Abstract
A high-throughput method for the determination of a variety of chemical hazards in poultry muscle and egg samples was established via ultra-performance liquid chromatography–tandem triple quadrupole mass spectrometry (UPLC–QqQ-MS). The sample preparation procedure was developed based on this quick, easy, cheap, effective, rugged, and safe (QuEChERS) method and validated for 280 chemical hazards potentially present in poultry products. The target compounds in poultry samples were extracted with a 1% formic acid–acetonitrile solution (15:85, v/v), and the metal ions in the matrix were chelated by adding ethylenediaminetetraacetic acid disodium salt (Na2EDTA). The supernatant was purified using Enhanced Matrix Removal (EMR) lipid sorbent. Chromatographic gradient separation was performed on an ACQUITY UPLC BEH C18 (2.1 mm × 100 mm, 1.7 μm) column with multiple reaction monitoring (MRM) under both negative- and positive-ion mode. Internal standard calibration or matrix-matched calibration was used for the quantitation. The results showed that good linearity was achieved for each target compound with correlation coefficients (R2) ≥ 0.99. The limits of detection (LODs) ranged from 0.05 to 10 µg/kg, and the acceptable limits of quantification (LOQs) were determined to be 0.1–20 µg/kg for all 280 compounds. Approximately 90% of the target compounds exhibited mean recoveries ranging from 60% to 120%, with relative standard deviations (RSDs) within 16.2%. This method can be used for the high-throughput rapid detection of prohibited drug residues in poultry eggs due to its easy operation and high accuracy. It was applied in real sample detection, and 43 chemicals including metronidazole were found in 211 poultry samples, with a concentration range of 0.11–638 μg/kg.
1. Introduction
Poultry products, including muscle and eggs, are common animal-derived foods in daily life due to being rich in protein, vitamins, minerals, and other nutrients. China is a major producer and consumer of poultry products. According to the report of the National Bureau of Statistics [1], the off-take volume nationwide of poultry in 2024 was 17.3 billion, which means that the quality of these products is closely related to consumer health and national interests. Poultry product safety has become a hot topic in recent years. The government has established standards to regulate the breeding industry. However, during poultry farming, the abuse or non-compliant use of veterinary drugs poses a risk of excessive residues in the final products (including, but not limited to, sulfonamides, quinolones, tetracyclines, and β-agonists). Additionally, the environmental pollution produced by farming sites and feed can lead to the pollution or accumulation of contaminants through the food chain, thereby threatening consumer health. The long-term consumption of products with excessive levels of chemical residues may cause chronic toxicological effects, such as drug resistance; allergic reactions; and even teratogenic, carcinogenic, or mutagenic effects [2,3]. Therefore, it is essential to establish a high-throughput detection method to monitor multiclass chemical hazards in poultry products.
The current methods for drug residue analysis in animal-derived foods mainly include gas chromatography [4,5], high-performance liquid chromatography [6,7], and liquid chromatography–tandem mass spectrometry (LC–MS/MS). LC–MS/MS is a common method for analyzing drug residues in animal-derived foods. It is characterized by high selectivity and sensitivity and low detection limits [8,9,10,11,12]. The widely applied pre-treatment methods for drug residue analysis in animal-derived foods mainly include solid-phase extraction [13,14,15] and the QuEChERS approach [16,17]. The former method requires enrichment, separation, and purification steps, which are costly and time-consuming; meanwhile, solid-phase extraction columns are mostly based on the principles of polar separation and ion-exchange separation, so they are not suitable for the analysis of a large number of compounds with widely varying properties [18,19]. In contrast, the QuEChERS method, known for its rapidity, low cost, and simplicity, is widely used for drug residue analysis in animal-derived foods and enables the high-throughput analysis of compounds with significantly different properties [20,21]. Therefore, this study aims to establish a QuEChERS ultra-performance liquid chromatography–tandem triple quadrupole mass spectrometry (UPLC–MS/MS) method for detecting 280 chemical hazards in poultry products, which will provide technical support for monitoring chemical residues and ensuring food safety.
2. Materials and Methods
2.1. Materials and Reagents
A total of 280 analytical standards and 44 isotope-labeled internal standards (listed in Supplementary Table S1), all with purity > 98%, were obtained from TRC (Toronto, ON, Canada) or Alta Scientific Co., Ltd. (Tianjin, China). Zirconia-bonded octadecyl silica (Z-sep/C18), octadecyl-bonded silica (C18), and N-propylethylenediamine-bonded solid phase (PSA) adsorbent materials were obtained from SUPELCO (Bellefonte, PA, USA); multiwalled carbon nanotube (MWNT, 8–15 nm) adsorbent materials were purchased from Xianfeng Nanomaterials Technology (Nanjing, China); and QuEChERS dSPE EMR-lipid purification packing material was purchased from Agilent (Santa Clara, CA, USA).
HPLC-grade acetonitrile and methanol were obtained from Dima Technology Ltd. (Beijing, China); formic acid was purchased from Acros Corporation (Lexington, MA, USA) with an available purity > 95%; disodium EDTA (Na2EDTA), sodium chloride, anhydrous sodium sulfate, and ammonia were purchased from Sinopharm Chemical Reagent Co. (Beijing, China); and ammonium fluoride (purity > 99%) was purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Ultrapure water was prepared using a Milli-Q system (Millipore, MA, USA).
2.2. Instruments and Equipment
The instruments and equipment used in this study were as follows: an ACQUITYTM Ultra Performance Liquid Chromatograph—Xevo® TQ-XS Tandem Mass Spectrometer (Waters, Milford, MA, USA); an Allegra X-30R Centrifuge (Beckman, Brea, CA, USA); a Vortex-Genie 2 Vortex Oscillator (Scientific Industries, Bohemia, NY, USA); and a Mettler Toledo XPE 105 Electronic Balance (Greifensee, Switzerland).
2.3. Sample Preparation
Blank samples of chicken muscle and eggs used for method development or validation were kindly donated by China Agricultural University, while the samples for detection are collected from local markets in Beijing and Ningxia.
Poultry muscle samples were prepared by removing connective tissues, dicing into small pieces, and homogenizing using a mechanical grinder. Egg samples were prepared by pooling and homogenizing 10 whole eggs. Each 2 g homogenized egg/muscle sample was accurately weighed out, placed in a 50 mL centrifuge tube, and spiked with 100 μL of 100 μg/L IS solution. Then, 1.5 mL of ultrapure water was added and vortexed for 30 s; following this, 8.5 mL of acetonitrile, 100 μL of formic acid, and 25 mg of ethylenediaminetetraacetic acid disodium salt were added to the centrifuge tubes and mixed by vortexing for 1 min. The mixture was ultrasonically extracted for 30 min. Afterward, the samples were centrifuged at 10,000 r/min for 10 min at 4 °C. The supernatant was transferred into a new centrifuge tube, 2 g of anhydrous sodium sulfate and 1 g of sodium chloride solid were added [22], and the mixture was vortexed for 1 min and then centrifuged at 10,000 r/min for 5 min at 4 °C. For the extract cleanup, 1 mL of supernatant was then vortexed by adding 50 mg of dSPE EMR-lipid [23,24] sorbent and vortexing for 1 min. After centrifugation at 4 °C at 14,000 r/min for 5 min, the supernatant was pipetted into a glass vial and injected into the UPLC–MS/MS for analysis.
2.4. Instrumental Conditions
The analytical column was an ACQUITY UPLC BEH C18 (2.1 mm × 100 mm, 1.7 μm; Waters, Milford, MA, USA) with a flow rate of 0.3 mL/min at 40 °C. In positive-ion mode, the phase A was methanol-acetonitrile (v/v, 1:1), and phase B was a 0.5 mmol/L ammonium fluoride—0.1% formic acid aqueous solution, with the gradient elution procedure as follows: 0~2 min, 3% A; 2~5 min, 3~15% A; 5~10 min, 15% A; 10~15 min, 15~30% A; 15~20 min, 30~50% A; 20~24 min, 50~100% A; 24~27 min, 100% A; 27~28 min, 100% A; 28~28.5 min, 100%~3% A; 28.5~30 min, 3% A. In negative-ion mode, the mobile phase A was acetonitrile, and phase B was a 1 mmol/L ammonium fluoride aqueous solution; the elution gradient was as follows: 0~1 min, 10~15% A; 1~2.5 min, 15~50% A; 2.5~6 min, 50~65% A; 6~7.5 min, 65~95% A; 7.5~8 min, 95~100% A; 8~8.5 min, 100% A. The injection volume was 3 µL in positive-ion mode and 5 µL in negative-ion mode.
MS/MS analyses were performed on a TQ-XS triple quadrupole mass spectrometer with electrospray ionization (ESI) in multiple-reaction-monitoring (MRM) mode. The ESI settings were as follows: capillary voltage, 2.5 kV; cone voltage, 30 V; ion source temperature, 150 °C; dissolvent gas temperature, 400 °C; dissolvent gas (N2) flow rate, 800 L/h; pressure of collision chamber, 3.2 × 10−3 mbar. The compound-dependent parameters like precursor ions, product ions, and collision energies are listed in Table S1 of the Supplementary.
2.5. Preparation of Standards and Working Solutions
Standard stock solutions at 1000 mg/L were prepared in methanol solution with 0.1% formic acid and stored at –20 °C prior to use. The standard working solutions were diluted with methanol to the desired concentration. A mixed IS solution of 44 isotope-labeled chemicals (Table S1 in Supplementary) was diluted to 100 μg/L.
2.6. Quality Assurance
A robust quality assurance design for LC–MS/MS ensures data integrity, regulatory compliance, and reliable results. In this study, the method was validated according to China National Standard GB/T 27417-2017 Conformity assessment—Guidance on validation and verification of chemical analytical methods [25]. Various parameters including linearity, accuracy, precision, limit of detection (LOD), and limit of quantification (LOQ) were evaluated during the course.
The performance of the LC–MS/MS analytical system was verified using the evaluated standard solutions, including the analytes and internal standards. With the calibration curve established, accuracy should be +15% for all points except the lowest calibrator (+20%). In addition, the curve slope should be r2 > 0.99. A spiked blank matrix sample was used as the quality control (QC) sample due to the lack of certified reference materials. A blank sample, as well as QC samples, was applied for every 20 injections in a batch analysis.
3. Results and Discussion
3.1. Optimization of UPLC–MS/MS
3.1.1. Mass Spectrometry Parameters
The mass spectrometry parameters were optimized for individual standards. A full scan of the primary mass spectrum was performed in positive- and negative-ion modes. Ultimately, satisfactory results were achieved for 262 substances in the positive-ion mode, while the remaining 18 substances performed better in the negative-ion mode. The cone voltage was determined by optimizing the response of the precursor ion; then, a product ion scan of the secondary mass spectrum was performed, the fragment ions with the highest response were selected as the quantitative ions, and the second-highest fragment ions were selected as the qualitative ions by changing the collision energy. The detailed mass spectrometry parameters are shown in Supplementary Table S1.
3.1.2. Chromatographic Conditions
The mobile phase composition was systematically optimized to achieve optimal response and separation for all 280 target compounds. Three organic phase compositions were evaluated: methanol, acetonitrile, and methanol-acetonitrile (1:1, v/v). Concurrently, two aqueous phase formulations were compared for use in positive-ion mode: (1) 0.1% formic acid and (2) 0.5 mmol/L ammonium fluoride containing 0.1% formic acid. The results (Figure 1A,B) demonstrated that the addition of 0.5 mmol/L ammonium fluoride into a 0.1% formic acid aqueous solution significantly improved the chromatographic resolution and enhanced the mass spectrometric response intensities for most target analytes compared to using a formic acid solution alone. In organic phase screening, the methanol-acetonitrile mixed system exhibited superior comprehensive performance (response intensity and resolution), markedly outperforming individual solvent systems, and was, therefore, selected as the optimal organic phase.
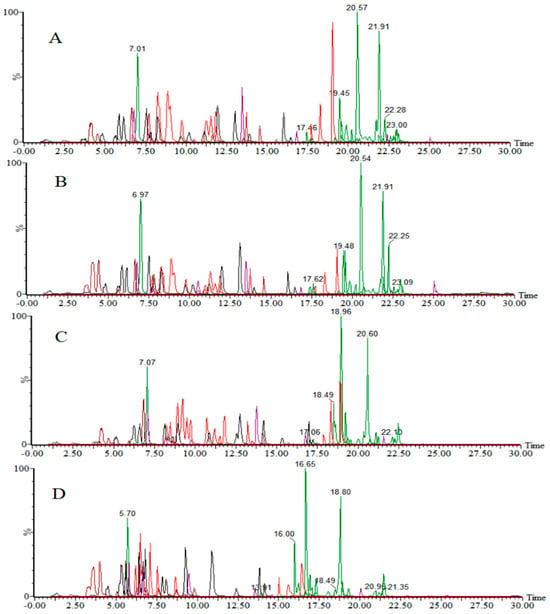
Figure 1.
Total ion chromatograms of target compounds using different mobile phases in positive-ion mode: (A) methanol-acetonitrile (1:1, v/v)—0.5 mmol/L ammonium fluoride—0.1% formic acid in water; (B) methanol-acetonitrile (1:1, v/v)—0.1% formic acid in water; (C) acetonitrile—0.5 mmol/L ammonium fluoride—0.1% formic acid in water; (D) methanol—0.5 mmol/L ammonium fluoride—0.1% formic acid in water.
Based on these findings, the final optimized mobile phase combination for positive-ion mode analysis was established as follows: methanol-acetonitrile (1:1, v/v) with a 0.5 mmol/L ammonium fluoride aqueous solution containing 0.1% formic acid.
Under the negative-ion mode (Figure 2), the aqueous phase was compared with three solutions, 1 mmol/L ammonium fluoride, water, and 0.01% ammonia, and it was seen that the response value was higher when the aqueous phase was ammonium fluoride and water. The organic phase was compared with methanol and acetonitrile. Finally, acetonitrile—1 mmol/L ammonium fluoride solution was used as the mobile phase in the negative analysis.
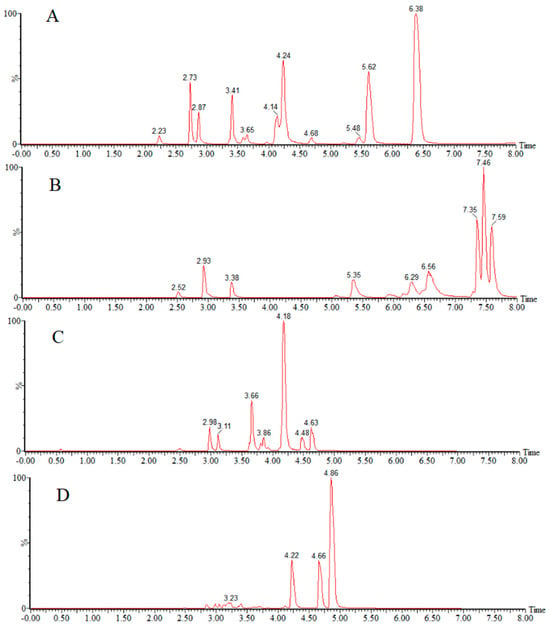
Figure 2.
Total ion chromatograms of target compounds using different mobile phases in negative-ion mode: (A) acetonitrile—1 mmol/L ammonium fluoride; (B) methanol—1 mmol/L ammonium fluoride; (C) acetonitrile—0.01% ammonium hydroxide in water; (D) acetonitrile-water.
3.2. Optimization of Sample Pretreatment
3.2.1. Extraction Solvents
Aqueous acetonitrile solution is the most commonly used extract in animal-derived food sample treatment due to its excellent protein precipitation effect and organic solubility. In this study, pure acetonitrile, 85% acetonitrile aqueous solution, and 50% acetonitrile aqueous solution were used for the chicken muscle and egg extraction. From a visual perspective (as shown in Supplementary Figure S1), the supernatant of the 50% acetonitrile aqueous solution remained turbid even after 10 min of high-speed centrifugation. In contrast, when extracted with pure acetonitrile or 85% acetonitrile, the supernatant appeared significantly clearer. However, pure acetonitrile caused the tight aggregation of muscle samples, resulting in relatively poor extraction efficiencies for the majority of target compounds. We also compared the extraction results of an initial homogenization with 1.5 mL of water followed by the addition of 8.5 mL of acetonitrile. This approach demonstrated a clear supernatant and improved sample dispersion. Additionally, acidic conditions have been proven to enhance the extraction efficiency and response of many drugs [26]. Consequently, 0.1% formic acid was subsequently introduced into the sample treatment process. The results indicated that the addition of formic acid enhanced the response values for approximately 48% of the target compounds. In conclusion, a mixture of 1.5 mL of water, 8.5 mL of acetonitrile, and 0.1% formic acid was selected as the extraction solvent in this study.
3.2.2. Purification Materials
Proteins, lipids, and other impurities in the sample extracts could interfere with the assay of the target compounds, and thus the extracts need further purification to reduce matrix interference before analysis. Currently, the dispersed purification materials commonly used in the QuEChERS approach include C18 [27,28], EMR [29,30], PSA [31,32], MWNTs [33], GCB [34], etc. In this experiment, the purification effects of five sorbents, namely, 50 mg of C18, 25 mg of PSA, 50 mg of Z-Sep/C18, 10 mg of MWNTs, and 50 mg of EMR-lipid, were compared. The dosage of different sorbents were determined upon the literature review or pre-experiment basis. In the chicken muscle matrix, the EMR-lipid sorbent showed optimal performance in terms of recovery rate, with 90% of the target compounds achieving recovery within the validated acceptable range (60–120%) and only 8% showing recovery below 60%, fulfilling all essential criteria for multiclass veterinary drug residue analysis. PSA ranked second, while MWNTs induced the worst recovery, with 49% of the targets achieving a recovery lower than 60%. Accordingly, the matrix effect (ME, Figure 3) of many targets was significantly weakened after the purification; among them, there were 14 targets with matrix inhibition in the PSA groups and 65 targets in the EMR-lipid group (ME < 80%), and the percentage of compounds with matrix inhibition in these two groups was significantly lower than that in other groups. The target recovery rates of EMR-lipid and PSA were further compared by plotting the 29 substances with the largest differences in recovery rates when using these two sorbents. Figure 4 demonstrates that the recovery rates of the 29 compounds through EMR-lipid purification were higher than those achieved with PSA. This difference can be attributed to the fact that EMR-lipid filler is a lipid-enhanced sorbent, which effectively removes impurities such as phospholipids in meat without compromising the recovery of target compounds. In contrast, PSA filler eliminates organic acids and other matrix impurities, and it is commonly used for pesticide residue analysis. However, in this experiment, PSA exhibited a matrix-enhancing effect (ME > 120%) and resulted in lower recoveries for many target compounds [35,36,37]. With respect to the recovery rate and matrix effect, EMR-lipid sorbent was selected for the chicken muscle purification. Similar comparisons were conducted for the treatment of egg extracts, and EMR-lipid sorbent was also proven to be the most effective option. Figure 5 and Figure 6 present statistical comparisons of target compounds demonstrating > 20% recovery differences between the two optimal purification sorbents (EMR-lipid and PSA) in chicken muscle and (EMR-lipid and Z-Sep/C18) egg matrices, respectively.
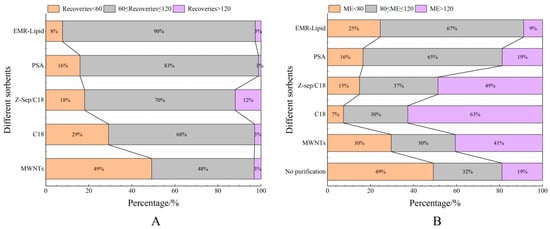
Figure 3.
Distribution of recovery (A) and matrix effects (B) of target compounds when using different sorbents in chicken muscle.
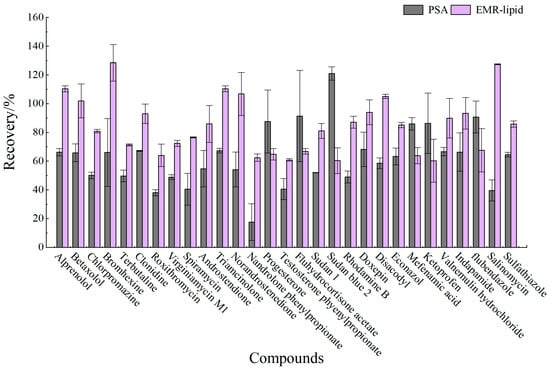
Figure 4.
Recovery of 29 targets in chicken muscle using EMR-lipid and PSA purification.
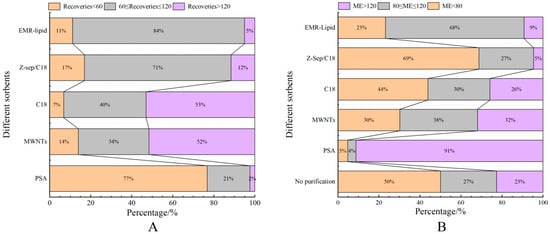
Figure 5.
Distribution of recovery (A) and matrix effects (ME, (B)) of target compounds when using different sorbents in egg samples.
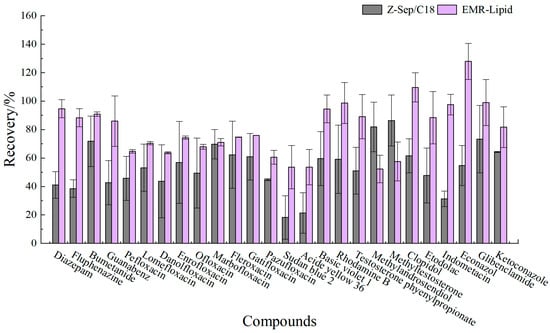
Figure 6.
Recovery of 26 targets in eggs using EMR-lipid and Z-Sep/C18 purification.
3.3. Method Validation
3.3.1. Matrix Effect
The matrix effect (ME) of the target compounds was evaluated by calculating the ratio of the slope of the matrix-matched standard curve to the slope of the solvent-based standard curve. A ratio within the range of 0.80–1.20 was considered negligible, while values greater than 1.20 indicated matrix enhancement and values less than 0.80 indicated matrix inhibition. As shown in Figure 7, the matrix effects for egg samples ranged from 0.53 to 1.44, with 54 target compounds exhibiting matrix inhibition. Among these, 11 targets, including Sudan 2, demonstrated a matrix effect of less than 0.60. Similarly, for chicken muscle, the matrix effects ranged from 0.54 to 1.49, with 62 target compounds showing matrix inhibition, of which 8 targets, including clenbuterol, had a matrix effect below 0.60.
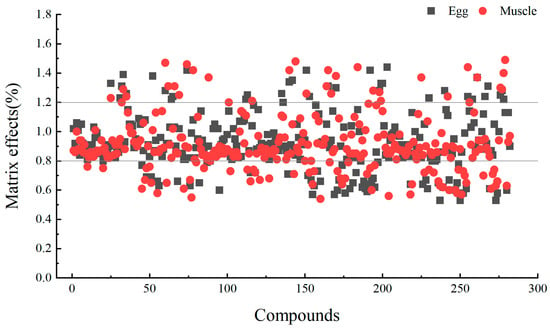
Figure 7.
Matrix effects of 280 targets in chicken egg and chicken muscle (the compound number is the same as that in Supplementary Table S1).
3.3.2. Linear Range and Method Limit of Quantification (LOQ)
IS-spiked standard calibration curves or matrix-matched calibration curves were prepared using matrix blank extracts with concentrations of 0.04, 0.1, 0.2, 0.4, 1, 2, 4, 10, 20, 40, and 100 μg/L (with a constant IS concentration of 1 μg/L). These samples were then analyzed under the optimized conditions described above. Linear regression analysis was performed using the mass concentration of the target compounds as the independent variable (x-axis, μg/L) and the corresponding peak area ratios of the external standards to the IS as the dependent variable (y-axis). As shown in Table S1 in the Supplementary Materials, the target showed a good linear relationship between mass concentration and peak area with a correlation coefficient R2 ≥ 0.99 within the linear range of 0.04~100 μg/L. The method demonstrated excellent sensitivity, with limits of detection (LODs, S/N ≥ 3) ranging from 0.05 to 10 μg/kg and limits of quantification (LOQs, S/N ≥ 10) ranging from 0.1 to 20 μg/kg. In total, 228 target compounds exhibited high sensitivity with LOQs between 0.1 and 2 μg/kg, while only 8 compounds had LOQs exceeding 10 μg/kg. Compared to the published literature focused on multiresidue detection in eggs and muscles, this study presented comparable or higher sensitivity (Table 1).

Table 1.
Summary of the literature on multidrug residues analysis in egg and chicken muscle matrices.
3.3.3. Spiking Recoveries and Precisions
Spiking experiments were conducted at three concentration levels (1 μg/kg, 10 μg/kg, and 50 μg/kg) using blank egg and chicken muscle samples. For each concentration level, six replicate groups were prepared. The results demonstrated that the recoveries of the target compounds in the egg matrix ranged from 55.4% to 139%, with relative standard deviations (RSDs) ranging from 1.14% to 16.2%. Similarly, in the chicken matrix, the average recoveries ranged from 57.9% to 137%, with RSDs between 0.98% and 15.9%. It needs to be noted that 253 of the target compounds exhibited mean recoveries ranging from 60% to 120% at the three spiked levels. Figure 8 depicts the distribution of the recoveries, and it can be seen that the median recoveries of the targets at different concentration spiked levels ranged from 84.3% to 103%, which is acceptable and meets the requirements for the analysis of multidrug residues.
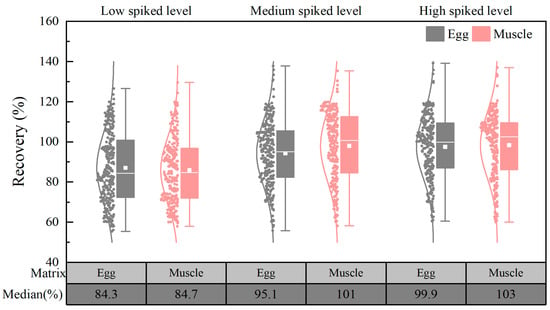
Figure 8.
Distribution of target recovery in chicken and egg matrices (n = 6).
3.4. Application
This newly developed high-throughput method for chemical residue determination in poultry products was applied to 144 poultry egg and 67 chicken muscle samples. In total, 114 poultry eggs (eggs and egg products: 63 eggs, 1 goose egg, 1 pigeon egg, 1 quail egg, 9 salted duck eggs, 9 preserved eggs, and 9 soft-boiled eggs) and 37 poultry muscle samples were collected in February, May, and July 2021 from supermarkets and grocery in Beijing, while 30 chicken muscle and 30 egg samples were collected in July 2021 in Ningxia. A total of 43 compounds were detected in poultry egg samples, including sulfonamides, nitroimidazoles, quinolones, sedatives, anticoccidials, anti-inflammatories, and hormones. Since the hormone substances presented in Table 2 such as progesterone, 17α-hydroxyprogesterone, hydrocortisone, cortisone, corticosterone, androstenedione, and testosterone have been reported to be naturally present in mammals with different ranges of content at different stages of growth [44], in general, these endogenously present hormone substances pose no health risk to consumers at the detected levels. Therefore, hormones are not included in the description below.

Table 2.
Occurrence of chemical hazards in poultry muscle and egg samples.
A total of 17 targets were detected in 67 chicken muscle samples at concentrations ranging from 0.11 to 333 μg/kg, with the maximum value observed for toltrazuril. A total of 27 targets were found in 144 poultry egg samples at concentrations ranging from 0.11 to 638 μg/kg, with the maximum value observed for sulfadiazine (Table 2). It is worth noting that 12 target compounds were detected in 18 egg products, of which 6 target compounds were detected in 1 salted duck egg sample simultaneously (Figure 9 ), indicating that the supervision of egg products needs to be strengthened.
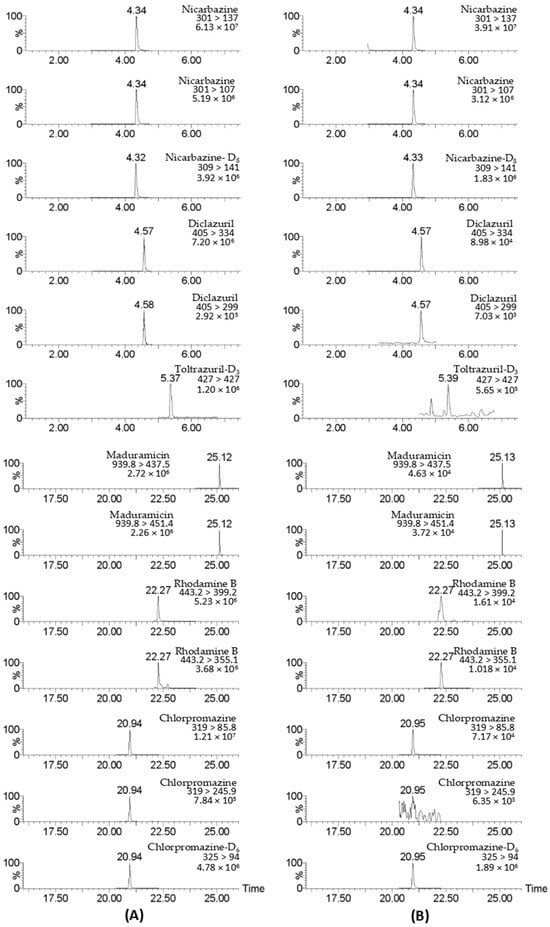
Figure 9.
UPLC–MS/MS chromatograms of detected drugs in standard solution (A) and a salted duck egg sample (B). The standard concentration was 5 μg/L.
4. Conclusions
An UPLC–MS/MS method was established for the simultaneous determination of 280 chemical hazards that may be present in poultry samples. Through the optimization of extraction solvents and purification materials, acidic acetonitrile solution and the EMR-lipid filler were selected as the extraction and purification agent, respectively, to reduce the influence of interferences in the matrix. All the target analytes exhibited linear responses (R2 ≥ 0.99) within their respective calibration ranges. The spiked recoveries ranged from 55.4% to 139% in the egg matrix and 57.6% to 137% in the chicken matrix, and the limits of quantification (LOQs) were between 0.1 and 20 μg/kg. Compared with the other reported methods, this method covers more types of compounds and achieved the high-throughput detection of chemical hazards. It has been applied to the detection of over 200 actual samples. This method is suitable for the routine monitoring of chemical hazards in poultry products, providing technical support and a valuable reference for ensuring the quality and safety of these products. While this method enables broad compound coverage, certain analytes demonstrate limited recovery efficiency, and the reliance on costly EMR-lipid sorbents may hinder large-scale implementation. Future studies should prioritize advanced sorbent development to improve recovery and automation-integrated workflows to boost throughput.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/foods14101660/s1. Table S1. Mass spectrometry parameters, linear ranges, and linear correlation coefficients of 280 targets and 44 internal standards. Figure S1. Precipitation results using different acetonitrile–water solutions for the extraction of chicken muscle (A) and eggs (B).
Author Contributions
R.C.: experiment preparation, methodology, figures, and writing—original draft preparation. L.C.: sample collection, method validation, data analysis, and writing—original draft preparation. J.Z.: study design, writing—review and editing, project administration, supervision, and funding acquisition. X.Y.: study design, writing—review and editing, and supervision. M.D.: literature search, figures, and data collection. Q.G.: data analysis and data interpretation. C.Z.: data analysis and data interpretation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Talent Development Plan for High-Llevel Public Health Technical Personnel Project (Gugan-01-033) from Beijing Municipal Health Commission and the National Key Research and Development Program of China (Grant 2019YFC1605700) from the Ministry of Science and Technology of PR China.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- China Economic Net. Agricultural Economy Shows Steady and Positive Development in 2024. Available online: http://www.ce.cn/xwzx/gnsz/gdxw/202501/17/t20250117_39269913.shtml (accessed on 10 February 2025).
- Getahun, M.; Abebe, R.B.; Sendekie, A.K.; Woldeyohanis, A.E.; Kasahun, A.E. Evaluation of antibiotics residues in milk and meat using different analytical methods. Int. J. Anal. Chem. 2023, 2023, 4380261. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ying, G.-G.; Deng, W.-J. Antibiotic residues in food: Extraction, analysis, and human health concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, E.; Chaurand, P.; Raghavan, V. Visualizing the distribution of strawberry plant metabolites at different maturity stages by MALDI-TOF imaging mass spectrometry. Food Chem. 2021, 345, 128838. [Google Scholar] [CrossRef] [PubMed]
- Manav, Ö.G.; Dinç-Zor, Ş.; Alpdoğan, G. Optimization of a modified QuEChERS method by means of experimental design for multiresidue determination of pesticides in milk and dairy products by GC-MS. Microchem. J. 2019, 144, 124–129. [Google Scholar] [CrossRef]
- Lozano, A.; Hernando, M.D.; Uclés, S.; Hakme, E.; Fernández-Alba, A.R. Identification and measurement of veterinary drug residues in beehive products. Food Chem. 2019, 274, 61–70. [Google Scholar] [CrossRef]
- Kang, J.; Park, S.J.; Park, H.C.; Hossain, M.A.; Kim, M.A.; Son, S.W.; Lim, C.M.; Kim, T.W.; Cho, B.H. Multiresidue screening of veterinary drugs in meat, milk, egg, and fish using liquid chromatography coupled with ion trap time-of-flight mass spectrometry. Appl. Biochem. Biotechnol. 2017, 182, 635–652. [Google Scholar] [CrossRef]
- Desmarchelier, A.; Fan, K.; Minh Tien, M.; Savoy, M.C.; Tarres, A.; Fuger, D.; Goyon, A.; Bessaire, T.; Mottier, P. Determination of 105 antibiotic, anti-inflammatory, antiparasitic agents and tranquilizers by LC-MS/MS based on an acidic QuEChERS-like extraction. Food Addit. Contam. Part A 2018, 35, 646–660. [Google Scholar] [CrossRef]
- Savoy, M.C.; Woo, P.M.; Ulrich, P.; Tarres, A.; Mottier, P.; Desmarchelier, A. Determination of 14 aminoglycosides by LC-MS/MS using molecularly imprinted polymer solid phase extraction for clean-up. Food Addit. Contam. Part A 2018, 35, 674–685. [Google Scholar] [CrossRef]
- Yu, X.; Wu, X.; Xie, Y.; Tong, K.; Wang, M.; Li, J.; Fan, C.; Chen, H. Development and validation of a method for determination of 43 antimicrobial drugs in western-style pork products by UPLC-MS/MS with the aid of experimental design. Molecules 2022, 27, 8206. [Google Scholar] [CrossRef]
- Mookantsa, S.O.S.; Dube, S.; Nindi, M.M. Multiclass determination of 87 mixed veterinary drugs, pesticides and mycotoxin residues in beef muscle samples by ionic liquid-based dispersive liquid-liquid microextraction and liquid chromatography tandem mass spectrometry. Foods 2025, 14, 720. [Google Scholar] [CrossRef]
- Fan, Z.; Li, Y.; Fan, X.; Wang, P.; Yang, R.; Xie, C. Simultaneous determination of three active forms of vitamin B12 in situ produced during fermentation by LC-MS/MS. Foods 2025, 14, 309. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef] [PubMed]
- Piatkowska, M.; Jedziniak, P.; Zmudzki, J. Multiresidue method for the simultaneous determination of veterinary medicinal products, feed additives and illegal dyes in eggs using liquid chromatography-tandem mass spectrometry. Food Chem. 2016, 197, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xie, K.; Lee, K. Veterinary drug residues in animal-derived foods: Sample preparation and analytical methods. Foods 2021, 10, 127767. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Peng, R.; Gan, P. Determination of 19 sulfonamides residues in pork samples by combining QuEChERS with dispersive liquid-liquid microextraction followed by UHPLC-MS/MS. J. Sep. Sci. 2017, 40, 1377–1384. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, J.J.; Cong, J.M.; Cai, Z.X.; Zhang, J.S.; Wang, J.L.; Ren, Y.P. Optimization for quick, easy, cheap, effective, rugged and safe extraction of mycotoxins and veterinary drugs by response surface methodology for application to egg and milk. J. Chromatogr. A 2018, 1532, 20–29. [Google Scholar] [CrossRef]
- Peters, R.J.; Bolck, Y.J.; Rutgers, P.; Stolker, A.A.; Nielen, M.W. Multi-residue screening of veterinary drugs in egg, fish and meat using high-resolution liquid chromatography accurate mass time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 8206–8216. [Google Scholar] [CrossRef]
- Chen, D.; Tao, Y.; Liu, Z.; Liu, Z.; Wang, Y.; Huang, L.; Yuan, Z. Development of a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the quantification of glucocorticoid residues in edible tissues of swine, cattle, sheep, and chicken. Food Addit. Contam. Part A 2010, 27, 1363–1371. [Google Scholar] [CrossRef]
- Jin, Q.; Xu, Q.; Zhao, Z.; Si, W.; Bai, B.; Chen, L.; Zhou, C. Simultaneous determination of six acidic herbicides and metabolites in plant origin matrices by QuEChERS-UPLC-MS/MS. Molecules 2025, 30, 852. [Google Scholar] [CrossRef]
- Huang, X.; Feng, R.; Hu, Q.; Mao, X.; Zhou, H. Contamination status and health risk assessment of 73 mycotoxins in four edible and medicinal plants using an optimized QuEChERS pretreatment coupled with LC-MS/MS. Toxins 2025, 17, 52. [Google Scholar] [CrossRef]
- Xu, X.; Xu, X.; Han, M.; Qiu, S.; Hou, X. Development of a modified QuEChERS method based on magnetic multiwalled carbon nanotubes for the simultaneous determination of veterinary drugs, pesticides and mycotoxins in eggs by UPLC-MS/MS. Food Chem. 2019, 276, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Rizzetti, T.M.; de Souza, M.P.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Optimization of sample preparation by central composite design for multi-class determination of veterinary drugs in bovine muscle, kidney and liver by ultra-high-performance liquid chromatographic-tandem mass spectrometry. Food Chem. 2018, 246, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Liu, X.; Kong, F.; Tang, L.; Wang, Q.; Li, W.; Xu, W.; Wen, S.; Chen, L.; Li, Y. Multi-residue determination of 325 pesticides in chicken eggs with EMR-Lipid clean-up by UHPLC-MS/MS and GC-MS/MS. Chromatographia 2020, 83, 593–599. [Google Scholar] [CrossRef]
- GB/T 27417-2017; Conformity assessment—Guidance on Validation and Verification of Chemical Analytical Methods. Standardization Administration of China: Beijing, China, 2017.
- Zhu, X.; Wang, M.; Hou, F.; He, Y.; Zhu, Y.; Dai, H.; Huang, M.; Yang, Y.; Wu, L. Multi-residue analysis of pesticides and veterinary drugs by ultra-performance liquid chromatography-tandem mass spectrometry using a modified QuEChERS method. Microchem. J. 2025, 208, 112384. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, D.; Yu, G.; Yu, H.; Pan, Y.; Wang, Y.; Huang, L.; Yuan, Z. Simultaneous determination of lincomycin and spectinomycin residues in animal tissues by gas chromatography-nitrogen phosphorus detection and gas chromatography-mass spectrometry with accelerated solvent extraction. Food Addit. Contam. Part A 2011, 28, 145–154. [Google Scholar] [CrossRef]
- Ahammed Shabeer, T.P.; Girame, R.; Utture, S.; Oulkar, D.; Banerjee, K.; Ajay, D.; Arimboor, R.; Menon, K.R.K. Optimization of multi-residue method for targeted screening and quantitation of 243 pesticide residues in cardamom (Elettaria cardamomum) by gas chromatography tandem mass spectrometry (GC-MS/MS) analysis. Chemosphere 2018, 193, 447–453. [Google Scholar] [CrossRef]
- Sun, D.; Jin, Y.; Zhao, Q.; Tang, C.; Li, Y.; Wang, H.; Qin, Y.; Zhang, J. Modified EMR-lipid method combined with HPLC-MS/MS to determine folates in egg yolks from laying hens supplemented with different amounts of folic acid. Food Chem. 2021, 337, 127767. [Google Scholar] [CrossRef]
- Long, Y.; Huang, Y.; Zhu, M.; Ma, Y.; Gan, B.; Wang, Y.; Yu, Q.; Xie, J.; Chen, Y. Development of QuEChERS clean-up based on EMR-lipid for simultaneous analysis of 9 mycotoxins, acrylamide and 5-hydroxymethylfurfural in biscuit by UHPLC-MS/MS. Food Chem. 2023, 409, 135265. [Google Scholar] [CrossRef]
- Chu, N.; Shu, X.; Yuan, L.; Zhang, X.; Tang, M.; Yang, J.; Li, D.; Wu, S. Determination of 52 hidden chemical pesticides in biopesticide products by GC-MS/MS and LC-MS/MS. J. Environ. Sci. Health B 2022, 57, 504–515. [Google Scholar] [CrossRef]
- Lin, Z.; Lin, Y.; Lin, J.; Zhang, Y.; Fang, S. Trace analysis of fenbutatin oxide in soil and plant- and animal-derived foods using modified QuEChERS coupled with HPLC-MS/MS. ACS Omega 2021, 6, 10260–10265. [Google Scholar] [CrossRef]
- Wu, Y.L.; Chen, R.X.; Xue, Y.; Yang, T.; Zhao, J.; Zhu, Y. Simultaneous determination of amantadine, rimantadine and memantine in chicken muscle using multi-walled carbon nanotubes as a reversed-dispersive solid phase extraction sorbent. J. Chromatogr. B 2014, 965, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Guo, Y.; Hu, X.; Yu, Y.; Chen, J.; Su, J.; Lian, W.; Wu, X.; Meng, X. Simultaneous detection of four pesticides in agricultural products by a modified QuEChERS method and LC-MS/MS. J. Environ. Sci. Health B 2023, 58, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Ly, T.K.; Ho, T.D.; Behra, P.; Nhu-Trang, T.T. Determination of 400 pesticide residues in green tea leaves by UPLC-MS/MS and GC-MS/MS combined with QuEChERS extraction and mixed-mode SPE clean-up method. Food Chem. 2020, 326, 126928. [Google Scholar] [CrossRef]
- Areo, O.M.; Olowoyo, J.O.; Sethoga, L.S.; Adebo, O.A.; Njobeh, P.B. Determination of pesticide residues in rooibos (Aspalathus linearis) teas in South Africa. Toxicol. Rep. 2022, 9, 852–857. [Google Scholar] [CrossRef]
- Sandín-España, P.; Mateo-Miranda, M.; López-Goti, C.; Seris-Barrallo, E.; Alonso-Prados, J.L. Analysis of pesticide residues by QuEChERS method and LC-MS/MS for a new extrapolation of maximum residue levels in persimmon minor crop. Molecules 2022, 27, 126928. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, Q.; Liao, G.; Qian, Y.; Qiu, J. Multi-residue determination of 244 chemical contaminants in chicken eggs by liquid chromatography-tandem mass spectrometry after effective lipid clean-up. Agriculture 2022, 12, 869. [Google Scholar] [CrossRef]
- de Souza, Y.P.; de Oliveira, A.C.; Bastos, L.H.P.; Sartori, A.V.; Spisso, B.F. Development and validation of a miniaturized two-step neutral and acidified acetonitrile extraction method for simultaneous determination of pesticides, veterinary drugs, and mycotoxins in eggs by UHPLC-MS/MS. Food Anal. Methods 2025, 14, 720. [Google Scholar] [CrossRef]
- Luo, P.; Liu, X.; Kong, F.; Chen, L.; Wang, Q.; Li, W.; Wen, S.; Tang, L.; Li, Y. Simultaneous determination of 169 veterinary drugs in chicken eggs with EMR-Lipid clean-up using ultra-high performance liquid chromatography tandem mass spectrometry. Anal. Methods 2019, 11, 1657–1662. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Yu, F.; Wang, Y.; Ye, D.; Hu, X.; Zhou, L.; Du, J.; Xia, X. Multi-class analysis of veterinary drugs in eggs using dispersive-solid phase extraction and ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2021, 334, 127598. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, W.; Guo, W.; Li, Y.; Jiang, R.; Li, H.; Wang, S.; Li, Z. Simultaneous screening and analysis of 155 veterinary drugs in livestock foods using ultra-high performance liquid chromatography tandem quadrupole linear-ion-trap mass spectrometry. Food Chem. 2022, 393, 133260. [Google Scholar] [CrossRef]
- Chen, D.; Yu, J.; Tao, Y.; Pan, Y.; Xie, S.; Huang, L.; Peng, D.; Wang, X.; Wang, Y.; Liu, Z.; et al. Qualitative screening of veterinary anti-microbial agents in tissues, milk, and eggs of food-producing animals using liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. B 2016, 1017–1018, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, H.F.; Sharkawy, A.A. Hormonal residues in chicken and cattle meat: A risk threat the present and future consumer health. Food Chem. Toxicol. 2023, 182, 114172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).