Physicochemical Composition and Bioactive Properties of Uruguayan Bee Pollen from Different Botanical Sources
Abstract
1. Introduction
2. Material and Methods
2.1. Bee Pollen Samples
2.2. Reagents
2.3. Floral Origin Determination
2.4. Physicochemical and Bioactive Properties of Pollen Samples
2.4.1. Proximate Composition
2.4.2. Fatty Acid Profile
2.4.3. Vitamin C
2.4.4. Analysis of Tocopherols
2.4.5. Total Carotenoids Determination
2.4.6. Instrumental Color
2.4.7. Total Phenol Content
2.4.8. Antioxidant Properties
2.4.9. Inhibition of Enzymes Involved in Carbohydrate and Fat Digestion
2.5. Statistical Analysis
3. Results and Discussion
3.1. Floral Origin
3.2. Physicochemical Analysis
3.3. In Vitro Bioactive Properties
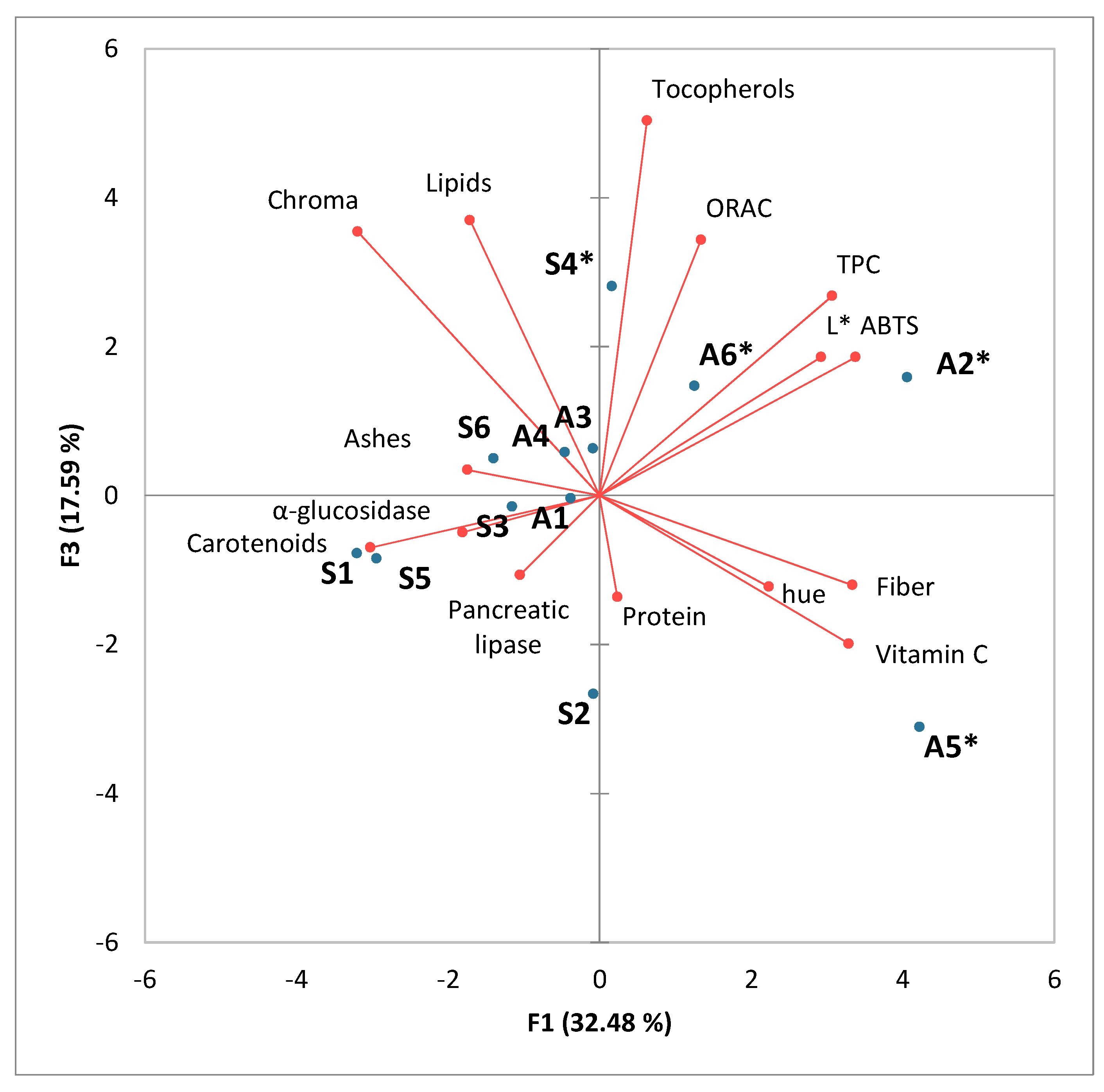
3.4. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aylanc, V.; Tomás, A.; Russo-Almeida, P.; Falcão, S.I.; Vilas-Boas, M. Assessment of Bioactive Compounds under Simulated Gastrointestinal Digestion of Bee Pollen and Bee Bread: Bioaccessibility and Antioxidant Activity. Antioxidants 2021, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Baky, M.H.; Abouelela, M.B.; Wang, K.; Farag, M.A. Bee Pollen and Bread as a Super-Food: A Comparative Review of Their Metabolome Composition and Quality Assessment in the Context of Best Recovery Conditions. Molecules 2023, 28, 715. [Google Scholar] [CrossRef]
- Keller, I.; Fluri, P.; Imdorf, A. Pollen Nutrition and Colony Development in Honey Bees: Part I. Bee World 2005, 86, 3–10. [Google Scholar] [CrossRef]
- Čeksteryte, V.; Kurtinaitiene, B.; Venskutonis, P.R.; Pukalskas, A.; Kazernavičiute, R.; Balžekas, J. Evaluation of Antioxidant Activity and Flavonoid Composition in Differently Preserved Bee Products. Czech J. Food Sci. 2016, 34, 133–142. [Google Scholar] [CrossRef]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and Bee Bread as New Health-Oriented Products: A Review. Trends Food Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Aylanc, V.; Larbi, S.; Calhelha, R.; Barros, L.; Rezouga, F.; Rodríguez-Flores, M.S.; Seijo, M.C.; El Ghouizi, A.; Lyoussi, B.; Falcão, S.I.; et al. Evaluation of Antioxidant and Anticancer Activity of Mono- and Polyfloral Moroccan Bee Pollen by Characterizing Phenolic and Volatile Compounds. Molecules 2023, 28, 835. [Google Scholar] [CrossRef]
- Hernández-Camacho, J.D.; Bernier, M.; López-Lluch, G.; Navas, P. Coenzyme Q10 Supplementation in Aging and Disease. Front. Physiol. 2018, 9, 316577. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Eid, N.; Abd El-Wahed, A.A.; Rateb, M.E.; Afifi, H.S.; Algethami, A.F.; Zhao, C.; Al Naggar, Y.; Alsharif, S.M.; Tahir, H.E.; et al. Honey Bee Products: Preclinical and Clinical Studies of Their Anti-Inflammatory and Immunomodulatory Properties. Front. Nutr. 2022, 8, 761267. [Google Scholar] [CrossRef]
- Dolezal, A.G.; Toth, A.L. Feedbacks between Nutrition and Disease in Honey Bee Health. Curr. Opin. Insect Sci. 2018, 26, 114–119. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Kabała-Dzik, A.; Kubina, R.; Jasik, K.; Kajor, M.; Wrześniok, D.; Stojko, J. Protective Effect of Polyphenol-Rich Extract from Bee Pollen in a High-Fat Diet. Molecules 2018, 23, 805. [Google Scholar] [CrossRef]
- El Ghouizi, A.; Bakour, M.; Laaroussi, H.; Ousaaid, D.; El Menyiy, N.; Hano, C.; Lyoussi, B. Bee Pollen as Functional Food: Insights into Its Composition and Therapeutic Properties. Antioxidants 2023, 12, 557. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Hu, J.; Huang, W.; Zhu, L.; Shao, M.; Dordoe, C.; Ahn, Y.J.; Wang, D.; Zhao, Y.; Xiong, Y.; et al. The Botanical Origin and Antioxidant. Anti-BACE1 and Antiproliferative Properties of Bee Pollen from Different Regions of South Korea. BMC Complement. Med. Ther. 2020, 20, 236. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, A.; Rodrigues, S.; Teixeira, A.; Feás, X.; Estevinho, L.M. Biological Activities of Commercial Bee Pollens: Antimicrobial. Antimutagenic. Antioxidant and Anti-Inflammatory. Food Chem. Toxicol. 2014, 63, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Komosinska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, L.; Olczyk, K. Bee Pollen: Chemical Composition and Therapeutic Application. Evid.-Based Complement. Altern. Med. 2015, 2015, 297425. [Google Scholar] [CrossRef]
- Ilie, C.I.; Oprea, E.; Geana, E.I.; Spoiala, A.; Buleandra, M.; Pircalabioru, G.G.; Badea, I.A.; Ficai, D.; Andronescu, E.; Ficai, A.; et al. Bee Pollen Extracts: Chemical Composition. Antioxidant Properties. and Effect on the Growth of Selected Probiotic and Pathogenic Bacteria. Antioxidants 2022, 11, 959. [Google Scholar] [CrossRef]
- Santos, E.; Invernizzi, C.; García, E.; Cabrera, C.; Landro, R.D.; Saadoun, A.; Daners, G. Crude Protein Content of Pollen from the Main Botanical Species Used by Honeybees in Uruguay. Agrociencia 2009, 13, 9–13. [Google Scholar] [CrossRef]
- Invernizzi, C.; Antúnez, K.; Arredondo, D.; Juri, P.; Mendoza, Y.; Nogueira, E.; Salvarrey, S. Sanitary Situation of Honey Bees in Uruguay: Novelties of the Last Decade. Veterinaria 2022, 58, 1–17. [Google Scholar] [CrossRef]
- Branchiccela, B.; Castelli, L.; Díaz-Cetti, S.; Invernizzi, C.; Mendoza, Y.; Santos, E.; Silva, C.; Zunino, P.; Antúnez, K. Can Pollen Supplementation Mitigate the Impact of Nutritional Stress on Honey Bee Colonies? J. Apic. Res. 2023, 62, 294–302. [Google Scholar] [CrossRef]
- Antúnez, K.; Anido, M.; Branchiccela, B.; Harriet, J.; Campa, J.; Invernizzi, C.; Santos, E.; Higes, M.; Martín-Hernández, R.; Zunino, P. Seasonal Variation of Honeybee Pathogens and Its Association with Pollen Diversity in Uruguay. Microb. Ecol. 2015, 70, 522–533. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- AOAC Official Method 925.09, Moisture—Vac Oven 100 (Food General). In AOAC Official Methods of Analysis; AOAC International: San Diego, CA, USA, 2023.
- AOAC Official Method 2001.11, Protein (Crude) in Animal Feed. Forage (Plant Tissue). Grain and Oilseeds. In AOAC Official Methods of Analysis; AOAC International: San Diego, CA, USA, 2019.
- Hara, A.; Radin, N.S. Lipid Extraction of Tissues with a Low-Toxicity Solvent. Anal. Biochem. 1978, 90, 420–426. [Google Scholar] [CrossRef] [PubMed]
- de Arruda, V.A.S.; Pereira, A.A.S.; de Freitas, A.S.; Barth, O.M.; de Almeida-Muradian, L.B. Dried Bee Pollen: B Complex Vitamins. Physicochemical and Botanical Composition. J. Food Compos. Anal. 2013, 29, 100–105. [Google Scholar] [CrossRef]
- AOAC Official Method 985.29, Total Dietary Fiber in Foods Enzymatic-Gravimetric Method. In AOAC Official Methods of Analysis; AOAC International: San Diego, CA, USA, 1997.
- MERCOSUR. MERCOSUR/GMC/RES. No46/03, Reglamento Técnico MERCOSUR Sobre El Rotulado Nutricional de Alimentos Envasados. 2003. Available online: https://www.argentina.gob.ar/normativa/nacional/resoluci%C3%B3n-46-2003-94928 (accessed on 7 May 2025).
- IUPAC Standard Method 2.301, Preparation of Fatty Acid Methyl Ester. In Standard Methods for Analysis of Oils. Fats and Derivatives; Blackwell: Oxford, UK, 1987.
- AOAC Official Method 967.21 Ascorbic Acid in Vitamin Preparations and Juices. In AOAC Official Methods of Analysis; AOAC International: San Diego, CA, USA, 2006.
- Andrikopoulos, N.K.; Brueschweiler, H.; Felber, H.; Taeschler, C. HPLC Analysis of Phenolic Antioxidants, Tocopherols and Triglycerides. J. Am. Oil Chem. Soc. 1991, 68, 359–364. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001; ISBN 1578810728. [Google Scholar]
- Machado De-Melo, A.A.; Fernandes Estevinho, M.L.M.; Gasparotto Sattler, J.A.; Rodrigues Souza, B.; da Silva Freitas, A.; Barth, O.M.; Almeida-Muradian, L.B. Effect of Processing Conditions on Characteristics of Dehydrated Bee-Pollen and Correlation between Quality Parameters. LWT—Food Sci. Technol. 2016, 65, 808–815. [Google Scholar] [CrossRef]
- Salazar-González, C.Y.; Rodríguez-Pulido, F.J.; Terrab, A.; Díaz-Moreno, C.; Fuenmayor, C.A.; Heredia, F.J. Analysis of Multifloral Bee Pollen Pellets by Advanced Digital Imaging Applied to Functional Food Ingredients. Plant Foods Hum. Nutr. 2018, 73, 328–335. [Google Scholar] [CrossRef]
- Fernández-Fernández, A.M.; Dellacassa, E.; Nardin, T.; Larcher, R.; Ibañez, C.; Terán, D.; Gámbaro, A.; Medrano-Fernandez, A.; Del Castillo, M.D. Tannat Grape Skin: A Feasible Ingredient for the Formulation of Snacks with Potential for Reducing the Risk of Diabetes. Nutrients 2022, 14, 419. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Santillan Cornejo, F.; Fernandez-Gomez, B.; Vera, G.; Guisantes-Batan, E.; Gomez Alonso, S.; San Andres, M.I.; Sanchez-Fortun, S.; Lopez-Gomez, L.; Uranga, J.A.; et al. Bioaccesibility. Metabolism. and Excretion of Lipids Composing Spent Coffee Grounds. Nutrients 2019, 11, 1411. [Google Scholar] [CrossRef]
- MSP Miel y Productos Relacionados. Disposiciones Particulares Para Polen. In Reglamento Bromatológico Nacional; Ministerio de Salud Pública: Montevideo, Uruguay, 1994. [Google Scholar]
- Szczesna, T.; Rybak-Chmielewska, H.; Chmielewski, W. Effect of Infestation of Pollen Loads with Acarid Mites on Amino Acid Content and Organoleptic Characteristics of the Product. Pszczel. Zesz. Nauk. 1999, 43, 235–245. [Google Scholar]
- Bogdanov, S.; Bieri, K.; Gremaud, G.; Iff, D.; Känzig, A.; Seiler, K.; Zürcher, K. Pollen Bienenprodukte. BAG. In Swiss Food Manual; Swiss Federal Office for Public Health: Berne, Switzerland, 2004. [Google Scholar]
- Ministério da Agricultura e Pecuária. Brasil Instrução Normativa SDA N° 03, de 19 de Janeiro de 2001—Regulamento Técnicos de Identidade e Qualidade de Apitoxina. Cera de Abelha. Geleia Real. Geleia Real Liofilizada. Polén Apícola. Propólis e Extrato de Propólis; Ministério da Agricultura e Pecuária: Brasília, Brazil, 2001.
- PN-R-78893; “Obnóza Pylkowe”—Polish Legislation for Bee-Pollen. Ministry of Agriculture and Rural Development: Warsaw, Poland, 2003.
- Campos, M.G.R.; Bogdanov, S.; de Almeida-Muradian, L.B.; Szczesna, T.; Mancebo, Y.; Frigerio, C.; Ferreira, F. Pollen Composition and Standardisation of Analytical Methods. J. Apic. Res. Bee World 2008, 47, 156–163. [Google Scholar] [CrossRef]
- Gasparotto Sattler, J.A.; Pereira de Melo, I.L.; Granato, D.; Araújo, E.; da Silva de Freitas, A.; Barth, O.M.; Sattler, A.; de Almeida-Muradian, L.B. Impact of Origin on Bioactive Compounds and Nutritional Composition of Bee Pollen from Southern Brazil: A Screening Study. Food Res. Int. 2015, 77, 82–91. [Google Scholar] [CrossRef]
- Gardana, C.; Del Bo, C.; Quicazán, M.C.; Corrrea, A.R.; Simonetti, P. Nutrients. Phytochemicals and Botanical Origin of Commercial Bee Pollen from Different Geographical Areas. J. Food Compos. Anal. 2018, 73, 29–38. [Google Scholar] [CrossRef]
- Castiglioni, S.; Astolfi, P.; Conti, C.; Monaci, E.; Stefano, M.; Carloni, P. Spectroscopic Properties of Bee Pollen Loads from Di Ff Erent Botanical Origin. Molecules 2019, 24, 3974. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Nanda, V. Composition and Functionality of Bee Pollen: A Review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Prdun, S.; Svečnjak, L.; Valentić, M.; Marijanović, Z.; Jerković, I. Characterization of Bee Pollen: Physico-Chemical Properties. Headspace Composition and Ftir Spectral Profiles. Foods 2021, 10, 2103. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Mărghitaş, L.A.; Dezmirean, D.S.; Dulf, F.V.; Bunea, A.; Socaci, S.A.; Bobiş, O. Predominant and Secondary Pollen Botanical Origins Influence the Carotenoid and Fatty Acid Profile in Fresh Honeybee-Collected Pollen. J. Agric. Food Chem. 2014, 62, 6306–6316. [Google Scholar] [CrossRef]
- Oroian, M.; Dranca, F.; Ursachi, F. Characterization of Romanian Bee Pollen—An Important Nutritional Source. Foods 2022, 11, 2633. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.C.L.S.; Moriya, M.; Azedo, R.A.B.; De Almeida-Muradian, L.B.; De, A.C.; Moreti, C.C. Relationship between Botanical Origin and Antioxidant Vitamins of Bee-Collected Pollen. Quim. Nova 2009, 32, 1099–1102. [Google Scholar] [CrossRef]
- Fatrcová-Šramková, K.; Nôžková, J.; Máriássyová, M.; Kačániová, M. Biologically Active Antimicrobial and Antioxidant Substances in the Helianthus annuus L. Bee Pollen. J. Environ. Sci. Health Part B 2016, 51, 176–181. [Google Scholar] [CrossRef]
- Song, X.D.; Mujumdar, A.S.; Law, C.L.; Fang, X.M.; Peng, W.J.; Deng, L.Z.; Wang, J.; Xiao, H.W. Effect of Drying Air Temperature on Drying Kinetics. Color. Carotenoid Content. Antioxidant Capacity and Oxidation of Fat for Lotus Pollen. Dry. Technol. 2020, 38, 1151–1164. [Google Scholar] [CrossRef]
- Abirached, C.; Bonifacino, C.; Dutto, E.; Velazco, L.; Jorge, F.; Vieitez, I. Study of Sesame Seeds Antioxidant and Emulsifying Properties: Original High-Quality Research Paper. J. Supercrit. Fluids 2020, 166, 104994. [Google Scholar] [CrossRef]
- Dauber, C.; Carreras, T.; González, L.; Gámbaro, A.; Valdés, A.; Ibañez, E.; Vieitez, I. Characterization and Incorporation of Extracts from Olive Leaves Obtained through Maceration and Supercritical Extraction in Canola Oil: Oxidative Stability Evaluation. LWT—Food Sci. Technol. 2022, 160, 113274. [Google Scholar] [CrossRef]
- Pereira De Melo, I.L.; Silva De Freitas, A.; Barth, O.M.; Bicudo De Almeida-Muradian, L. Correlation between Nutritional Composition and Floral Origin of Dried Bee Pollen. Rev. Inst. Adolfo Lutz 2009, 68, 346–353. [Google Scholar]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- See, X.Z.; Yeo, W.S.; Saptoro, A. A Comprehensive Review and Recent Advances of Vitamin C: Overview. Functions. Sources. Applications. Market Survey and Processes. Chem. Eng. Res. Des. 2024, 206, 108–129. [Google Scholar] [CrossRef]
- Bleha, R.; Shevtsova, T.; Kružík, V.; Brindza, J.; Sinica, A. Morphology. Physicochemical Properties and Antioxidant Capacity of Bee Pollens. Czech J. Food Sci. 2019, 37, 1–8. [Google Scholar] [CrossRef]
- Sebii, H.; Karra, S.; Bchir, B.; Ghribi, A.; Danthine, S.; Blecker, C.; Attia, H.; Besbes, S. Physico-Chemical. Surface and Thermal Properties of Date Palm Pollen as a Novel Nutritive Ingredient. Adv. Food Technol. Nutr. Sci. 2019, 5, 84–91. [Google Scholar] [CrossRef]
- Salazar-González, C.Y.; Stinco, C.M.; Rodríguez-Pulido, F.J.; Díaz-Moreno, C.; Fuenmayor, C.; Heredia, F.J.; González-Miret, M.L. Characterization of Carotenoid Profile and α-Tocopherol Content in Andean Bee Pollen Influenced by Harvest Time and Particle Size. LWT 2022, 170, 114065. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Stranț, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef]
- Lawag, I.L.; Yoo, O.; Lim, L.Y.; Hammer, K.; Locher, C. Optimisation of Bee Pollen Extraction to Maximise Extractable Antioxidant Constituents. Antioxidants 2021, 10, 1113. [Google Scholar] [CrossRef]
- Abdelsalam, E.; Foda, H.S.; Abdel-Aziz, M.S.; Abd El-Hady, F.K. Antioxidant and Antimicrobial Activities of Egyptian Bee Pollen. Middle East J. Appl. Sci. 2018, 8, 1248–1255. [Google Scholar]
- Castiglioni, S.; Stefano, M.; Astolfi, P.; Pisani, M.; Carloni, P. Characterisation of Bee Pollen from the Marche Region (Italy) According to the Botanical and Geographical Origin with Analysis of Antioxidant Activity and Colour. Using a Chemometric Approach. Molecules 2022, 27, 7996. [Google Scholar] [CrossRef] [PubMed]
- Feas, X.; Vazquez-Tato, M.P.; Estevinho, L.; Seijas, J.A.; Iglesias, A. Organic Bee Pollen: Botanical Origin. Nutritional Value. Bioactive Compounds. Antioxidant Activity and Microbiological Quality. Molecules 2012, 17, 8359–8377. [Google Scholar] [CrossRef] [PubMed]
- Soares de Arruda, V.A.; Vieria dos Santos, A.; Figueiredo Sampaio, D.; da Silva Araújo, E.; de Castro Peixoto, A.L.; Estevinho, L.M.; de Almeida-Muradian, L.B. Brazilian Bee Pollen: Phenolic Content. Antioxidant Properties and Antimicrobial Activity. J. Apic. Res. 2021, 60, 775–783. [Google Scholar] [CrossRef]
- Gabriele, M.; Parri, E.; Felicioli, A.; Sagona, S.; Pozzo, L.; Biondi, C.; Domenici, V.; Pucci, L. Phytochemical composition and antioxidant activity of tuscan bee pollen of different botanic origins. Ital. J. Food Sci. 2015, 27, 248–259. [Google Scholar]
- Gonçalves, A.C.; Lahlou, R.A.; Alves, G.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Potential Activity of Abrantes Pollen Extract: Biochemical and Cellular Model Studies. Foods 2021, 10, 2804. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.G.; Nahar, L.; Sarker, S.D.; Guo, Z.; Cao, W.; Zou, X.; et al. Bee Pollen: Current Status and Therapeutic Potential. Nutrients 2021, 13, 1876. [Google Scholar] [CrossRef]
- Laaroussi, H.; Ferreira-Santos, P.; Genisheva, Z.; Bakour, M.; Ousaaid, D.; El Ghouizi, A.; Teixeira, J.A.; Lyoussi, B. Unveiling the Techno-Functional and Bioactive Properties of Bee Pollen as an Added-Value Food Ingredient. Food Chem. 2023, 405, 134958. [Google Scholar] [CrossRef]
- Fernández-Fernández, A.M.; Dellacassa, E.; Nardin, T.; Larcher, R.; Gámbaro, A.; Medrano-Fernandez, A.; Del Castillo, M.D. In Vitro Bioaccessibility of Bioactive Compounds from Citrus Pomaces and Orange Pomace Biscuits. Molecules 2021, 26, 3480. [Google Scholar] [CrossRef]
- Herrera, T.; Iriondo-DeHond, M.; Ramos Sanz, A.; Bautista, A.I.; Miguel, E. Effect of Wild Strawberry Tree and Hawthorn Extracts Fortification on Functional. Physicochemical. Microbiological. and Sensory Properties of Yogurt. Foods 2023, 12, 3332. [Google Scholar] [CrossRef]
- Fernández-Fernández, A.M.; Iriondo-DeHond, A.; Dellacassa, E.; Medrano-Fernandez, A.; del Castillo, M.D. Assessment of Antioxidant. Antidiabetic. Antiobesity. and Anti-Inflammatory Properties of a Tannat Winemaking by-Product. Eur. Food Res. Technol. 2019, 245, 1539–1551. [Google Scholar] [CrossRef]
- Salonen, A.; Lavola, A.; Virjamo, V.; Julkunen-Tiitto, R. Protein and Phenolic Content and Antioxidant Capacity of Honey Bee-Collected Unifloral Pollen Pellets from Finland. J. Apic. Res. 2021, 60, 744–750. [Google Scholar] [CrossRef]
- Serea, D.; Condurache, N.N.; Aprodu, I.; Constantin, O.E.; Bahrim, G.E.; Stănciuc, N.; Stanciu, S.; Rapeanu, G. Thermal Stability and Inhibitory Action of Red Grape Skin Phytochemicals against Enzymes Associated with Metabolic Syndrome. Antioxidants 2022, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Quiles, J.L.; Cianciosi, D.; Forbes-Hernández, T.Y.; Orantes-Bermejo, F.J.; Alvarez-Suarez, J.M.; Battino, M. Bee Products: An Emblematic Example of Underutilized Sources of Bioactive Compounds. J. Agric. Food Chem. 2022, 70, 6833–6848. [Google Scholar] [CrossRef]
- Irazusta, A.; Caccavello, R.; Panizzolo, L.; Gugliucci, A.; Medrano, A. The Potential Use of Mentha x Piperita L. Peumus Boldus Mol. and Baccharis Trimera Iless. Extracts as Functional Food Ingredients. Int. J. Food Nutr. Res. 2018, 2, 14. [Google Scholar] [CrossRef][Green Version]
- Naguib, Y.M.; Hari, S.P.; Passwater, R., Jr.; Huang, D. Antioxidant Activities of Natural Vitamin E Formulations. J. Nutr. Sci. Vitaminol. 2003, 49, 217–220. [Google Scholar] [CrossRef]
- Sarungallo, Z.L.; Hariyadi, P.; Andarwulan, N.; Purnomo, E.H.; Wada, M. Analysis of α-Cryptoxanthin. β-Cryptoxanthin. α -Carotene. and β-Carotene of Pandanus Conoideus Oil by High-Performance Liquid Chromatography (HPLC). Procedia Food Sci. 2015, 3, 231–243. [Google Scholar] [CrossRef]

| Botanical Family | Common Name | Scientific Name | A1 | A2 * | A3 | A4 | A5 * | A6 * |
|---|---|---|---|---|---|---|---|---|
| Asteraceae 1 | Chirca/Carqueja | Baccharis sp. | 18.5 | 9.5 | 24.3 | 31.2 | 8.1 | |
| Asteraceae 2 | Chirca/Carqueja | Baccharis sp. | 19.5 | 16.1 | 28.4 | 9.3 | ||
| Asteraceae 3 | Chirca/Carqueja | T. Eupatorium buniifolium | 30.3 | 14.7 | 28.8 | |||
| Myrtaceae | T. Eucalyptus | T. Eucalyptus spp. | 8.5 | 100.0 | 75.2 | |||
| Apiaceae | T. Caraguatá | T. Eryngium sp. | 2.2 | 3.6 | ||||
| Asteraceae | Picris | Picris echioides | 21.0 | 5.2 | 7.1 | |||
| Asteraceae | Dandelion | Taraxacum officinale | 8.4 | |||||
| Arecaceae | Palm | Butia capitata | 15.4 | 28.8 | 7.2 | |||
| Lamiaceae | Mint | T. Mentha piperita | 2.4 | |||||
| Casuarinaceae | Casuarina | Casuarina cunninghamiana | 75.1 | |||||
| Brassicaceae | Raddish | Raphanus raphanistrum | 7.3 | 1.2 | ||||
| Poaceae | Grass | - | 0.8 | 3.2 | ||||
| Caprifoliaceae | Honeysuckle | Lonicera japonica | 4.5 | |||||
| Fabaceae | Ibirapitá | Peltophorum dubium | 7.3 | |||||
| Total General (%) | 100 | 100 | 100 | 100 | 100 | 100 |
| Botanical Family | Common Name | Scientific Name | S1 | S2 | S3 | S4 * | S5 | S6 |
|---|---|---|---|---|---|---|---|---|
| Fabaceae | Lotus | Lotus sp. | 15.5 | 2.0 | 10.2 | |||
| Boraginaceae | Borage | Echium plantagineum | 7.4 | 4.5 | ||||
| Asteraceae | Senecio | Senecio spp. | 8.1 | 4.8 | 6.7 | |||
| Myrtaceae | Eucalyptus | Eucalyptus sp. | 15.6 | 25.6 | 28.3 | 12.1 | 15.9 | |
| Fabaceae | Red Clover | Trifolium pratense | 1.9 | 4.5 | ||||
| Fabaceae | White Clover | Trifolium repens | 18.2 | 17.5 | 1.0 | 6.3 | ||
| Caprifoliaceae | Honeysuckle | Lonicera japonica | 5.7 | 2.4 | 4.5 | 3.2 | ||
| Anacardiaceae | Molle | Schinus longifolius | 14.2 | 6.4 | ||||
| Arecaceae | Palm | - | 3 | 4.7 | 7.8 | |||
| Rosaceae | - | - | 8.3 | 3.1 | ||||
| Asteraceae | Chicory | Cichorium intybus | 2.1 | |||||
| Asteraceae | Carqueja | Baccharis sp. | 10.2 | |||||
| Asteraceae 1 | Carqueja | Baccharis sp. | 12.0 | 5.8 | 9.1 | 7.6 | ||
| Asteraceae 2 | Carqueja | Baccharis sp. | 5.2 | |||||
| Fabaceae | Acacia de Chaucha | Gleditsia triacanthos | 10.4 | 20.2 | 9.8 | |||
| Salicaceae | Willow | Salix spp. | 28.7 | 16.7 | ||||
| Brassicaceae | Raddish | Raphanus raphanistrum | 6.3 | |||||
| Asteraceae | Thistle | T. Cirsium vulgare | 3.4 | 2.0 | ||||
| Unidentified | - | - | 3.5 | |||||
| Fabaceae | Cina cina | Parkinsonia aculeata | 4.8 | |||||
| Apiaceae | Caraguatá | T. Eryngium horridum. | 8.9 | |||||
| Brassicaceae | Rapeseed | Brassica spp. | 100.0 | |||||
| Liliaceae | - | - | 9.0 | |||||
| Onagraceae | Water Flower | Ludwigia peploides | 2.3 | |||||
| Asteraceae | Picris | Picris echioides | 12.3 | |||||
| Sapindaceae | Chal-chal | Allophylus edulis | 8.2 | 10.2 | ||||
| Cannabaceae | Tala | Celtis sp. | 6.4 | |||||
| Myrtaceae | Pitanga | Eugenia uniflora | 13.1 | |||||
| Fabaceae | Ñapinda | Acacia bonariensis | 2.4 | |||||
| Total General | 100 | 100 | 100 | 100 | 100 | 100 |
| Sample | Moisture (%) | Protein (%) | Lipids (%) | Ash (%) | Total Fiber (%) |
|---|---|---|---|---|---|
| A1 | 7.69 ± 0.02 f | 16.87 ± 0.22 b | 8.49 ± 0.06 ef | 1.95 ± 0.02 ab | 14.98 ± 0.15 e |
| A2 * | 6.05 ± 0.05 a | 16.77 ± 0.44 b | 9.15 ± 0.25 g | 1.94 ± 0.01ab | 16.76 ± 0.11 f |
| A3 | 7.44 ± 0.19 ef | 16.87 ± 0.37 b | 8.41 ± 0.23 def | 2.02 ± 0.03 ab | 13.10 ± 0.21 cd |
| A4 | 7.45 ± 0.16 ef | 17.43 ± 0.20 b | 9.01 ± 0.12 fg | 1.90 ± 0.02 a | 12.95 ± 0.12 bc |
| A5 * | 6.80 ± 0.08 bc | 23.26 ± 0.12 c | 4.37 ± 0.34 a | 2.06 ± 0.03 bc | 18.60 ± 0.16 g |
| A6 * | 6.73 ± 0.12 bc | 24.21 ± 0.27 cd | 6.90 ± 0.38 b | 2.18 ± 0.01cd | 14.55 ± 0.15 e |
| S1 | 7.40 ± 0.18 def | 24.72 ± 0.55 d | 8.24 ± 0.22 cde | 2.70 ± 0.02 f | 13.47 ± 0.12 cd |
| S2 | 6.51 ± 0.21 b | 23.54 ± 0.20 cd | 7.69 ± 0.13 c | 2.40 ± 0.03e | 13.12 ± 0.13 cd |
| S3 | 7.35 ± 0.16 def | 24.40 ± 0.10 cd | 8.73 ± 0.11 efg | 2.93 ± 0.05 g | 10.18 ± 0.19 a |
| S4 * | 7.06 ± 0.20 cd | 23.31 ± 0.12 c | 13.17 ± 0.20 i | 2.80 ± 0.01 fg | 13.14 ± 0.17 cd |
| S5 | 6.67 ± 0.03 b | 17.25 ± 0.38 b | 10.73 ± 0.12 h | 2.17 ± 0.08 cd | 12.46 ± 0.22 b |
| S6 | 7.24 ± 0.12 de | 13.62 ± 0.15 a | 7.86 ± 0.23 cd | 2.20 ± 0.04 d | 13.52 ± 0.18 d |
| Significance Level | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Fatty Acid | Sample | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 * | A3 | A4 | A5 * | A6 * | S1 | S2 | S3 | S4 * | S5 | S6 | |
| 4:0 | 0.1 | - | 0.2 | 0.3 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 |
| 6:0 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.3 | 0.2 | 0.1 | 0.2 | 0.2 |
| 8:0 | 2.0 | 0.2 | 1.0 | 1.3 | 0.2 | 0.5 | 0.2 | 0.9 | 0.3 | 0.1 | 0.8 | 1.2 |
| 10:0 | 0.4 | 0.1 | 0.2 | 0.8 | 0.8 | 0.5 | 0.8 | 0.8 | 0.5 | 0.2 | 0.4 | 0.2 |
| 12:0 | 1.2 | 0.2 | 0.3 | 0.7 | 0.6 | 2.8 | 2.0 | 1.3 | 1.0 | 0.4 | 2.4 | 0.5 |
| 14:0 | 0.6 | 0.3 | 0.6 | 1.3 | 1.8 | 0.9 | 1.7 | 3.3 | 2.4 | 2.5 | 0.3 | 1.0 |
| 16:0 | 22.6 | 27.9 | 22.1 | 22.8 | 10.4 | 16.2 | 19.3 | 22.5 | 18.8 | 17.9 | 21.3 | 22.5 |
| 17:0 | 1.3 | 0.2 | 1.0 | 1.1 | 1.3 | 2.4 | 3.8 | 2.6 | 2.2 | 5.8 | 2.7 | 1.1 |
| 18:0 | 2.0 | 2.5 | 2.9 | 3.5 | 4.2 | 2.0 | 3.4 | 4.5 | 3.1 | 2.1 | 2.6 | 6.8 |
| 18:1 n-9 | 8.6 | 10.6 | 15.5 | 9.9 | 27.9 | 13.0 | 11.2 | 12.1 | 12.1 | 9.1 | 6.1 | 11.3 |
| 18:2 trans | 1.8 | - | 2.2 | 1.9 | 5.6 | 3.5 | 1.2 | 1.1 | 3.9 | 1.5 | 0.9 | 2.9 |
| 18:2 c n-6 | 19.0 | 39.5 | 17.5 | 19.7 | 15.7 | 16.3 | 15.8 | 14.4 | 11.4 | 5.6 | 14.6 | 24.3 |
| 20:0 | 0.5 | 0.4 | 0.6 | 0.4 | - | 1.0 | 1.0 | 0.5 | 0.5 | 0.4 | 0.9 | 0.7 |
| 18:3 n-3 | 19.2 | 16.2 | 22.0 | 25.4 | 4.8 | 21.3 | 25.2 | 24.4 | 35.7 | 50.7 | 32.3 | 18.8 |
| 20:2 n6 | 0.8 | - | - | - | 1.7 | 2.0 | 1.1 | 0.8 | 0.6 | - | 1.3 | 1.1 |
| 22:0 | 0.6 | 0.3 | 0.7 | 0.7 | - | 0.6 | 0.8 | 0.3 | 0.3 | 0.1 | 0.7 | 0.9 |
| 20:3 n-6 | 0.4 | - | 0.6 | - | 0.4 | 0.5 | - | - | - | - | - | - |
| 20:3 n-3 | 2.2 | 0.1 | 0.7 | 0.4 | - | - | 0.7 | 0.3 | 0.1 | 0.6 | 0.9 | 0.8 |
| 23:0 | 0.4 | 0.1 | 0.5 | 0.4 | 0.4 | 0.6 | 0.8 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| 22:2 n6 | 1.6 | 0.1 | 0.8 | 0.8 | 0.7 | 2.4 | 1.2 | 0.1 | 0.3 | 0.1 | 0.3 | 0.4 |
| 24:0 | 2.9 | 0.2 | 1.2 | 0.7 | 0.1 | 0.3 | 0.6 | 0.8 | 0.2 | 0.1 | 0.7 | 1.2 |
| 20:5 n-3 | 0.3 | - | 0.4 | 0.2 | 0.7 | 0.4 | 0.9 | 0.4 | 0.2 | 1.0 | 0.3 | 0.2 |
| 24:1 n-9 | 1.2 | 0.1 | 0.2 | 0.1 | 0.1 | 0.3 | 0.6 | 0.5 | 0.1 | 0.2 | 0.4 | 0.2 |
| 22:6 n-3 | 0.6 | 0.1 | 0.9 | 1.2 | 2.0 | 1.2 | 0.4 | 0.4 | 0.1 | 0.1 | 0.5 | 0.4 |
| Total identified | 90.6 | 99.2 | 92.5 | 93.8 | 91.4 | 90.0 | 93.1 | 92.7 | 94.3 | 98.8 | 91.8 | 96.8 |
| Unidentified | 9.4 | 0.8 | 7.5 | 6.2 | 8.6 | 10.0 | 6.9 | 7.3 | 5.7 | 1.2 | 8.2 | 3.2 |
| SFA (%) | 34.9 | 32.3 | 31.6 | 34.2 | 31.9 | 27.9 | 34.9 | 38.1 | 29.8 | 30.0 | 33.4 | 36.5 |
| MUFA (%) | 9.8 | 10.8 | 15.8 | 9.9 | 28.0 | 14.6 | 11.8 | 12.6 | 12.2 | 9.3 | 6.5 | 11.5 |
| PUFA (%) | 41.9 | 55.9 | 42.2 | 47.4 | 25.9 | 44.1 | 45.2 | 40.7 | 48.2 | 57.9 | 49.9 | 45.5 |
| Sample | L* | Chroma (C*ab) | Hue (hab) | Total Carotenoid (µg/g) | Tocopherols (µg α-Tocopherol/g) | Vitamin C (mg AA/g) |
|---|---|---|---|---|---|---|
| A1 | 62.49 ± 0.97 fg | 54.46 ± 0.85 cde | 1.43 ± 004 c | 95.27 ± 6.04 e | 3.54 ± 0.26 f | 0.27 ± 0.01 d |
| A2 * | 63.58 ± 0.49 fgh | 45.29 ± 0.23 b | 1.49 ± 0.01 fg | 50.77 ± 3.48 c | 3.97 ± 0.24 g | 0.48 ± 0.01 g |
| A3 | 63.92 ± 0.77 fgh | 53.29 ± 0.13 cde | 1.46 ± 0.05 de | 99.33 ± 4.85 e | 3.27 ± 0.28 e | 0.27 ± 0.01 d |
| A4 | 61.48 ± 1.28 ef | 53.30 ± 1.04 cde | 1.44 ± 0.03 cd | 78.60 ± 4.60 d | 3.97 ± 0.32 g | 0.27 ± 0.01 d |
| A5 * | 64.09 ± 0.36 gh | 34.20 ± 0.50 a | 1.53 ± 0.04 h | 0.67 ± 0.01 a | 1.25 ± 0.11 a | 0.49 ± 0.01 g |
| A6 * | 58.84 ± 0.18 d | 50.97 ± 0.60 c | 1.37 ± 0.05 b | 334.70 ± 7.33 h | 5.84 ± 0.28 i | 0.40 ± 0.01 f |
| S1 | 52.77 ± 0.77 a | 52.94 ± 0.80 cd | 1.35 ± 0.05 ab | 690.53 ± 15.55 k | 2.61 ± 0.23 c | 0.13 ± 0.01 b |
| S2 | 59.16 ± 0.20 de | 43.68 ± 0.34 b | 1.48 ± 0.09 ef | 168.13 ± 5.60 f | 1.61 ± 0.15 b | 0.40 ± 0.01 f |
| S3 | 57.27 ± 0.43 cd | 51.07 ± 0.05 c | 1.37 ± 0.04 b | 210.30 ± 5.50 g | 3.23 ± 0.27 e | 0.36 ± 0.01 e |
| S4 * | 65.58 ± 0.96 h | 55.96 ± 0.56 de | 1.51 ± 0.06 gh | 8.03 ± 2.03 b | 4.77 ± 0.38 h | 0.06 ± 0.01 a |
| S5 | 55.55 ± 0.23 bc | 56.51 ± 0.31 de | 1.37 ± 0.05 b | 477.97 ± 12.09 j | 1.64 ± 0.17 b | 0.28 ± 0.01 d |
| S6 | 53.46 ± 0.74 ab | 57.06 ± 0.64 e | 1.33 ± 0.04 a | 455.50 ± 10.67 i | 2.85 ± 0.205 d | 0.16 ± 0.01 c |
| Significance Level | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Sample | TPC (mg GAE/g) | ABTS (µmol TE/g) | ORAC-FL (µmol TE/g) | α-Glucosidase (IC50·mg/mL) | Pancreatic Lipase (IC50·mg/mL) |
|---|---|---|---|---|---|
| A1 | 5.02 ± 0.17 abc | 73.74 ± 8.90 bcd | 117.95 ± 12.46 bc | 8.38 ± 0.70 h | 28.34 ± 1.35 fg |
| A2 * | 8.49 ± 0.27 f | 106.03 ± 5.23 f | 154.15 ± 10.93 de | 3.88 ± 0.27 a | 15.49 ± 0.80 ab |
| A3 | 5.16 ± 0.30 abcd | 71.61 ± 4.94 bc | 182.42 ± 15.67 e | 7.24 ± 0.54 g | 30.45 ± 2.45 g |
| A4 | 4.85 ± 0.05 ab | 67.40 ± 0.44 ab | 170.20 ± 16.10 e | 5.71 ± 0.29 ef | 40.25 ± 3.74 h |
| A5 * | 5.70 ± 0.31 cde | 87.19 ± 3.75 e | 135.53 ± 10.29 cd | 4.53 ± 0.22 ab | 21.87 ± 1.77 cd |
| A6 * | 6.32 ± 0.45 e | 89.53 ± 7.02 e | 184.80 ± 6.33 e | 5.12 ± 0.55 bcde | 15.84 ± 1.67 ab |
| S1 | 4.47 ± 0.12 a | 65.36 ± 2.67 ab | 139.79 ± 13.99 cd | 6.31 ± 0.60 fg | 22.11 ± 1.46 cd |
| S2 | 4.88 ± 0.20 ab | 73.00 ± 2.72 bc | 81.64 ± 7.99 a | 5.54 ± 0.43 cdef | 23.99 ± 2.55 de |
| S3 | 5.31 ± 0.24 bcd | 81.07 ± 2.31 cde | 113.40 ± 9.90 bc | 4.72 ± 0.43 abcd | 22.35 ± 2.17 fg |
| S4 * | 5.80 ± 0.34 de | 81.85 ± 1.41 cde | 160.08 ± 8.06 de | 4.76 ± 0.53 abcde | 18.60 ± 1.31 bc |
| S5 | 4.44 ± 0.04 a | 56.15 ± 2.08 a | 110.60 ± 9.33 abc | 5.67 ± 0.48 ef | 26.40 ± 1.14 ef |
| S6 | 6.21 ± 0.34 e | 86.72 ± 2.82 de | 100.19 ± 4.71 ab | 4.63 ± 0.42 abc | 12.32 ± 0.90 a |
| Significance Level | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gámbaro, A.; Miraballes, M.; Urruzola, N.; Kniazev, M.; Dauber, C.; Romero, M.; Fernández-Fernández, A.M.; Medrano, A.; Santos, E.; Vieitez, I. Physicochemical Composition and Bioactive Properties of Uruguayan Bee Pollen from Different Botanical Sources. Foods 2025, 14, 1689. https://doi.org/10.3390/foods14101689
Gámbaro A, Miraballes M, Urruzola N, Kniazev M, Dauber C, Romero M, Fernández-Fernández AM, Medrano A, Santos E, Vieitez I. Physicochemical Composition and Bioactive Properties of Uruguayan Bee Pollen from Different Botanical Sources. Foods. 2025; 14(10):1689. https://doi.org/10.3390/foods14101689
Chicago/Turabian StyleGámbaro, Adriana, Marcelo Miraballes, Nahir Urruzola, Maximiliano Kniazev, Cecilia Dauber, Melissa Romero, Adriana Maite Fernández-Fernández, Alejandra Medrano, Estela Santos, and Ignacio Vieitez. 2025. "Physicochemical Composition and Bioactive Properties of Uruguayan Bee Pollen from Different Botanical Sources" Foods 14, no. 10: 1689. https://doi.org/10.3390/foods14101689
APA StyleGámbaro, A., Miraballes, M., Urruzola, N., Kniazev, M., Dauber, C., Romero, M., Fernández-Fernández, A. M., Medrano, A., Santos, E., & Vieitez, I. (2025). Physicochemical Composition and Bioactive Properties of Uruguayan Bee Pollen from Different Botanical Sources. Foods, 14(10), 1689. https://doi.org/10.3390/foods14101689









