Poly-β-hydroxybutyrate Production from Bread Waste via Sequential Dark Fermentation and Photofermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Inoculum and Dark Fermentation Assay Using Bread Waste
2.3. Inoculum and Batch Photofermentation Assays Using Fermented Bread Broth
2.4. Photobioreactor Design and Scale-Up of PHB Production
2.5. Analytical Methods
2.5.1. FBB Chemical Characterization
2.5.2. C/N Ratio and Organic Acids Yield Calculation
2.5.3. Monitoring of PNSB Growth
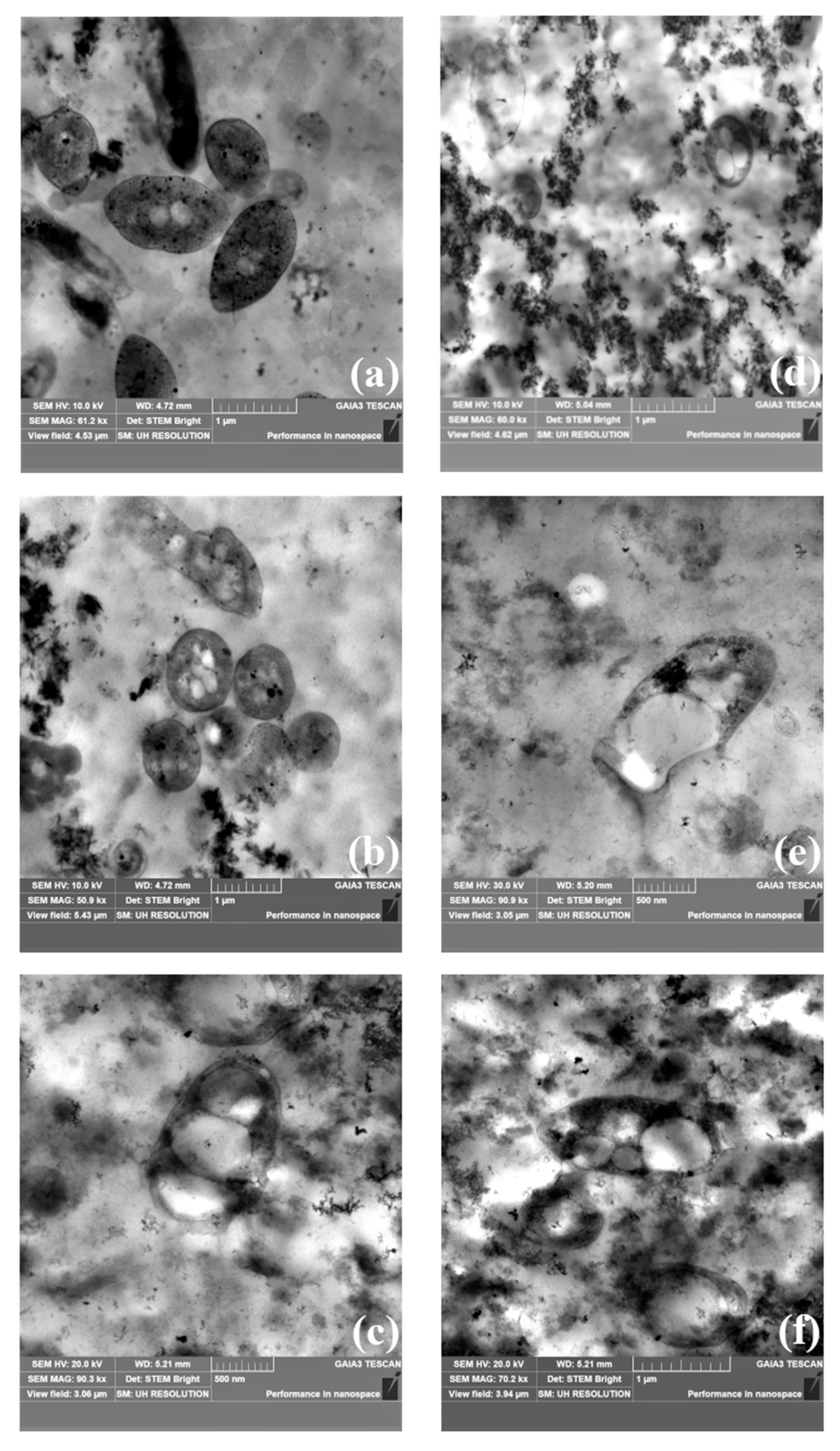
2.5.4. Transmission Electron Microscopy (TEM) Analysis
2.5.5. PHB Quantification
2.6. Statistical Analysis
3. Results
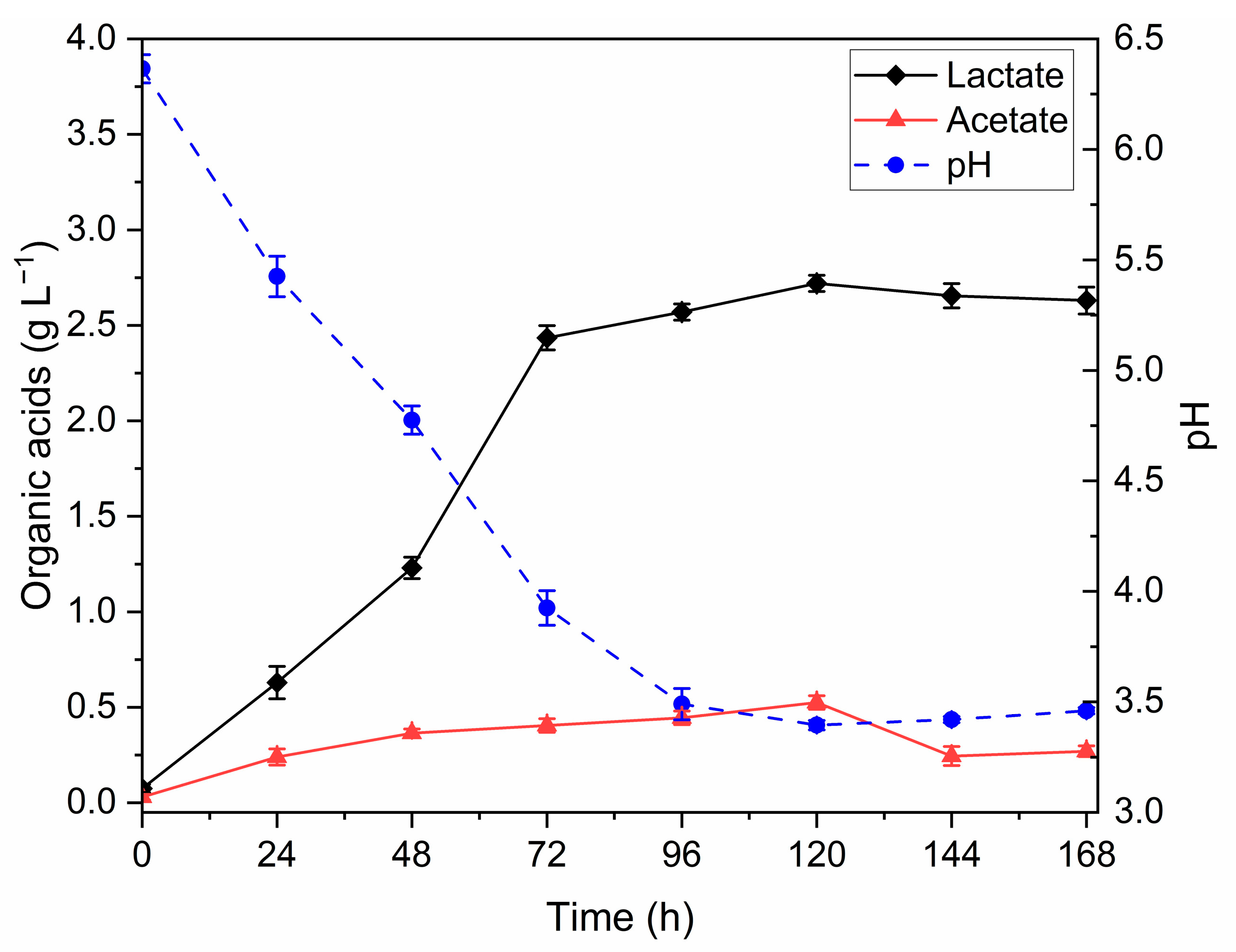
3.1. Dark Fermentation (DF) Optimization
3.2. FBB Chemical Composition
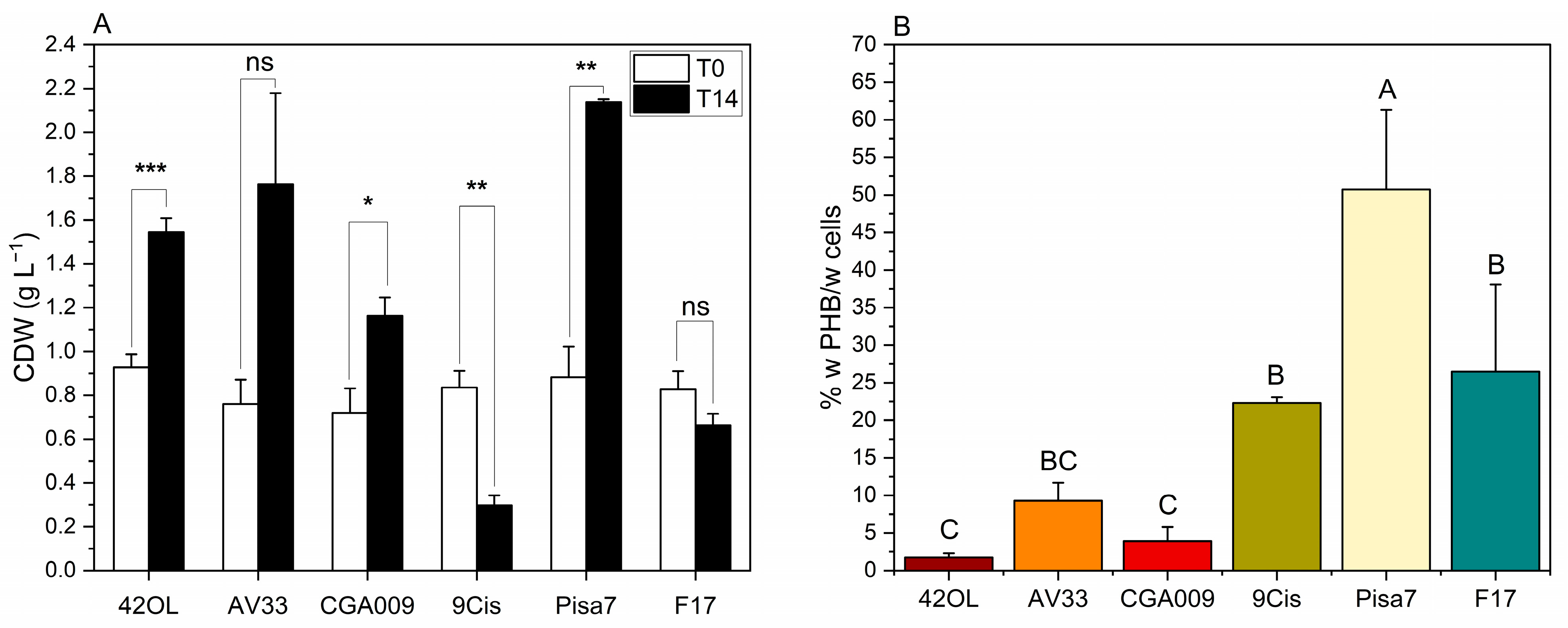
3.3. Screening of PNSB Strains for Growth and PHB Production on FBB
3.4. Scale-Up of PHB Production in a 5 L Photobioreactor
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PHAs | Polyhydroxyalkanoates |
| PHB | Poly-β-hydroxybutyrate |
| Scl-PHA | Short-chain-length polyhydroxyalkanoate |
| PNSB | Purple non-sulfur bacteria |
| PF | Photofermentation |
| Bio-H2 | Biohydrogen |
| DF | Dark fermentation |
| LAB | Lactic acid bacteria |
| BChls | Bacteriochlorophylls |
| FBB | Fermented bread broth |
| AD | Anaerobic digestion |
| HPLC | High-performance liquid chromatography |
| CDW | Cell dry weight |
| TEM | Transmission electron microscopy |
| OD660 | Optical density at 660 nm |
| BChla | Bacteriochlorophyll a |
References
- He, Y.; Deng, X.; Jiang, L.; Hao, L.; Shi, Y.; Lyu, M.; Zhang, L.; Wang, S. Current advances, challenges and strategies for enhancing the biodegradation of plastic waste. Sci. Total Environ. 2024, 906, 167850. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Walker, T.R. Plastic recycling: A panacea or environmental pollution problem. Npj Mater. Sustain. 2024, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Luengo, J.M.; García, B.; Sandoval, A.; Naharro, G.; Olivera, E.R. Bioplastics from microorganisms. Curr. Opin. Microbiol. 2003, 6, 251–260. [Google Scholar] [CrossRef]
- Singh, N.; Ogunseitan, O.A.; Wong, M.H.; Tang, Y. Sustainable materials alternative to petrochemical plastics pollution: A review analysis. Sustain. Horiz. 2022, 2, 100016. [Google Scholar] [CrossRef]
- Bishop, G.; Styles, D.; Lens, P.N. Environmental performance comparison of bioplastics and petrochemical plastics: A review of life cycle assessment (LCA) methodological decisions. Resour. Conserv. Recycl. 2021, 168, 105451. [Google Scholar] [CrossRef]
- Kumar, R.; Lalnundiki, V.; Shelare, S.D.; Abhishek, G.J.; Sharma, S.; Sharma, D.; Kumar, A.; Abbas, M. An investigation of the environmental implications of bioplastics: Recent advancements on the development of environmentally friendly bioplastics solutions. Environ. Res. 2024, 244, 117707. [Google Scholar] [CrossRef] [PubMed]
- Monroy, I.; Buitrón, G. Production of polyhydroxybutyrate by pure and mixed cultures of purple non-sulfur bacteria: A review. J. Biotechnol. 2020, 317, 39–47. [Google Scholar] [CrossRef]
- Corneli, E.; Adessi, A.; Dragoni, F.; Ragaglini, G.; Bonari, E.; De Philippis, R. Agroindustrial residues and energy crops for the production of hydrogen and poly-β-hydroxybutyrate via photofermentation. Bioresour. Technol. 2016, 216, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Rehm, B.H. Bacterial biopolymers: From pathogenesis to advanced materials. Nat. Rev. Microbiol. 2020, 18, 195–210. [Google Scholar] [CrossRef]
- Obruca, S.; Sedlacek, P.; Slaninova, E.; Fritz, I.; Daffert, C.; Meixner, K.; Sedrlova, Z.; Koller, M. Novel unexpected functions of PHA granules. Appl. Microbiol. Biotechnol. 2020, 104, 4795–4810. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Di Donato, P.; Abbamondi, G.R.; Nicolaus, B. Synthesis, production, and biotechnological applications of exopolysaccharides and polyhydroxyalkanoates by archaea. Archaea 2011, 2011, 693253. [Google Scholar] [CrossRef] [PubMed]
- Thu, N.T.T.; Hoang, L.H.; Cuong, P.K.; Viet-Linh, N.; Nga, T.T.H.; Kim, D.D.; Leong, Y.K.; Nhi-Cong, L.T. Evaluation of polyhydroxyalkanoate (PHA) synthesis by Pichia sp. TSLS24 yeast isolated in Vietnam. Sci. Rep. 2023, 13, 3137. [Google Scholar] [CrossRef] [PubMed]
- Amadu, A.A.; Qiu, S.; Ge, S.; Addico, G.N.D.; Ameka, G.K.; Yu, Z.; Xia, W.; Abbew, A.-W.; Shao, D.; Champagne, P.; et al. A review of biopolymer (Poly-β-hydroxybutyrate) synthesis in microbes cultivated on wastewater. Sci. Total Environ. 2021, 756, 143729. [Google Scholar] [CrossRef]
- Koller, M.; Mukherjee, A. A new wave of industrialization of PHA biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Muneer, F.; Rasul, I.; Azeem, F.; Siddique, M.H.; Zubair, M.; Nadeem, H. Microbial Polyhydroxyalkanoates (PHAs): Efficient Replacement of Synthetic Polymers. J. Polym. Environ. 2020, 28, 2301–2323. [Google Scholar] [CrossRef]
- Adessi, A.; Corneli, E.; De Philippis, R. Photosynthetic Purple Non Sulfur Bacteria in Hydrogen Producing Systems: New Approaches in the Use of Well Known and Innovative Substrates. In Modern Topics in the Phototrophic Prokaryotes; Hallenbeck, P., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Alsiyabi, A.; Immethun, C.M.; Saha, R. Modeling the Interplay between Photosynthesis, CO2 Fixation, and the Quinone Pool in a Purple Non-Sulfur Bacterium. Sci. Rep. 2019, 9, 12638. [Google Scholar] [CrossRef]
- McKinlay, J.B. Systems biology of photobiological hydrogen production by purple non-sulfur bacteria. In Microbial Bioenergy: Hydrogen Production; Springer: Dordrecht, The Netherlands, 2014; pp. 155–176. [Google Scholar] [CrossRef]
- Montiel-Corona, V.; Buitrón, G. Polyhydroxyalkanoates from organic waste streams using purple non-sulfur bacteria. Bioresour. Technol. 2021, 323, 124610. [Google Scholar] [CrossRef]
- Wu, S.C.; Liou, S.Z.; Lee, C.M. Correlation between bio-hydrogen production and polyhydroxybutyrate (PHB) synthesis by Rhodopseudomonas palustris WP3-5. Bioresour. Technol. 2012, 113, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Argun, H.; Kargi, F. Bio-hydrogen production by different operational modes of dark and photo-fermentation: An overview. Int. J. Hydrogen Energy 2011, 36, 7443–7459. [Google Scholar] [CrossRef]
- Hördt, A.; López, M.G.; Meier-Kolthoff, J.P.; Schleuning, M.; Weinhold, L.M.; Tindall, B.J.; Gronow, S.; Kyrpides, N.C.; Woyke, T.; Göker, M. Analysis of 1,000+ type-strain genomes substantially improves taxonomic classification of Alphaproteobacteria. Front. Microbiol. 2020, 11, 468. [Google Scholar] [CrossRef]
- Gahlawat, G.; Kumari, P.; Bhagat, N.R. Technological advances in the production of polyhydroxyalkanoate biopolymers. Curr. Sustain./Renew. Energy Rep. 2020, 7, 73–83. [Google Scholar] [CrossRef]
- Gowda, V.; Shivakumar, S. Agrowaste-based Polyhydroxyalkanoate (PHA) production using hydrolytic potential of Bacillus thuringiensis IAM 12077. Braz. Arch. Biol. Technol. 2014, 57, 55–61. [Google Scholar] [CrossRef]
- Sirohi, R.; Pandey, J.P.; Gaur, V.K.; Gnansounou, E.; Sindhu, R. Critical overview of biomass feedstocks as sustainable substrates for the production of polyhydroxybutyrate (PHB). Bioresour. Technol. 2020, 311, 123536. [Google Scholar] [CrossRef] [PubMed]
- Adessi, A.; Venturi, M.; Candeliere, F.; Galli, V.; Granchi, L.; De Philippis, R. Bread wastes to energy: Sequential lactic and photo-fermentation for hydrogen production. Int. J. Hydrogen Energy 2018, 43, 9569–9576. [Google Scholar] [CrossRef]
- Verni, M.; Minisci, A.; Convertino, S.; Nionelli, L.; Rizzello, C.G. Wasted bread as substrate for the cultivation of starters for the food industry. Front. Microbiol. 2020, 11, 293. [Google Scholar] [CrossRef]
- Ike, A.; Toda, N.; Tsuji, N.; Hirata, K.; Miyamoto, K. Hydrogen photoproduction from CO2-fixing microalgal biomass: Application of halotolerant photosynthetic bacteria. J. Ferment. Bioeng. 1997, 84, 606–609. [Google Scholar] [CrossRef]
- Oda, Y.; Park, B.S.; Moon, K.H.; Tonomura, K. Recycling of bakery wastes using an amylolytic lactic acid bacterium. Bioresour. Technol. 1997, 60, 101–106. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Hashimoto, K.; Hirata, K.; Miyamoto, K. H2 production from algal biomass by a mixed culture of Rhodobium marinum A-501 and Lactobacillus amylovorus. J. Biosci. Bioeng. 2001, 91, 277–282. [Google Scholar] [CrossRef]
- Trontel, A.; Baršić, V.; Slavica, A.; Šantek, B.; Novak, S. Modelling the effect of different substrates and temperature on the growth and lactic acid production by Lactobacillus amylovorus DSM 20531T in batch process. Food Technol. Biotechnol. 2010, 48, 352–361. [Google Scholar]
- Adessi, A.; De Philippis, R. Photobioreactor design and illumination systems for H2 production with anoxygenic photosynthetic bacteria: A review. Int. J. Hydrogen Energy 2014, 39, 3127–3141. [Google Scholar] [CrossRef]
- Leibniz Institute DSMZ German Collection of Microorganisms and Cell Cultures GmbH. Available online: https://www.dsmz.de/ (accessed on 25 March 2025).
- Mugnai, G.; Bernabò, L.; Daly, G.; Corneli, E.; Adessi, A. Photofermentative production of poly-β-hydroxybutyrate (PHB) by purple non-sulfur bacteria using olive oil by-products. Bioresour. Bioprocess. 2025, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Adessi, A.; Spini, G.; Presta, L.; Mengoni, A.; Viti, C.; Giovannetti, L.; Fani, R.; De Philippis, R. Draft genome sequence and overview of the purple non sulfur bacterium Rhodopseudomonas palustris 42OL. Stand. Genom. Sci. 2016, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Mannelli, F.; Viti, C.; Adessi, A.; De Philippis, R. Hydrogen-producing purple non-sulfur bacteria isolated from the trophic lake Averno (Naples, Italy). Int. J. Hydrogen Energy 2010, 35, 12216–12223. [Google Scholar] [CrossRef]
- Kim, M.K.; Harwood, C.S. Regulation of benzoate-CoA ligase in Rhodopseudomonas palustris. FEMS Microbiol. Lett. 1991, 83, 199–203. [Google Scholar] [CrossRef]
- Carlozzi, P.; Sacchi, A. Biomass production and studies on Rhodopseudomonas palustris grown in an outdoor, temperature controlled, underwater tubular photobioreactor. J. Biotechnol. 2001, 88, 239–249. [Google Scholar] [CrossRef]
- De Philippis, R.; Sili, C.; Vincenzini, M. Glycogen and poly-β-hydroxybutyrate synthesis in Spirulina maxima. Microbiology 1992, 138, 1623–1628. [Google Scholar] [CrossRef]
- Sali, S.; Mackey, H.R. The application of purple non-sulfur bacteria for microbial mixed culture polyhydroxyalkanoates production. Rev. Environ. Sci. Bio/Technol. 2021, 20, 959–983. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Brandl, H.; Gross, R.A.; Lenz, R.W.; Lloyd, R.; Fuller, R.C. The accumulation of poly (3-hydroxyalkanoates) in Rhodobacter sphaeroides. Arch. Microbiol. 1991, 155, 337–340. [Google Scholar] [CrossRef]
- Fradinho, J.C.; Oehmen, A.; Reis, M.A.M. Photosynthetic mixed culture polyhydroxyalkanoate (PHA) production from individual and mixed volatile fatty acids (VFAs): Substrate preferences and co-substrate uptake. J. Biotechnol. 2014, 185, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Liebergesell, M.; Hustede, E.; Timm, A.; Steinbüchel, A.; Fuller, R.C.; Lenz, R.W.; Schlegel, H.G. Formation of poly (3-hydroxyalkanoates) by phototrophic and chemolithotrophic bacteria. Arch. Microbiol. 1991, 155, 415–421. [Google Scholar] [CrossRef]
- Häfner, F.; Hartung, J.; Möller, K. Digestate composition affecting N fertiliser value and C mineralisation. Waste Biomass Valorization 2022, 13, 3445–3462. [Google Scholar] [CrossRef]
- Demiriz, B.Ö.; Kars, G.; Yücel, M.; Eroğlu, İ.; Gündüz, U. Hydrogen and poly-β-hydroxybutyric acid production at various acetate concentrations using Rhodobacter capsulatus DSM 1710. Int. J. Hydrogen Energy 2019, 44, 17269–17277. [Google Scholar] [CrossRef]
- Alsafadi, D.; Al-Mashaqbeh, O. A one-stage cultivation process for the production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) from olive mill wastewater by Haloferax mediterranei. New Biotechnol. 2017, 34, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Carlozzi, P.; Seggiani, M.; Cinelli, P.; Mallegni, N.; Lazzeri, A. Photofermentative poly-3-hydroxybutyrate production by Rhodopseudomonas sp. S16-VOGS3 in a novel outdoor 70-L photobioreactor. Sustainability 2018, 10, 3133. [Google Scholar] [CrossRef]
- Khatipov, E.; Miyake, M.; Miyake, J.; Asada, Y. Accumulation of poly-β-hydroxybutyrate by Rhodobacter sphaeroides on various carbon and nitrogen substrates. FEMS Microbiol. Lett. 1998, 162, 39–45. [Google Scholar] [CrossRef]
- Brown, B.; Immethun, C.; Alsiyabi, A.; Long, D.; Wilkins, M.; Saha, R. Heterologous phasin expression in Rhodopseudomonas palustris CGA009 for bioplastic production from lignocellulosic biomass. Metab. Eng. Commun. 2022, 14, e00191. [Google Scholar] [CrossRef]
- Carlozzi, P.; Touloupakis, E.; Di Lorenzo, T.; Giovannelli, A.; Seggiani, M.; Cinelli, P.; Lazzeri, A. Whey and molasses as in-expensive raw materials for parallel production of biohydrogen and polyesters via a two-stage bioprocess: New routes towards a circular bioeconomy. J. Biotechnol. 2019, 303, 37–45. [Google Scholar] [CrossRef]
- Ghimire, A.; Valentino, S.; Frunzo, L.; Pirozzi, F.; Lens, P.N.; Esposito, G. Concomitant biohydrogen and poly-β-hydroxybutyrate production from dark fermentation effluents by adapted Rhodobacter sphaeroides and mixed pho-tofermentative cultures. Bioresour. Technol. 2016, 217, 157–164. [Google Scholar] [CrossRef]
- Luongo, V.; Ghimire, A.; Frunzo, L.; Fabbricino, M.; d’Antonio, G.; Pirozzi, F.; Esposito, G. Photofermentative production of hydrogen and poly-β-hydroxybutyrate from dark fermentation products. Bioresour. Technol. 2017, 228, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Corona, V.M.; Le Borgne, S.; Revah, S.; Morales, M. Effect of light-dark cycles on hydrogen and poly-β-hydroxybutyrate production by a photoheterotrophic culture and Rhodobacter capsulatus using a dark fermentation effluent as substrate. Bioresour. Technol. 2017, 226, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Corona, V.; Revah, S.; Morales, M. Hydrogen production by an enriched photoheterotrophic culture using dark fer-mentation effluent as substrate: Effect of flushing method, bicarbonate addition, and outdoor–indoor conditions. Int. J. Hydrogen Energy 2015, 40, 9096–9105. [Google Scholar] [CrossRef]
- Allegue, L.D.; Puyol, D.; Melero, J.A. Food waste valorization by purple phototrophic bacteria and anaerobic digestion after thermal hydrolysis. Biomass Bioenergy 2020, 142, 105803. [Google Scholar] [CrossRef]

| Species | Origin | Reference |
|---|---|---|
| Rhodopseudomonas palustris strain 42OL | Sugar refinery waste treatment pond; Castiglion Fiorentino (AR), Italy. | [36] |
| Rhodopseudomonas palustris strain AV33 | Trophic lake; Pozzuoli (NA), Italy. | [37] |
| Rhodopseudomonas palustris strain CGA009 | Chloramphenicol-resistant derivative of R. palustris CGA001; courtesy of Prof. C. S. Harwood, University of Washington. | [38] |
| Cereibacter johrii strain 9Cis | Dairy effluent; Bologna (BO), Italy. | [35] |
| Cereibacter johrii strain Pisa7 | Lake; San Rossore (PI), Italy. | [35] |
| Cereibacter sphaeroides strain F17 | Sewage treatment pond; Florence (FI), Italy. | [35] |
| Compounds | Concentration (g L−1) |
|---|---|
| B | nd |
| Ca | 1.27 (±0.53) |
| Cd | nd |
| Co | nd |
| Cr | nd |
| Cu | nd |
| Fe | nd |
| K | 2.74 (±0.24) |
| Mg | 0.66 (±0.012) |
| Mn | nd |
| Mo | nd |
| Na | 8.76 (±2.14) |
| Ni | nd |
| S | 0.14 (±0.007) |
| Si | 0.042 (±0.003) |
| Zn | nd |
| Compounds | FBB for the Screening Test | FBB for Run_1 | FBB for Run_2 |
|---|---|---|---|
| Lactate (g L−1) | 2.64 (±0.06) a | 3.12 (±0.30) a | 2.96 (±0.01) a |
| Acetate (g L−1) | 0.20 (±0.04) a | 0.19 (±0.05) a | 0.20 (±0.05) a |
| Ammonium (mg L−1) | 25.32 (±1.95) a | 25.66 (±2.90) a | 27.90 (±4.96) a |
| C/N ratio | 67.27 (±2.52) a | 77.50 (±6.70) a | 67.95 (±1.37) a |
| Organic acids yield (%) | 5.89 (±0.22) a | 6.91 (±0.57) a | 6.56 (±0.13) a |
| Strain/Run | mg PHB Lcult−1 | mg PHB Lcult−1 h−1 | mg PHB Lcult−1 d−1 |
|---|---|---|---|
| R. palustris 42OL | 27.11 (±8.36) b | 0.08 (±0.02) b | 1.94 (±0.60) b |
| R. palustris AV33 | 159.68 (±42.02) b | 0.48 (±0.13) b | 11.41 (±3.00) b |
| R. palustris CGA009 | 44.66 (±17.66) b | 0.13 (±0.05) b | 3.19 (±1.26) b |
| C. johrii 9Cis | 66.13 (±8.51) b | 0.20 (±0.03) b | 4.72 (±0.61) b |
| C. johrii Pisa7 | 1083.76 (±220.05) a | 3.23 (±0.65) a | 77.41 (±15.72) a |
| C. sphaeroides F17 | 179.47 (±92.29) b | 0.53 (±0.27) b | 12.82 (±6.59) b |
| Run_1 | 744.22 (±61.22) A | 2.03 (±0.18) A | 48.82 (±4.34) A |
| Run_2 | 352.01 (±57.78) B | 0.89 (±0.19) B | 21.33 (±4.55) B |
| Compound | Run | T0 (0 h) | T7 (168 h) | T14 (336 h) |
|---|---|---|---|---|
| Maltotriose (g L−1) | Run_1 | 0.73 (±0.17) a, A | 0.16 (±0.04) b, A | nd |
| Run_2 | 0.67 (±0.11) a, A | 0.09 (±0.05) b, A | 0.02 (±0.01) b | |
| Maltose (g L−1) | Run_1 | 0.28 (±0.07) a, A | 0.17 (±0.02) b, A | nd |
| Run_2 | 0.24 (±0.10) a, A | 0.04 (±0.02) b, B | nd | |
| Lactate (g L−1) | Run_1 | 3.12 (±0.30) a, A | 2.15 (±0.03) b, A | 1.98 (±0.06) b, A |
| Run_2 | 2.96 (±0.01) a, A | 1.99 (±0.41) b, A | 2.27 (±0.53) b, A | |
| Acetate (g L−1) | Run_1 | 0.19 (±0.05) a, A | 0.31 (±0.02) b, A | 0.75 (±0.03) c, A |
| Run_2 | 0.20 (±0.05) a, A | 0.36 (±0.09) b, A | 0.97 (±0.25) c, A | |
| Ethanol (g L−1) | Run_1 | 1.21 (±0.12) a, A | 1.24 (±0.12) ab, A | 1.58 (±0.00) b, A |
| Run_2 | 0.95 (±0.11) a, A | 1.29 (±0.28) ab, A | 2.21 (±0.52) b, A | |
| Ammonium (mg L−1) | Run_1 | 25.66 (±2.90) a, A | 25.33 (±2.65) a, A | 15.53 (±0.32) b, A |
| Run_2 | 27.90 (±4.96) a, A | 27.63 (±0.76) a, A | 11.60 (±2.18) b, A |
| Inoculum | Substrate | Light | PHB (or PHA) (% CDW) | PHB (mg Lcult−1) | WV (mL) | Ref. |
|---|---|---|---|---|---|---|
| Rhodopseudomonas capsulatus | DF fruit and vegetable waste | White | 24 | - | 76 | [55] |
| Rhodopseudomonas capsulatus | DF fruit and vegetable waste | White | 5 | - | 76 | [56] |
| Rhodobacter sphaeroides AV1b | DF food waste | White | 80 | 800 | 400 | [53] |
| Rhodobacter sphaeroides AV1b | DF municipal organic waste | White | 83 | 882 | 400 | [54] |
| Rhodopseudomonas sp. S16-VOGS3 | DF cheese whey | White | 18 | 232 | 4000 | [52] |
| Mixed photosynthetic culture | DF food waste | Infrared | 19 (PHA) | - | 100 | [57] |
| C. johrii Pisa7 | DF bread waste (FBB) | White | 50.73 | 1083.76 | 100 | This study |
| C. johrii Pisa7 (Run_1) | DF bread waste (FBB) | Selected (593 and 860 nm) | 15.17 | 744.22 | 5000 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernabò, L.; Daly, G.; Mugnai, G.; Galli, V.; Corneli, E.; Granchi, L.; Adessi, A. Poly-β-hydroxybutyrate Production from Bread Waste via Sequential Dark Fermentation and Photofermentation. Foods 2025, 14, 1659. https://doi.org/10.3390/foods14101659
Bernabò L, Daly G, Mugnai G, Galli V, Corneli E, Granchi L, Adessi A. Poly-β-hydroxybutyrate Production from Bread Waste via Sequential Dark Fermentation and Photofermentation. Foods. 2025; 14(10):1659. https://doi.org/10.3390/foods14101659
Chicago/Turabian StyleBernabò, Luca, Giulia Daly, Gianmarco Mugnai, Viola Galli, Elisa Corneli, Lisa Granchi, and Alessandra Adessi. 2025. "Poly-β-hydroxybutyrate Production from Bread Waste via Sequential Dark Fermentation and Photofermentation" Foods 14, no. 10: 1659. https://doi.org/10.3390/foods14101659
APA StyleBernabò, L., Daly, G., Mugnai, G., Galli, V., Corneli, E., Granchi, L., & Adessi, A. (2025). Poly-β-hydroxybutyrate Production from Bread Waste via Sequential Dark Fermentation and Photofermentation. Foods, 14(10), 1659. https://doi.org/10.3390/foods14101659







