The Preparation and Characterization of Antioxidant Films Based on Hazelnut Shell-Based Vegetable Carbon Black/Chitosan/Gelatin and the Application on Soybean Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Preparation of Hazelnut Shell-Based Vegetable Carbon Black (HCB)
2.3. The Preparation of CS-GEL-HCB Composite Film
2.3.1. The Preparation of CS Solution and GEL Solution
2.3.2. The Preparation of HCB Suspension
2.3.3. The Preparation of Composite Films
2.4. Films’ Structural Characterization
2.4.1. Scanning Electron Microscope (SEM)
2.4.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4.3. X-Ray Diffraction Pattern (XRD)
2.5. Optical Properties of the Films
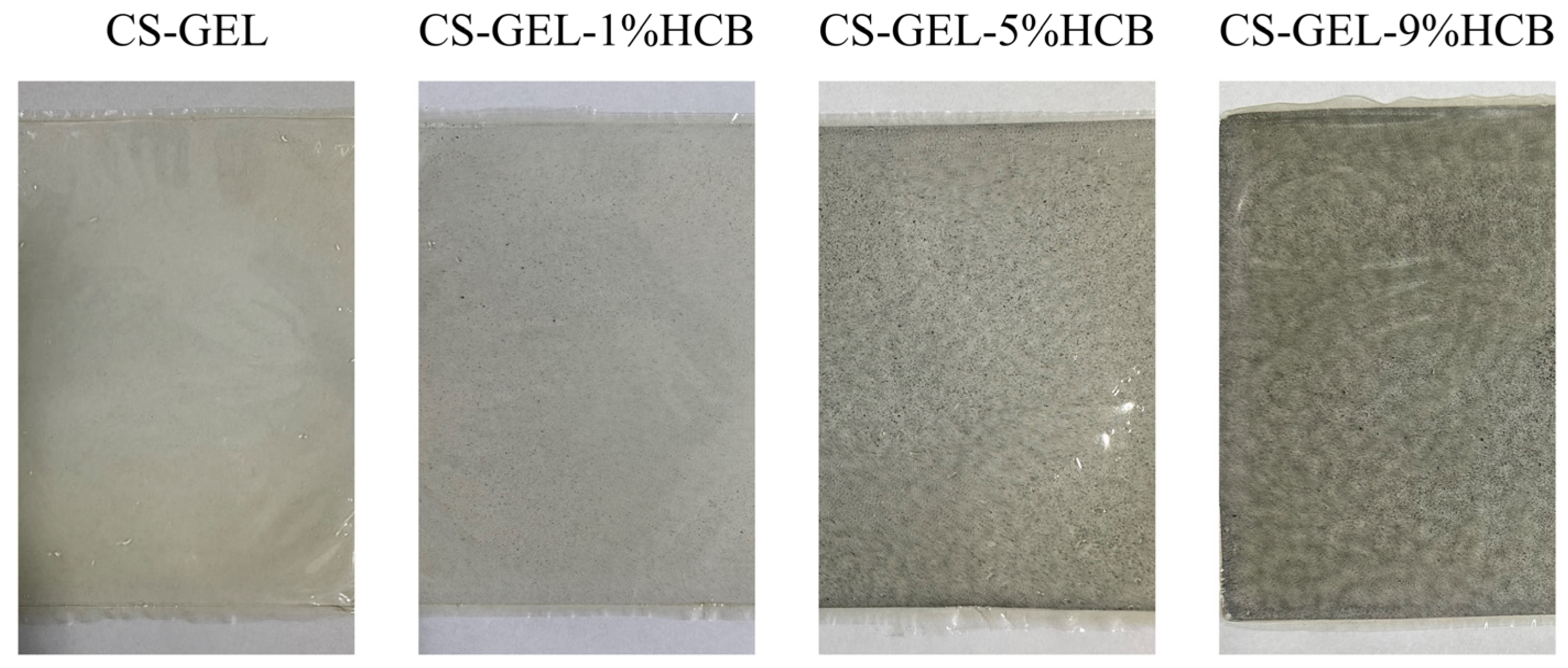
2.5.1. The Appearance and Color of the Films
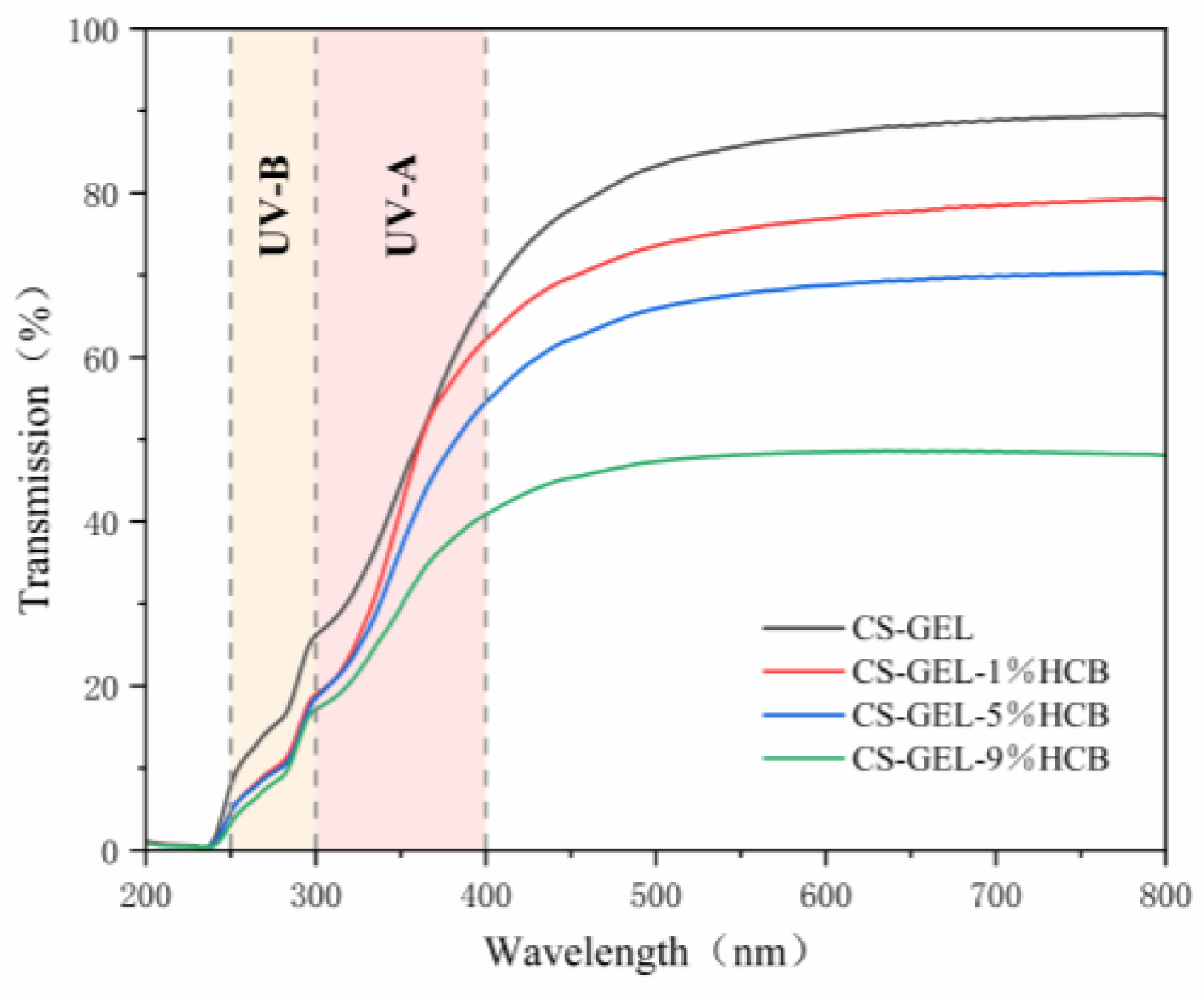
2.5.2. Transmittance Test
2.5.3. Opacity
2.6. Physical Properties of the Films
2.6.1. Thickness of Films
2.6.2. Mechanical Properties of Composite Films
2.6.3. The Moisture Content (MC) and Water Solubility (WS) of Composite Films
2.6.4. WVP of Composite Films
2.6.5. The Oxygen Transmission Rate of Composite Films (OTR)
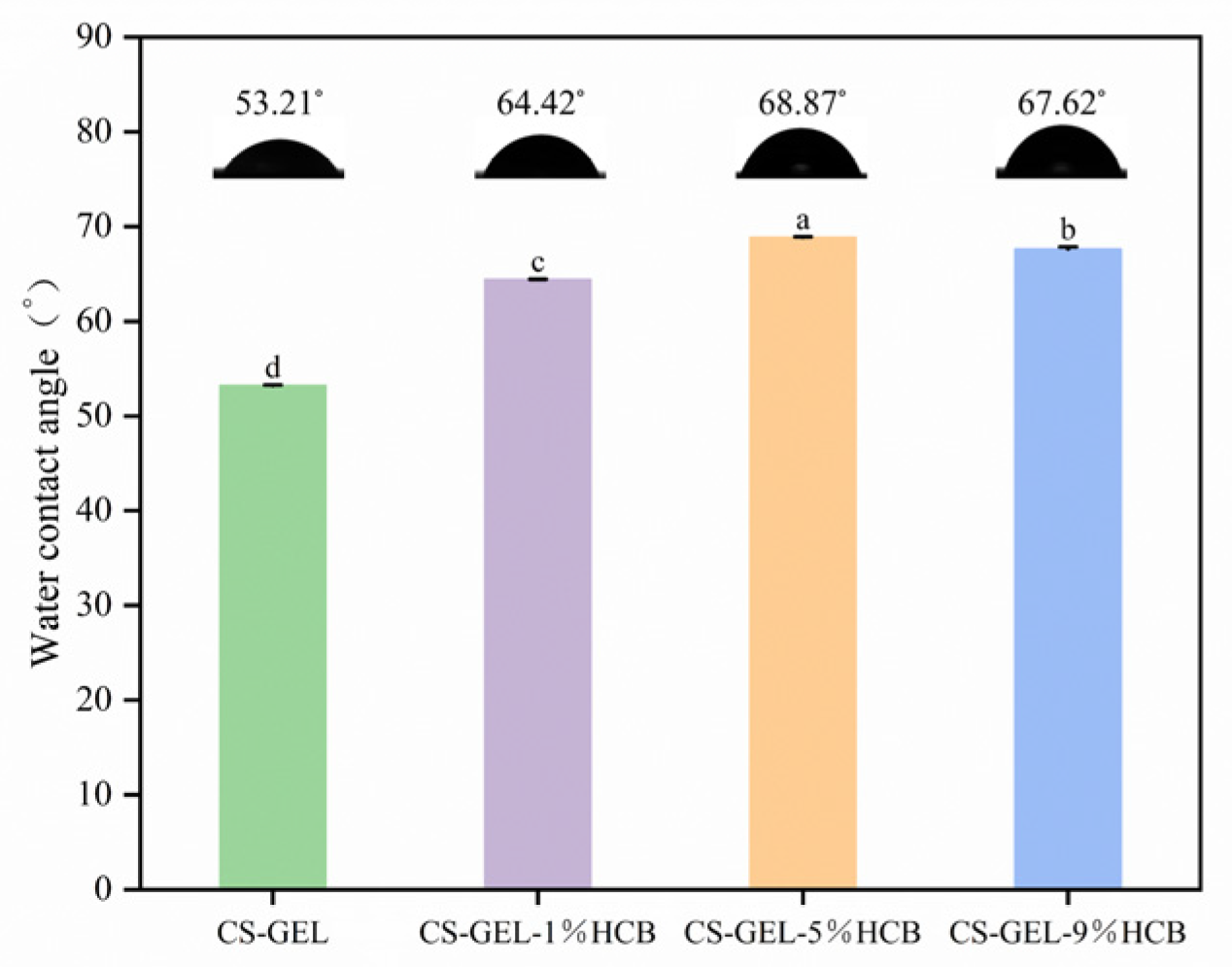
2.6.6. The Water Contact Angle of Composite Film (WCA)
2.7. The Antimicrobial Activity of Composite Films
2.8. Application in Inhibiting the Oxidation of Soybean Oil
2.9. Statistical Analysis
3. Results and Discussion
3.1. The Characterization of Carbon Black in Hazelnut Shell-Based Vegetables
3.2. The Structural Characterization of Composite Films
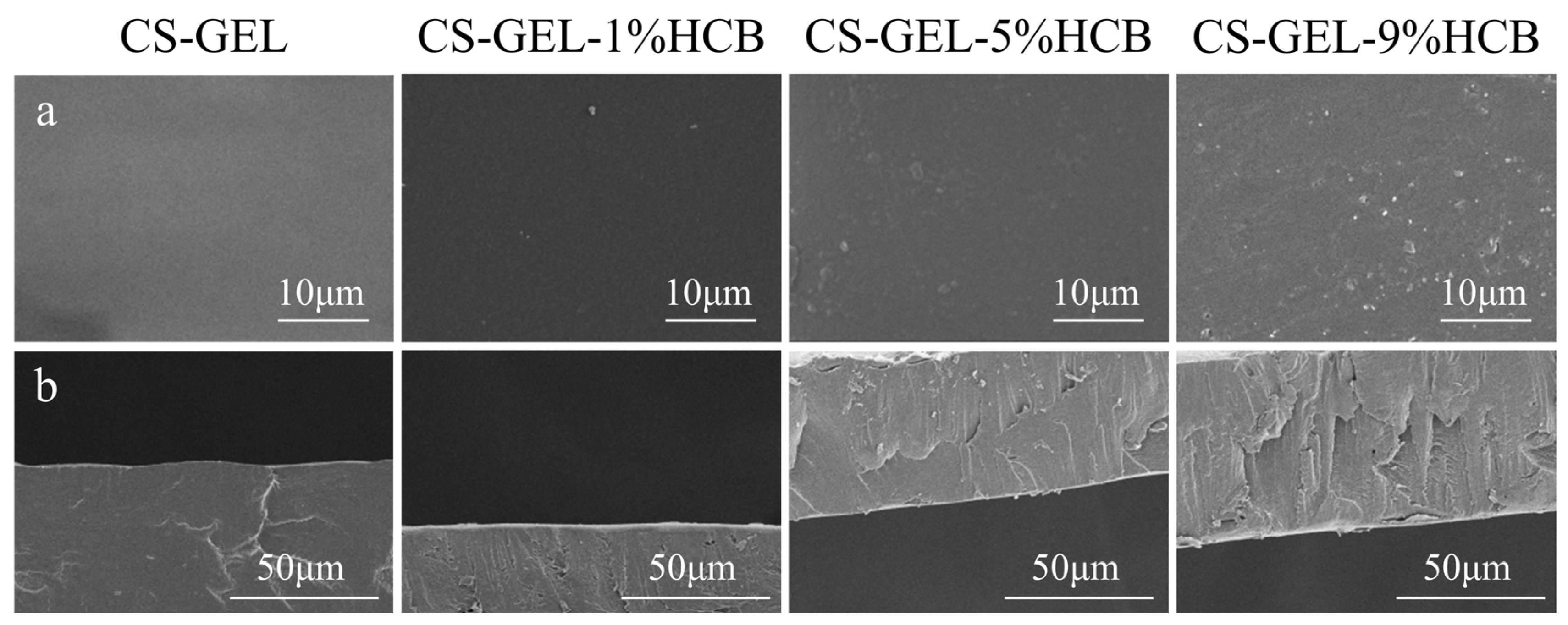
3.2.1. Microstructure
3.2.2. X-Ray Diffraction Analysis
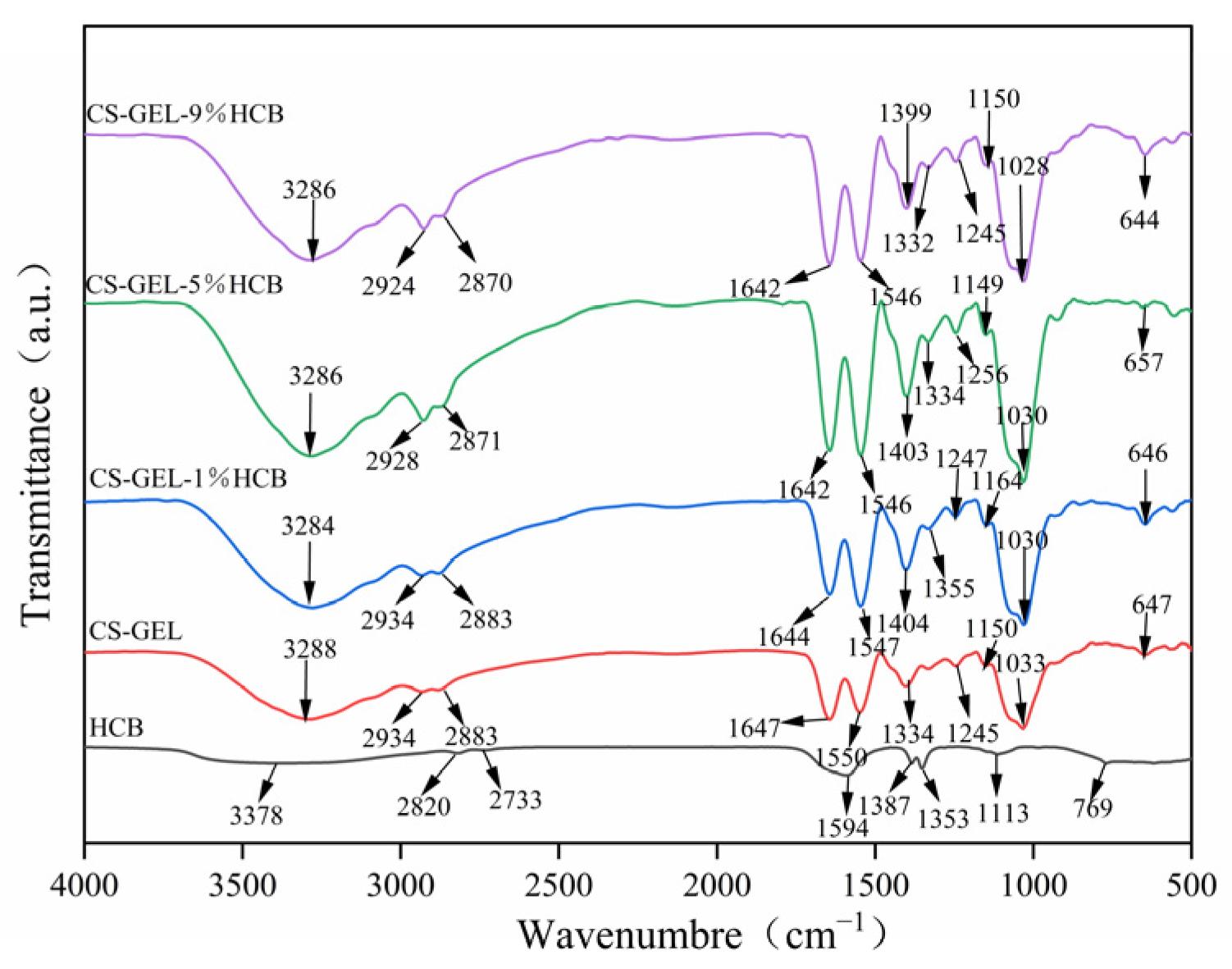
3.2.3. FT-IR Spectroscopy Analysis
3.3. Optical Properties of the Film
3.4. Physical Properties of the Film
3.4.1. Film Thickness and Mechanical Properties
3.4.2. The MC, WS, WVP, and OTR of Films
3.5. The Ability of Film to Inhibit Microbial Activity
3.6. The Application of Composite Film in Inhibiting the Oxidation of Soybean Oil
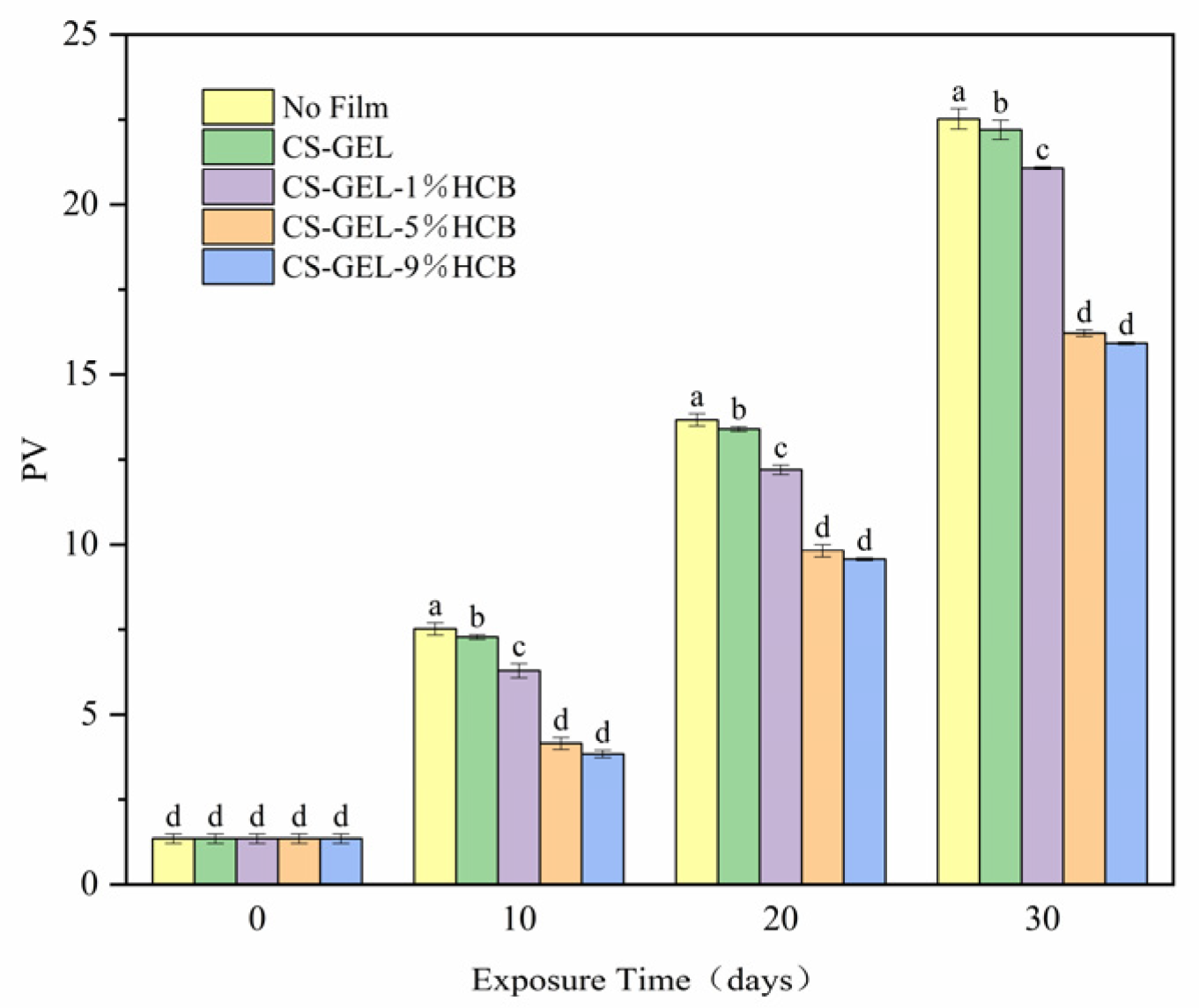
3.6.1. The Change of Peroxide Value (PV)
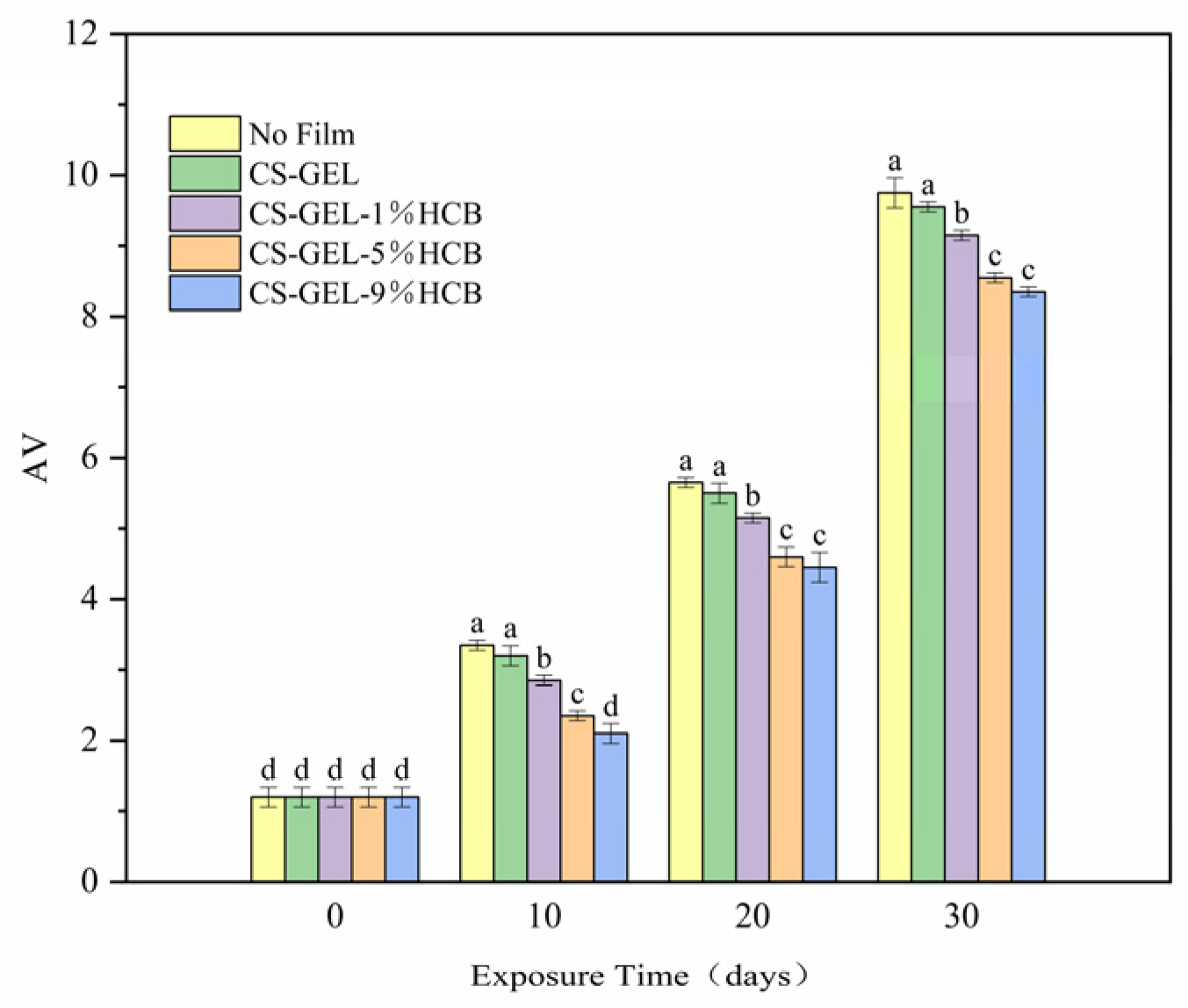
3.6.2. Change of P-Anisidine Value (AV)
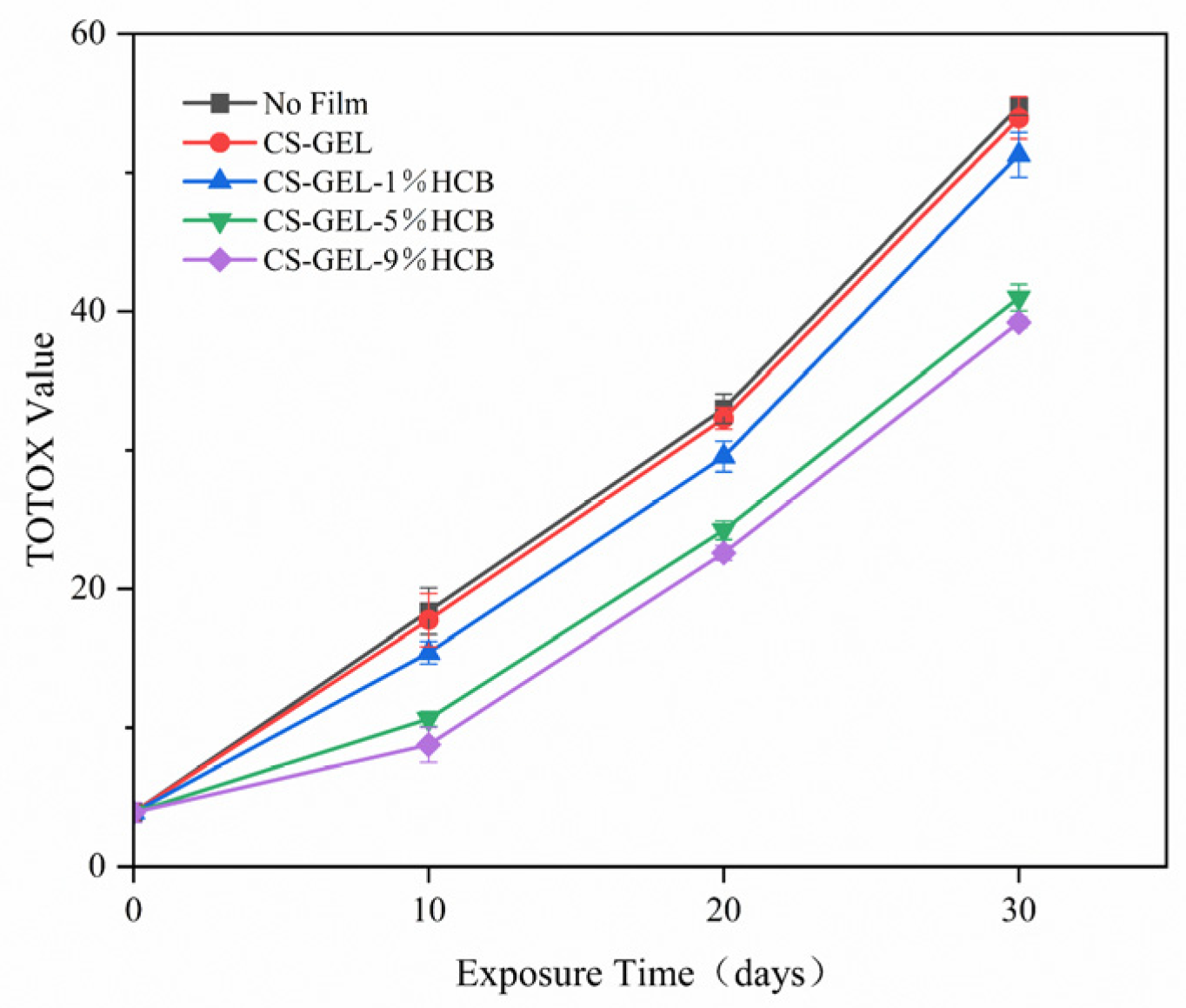
3.6.3. Changes in Total Peroxide Value (TOTOX)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, L.; Wang, F.; Xie, X.; Xie, C.; Li, A.; Xia, N.; Gong, X.; Zhang, H. Development and characterization of chitosan/guar gum active packaging containing walnut green husk extract and its application on fresh-cut apple preservation. Int. J. Biol. Macromol. 2022, 209, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, F.; Yong, H.; Chen, D.; Tang, C.; Kan, J.; Liu, J. Recent advances in chitosan-based active and intelligent packaging films incorporated with flavonoids. Food Chem. X 2025, 25, 102200. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, H.; Julian McClements, D.; Chen, L.; Jiao, A.; Tian, Y.; Miao, M.; Jin, Z. Recent advances in intelligent food packaging materials: Principles, preparation and applications. Food Chem. 2022, 375, 131738. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, Protein and Lipid-Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Nurul Syahida, S.; Ismail-Fitry, M.R.; Ainun, Z.M.A.A.; Nur Hanani, Z.A. Effects of palm wax on the physical, mechanical and water barrier properties of fish gelatin films for food packaging application. Food Packag. Shelf Life 2020, 23, 100437. [Google Scholar] [CrossRef]
- Pattarasiriroj, K.; Kaewprachu, P.; Rawdkuen, S. Properties of rice flour-gelatine-nanoclay film with catechin-lysozyme and its use for pork belly wrapping. Food Hydrocoll. 2020, 107, 105951. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Chen, C.; Fan, H.; Fang, J.; Wu, X.; Qi, J.; Li, H. Chitosan/PVA composite film enhanced by ZnO/lignin with high-strength and antibacterial properties for food packaging. Int. J. Biol. Macromol. 2025, 306, 141658. [Google Scholar] [CrossRef]
- Parveen, S.; Nazeer, S.; Chotana, G.A.; Kanwal, A.; Batool, B.; Bukhari, N.; Yaqoob, A.; Talib, F. Designing of chitosan/gelatin based nanocomposite films integrated with Vachellia nilotica gum carbon dots for smart food packaging applications. Int. J. Biol. Macromol. 2024, 264, 130208. [Google Scholar] [CrossRef]
- Li, C.; Yang, Y.; Zhang, R.; Wang, J.; Zhong, S.; Cui, X. Chitosan-gelatin composite hydrogel antibacterial film for food packaging. Int. J. Biol. Macromol. 2025, 285, 138330. [Google Scholar] [CrossRef]
- Bertolo, M.R.V.; Dias, L.D.; Oliveira Filho, J.G.D.; Alves, F.; Marangon, C.A.; Amaro Martins, V.d.C.; Ferreira, M.D.; Bagnato, V.S.; Guzzi Plepis, A.M.D.; Bogusz, S. Central composite design optimization of active and physical properties of food packaging films based on chitosan/gelatin/pomegranate peel extract. Food Packag. Shelf Life 2022, 34, 100986. [Google Scholar] [CrossRef]
- Eranda, D.H.U.; Chaijan, M.; Panpipat, W.; Karnjanapratum, S.; Cerqueira, M.A.; Castro-Muñoz, R. Gelatin-chitosan interactions in edible films and coatings doped with plant extracts for biopreservation of fresh tuna fish products: A review. Int. J. Biol. Macromol. 2024, 280, 135661. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ge, Y.; Ding, H.; Yan, Y.; Wang, L. Vanillin cross-linked chitosan/gelatin bio-polymer film with antioxidant, water resistance and ultraviolet-proof properties. Int. J. Biol. Macromol. 2023, 253, 126726. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Chen, M.; Warner, R.D.; Fang, Z. Incorporating nisin and grape seed extract in chitosan-gelatine edible coating and its effect on cold storage of fresh pork. Food Control. 2020, 110, 107018. [Google Scholar] [CrossRef]
- Fu, B.; Liu, Q.; Liu, M.; Chen, X.; Lin, H.; Zheng, Z.; Zhu, J.; Dai, C.; Dong, X.; Yang, D.-P. Carbon dots enhanced gelatin/chitosan bio-nanocomposite packaging film for perishable foods. Chin. Chem. Lett. 2022, 33, 4577–4582. [Google Scholar] [CrossRef]
- Fan, S.; Wang, D.; Wen, X.; Li, X.; Fang, F.; Richel, A.; Xiao, N.; Fauconnier, M.-L.; Hou, C.; Zhang, D. Incorporation of cinnamon essential oil-loaded Pickering emulsion for improving antimicrobial properties and control release of chitosan/gelatin films. Food Hydrocoll. 2023, 138, 108438. [Google Scholar] [CrossRef]
- Ding, J.; Wu, X.; Qi, X.; Guo, H.; Liu, A.; Wang, W. Impact of nano/micron vegetable carbon black on mechanical, barrier and anti-photooxidation properties of fish gelatin film. J. Sci. Food Agric. 2017, 98, 2632–2641. [Google Scholar] [CrossRef]
- Pérez-Armada, L.; Rivas, S.; González, B.; Moure, A. Extraction of phenolic compounds from hazelnut shells by green processes. J. Food Eng. 2019, 255, 1–8. [Google Scholar] [CrossRef]
- Allegrini, A.; Salvaneschi, P.; Schirone, B.; Cianfaglione, K.; Di Michele, A. Multipurpose plant species and circular economy: Corylus avellana L. as a study case. Front. Biosci.-Landmark 2022, 27, 11. [Google Scholar] [CrossRef]
- Yuan, B.; Lu, M.; Eskridge, K.M.; Isom, L.D.; Hanna, M.A. Extraction, identification, and quantification of antioxidant phenolics from hazelnut (Corylus avellana L.) shells. Food Chem. 2018, 244, 7–15. [Google Scholar] [CrossRef]
- Cabo, S.; Aires, A.; Carvalho, R.; Pascual-Seva, N.; Silva, A.P.; Gonçalves, B. Corylus avellana L. husks an underutilized waste but a valuable source of polyphenols. Waste Biomass Valorization 2021, 12, 3629–3644. [Google Scholar] [CrossRef]
- Ozpinar, P.; Dogan, C.; Demiral, H.; Morali, U.; Erol, S.; Samdan, C.; Yildiz, D.; Demiral, I. Activated carbons prepared from hazelnut shell waste by phosphoric acid activation for supercapacitor electrode applications and comprehensive electrochemical analysis. Renew. Energy 2022, 189, 535–548. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Baykan, A.; Yaman, S.; Kucukbayrak, S. Effects of fragmentation and particle size on the fuel properties of hazelnut shells. Fuel 2013, 112, 326–330. [Google Scholar] [CrossRef]
- Mohammad Zadeh, E.; O’Keefe, S.F.; Kim, Y.T. Lignin-Based Biopolymeric Active Packaging System for Oil Products. J. Food Sci. 2019, 84, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Rodsamran, P.; Sothornvit, R. Lime peel pectin integrated with coconut water and lime peel extract as a new bioactive film sachet to retard soybean oil oxidation. Food Hydrocoll. 2019, 97, 105173. [Google Scholar] [CrossRef]
- Sun, X.; Li, Q.; Wu, H.; Zhou, Z.; Feng, S.; Deng, P.; Zou, H.; Tian, D.; Lu, C. Sustainable Starch/Lignin Nanoparticle Composites Biofilms for Food Packaging Applications. Polymers 2023, 15, 1959. [Google Scholar] [CrossRef]
- König, J.; Aguilar, F.; Dusemund, B.; Galtier, P.; Gott, D.M.; Lambre, C.; Leblanc, J.C.; Parent-Massin, D.; Stankovic, I.; Tobback, P.; et al. Scientific Opinion on the re-evaluation of vegetable carbon (E 153) as a food additive. EFSA J. 2012, 10, 2592. [Google Scholar] [CrossRef]
- Song, S.; Ji, R.; Xu, J.; Yang, X.; An, Q.; Zhang, X.; Zhang, W. Preparation and characterization of highly stable pH-sensitive multifunctional films based on co-pigment-anthocyanin conjugate system for pork monitoring and preservation. Food Hydrocoll. 2025, 164, 111151. [Google Scholar] [CrossRef]
- Zhu, B.; Zhao, S.; Guo, J.; Song, K.; He, J.; Liu, S.; Zhou, X. Enhancing the mechanical properties of polylactic acid (PLA) composite films using Pueraria lobata root microcrystalline cellulose. Int. J. Biol. Macromol. 2024, 279, 135579. [Google Scholar] [CrossRef]
- Li, R.; Feng, H.; Wang, S.; Zhuang, D.; Zhu, J. A colorimetry-enhanced tri-functional film with high stability by polyphenol-anthocyanin co-pigmentation/conjugate: New prospect for active intelligent food packaging. Food Chem. 2024, 447, 138927. [Google Scholar] [CrossRef]
- Azaza, Y.B.; Hamdi, M.; Charmette, C.; Jridi, M.; Li, S.; Nasri, M.; Nasri, R. Development and characterization of active packaging films based on chitosan and sardinella protein isolate: Effects on the quality and the shelf life of shrimps. Food Packag. Shelf Life 2022, 31, 100796. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Lv, J.; Yang, J.; Liu, X.; Zhang, X.; Zhang, W. Fabrication and characterization of blue honeysuckle anthocyanins-loaded nanocomposite films and the application in pork preservation. Food Hydrocoll. 2024, 149, 109600. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C. A smart film incorporating anthocyanins and tea polyphenols into sodium carboxymethyl cellulose/polyvinyl alcohol for application in mirror carp. Int. J. Biol. Macromol. 2022, 223, 404–417. [Google Scholar] [CrossRef]
- Li, T.; Xia, N.; Xu, L.; Zhang, H.; Zhang, H.; Chi, Y.; Zhang, Y.; Li, L.; Li, H. Preparation, characterization and application of SPI-based blend film with antioxidant activity. Food Packag. Shelf Life 2021, 27, 100614. [Google Scholar] [CrossRef]
- Hu, J.; Li, D.; Huai, Q.; Geng, M.; Sun, Z.; Wang, M.; Wang, S.; Li, Y.; Zheng, H. Development and evaluation of soybean protein isolate–based antibacterial nanocomposite films containing nano-TiO2. Ind. Crops Prod. 2023, 197, 116620. [Google Scholar] [CrossRef]
- Wang, F.; Xie, C.; Tang, H.; Hao, W.; Wu, J.; Sun, Y.; Sun, J.; Liu, Y.; Jiang, L. Development, characterization and application of intelligent/active packaging of chitosan/chitin nanofibers films containing eggplant anthocyanins. Food Hydrocoll. 2023, 139, 108496. [Google Scholar] [CrossRef]
- Wang, F.; Xie, C.; Ye, R.; Tang, H.; Jiang, L.; Liu, Y. Development of active packaging with chitosan, guar gum and watermelon rind extract: Characterization, application and performance improvement mechanism. Int. J. Biol. Macromol. 2023, 227, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Hanani, Z.A.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Gu, X.; Huseien, G.F.; Kar, T.; Sarı, A.; Karaahmet, Z.; Gencel, O.; Hekimoğlu, G.; Çakır, E. Hazelnut shell-based activated carbon/carbon nanotubes/palmityl alcohol as new form-stable phase change material with enhanced energy storage capacity and thermal conductivity. J. Energy Storage 2024, 103, 114346. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Wang, R.; Ge, Y.; Wang, L. Tannic acid crosslinked chitosan/gelatin/SiO2 biopolymer film with superhydrophobic, antioxidant and UV resistance properties for prematuring fruit packaging. Int. J. Biol. Macromol. 2024, 275, 133368. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, J.; Zhou, H.; Zhou, S.; Lv, Y.; Cheng, Y.; Tao, Y.; Lu, J.; Wang, H. Biodegradable intelligent film for food preservation and real-time visual detection of food freshness. Food Hydrocoll. 2022, 129, 107665. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Chen, Y.; Zhang, L.; Kan, J.; Jin, C. Physical, mechanical and antioxidant properties of chitosan films grafted with different hydroxybenzoic acids. Food Hydrocoll. 2017, 71, 176–186. [Google Scholar] [CrossRef]
- Ji, R.; Zhang, X.; Chen, Z.; Song, S.; Li, Y.; Zhang, X.; Zhang, W. Effect of metal cation crosslinking on the mechanical properties and shrimp freshness monitoring sensitivity of pectin/carboxymethyl cellulose sodium/anthocyanin intelligent films. Carbohydr. Polym. 2024, 340, 122285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.; Yong, H.; Qin, Y.; Liu, J.; Jin, C. Development of antioxidant and antimicrobial packaging films based on chitosan and mangosteen (Garcinia mangostana L.) rind powder. Int. J. Biol. Macromol. 2020, 145, 1129–1139. [Google Scholar] [CrossRef]
- Cao, J.; Wang, C.; Zou, Y.; Xu, Y.; Wang, S.; Jiang, C.; Liu, T.; Zhou, X.; Zhang, Q.; Li, S. Colorimetric and antioxidant films based on biodegradable polymers and black nightshade (Solanum nigrum L.) extract for visually monitoring Cyclina sinensis freshness. Food Chem. X 2023, 18, 100661. [Google Scholar] [CrossRef]
- Asif, F.; Mahmood, H.; Jiangtao, L.; Ce, S.; Haiying, C.; Yuxiao, W.; Rentang, Z.; Yaqoob, S.; Jianbo, X.; Lin, L.; et al. Development of eco-friendly chitosan films incorporated with pomelo peel (Citrus Paradisi cv. Changshanhuyou) extract and application to prolong the shelf life of grapes. Int. J. Biol. Macromol. 2025, 304, 140547. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xie, C.; Tang, H.; Li, H.; Hou, J.; Zhang, R.; Liu, Y.; Jiang, L. Intelligent packaging based on chitosan/fucoidan incorporated with coleus grass (Plectranthus scutellarioides) leaves anthocyanins and its application in monitoring the spoilage of salmon (Salmo salar L.). Int. J. Biol. Macromol. 2023, 252, 126423. [Google Scholar] [CrossRef]
- Tagrida, M.; Nilsuwan, K.; Gulzar, S.; Prodpran, T.; Benjakul, S. Fish gelatin/chitosan blend films incorporated with betel (Piper betle L.) leaf ethanolic extracts: Characteristics, antioxidant and antimicrobial properties. Food Hydrocoll. 2022, 137, 108316. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Ran, L.; Xu, D.; Sun, H.; Yang, J.; Zhu, B. Effects of chestnut shell extract and citric acid on the properties of navel orange pomace/chitosan composite films. Int. J. Biol. Macromol. 2024, 283, 137575. [Google Scholar] [CrossRef]
- Kadam, A.A.; Singh, S.; Gaikwad, K.K. Chitosan based antioxidant films incorporated with pine needles (Cedrus deodara) extract for active food packaging applications. Food Control. 2021, 124, 107877. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, X.; Bai, R.; Liu, Y.; Liu, J.; Liu, J. Preparation and characterization of antioxidant and antimicrobial packaging films based on chitosan and proanthocyanidins. Int. J. Biol. Macromol. 2019, 134, 11–19. [Google Scholar] [CrossRef]
- Fabra, M.J.; Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A. Antiviral and antioxidant properties of active alginate edible films containing phenolic extracts. Food Hydrocoll. 2018, 81, 96–103. [Google Scholar] [CrossRef]
- Mirbagheri, V.S.; Alishahi, A.; Ahmadian, G.; Hashemi Petroudi, S.H.; Ojagh, S.M.; Romanazzi, G. Toward understanding the antibacterial mechanism of chitosan: Experimental approach and in silico analysis. Food Hydrocoll. 2024, 147, 109382. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Khezerlou, A.; Rezvani-Ghalhari, M.; McClements, D.J.; Varma, R.S. Advanced carbon-based nanomaterials: Application in the development of multifunctional next-generation food packaging materials. Adv. Colloid Interface Sci. 2025, 339, 103422. [Google Scholar] [CrossRef] [PubMed]
- Raul, P.K.; Thakuria, A.; Das, B.; Devi, R.R.; Tiwari, G.; Yellappa, C.; Kamboj, D.V. Carbon Nanostructures As Antibacterials and Active Food-Packaging Materials: A Review. ACS Omega 2022, 7, 11555–11559. [Google Scholar] [CrossRef]
- Saeed, R.; Naz, S. Effect of heating on the oxidative stability of corn oil and soybean oil. Grasas Y Aceites 2019, 70, e303. [Google Scholar] [CrossRef]
- Huang, S.; Tu, Z.; Sha, X.; Hu, Y.; Chen, N.; Wang, H. Fabrication and performance evaluation of pectin–fish gelatin–resveratrol preservative films. Food Chem. 2021, 361, 129832. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, M.; Lyu, C.; Li, B.; Meng, X.; Si, X.; Shu, C. Effect of heat treatment on oxidation of hazelnut oil. J. Oleo Sci. 2022, 71, 1711–1723. [Google Scholar] [CrossRef]


| Films | L | a | b | ∆E | WI |
|---|---|---|---|---|---|
| CS-GEL | 89.91 ± 0.11 a | 0.12 ± 0.02 d | −8.33 ± 0.14 d | 3.75 ± 0.13 d | 86.91 ± 0.02 a |
| CS-GEL-1%HCB | 82.01 ± 0.16 b | 0.18 ± 0.02 c | −6.38 ± 0.06 c | 9.90 ± 0.10 c | 80.91 ± 0.18 b |
| CS-GEL-5%HCB | 74.55 ± 0.27 c | 0.26 ± 0.03 b | −5.17 ± 0.12 b | 17.04 ± 0.25 b | 74.03 ± 0.26 c |
| CS-GEL-9%HCB | 62.41 ± 0.17 d | 0.37 ± 0.02 a | −3.28 ± 0.12 a | 29.09 ± 0.12 a | 62.26 ± 0.17 d |
| Films | Thickness (mm) | TS (MPa) | EAB (%) | Opacity Value (mm−1) |
|---|---|---|---|---|
| CS-GEL | 0.087 ± 0.007 c | 15.83 ± 0.40 d | 49.51 ± 1.03 a | 0.67 ± 0.02 d |
| CS-GEL-1%HCB | 0.101 ± 0.003 b | 21.26 ± 0.90 c | 48.49 ± 0.97 a | 0.73 ± 0.01 c |
| CS-GEL-5%HCB | 0.111 ± 0.004 a | 32.06 ± 0.61 a | 37.11 ± 1.22 b | 1.52 ± 0.03 b |
| CS-GEL-9%HCB | 0.117 ± 0.002 a | 25.83 ± 0.42 b | 32.90 ± 1.20 c | 3.85 ± 0.03 a |
| Films | MC (%) | WS (%) | WVP (×10−7 g m−1 h−1 Pa−1) | OTR (g/d·m2) |
|---|---|---|---|---|
| CS-GEL | 16.27 ± 0.44 a | 24.84 ± 0.45 a | 5.51 ± 0.07 a | 0.55 ± 0.03 a |
| CS-GEL-1%HCB | 15.58 ± 0.52 a | 23.37 ± 0.67 b | 5.14 ± 0.05 b | 0.42 ± 0.03 b |
| CS-GEL-5%HCB | 12.27 ± 0.28 b | 20.75 ± 0.57 c | 3.79 ± 0.16 d | 0.23 ± 0.02 c |
| CS-GEL-9%HCB | 11.76 ± 0.22 b | 20.10 ± 0.45 c | 4.51 ± 0.26 c | 0.15 ± 0.02 d |
| Films | Diameter of Inhibition Zone (mm) | |
|---|---|---|
| Escherichia coli | Staphylococcus aureus | |
| CS-GEL | 15.81 ± 0.03 c | 18.89 ± 0.06 c |
| CS-GEL-1%HCB | 16.64 ± 0.09 b | 19.58 ± 0.06 b |
| CS-GEL-5%HCB | 19.56 ± 0.09 a | 22.84 ± 0.07 a |
| CS-GEL-9%HCB | 15.92 ± 0.01 c | 18.91 ± 0.07 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, M.; Wang, J.; Xun, Z.; Liu, M.; Li, H.; Wang, W.; Wang, Y.; Guo, C.; Li, H.; Xu, N.; et al. The Preparation and Characterization of Antioxidant Films Based on Hazelnut Shell-Based Vegetable Carbon Black/Chitosan/Gelatin and the Application on Soybean Oils. Foods 2025, 14, 1678. https://doi.org/10.3390/foods14101678
Niu M, Wang J, Xun Z, Liu M, Li H, Wang W, Wang Y, Guo C, Li H, Xu N, et al. The Preparation and Characterization of Antioxidant Films Based on Hazelnut Shell-Based Vegetable Carbon Black/Chitosan/Gelatin and the Application on Soybean Oils. Foods. 2025; 14(10):1678. https://doi.org/10.3390/foods14101678
Chicago/Turabian StyleNiu, Mengyuan, Jiaxin Wang, Zhaoying Xun, Mengzhuo Liu, He Li, Weiyi Wang, Yuchen Wang, Chao Guo, Hanyu Li, Ning Xu, and et al. 2025. "The Preparation and Characterization of Antioxidant Films Based on Hazelnut Shell-Based Vegetable Carbon Black/Chitosan/Gelatin and the Application on Soybean Oils" Foods 14, no. 10: 1678. https://doi.org/10.3390/foods14101678
APA StyleNiu, M., Wang, J., Xun, Z., Liu, M., Li, H., Wang, W., Wang, Y., Guo, C., Li, H., Xu, N., Zhang, H., & Xia, N. (2025). The Preparation and Characterization of Antioxidant Films Based on Hazelnut Shell-Based Vegetable Carbon Black/Chitosan/Gelatin and the Application on Soybean Oils. Foods, 14(10), 1678. https://doi.org/10.3390/foods14101678







