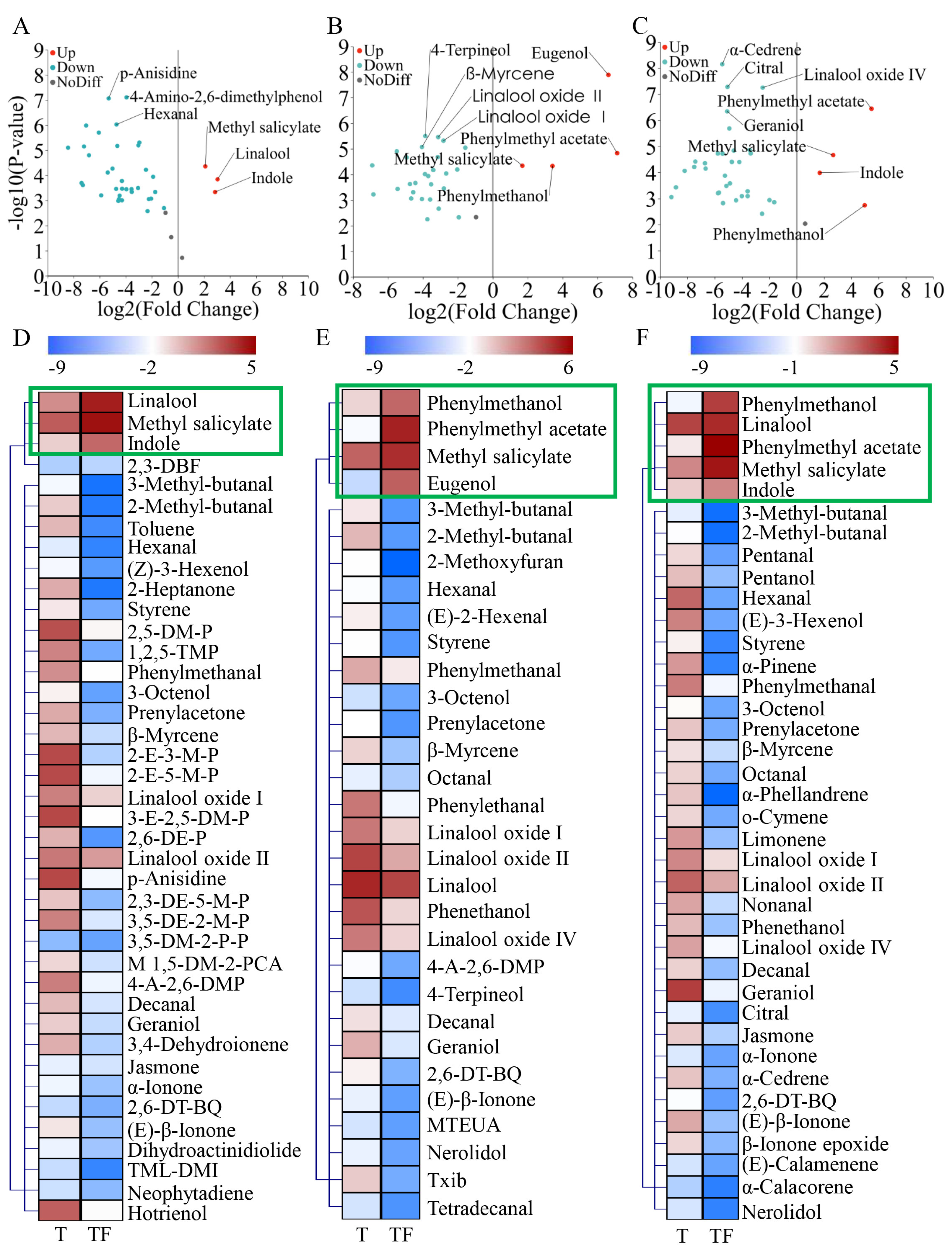
3.7. Dynamic Changes in Volatiles of Three Chimonanthus Teas During Storage
3.8. Changes in Different Volatile Compounds in Three Chimonanthus Teas During Storage After Scenting
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, N.; Mukhtar, H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, P.; Xia, T.; Wan, X. Exploring plant metabolic genomics: Chemical diversity, metabolic complexity in the biosynthesis and transport of specialized metabolites with the tea plant as a model. Crit. Rev. Biotechnol. 2020, 40, 667–688. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Q.; Qin, M.; Xu, W.; Wang, X.; Zhou, J.; He, C.; Chen, Y.; Yu, Z.; Ni, D. Study on color, aroma, and taste formation mechanism of large-leaf yellow tea during an innovative manufacturing process. Food Chem. 2024, 438, 138062. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, M.; Zhao, J.; Wang, Y.H.; Tang, Q.; Khan, I.A. Yellow tea (Camellia sinensis L.), a promising Chinese tea: Processing, chemical constituents and health benefits. Food Res. Int. 2018, 107, 567–577. [Google Scholar] [CrossRef]
- Zhao, G.; Teng, J.; Dong, R.; Ban, Q.; Yang, L.; Du, K.; Wang, Y.; Pu, H.; Yang, C.S.; Ren, Z. Alleviating effects and mechanisms of action of large-leaf yellow tea drinking on diabetes and diabetic nephropathy in mice. Food Sci. Hum. Wellness. 2023, 12, 1660–1673. [Google Scholar] [CrossRef]
- Meng, X.; Wang, J.Q.; Wang, F.; Gao, Y.; Fu, C.H.; Du, Q.; Feng, Z.H.; Chen, J.X.; Yin, J.F.; Xu, Y.Q. Moisture content of tea dhool for the scenting process affects the aroma quality and volatile compounds of osmanthus black tea. Food Chem. 2024, 438, 138051. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Q.; Cui, H.; Wang, L.; Deng, Y.; Yuan, H.; Zhu, J.; Yang, Y.; Jiang, Y. Characterization of Gardenia tea based on aroma profiles using GC-E-Nose, GC-O-MS and GC × GC-TOFMS combined with chemometrics. Bev. Plant Res. 2024, 4, e001. [Google Scholar] [CrossRef]
- Zhou, J.; Ni, D. Changes in flower aroma compounds of cultivars of Chimonanthus praecox (L.) Link and at different stages relative to Chimonanthus tea quality. Acta Hortic. Sin. 2010, 37, 1621–1628. [Google Scholar]
- Zhou, L.; Wu, S.; Chen, Y.; Huang, R.; Cheng, B.; Mao, Q.; Liu, T.; Liu, Y.; Zhao, K.; Pan, H.; et al. Multi-omics analyzes of Rosa gigantea illuminate tea scent biosynthesis and release mechanisms. Nat. Commun. 2024, 15, 8469. [Google Scholar] [CrossRef]
- Ito, Y.; Sugimoto, A.; Kakuda, T.; Kubota, K. Identification of potent odorants in Chinese jasmine green tea scented with flowers of Jasminum sambac. J. Agric. Food Chem. 2002, 50, 4878–4884. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Chen, L.; Xia, W.; Zhang, W. Phytochemical profiles and antioxidant activities of different varieties of Chimonanthus Praecox. Ind. Crop Prod. 2016, 85, 11–21. [Google Scholar] [CrossRef]
- Zuo, H.; Si, X.; Tan, M.; Li, W.; Xie, J.; Yang, W.; Chen, Z.; Zhu, M.; Zhou, Z.; Chen, C.; et al. Novel Chimonanthus teas made from scenting different tea dhools with Chimonanthus praecox flowers. Food Chem. 2025, 482, 144118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, M.; Yang, S.; Fan, W.; Lin, H.; Jin, S.; Wang, P.; Ye, N. Dynamic aroma characteristics of jasmine tea scented with single-petal jasmine “Bijian”: A comparative study with traditional double-petal jasmine. Food Chem. 2025, 464, 141735. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Y.; An, H.; Li, J.; Li, Q.; Huang, J.; Liu, Z. Analysis of volatile components of Jasmine and Jasmine tea during scenting process. Molecules. 2022, 27, 479. [Google Scholar] [CrossRef]
- Tang, M.; Liao, X.; Gu, M.; Li, P.; Hong, Y.; Xu, M.; Jin, S.; Ye, N.; Zhang, J. Analysis of aroma components in three types of Osmanthus tea scented with tea dhool based on HS-SPME-GC-MS. Mod. Food Sci. Technol. 2024, 40, 247–258. [Google Scholar]
- Guo, L.; Hu, X.; Hu, W.; Zhang, Y.; Zhu, Y.; Dai, W.; Hu, S.; Lin, Z. Volatile cmpounds and aroma characteristics of Jingui green tea and Jingui flowers. Sci. Technol. Food Ind. 2022, 43, 276–283. [Google Scholar]
- Motohashi, N.; Seetharamappa, J.; Naik, R.S. Medicinal phytochemicals and health effects of Chimonanthus Praecox (wintersweet) based on their evidences. In Occurrences, Structure, Biosynthesis, and Health Benefits Based on Their Evidences of Medicinal Phytochemicals in Vegetables and Fruits; Motohashi, N., Ed.; Nova Science Publishers: New York, NY, USA, 2018; Volume 9. [Google Scholar]
- Du, L.; Wang, C.; Li, J.; Xiao, D.; Li, C.; Xu, Y. Optimization of headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry for detecting methoxyphenolic compounds in pu-erh tea. J. Agric. Food Chem. 2013, 61, 561–568. [Google Scholar] [CrossRef]
- Meng, L.; Shi, R.; Wang, Q.; Wang, S. Analysis of floral fragrance compounds of Chimonanthus praecox with different floral colors in Yunnan, China. Separations 2021, 8, 122. [Google Scholar] [CrossRef]
- Chen, T.; Ge, Z.; Yang, X.; Wang, X.; Zuo, H.; Liao, Y.; Chen, Z.; Zhang, Z.; Chen, M.; Zhao, J.; et al. Characterization of a new Camellia plant resource with low caffeine and high theobromine for production of a novel natural low-caffeine tea. Food Chem. X. 2024, 23, 101586. [Google Scholar] [CrossRef]
- Feng, W.; Zhou, H.; Xiong, Z.; Sheng, C.; Xia, D.; Zhang, J.; Li, T.; Wei, Y.; Deng, W.W.; Ning, J. Exploring the effect of different tea varieties on the quality of Lu’an Guapian tea based on metabolomics and molecular sensory science. Food Chem. X 2024, 23, 101534. [Google Scholar] [CrossRef]
- Göksu Sürücü, C.; Tolun, A.; Halisçelik, O.; Artık, N. Brewing method-dependent changes of volatile aroma constituents of green tea (Camellia sinensis L.). Food Sci. Nutr. 2024, 12, 7186–7201. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Zhu, X.; Ouyang, W.; Yu, Y.; Chen, M.; Wang, J.; Yuan, H.; Jiang, Y. Non-target and target quantitative metabolomics with quantitative aroma evaluation reveal the influence mechanism of withering light quality on tea aroma and volatile metabolites evolution. Food Res. Int. 2024, 192, 114773. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hao, C.; Jia, H.; Zhang, J.; Wu, H.; Ning, J.; Wang, R.; Deng, W.-W. Aroma characterization and their changes during the processing of black teas from the cultivar, Camellia sinensis (L.) O. Kuntze cv. Jinmudan. J. Food Compos. Anal. 2022, 108, 104449. [Google Scholar] [CrossRef]
- Ma, L.; Gao, M.; Zhang, L.; Qiao, Y.; Li, J.; Du, L.; Zhang, H.; Wang, H. Characterization of the key aroma-active compounds in high-grade Dianhong tea using GC-MS and GC-O combined with sensory-directed flavor analysis. Food Chem. 2022, 378, 132058. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-G.; Cao, H.; Lee, M.-R.; Shen, D.-L. Analysis of volatile compounds emitted from Chimonanthus praecox (L.) Link in different florescence and QSRR study of GC retention indices. Chromatographia 2009, 70, 1153–1162. [Google Scholar] [CrossRef]
- Liu, P.; Xu, Y.-Q.; Yin, J.; Chen, G.; Wang, F.; Yuan, H.; Liu, Y. Effect of main water quality factors on volatile components of Huangshan maofeng tea with faint scent. J. Chin. Inst. Food Sci. Technol. 2016, 16, 245–257. [Google Scholar]
- Xu, L.; Ma, C.; Chen, X.; Du, Q.; Song, C.; Li, X.; Xu, Y.-Q. Theories and applications of tea residue adsorbing aroma compounds: A review. Bev. Plant Res. 2024, 4, e030. [Google Scholar] [CrossRef]
- Ayotte, P.; Smith, R.S.; Stevenson, K.P.; Dohnálek, Z.; Kimmel, G.A.; Kay, B.D. Effect of porosity on the adsorption, desorption, trapping, and release of volatile gases by amorphous solid water. J. Geophys. Res.-Planet. 2001, 106, 33387–33392. [Google Scholar] [CrossRef]
- Guo, Y.; Nie, Z.; Cao, M.; Yang, T.; Tao, G.; Song, L.; Liu, R.; Chang, M.; Wang, X. Exploring the characteristics, digestion behaviors, and nutraceutical potential of the underutilized Chimonanthus praecox (L.) link kernel oil: A combined in vitro and in vivo study. Food Chem. 2024, 455, 139898. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Xu, Z.; Wu, M.; Xia, W.; Zhang, W. Chimonanthus praecox extract/cyclodextrin inclusion complexes: Selective inclusion, enhancement of antioxidant activity and thermal stability. Ind. Crops Prod. 2017, 95, 60–65. [Google Scholar] [CrossRef]
- Bartels-Rausch, T.; Wren, S.; Schreiber, S.; Riche, F.; Schneebeli, M.; Ammann, M. Diffusion of volatile organics through porous snow: Impact of surface adsorption and grain boundaries. Atmos. Chem. Phys. 2013, 13, 6727–6739. [Google Scholar] [CrossRef]
- Guo, R.; Jiang, S.; Hu, M.; Zhan, Y.; Cheng, K.; Duan, G. Adsorption of volatile benzene series compounds by surface-modified glass fibers: Kinetics, thermodynamic adsorption efficiencies, and mechanisms. Environ. Sci. Pollut. Res. Int. 2021, 28, 30898–30907. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, X.; Zuo, H.; Li, P.; Tan, Y.; Tan, M.; Chen, Z.; Chen, C.; Chen, T.; Liu, Z.; Zhao, J. Dynamic Change of Aroma Components in Chimonanthus praecox Flower Scented Teas During Absorption and Storage. Foods 2025, 14, 1696. https://doi.org/10.3390/foods14101696
Si X, Zuo H, Li P, Tan Y, Tan M, Chen Z, Chen C, Chen T, Liu Z, Zhao J. Dynamic Change of Aroma Components in Chimonanthus praecox Flower Scented Teas During Absorption and Storage. Foods. 2025; 14(10):1696. https://doi.org/10.3390/foods14101696
Chicago/Turabian StyleSi, Xiongyuan, Hao Zuo, Penghui Li, Ye Tan, Mangmang Tan, Zhihui Chen, Changsong Chen, Taolin Chen, Zhonghua Liu, and Jian Zhao. 2025. "Dynamic Change of Aroma Components in Chimonanthus praecox Flower Scented Teas During Absorption and Storage" Foods 14, no. 10: 1696. https://doi.org/10.3390/foods14101696
APA StyleSi, X., Zuo, H., Li, P., Tan, Y., Tan, M., Chen, Z., Chen, C., Chen, T., Liu, Z., & Zhao, J. (2025). Dynamic Change of Aroma Components in Chimonanthus praecox Flower Scented Teas During Absorption and Storage. Foods, 14(10), 1696. https://doi.org/10.3390/foods14101696






