Microbiological and Chemical Profiles of Kiwi Kefir-like Beverages Produced Using Different Agitation Speeds and Kefir Grain Weights
Abstract
1. Introduction
2. Materials and Methods
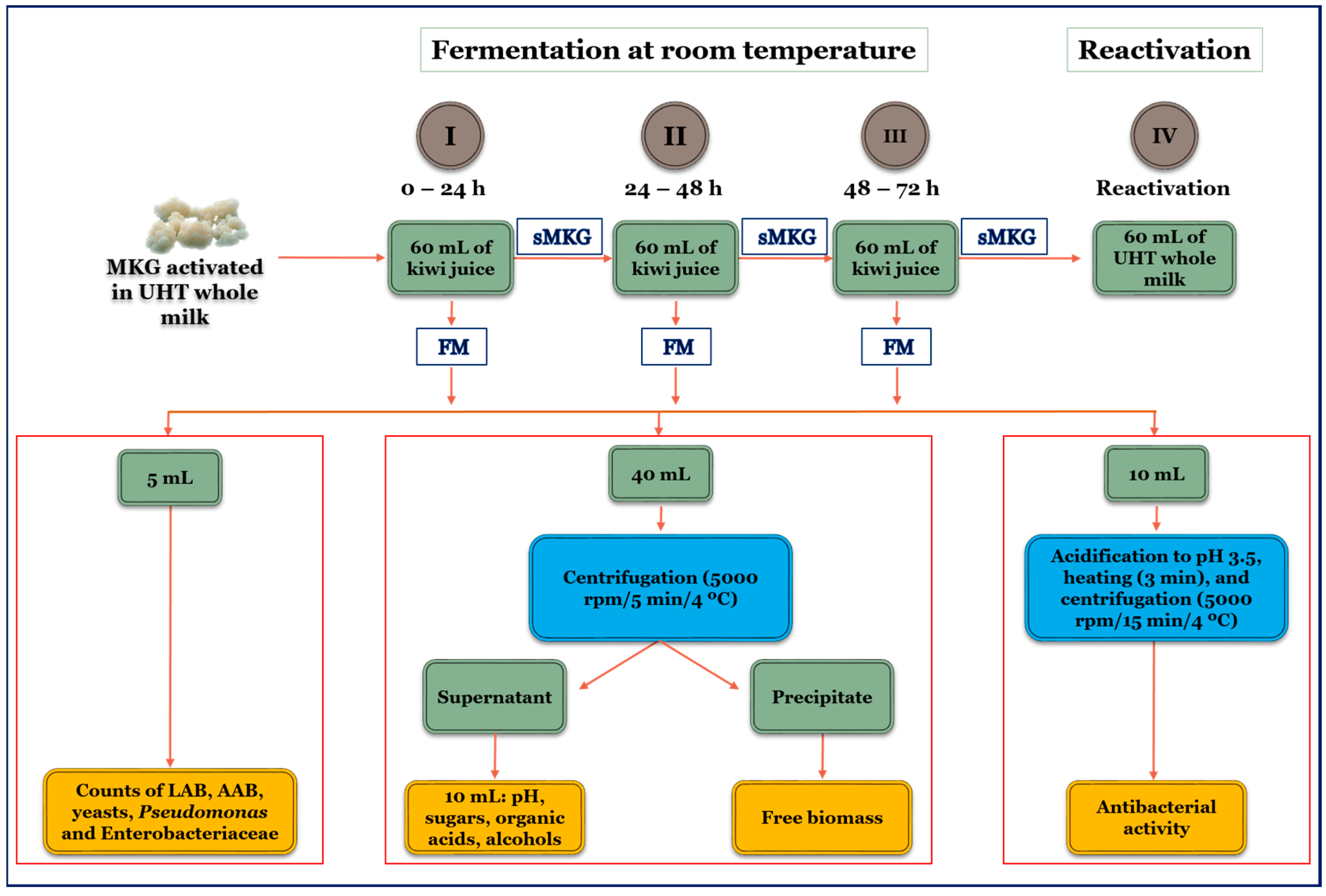
2.1. Preparation of Kefir Grains and Kiwi Juice
2.2. Fermentation Conditions
2.3. Microbiological and Chemical Analysis of Unfermented Kiwi Juice and Kiwi Kefir-like Beverages
2.4. Antibacterial Activity of Kiwi Kefir-like Beverages
2.5. Statistical Methods
2.5.1. Experimental Design
2.5.2. Principal Component Analysis
3. Results and Discussion
3.1. Effect of Agitation and Kefir Grain Weight on Kinetics of Kiwi Juice Fermentation over Three Successive Kefir Grain Passages (0–24 h, 24–48 h, and 48–72 h)
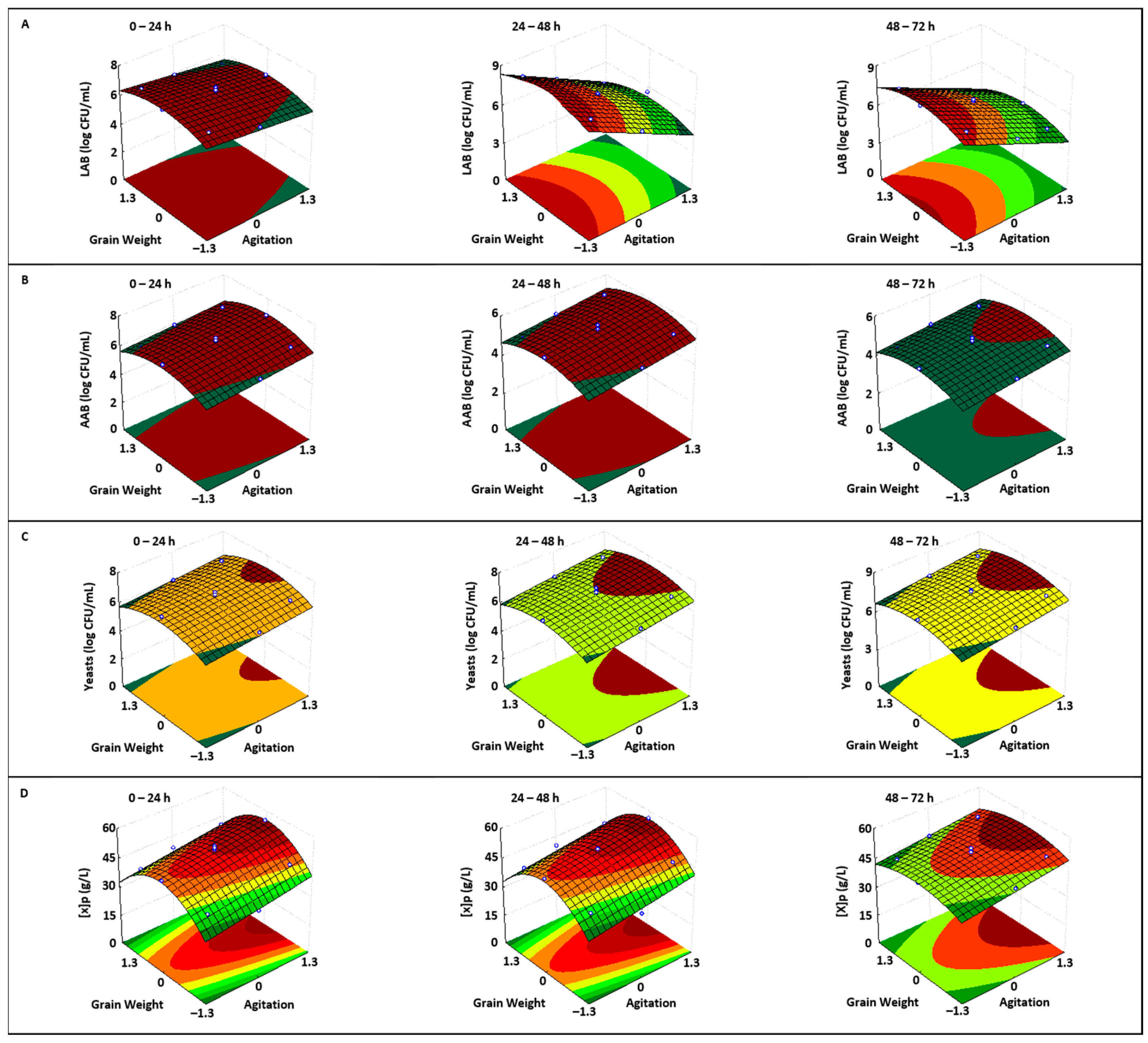
3.1.1. Nutrient Consumption
3.1.2. Microbial Growth
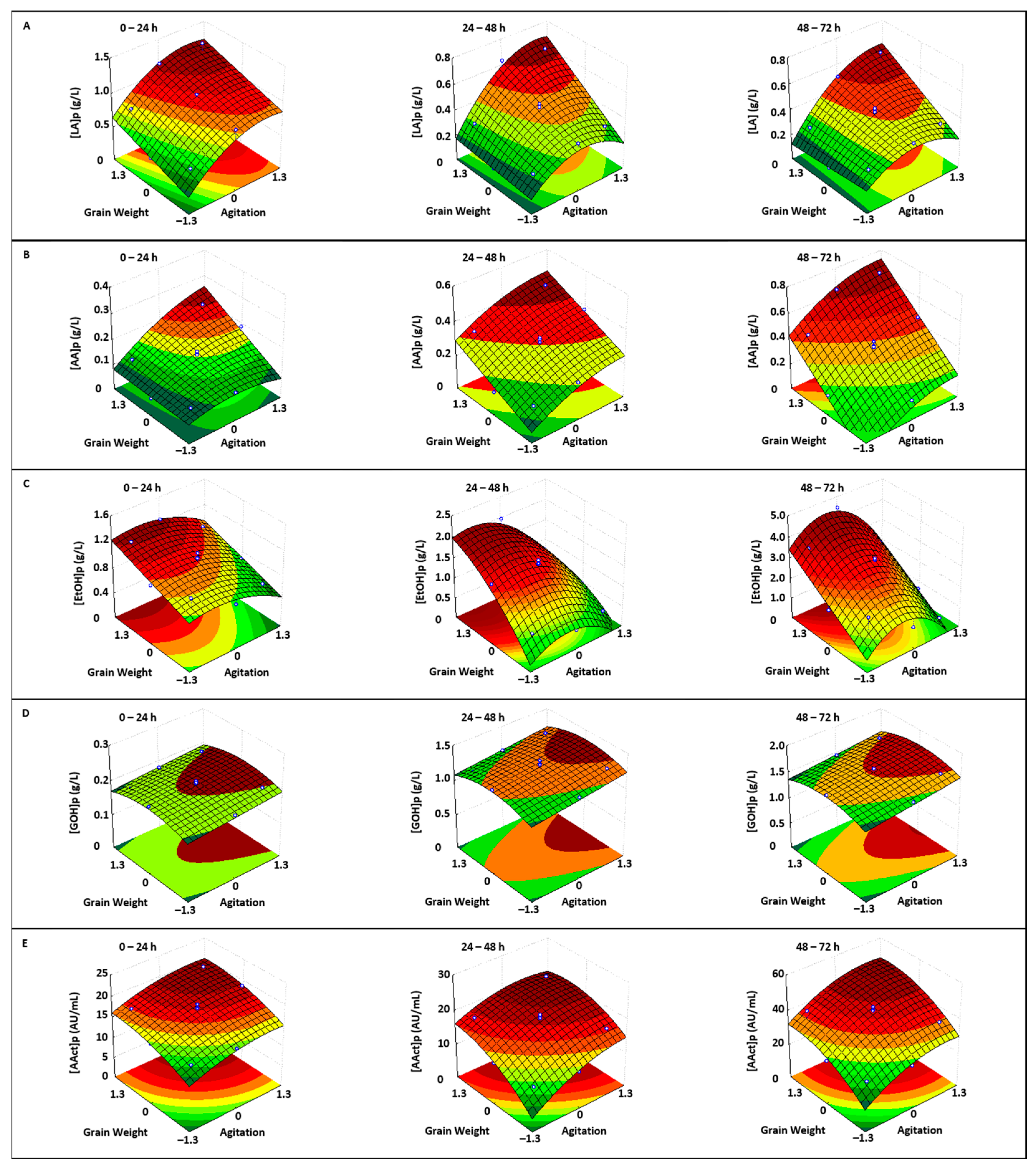
3.1.3. Metabolite Production
3.1.4. Comparison of Maximum Levels Achieved for Independent Variables Under Both Optimal and Cost-Effective Conditions
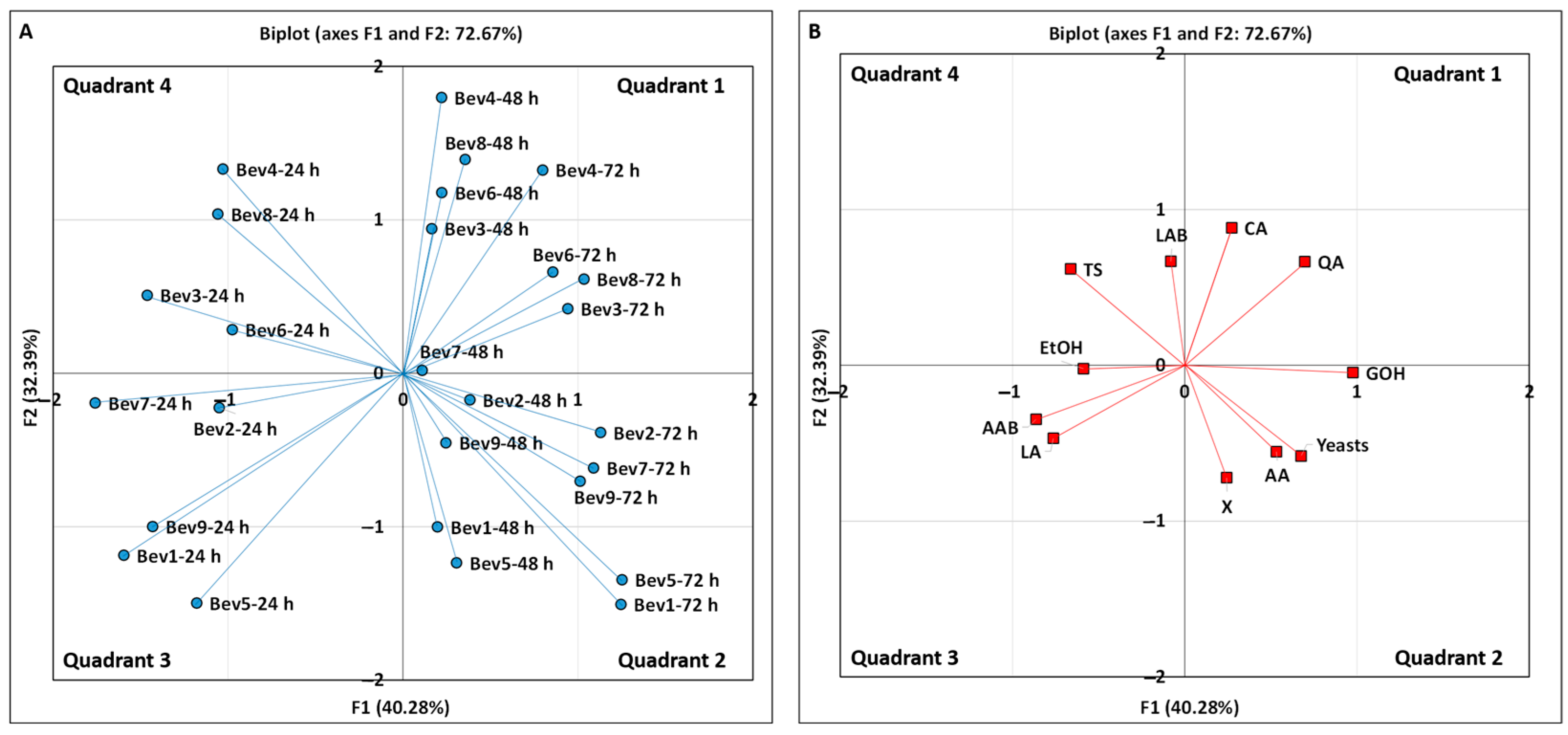
3.1.5. Principal Component Analysis of Kiwi Kefir-like Beverages Based on Their Microbiological and Chemical Compositions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lyu, X.; Peng, X.; Wang, S.; Yang, B.; Wang, X.; Yang, H.; Xiao, Y.; Baloch, A.B.; Xia, X. Quality and Consumer Acceptance of Radio Frequency and Traditional Heat Pasteurised Kiwi Puree During Storage. Int. J. Food Sci. Technol. 2018, 53, 209–218. [Google Scholar] [CrossRef]
- Pinto, T.; Vilela, A. Kiwifruit, a Botany, Chemical and Sensory Approach a Review. Adv. Plants Agric. Res. 2018, 8, 383–390. [Google Scholar] [CrossRef]
- Huang, H. Domestication and Commercialization of Actinidia. In The Genus Actinidia; Huang, H., Ed.; Academic Press: San Diego, CA, USA, 2016; Chapter 4; pp. 191–210. [Google Scholar]
- Parkar, S.G.; Simmons, L.; Herath, T.D.; Phipps, J.E.; Trower, T.M.; Hedderley, D.I.; McGhie, T.K.; Blatchford, P.; Ansell, J.; Sutton, K.H.; et al. Evaluation of the Prebiotic Potential of Five Kiwifruit Cultivars after Simulated Gastrointestinal Digestion and Fermentation with Human Faecal Bacteria. Int. J. Food Sci. Technol. 2018, 53, 1203–1210. [Google Scholar] [CrossRef]
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The Nutritional and Health Attributes of Kiwifruit: A Review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef]
- Satpal, D.; Kaur, J.; Bhadariya, V.; Sharma, K. Actinidia Deliciosa (Kiwi fruit): A Comprehensive Review on the Nutritional Composition, Health Benefits, Traditional Utilization, and Commercialization. J. Food Process. Preserv. 2021, 45, e15588. [Google Scholar] [CrossRef]
- MINISTERIO DE AGRICULTURA, PESCA Y ALIMENTACIÓN (MAPA), Gobierno de España. Subsecretaría de Agricultura, Pesca y Alimentación Subdirección General de Análisis, Coordinación y Estadística. Secretaría General Técnica. Boletín Mensual de Estadística. 2025. Available online: https://www.mapa.gob.es/es/estadistica/temas/novedades/bme-2025-02-febrero_tcm30-704223.pdf (accessed on 20 February 2025).
- Randazzo, W.; Corona, O.; Guarcello, R.; Francesca, N.; Germanà, M.A.; Erten, H.; Moschetti, G.; Settanni, L. Development of new non-dairy beverages from Mediterranean fruit juices fermented with water kefir microorganisms. Food Microbiol. 2016, 54, 40–51. [Google Scholar] [CrossRef]
- Dikmetas, D.N.; Acar, E.G.; Ceylan, F.D.; İlkadım, F.; Özer, H.; Karbancioglu-Guler, F. Functional Fermented Fruit Juice Production and Characterization by Using Water Kefir Grains. J. Food Sci. Technol. 2025, 1–14. [Google Scholar] [CrossRef]
- Bazán, D.L.; del Río, P.G.; Domínguez, J.M.; Cortés–Diéguez, S.; Mejuto, J.C.; Pérez–Guerra, N. The Chemical, Microbiological and Volatile Composition of Kefir–like Beverages Produced from Red Table Grape Juice in Repeated 24–h Fed–Batch Subcultures. Foods 2022, 11, 3117. [Google Scholar] [CrossRef]
- Afonso, M.J.; Ramalhosa, E.; del Río, P.G.; Martins, F.; Baptista, P.; Pereira, E.L.; Guerra, N.P. Production of Nondairy Fermented Products with Chestnut Puree as Substrate and Milk Kefir Grains or Two Lactic Acid Bacteria. Food Sci. 2025, 90, e17474. [Google Scholar] [CrossRef]
- Sun, M.C.; Fan, X.J.; Wang, J.T.; Yang, F.S.; Yang, L.; Li, Z.; Fei, P.; Zhang, T.; Zhao, C. Ex-ploring the Mechanism of Milk Kefir Grain Fermentation to Improve the Palatability of Chokeberry Juice. LWT-Food Sci. Technol. 2024, 213, 117074. [Google Scholar] [CrossRef]
- Gao, J.; Gu, F.; Ruan, H.; Chen, Q.; He, J.; He, G. Culture Conditions Optimization of Tibetan Kefir Grains by Response Surface Methodology. Proc. Eng. 2012, 37, 132–136. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Gharibzahedi, S.M.T. Enhanced Production of Iranian Kefir Grain Biomass by Optimization and Empirical Modeling of Fermentation Conditions Using Response Surface Methodology. Food Bioproc. Technol. 2012, 5, 3230–3235. [Google Scholar] [CrossRef]
- Vieira, C.P.; Álvares, T.S.; Gomes, L.S.; Torres, A.G.; Paschoalin, V.M.F.; Conte-Junior, C.A. Kefir Grains Change Fatty Acid Profile of Milk During Fermentation and Storage. PLoS ONE 2015, 10, e0139910. [Google Scholar] [CrossRef] [PubMed]
- Corona, O.; Randazzo, W.; Miceli, A.; Guarcello, R.; Francesca, N.; Erten, H.; Moschetti, G.; Settanni, L. Characterization of Kefir-like Beverages Produced from Vegetable Juices. LWT-Food Sci. Technol. 2016, 66, 572–581. [Google Scholar] [CrossRef]
- McGovern, C.J.; González-Orozco, B.D.; Jiménez-Flores, R. Evaluation of Kefir Grain Microbiota, Grain Viability, and Bioactivity from Fermenting Dairy Processing By-Products. J. Dairy Sci. 2024, 107, 4259–4276. [Google Scholar] [CrossRef]
- Schoevers, A.; Britz, T.J. Influence of Different Culturing Conditions on Kefir Grain Increase. Int. J. Dairy Technol. 2003, 56, 183–187. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.; Wyffels, J.T.; Seydim, A.C.; Greene, A.K. Turkish Kefir and Kefir Grains: Microbial Enumeration and Electron Microscobic Observation. Int. J. Dairy Technol. 2005, 58, 25–29. [Google Scholar] [CrossRef]
- Zajšek, K.; Goršek, A.; Kolar, M. Cultivating Conditions Effects on Kefiran Production by the Mixed Culture of Lactic Acid Bacteria Imbedded within Kefir Grains. Food Chem. 2013, 139, 970–977. [Google Scholar] [CrossRef]
- Pop, C.; Apostu, S.; Salanţă, L.; Rotar, A.M.; Sindic, M.; Mabon, N.; Socaciu, C. Influence of Different Growth Conditions on the Kefir Grains Production, used in the Kefiran Synthesis. Bull. UASVM Food Sci. Technol. 2014, 71, 147–153. [Google Scholar] [CrossRef][Green Version]
- Ismaiel, A.A.; Ghaly, M.F.; El-Naggar, A.K. Some Physicochemical Analyses of Kefir Produced Under Different Fermentation Conditions. J. Sci. Ind. Res. 2011, 70, 365–372. [Google Scholar]
- Blandón, L.M.; Noseda, M.D.; Islan, G.A.; Castro, G.R.; Pereira, G.V.M.; Thomaz-Soccol, V.; Soccol, C.R. Optimization of Culture Conditions for Kefiran Production in Whey: The Structural and Biocidal Properties of the Resulting Polysaccharide. Bioact. Carbohydr. Diet. Fibre 2018, 16, 14–21. [Google Scholar] [CrossRef]
- Fontán, M.C.G.; Martínez, S.; Franco, I.; Carballo, J. Microbiological and Chemical Changes During the Manufacture of Kefir made from Cows’ Milk, using a Commercial Starter Culture. Int. Dairy J. 2006, 16, 762–767. [Google Scholar] [CrossRef]
- Laureys, D.; De Vuyst, L. The Water Kefir Grain Inoculum determines the Characteristics of the Resulting Water Kefir Fermentation Process. J. Appl. Microbiol. 2016, 122, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.R.; Pedreira, A.J.R.M.; Cristianini, L.B.; Maria Guidi, F.; Capato, M.O.; Ávila, P.F.; Goldbeck, R.; Kamimura, E.S. Application of Soluble Fibres in the Osmotic Dehydration of Pineapples and Reuse of Effluent in a Beverage Fermented by Water Kefir. LWT-Food Sci. Technol. 2020, 132, 109819. [Google Scholar] [CrossRef]
- Bazán, D.L.; Del–Río, P.G.; Cortés Diéguez, S.; Domínguez, J.M.; Pérez Guerra, N. Main Composition and Visual Appearance of Milk Kefir Beverages Obtained from Four Consecutive 24– and 48–h Batch Subcultures. Processes 2024, 12, 1419. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Havilah, E.J.; Wallis, D.M.; Morris, R.; Woolnouugh, J.A. A Microcolorimetric Method for Determination of Ammonia in Kjeldahl Digests with a Manual Spectrofotometer. Lab. Pract. 1977, 26, 545–547. [Google Scholar]
- Costas, M.; Alonso, E.; Bazán, D.L.; Bendaña, R.J.; Guerra, N.P. Batch and Fed–Batch Production of Probiotic Biomass and Nisin in Nutrient–Supplemented Whey Media. Braz. J. Microbiol. 2019, 50, 915–925. [Google Scholar]
- Rodmui, A.; Kongkiattikajorn, J.; Dandusitapun, Y. Optimization of Agitation Conditions for Maximum Ethanol Production by Coculture. Nat. Sci. 2008, 42, 285–293. [Google Scholar]
- Tissot, S.; Michel, P.O.; Douet, C.J.; Grezet, S.; Baldi, L.; Hacker, D.L.; Wurm, F.M. Helical-Track Bioreactors for Bacterial, Mammalian and Insect Cell Cultures. Processes 2013, 1, 3–11. [Google Scholar] [CrossRef]
- Hurst, L. Bacteriology, 1st ed.; Ed-Tech Press: Waltham Abbey Essex, UK, 2019; pp. 273–274. [Google Scholar]
- Zhong, J.J. Bioreactor Engineering. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 2, pp. 165–177. [Google Scholar]
- Soccol, C.R.; Vandenberghe, L.P.S.; Rodrigues, C.; Pandey, A. New Perspectives for Citric Acid Production and Application. Food Technol. Biotechnol. 2006, 44, 141–149. [Google Scholar]
- Yalçin, S.K.; Bozdemir, M.T.; Özbaş, Z.Y. Citric Acid Production by Yeasts: Fermentation Conditions, Process Optimization and Strain Improvement. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology, 1st ed.; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2010; Volume 2, pp. 1374–1382. [Google Scholar]
- Torija, M.J.; Beltran, G.; Novo, M.; Poblet, M.; Rozes, N.; Mas, A.; Guillamón, J.M. Effect of Organic Acids and Nitrogen Source on Alcoholic Fermentation: Study of their Buffering Capacity. J. Agric. Food Chem. 2003, 51, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, Z.E.; Mousavi, S.M.; Razavi, S.H.; Emam-Djomeh, Z.; Kiani, H. Fermentation of Pomegranate Juice by Probiotic Lactic Acid Bacteria. World J. Microbiol. Biotechnol. 2011, 27, 123–128. [Google Scholar] [CrossRef]
- Mamlouk, D.; Gullo, M. Acetic Acid Bacteria: Physiology and Carbon Sources Oxidation. Indian J. Microbiol. 2013, 53, 377–384. [Google Scholar] [CrossRef]
- Patil, S.S.; Kadam, S.R.; Patil, S.S.; Bastawde, K.B.; Khire, J.M.; Gokhale, D.V. Production of Lactic Acid and Fructose from Media with Cane Sugar using Mutant of Lactobacillus Delbrueckii NCIM 2365. Lett. Appl. Microbiol. 2006, 43, 53–57. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Mendes-Faia, A. The Role of Yeasts and Lactic Acid Bacteria on the Metabolism of Organic Acids during Winemaking. Foods 2020, 9, 1231. [Google Scholar] [CrossRef]
- Wang, B.; Shao, Y.; Chen, F. Overview on Mechanisms of Acetic Acid Resistance in Acetic Acid Bacteria. World J. Microbiol. Biotechnol. 2015, 31, 255–263. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Wilkinson, S.; Daenen, L.; Arendt, E.K. Physiology of Acetic Acid Bacteria and their Role in Vinegar and Fermented Beverages. Compr. Rev. Food Sci. Food Saf. 2019, 18, 587–625. [Google Scholar] [CrossRef]
- Silva, J.P.A.; Mussatto, S.I.; Roberto, I.C. The Influence of Initial Xylose Concentration, Agitation, and aeration on Ethanol Production by Pichia Stipites from Rice Straw Hemicellulosic Hydrolysate. Appl. Biochem. Biotechnol. 2010, 162, 1306–1315. [Google Scholar] [CrossRef]
- Agbogbo, F.K.; Wenger, K.S. Production of Ethanol from Corn Stover Hemicellulose Hydrolyzate using Pichia Stipitis. J. Ind. Microbiol. Biotechnol. 2007, 34, 723–727. [Google Scholar] [CrossRef]
- Telli-Okur, M.; Eken-Saraçoğlu, N. Fermentation of Sunflower Seed Hull Hydrolysate to Ethanol by Pichia Stipitis. Biores. Technol. 2008, 99, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.; Leyton, Y.; Riquelme, C. Probiotic Bacteria as a Healthy Alternative for Fish Aquaculture. In Antibiotic Use in Animals, 1st ed.; Savic, S., Ed.; InTechOpen Limited: London, UK, 2018; Chapter 7; pp. 115–132. [Google Scholar]
- Guerra, N.P.; Bernárdez, P.F.; Castro, L.P. Modelling the Stress Inducing Biphasic Growth and Pediocin Production by Pediococcus Acidilactici NRRL B-5627 in Re-alkalized Fed-Batch Cultures. Biochem. Eng. J. 2008, 40, 465–472. [Google Scholar] [CrossRef]
- González-Sáiz, J.M.; Garrido-Vidal, D.; Pizarro, C. Modelling the Industrial Production of Vinegar in Aerated-Stirred Fermentors in Terms of Process Variables. J. Food Eng. 2009, 91, 183–196. [Google Scholar] [CrossRef]
- Garrido-Vidal, D.; Pizarro, C.; González-Sáiz, J.M. Study of Process Variables in Industrial Acetic Fermentation by a Continuous Pilot Fermentor and Response Surfaces. Biotechnol. Prog. 2003, 19, 1468–1479. [Google Scholar] [CrossRef]
- Aslankoohi, E.; Rezaei, M.N.; Vervoort, Y.; Courtin, C.M.; Verstrepen, K.J. Glycerol Production by Fermenting Yeast Cells Is Essential for Optimal Bread Dough Fermentation. PLoS ONE 2015, 10, e0119364. [Google Scholar] [CrossRef]
- Klein, M.; Swinnen, S.; Thevelein, J.M.; Nevoigt, E. Glycerol Metabolism and Transport in Yeast and Fungi: Established Knowledge and Ambiguities. Environ. Microbiol. 2017, 19, 878–893. [Google Scholar] [CrossRef] [PubMed]
- Guerra, N.P.; Pastrana, L. Enhancement of Nisin Production by Lactococcus Lactis in Periodically Re-alkalized Cultures. Biotechnol. Appl. Biochem. 2003, 38, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Chinachoti, N.; Zaima, T.; Matsusaki, H.; Sonomoto, K.; Ishizaki, A. Relationship between Nisin Z Fermentative Production and Aeration Condition using Lactococcus Lactis 10-1. J. Fac. Agric. Kyushu Univ. 1997, 43, 437–448. [Google Scholar]
- Tafreshi, S.H.; Mirdamadi, S.; Norouzian, D.; Khatami, S.; Sardari, S. Effect of Non-Nutritional Factors on Nisin Production. Afr. J. Biotechnol. 2010, 9, 1382–1391. [Google Scholar]

| Points | Codified Values | Natural Values | |||
|---|---|---|---|---|---|
| A | GW | A (rpm) | GW (g) | Designation | |
| Factorial | 1 | 1 | 134 | 2.60 | Bev1 |
| 1 | −1 | 134 | 1.00 | Bev2 | |
| −1 | 1 | 38 | 2.60 | Bev3 | |
| −1 | −1 | 38 | 1.00 | Bev4 | |
| Axial | 1.267 | 0 | 147 | 1.80 | Bev5 |
| −1.267 | 0 | 25 | 1.80 | Bev6 | |
| 0 | 1.267 | 86 | 2.81 | Bev7 | |
| 0 | −1.267 | 86 | 0.79 | Bev8 | |
| * Center | 0 | 0 | 86 | 1.80 | Bev9 |
| Variable * | Lmax | Lest | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| [TS]c (g/L) | 57.23 | 73.43 | 93.48 | 23.32 | 29.34 | 53.97 | 59.2 | 60.0 | 42.3 |
| [CA]c (g/L) | 13.83 | 12.40 | 11.62 | 9.16 | 8.15 | 7.84 | 33.8 | 34.3 | 32.5 |
| [QA]c (g/L) | 4.37 | 1.70 | 3.02 | 2.48 | 0.79 | 1.08 | 43.2 | 53.5 | 64.2 |
| [LA]p (g/L) | 1.22 | 0.66 | 0.60 | 0.43 | 0.13 | 0.14 | 64.7 | 80.3 | 76.7 |
| [AA]p (g/L) | 0.26 | 0.47 | 0.72 | 0.08 | 0.18 | 0.15 | 69.2 | 61.7 | 79.2 |
| [EtOH]p (g/L) | 2.04 | 4.36 | 1.27 | 1.36 | 2.06 | 1.00 | 33.3 | 52.7 | 21.3 |
| [GOH]p (g/L) | 0.22 | 1.36 | 1.71 | 0.19 | 1.21 | 1.52 | 13.6 | 11.0 | 11.1 |
| [AAct]p (AU/mL) | 20.02 | 20.92 | 48.60 | 14.92 | 13.02 | 28.47 | 25.5 | 37.8 | 41.4 |
| LAB (log(CFU/mL)) | 7.02 | 8.99 | 8.28 | 7.02 | 8.99 | 8.28 | 0 | 0 | 0 |
| AAB (log(CFU/mL)) | 6.93 | 5.88 | 5.28 | 6.40 | 5.26 | 4.73 | 7.6 | 10.5 | 10.4 |
| Yeasts (log(CFU/mL)) | 7.10 | 7.43 | 8.38 | 6.50 | 6.61 | 7.42 | 8.4 | 11.0 | 11.1 |
| [X]p (g/L) | 55.21 | 55.23 | 53.76 | 47.24 | 47.13 | 47.29 | 14.4 | 14.7 | 12.0 |
| Beverage | Passage | LAB * | AAB * | Yeasts * | [X] * | [TS] * | [CA] * | [QA] * | [LA] * | [AA] * | [EtOH] * | [GOH] * | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Be1-24 h | 1st | 5.85 | 6.42 | 6.64 | 45.71 | 71.10 | 5.60 | 5.51 | 1.31 | 0.22 | 2.32 | 0.20 | 3.19 |
| Be2-24 h | 1st | 5.80 | 6.43 | 6.66 | 45.69 | 87.14 | 8.88 | 6.58 | 0.86 | 0.09 | 0.36 | 0.20 | 3.11 |
| Be3-24 h | 1st | 6.51 | 5.92 | 5.96 | 39.81 | 90.71 | 9.87 | 6.90 | 0.78 | 0.12 | 3.50 | 0.18 | 3.08 |
| Be4-24 h | 1st | 6.53 | 5.88 | 5.92 | 39.64 | 107.53 | 13.02 | 7.92 | 0.49 | 0.09 | 2.11 | 0.18 | 3.01 |
| Be5-24 h | 1st | 6.22 | 6.93 | 7.09 | 55.79 | 72.37 | 5.55 | 5.49 | 1.02 | 0.19 | 0.65 | 0.21 | 3.21 |
| Be6-24 h | 1st | 6.86 | 6.57 | 6.88 | 47.32 | 102.66 | 8.86 | 7.32 | 0.41 | 0.07 | 1.66 | 0.20 | 3.12 |
| Be7-24 h | 1st | 5.94 | 5.99 | 6.06 | 39.28 | 75.78 | 8.25 | 6.37 | 1.15 | 0.18 | 4.50 | 0.18 | 3.11 |
| Be8-24 h | 1st | 6.01 | 5.92 | 6.13 | 34.76 | 98.73 | 12.54 | 7.77 | 0.89 | 0.11 | 1.04 | 0.18 | 3.00 |
| Be9-24 h | 1st | 6.63 | 6.66 | 6.79 | 51.23 | 71.40 | 5.88 | 5.92 | 0.93 | 0.16 | 2.94 | 0.20 | 3.20 |
| Be1-48 h | 2nd | 5.12 | 5.46 | 6.90 | 46.15 | 49.66 | 7.52 | 6.56 | 0.65 | 0.44 | 0.99 | 1.27 | 3.13 |
| Be2-48 h | 2nd | 5.06 | 5.48 | 6.92 | 46.97 | 65.63 | 10.26 | 7.43 | 0.33 | 0.25 | 0.66 | 1.27 | 3.07 |
| Be3-48 h | 2nd | 8.27 | 4.92 | 6.20 | 40.56 | 76.37 | 11.08 | 7.56 | 0.30 | 0.35 | 1.22 | 1.14 | 3.04 |
| Be4-48 h | 2nd | 8.31 | 4.87 | 6.15 | 40.18 | 92.32 | 13.72 | 8.70 | 0.22 | 0.15 | 0.96 | 1.13 | 2.99 |
| Be5-48 h | 2nd | 5.66 | 5.75 | 7.27 | 56.91 | 65.26 | 6.48 | 6.55 | 0.42 | 0.38 | 0.70 | 1.34 | 3.17 |
| Be6-48 h | 2nd | 9.32 | 5.28 | 6.60 | 48.47 | 94.38 | 10.09 | 8.49 | 0.14 | 0.13 | 0.92 | 1.21 | 3.07 |
| Be7-48 h | 2nd | 6.36 | 5.04 | 6.30 | 40.37 | 53.89 | 9.73 | 7.18 | 0.64 | 0.40 | 1.26 | 1.16 | 3.06 |
| Be8-48 h | 2nd | 6.46 | 4.99 | 6.37 | 33.46 | 76.22 | 13.32 | 8.82 | 0.37 | 0.21 | 0.69 | 1.17 | 2.98 |
| Be9-48 h | 2nd | 7.03 | 5.57 | 7.02 | 51.20 | 65.82 | 7.08 | 7.37 | 0.43 | 0.31 | 1.02 | 1.29 | 3.14 |
| Be1-72 h | 3rd | 4.28 | 4.88 | 7.84 | 49.73 | 34.13 | 8.04 | 7.71 | 0.62 | 0.69 | 0.99 | 1.60 | 3.07 |
| Be2-72 h | 3rd | 4.77 | 4.90 | 7.86 | 49.89 | 54.93 | 10.64 | 8.15 | 0.34 | 0.22 | 0.35 | 1.60 | 3.05 |
| Be3-72 h | 3rd | 7.31 | 4.39 | 7.03 | 44.64 | 56.56 | 11.42 | 8.29 | 0.26 | 0.44 | 1.82 | 1.43 | 3.02 |
| Be4-72 h | 3rd | 7.40 | 4.35 | 6.98 | 44.29 | 71.63 | 13.91 | 8.72 | 0.25 | 0.00 | 0.65 | 1.42 | 2.99 |
| Be5-72 h | 3rd | 4.75 | 5.14 | 8.25 | 52.34 | 44.67 | 7.06 | 7.70 | 0.38 | 0.45 | 0.46 | 1.68 | 3.12 |
| Be6-72 h | 3rd | 8.04 | 4.72 | 7.49 | 46.26 | 68.00 | 9.96 | 8.64 | 0.14 | 0.15 | 1.46 | 1.52 | 3.07 |
| Be7-72 h | 3rd | 5.40 | 4.51 | 7.15 | 45.37 | 31.13 | 10.14 | 8.07 | 0.51 | 0.64 | 1.98 | 1.46 | 3.02 |
| Be8-72 h | 3rd | 5.89 | 4.45 | 7.23 | 45.89 | 58.88 | 13.53 | 8.65 | 0.36 | 0.15 | 0.44 | 1.47 | 2.98 |
| Be9-72 h | 3rd | 6.55 | 5.00 | 7.92 | 50.56 | 45.86 | 7.63 | 8.09 | 0.41 | 0.37 | 1.45 | 1.62 | 3.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazán, D.L.; Del-Río, P.G.; Pérez-Guerra, N. Microbiological and Chemical Profiles of Kiwi Kefir-like Beverages Produced Using Different Agitation Speeds and Kefir Grain Weights. Foods 2025, 14, 1681. https://doi.org/10.3390/foods14101681
Bazán DL, Del-Río PG, Pérez-Guerra N. Microbiological and Chemical Profiles of Kiwi Kefir-like Beverages Produced Using Different Agitation Speeds and Kefir Grain Weights. Foods. 2025; 14(10):1681. https://doi.org/10.3390/foods14101681
Chicago/Turabian StyleBazán, Delicia L., Pablo G. Del-Río, and Nelson Pérez-Guerra. 2025. "Microbiological and Chemical Profiles of Kiwi Kefir-like Beverages Produced Using Different Agitation Speeds and Kefir Grain Weights" Foods 14, no. 10: 1681. https://doi.org/10.3390/foods14101681
APA StyleBazán, D. L., Del-Río, P. G., & Pérez-Guerra, N. (2025). Microbiological and Chemical Profiles of Kiwi Kefir-like Beverages Produced Using Different Agitation Speeds and Kefir Grain Weights. Foods, 14(10), 1681. https://doi.org/10.3390/foods14101681









