Goat’s Milk Powder Enriched with Red (Lycium barbarum L.) and Black (Lycium ruthenicum Murray) Goji Berry Extracts: Chemical Characterization, Antioxidant Properties, and Prebiotic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Goji Berry Extracts
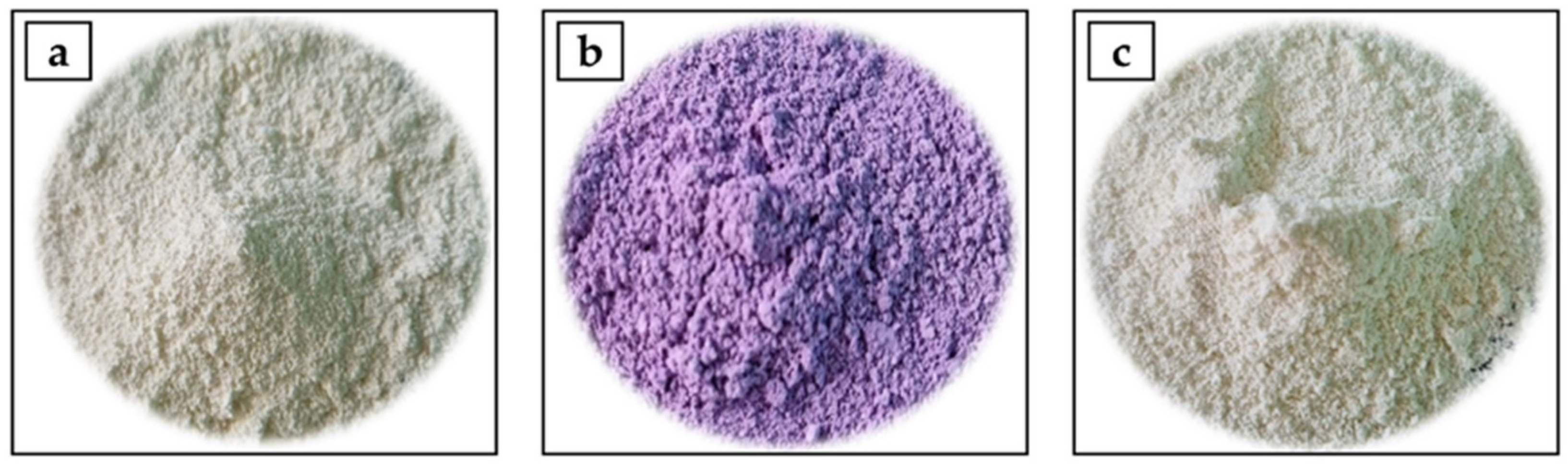
2.2. Preparation of Goat′s Milk and Goat′s Milk/Goji Extract Powders
2.3. Proximate Analysis
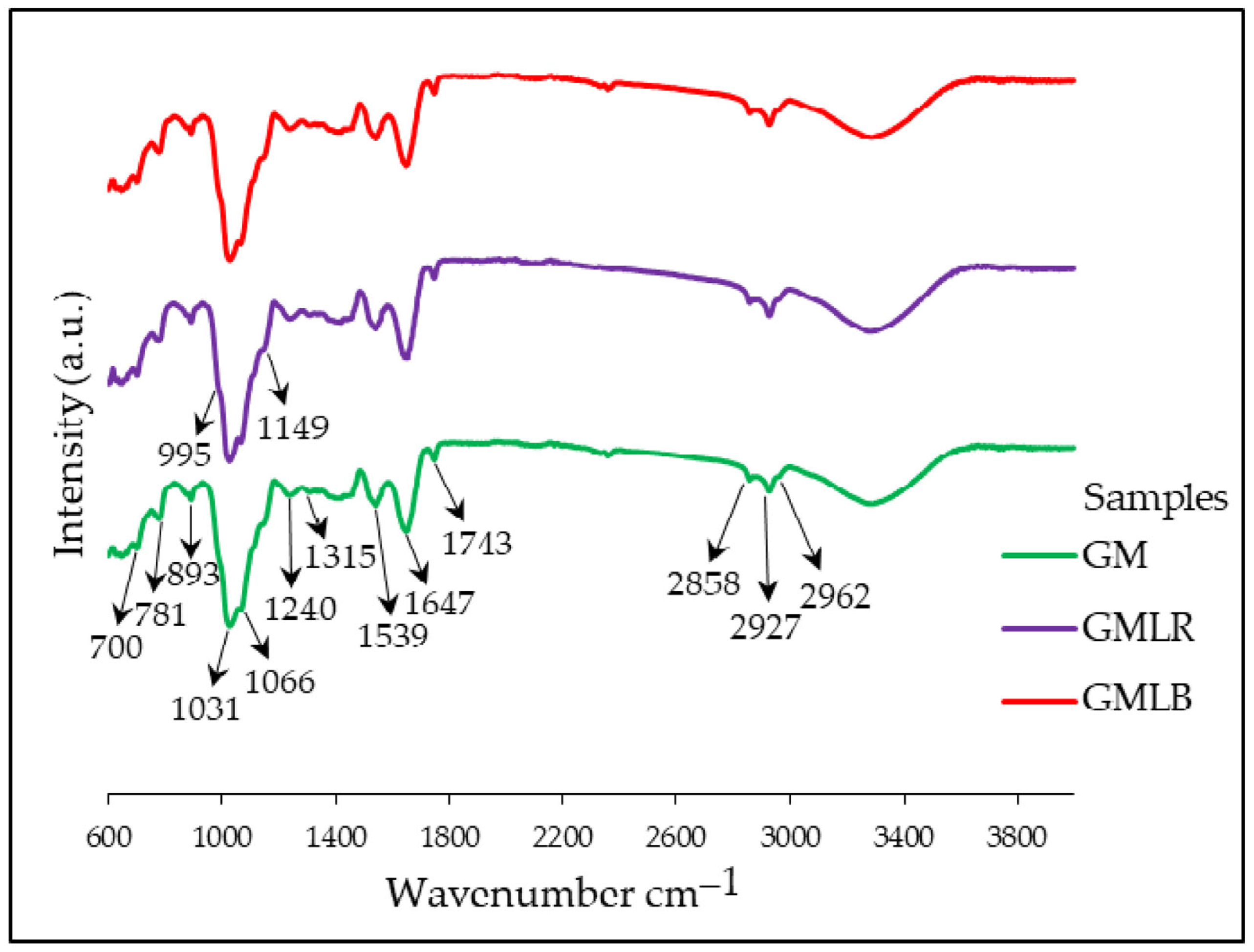
2.4. FTIR-ATR Spectroscopy of Powders
2.5. UHPLC Q-ToF MS Analysis
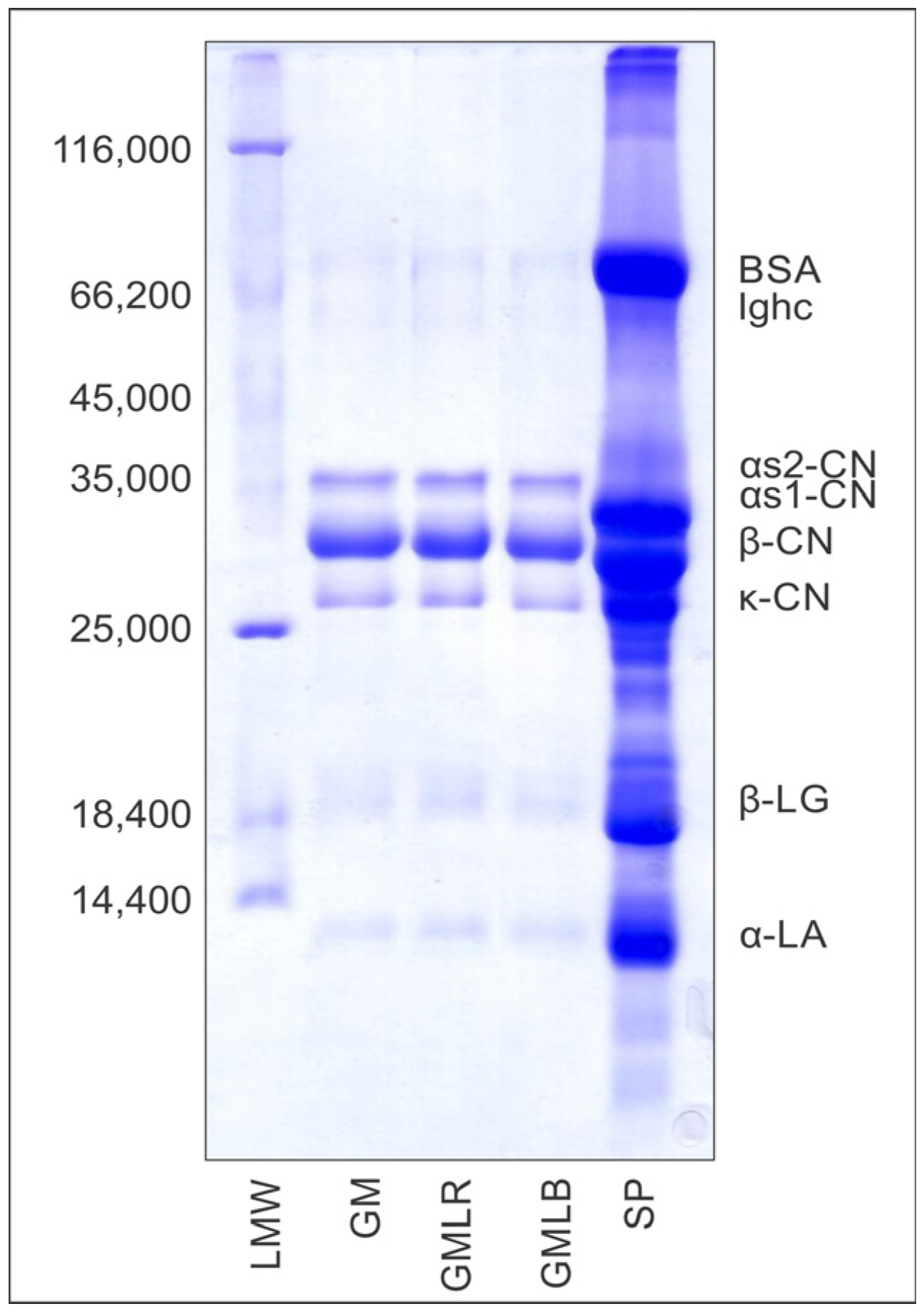
2.6. Electrophoretic Analysis of Powders
2.7. Total Phenolics, Total Proteins, and Antioxidant Properties of Powders
2.8. Prebiotic Activity of Powders
2.9. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition
3.2. FTIR-ATR Characterization of Powders
3.3. UHPLC Q-ToF MS Characterization of Phenolic Compounds in Methanol Extracts of Powders
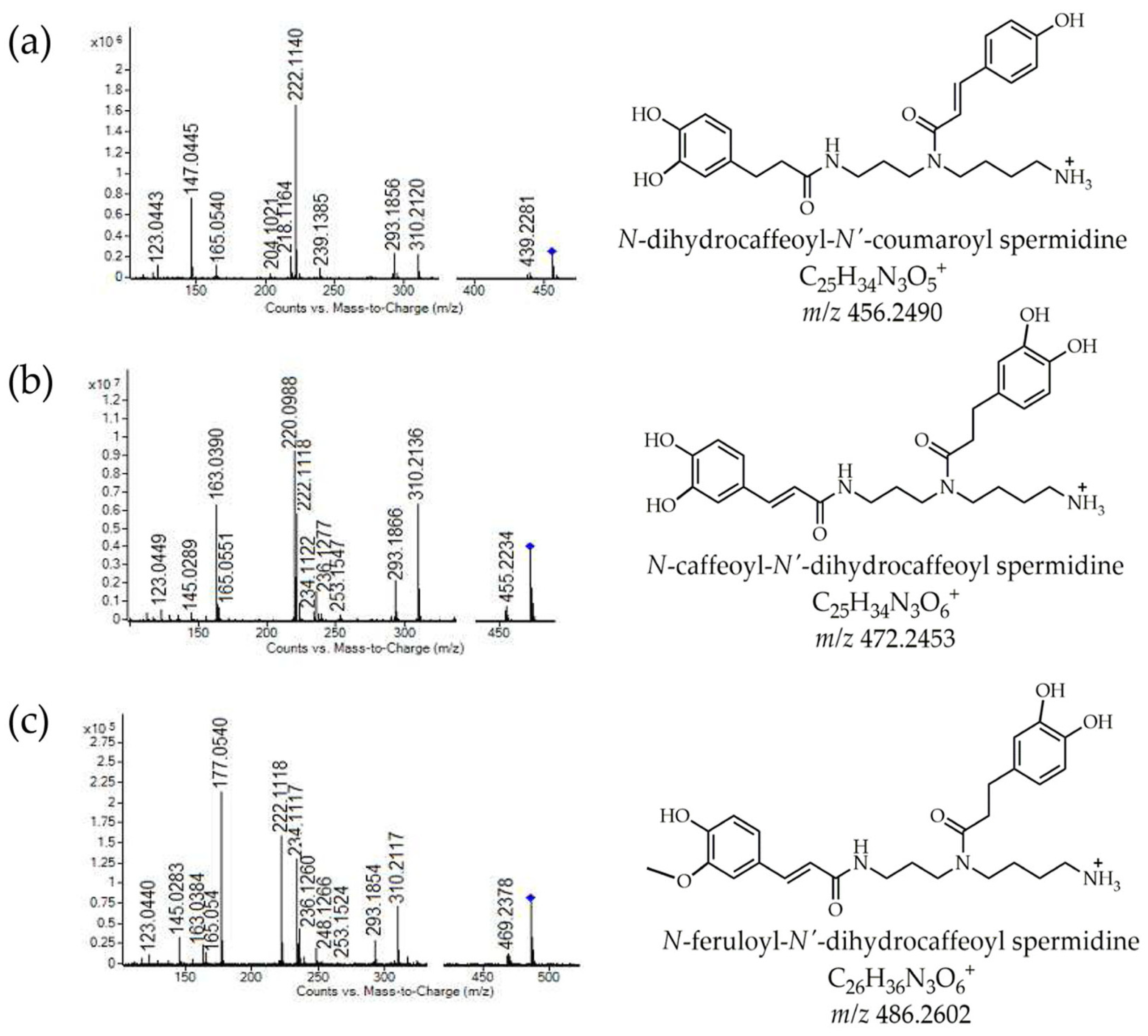
3.4. UHPLC Q-ToF MS Profile of Lycium Phenylamides in Methanolic Extracts of Powders
3.5. Electrophoretic Analysis of GM, GMLR, and GMLB Powders
3.6. Total Phenolic, Total Protein Content, and Antioxidant Properties
3.7. Prebiotic Activity of Powders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, B.A.; Lu, C.D. Current status of global dairy goat production: An overview. Asian-Australas J. Anim. Sci. 2019, 32, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Getaneh, G.; Mebrat, A.; Wubie, A.; Kendie, H. Review on goat milk composition and its nutritive value. J. Nutr. Health Sci. 2016, 3, 401–410. [Google Scholar] [CrossRef]
- Martinez-Ferez, A.; Rudloff, S.; Guadix, A.; Henkel, C.A.; Pohlentz, G.; Boza, J.J.; Guadix, E.M.; Kunz, C. Goats’ milk as a natural source of lactose-derived oligosaccharides: Isolation by membrane technology. Int. Dairy J. 2006, 16, 173–181. [Google Scholar] [CrossRef]
- Verruck, S.; Dantas, A.; Prudencio, E.S. Functionality of the components from goat’s milk, recent advances for functional dairy products development and its implications on human health. J. Funct. Foods. 2019, 52, 243–257. [Google Scholar] [CrossRef]
- Sousa, Y.R.F.; Medeiros, L.B.; Pintado, M.M.E.; Queiroga, R.C.R.E. Goat milk oligosaccharides: Composition, analytical methods and bioactive and nutritional properties. Trends Food Sci. Technol. 2019, 92, 152–161. [Google Scholar] [CrossRef]
- van Leeuwen, S.S.; Te Poele, E.M.; Chatziioannou, A.C.; Benjamins, E.; Haandrikman, A.; Dijkhuizen, L. Goat milk oligosaccharides: Their diversity, quantity, and functional properties in comparison to human milk oligosaccharides. J. Agric. Food Chem. 2020, 68, 13469–13485. [Google Scholar] [CrossRef]
- Hammam, A.R.; Salman, S.M.; Elfaruk, M.S.; Alsaleem, K.A. Goat milk: Compositional, technological, nutritional, and therapeutic aspects. Asian J. Dairy Res. 2022, 41, 367–376. [Google Scholar] [CrossRef]
- dos Santos, W.M.; Guimarães Gomes, A.C.; de Caldas Nobre, M.S.; de Souza Pereira, Á.M.; dos Santos Pereira, E.V.; dos Santos, K.M.O.; Florentino, E.R.; Alonso Buriti, F.C. Goat milk as a natural source of bioactive compounds and strategies to enhance the amount of these beneficial components. Int. Dairy J. 2023, 137, 105515. [Google Scholar] [CrossRef]
- Thakur, R.; Biswal, P.; Sari, T.P.; Kumar, D.; Sagar, N.A.; Bhardwaj, S.; Pandey, H.O.; Chandratre, G.A.; Tarafdar, A. Therapeutic effect of goat milk and its value-addition: Current status and way forward. J. Food Sci. Technol. 2024, 61, 1621–1631. [Google Scholar] [CrossRef]
- ALKaisy, Q.H.; Al-Saadi, J.S.; Al-Rikabi, A.K.J.; Altemimi, A.B.; Hesarinejad, M.A.; Abedelmaksoud, T.G. Exploring the health benefits and functional properties of goat milk proteins. Food Sci. Nutr. 2023, 11, 5641–5656. [Google Scholar] [CrossRef]
- Qin, Y.S.; Jiang, H.; Wang, C.F.; Cheng, M.; Wang, L.L.; Huang, M.Y.; Zhao, Q.X.; Jiang, H.H. Physicochemical and functional properties of goat milk whey protein and casein obtained during different lactation stages. J. Dairy Sci. 2021, 104, 3936–3946. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Wang, C.N.; Cheng, J.J.; Wang, H.; Guo, M.R. Preparation and characterization of soy isoflavones nanoparticles using polymerized goat milk whey protein as wall material. Foods 2020, 9, 1198. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Sun, X.; Cheng, J.; Guo, M. Physicochemical and Functional properties of thermal-induced polymerized goat milk whey protein. Foods 2023, 12, 3626. [Google Scholar] [CrossRef] [PubMed]
- Komes, D.; Bušić, A.; Belščak-Cvitanović, A.; Brnčić, M.; Bosiljkov, T.; Vojvodić, A.; Dujmić, F. Novel approach to the development of functional goat’s milk-based beverages using medicinal plant extracts in combination with high intensity ultrasound treatment. Food Technol. Biotechnol. 2017, 55, 484–495. [Google Scholar] [CrossRef]
- Sharma, H.; Singh, A.K.; Deshwal, G.K.; Rao, P.S.; Kumar, M.D. Functional Tinospora cordifolia (giloy) based pasteurized goat milk beverage: Impact of milk protein-polyphenol interaction on bioactive compounds, anti-oxidant activity and microstructure. Food Biosci. 2021, 42, 101101. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Stanisavljević, N.S.; Gašić, U.M.; Lević, S.; Kojić, M.O.; Lj. Tešić, Ž.; Nedović, V.; Barać, M.B.; Pešić, M.B. Polyphenol bioaccessibility and antioxidant properties of in vitro digested spray-dried thermally-treated skimmed goat milk enriched with pollen. Food Chem. 2021, 351, 129310. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Kostić, A.Ž.; Kolašinac, S.; Rac, V.; Banjac, N.; Lađarević, J.; Lević, S.; Pavlović, V.B.; Stanojević, S.P.; Nedović, V.A.; et al. Goat milk powders enriched with grape pomace seed extract: Physical and techno-functional properties. Food Hydrocoll. 2024, 146, 109293. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.Ž.; Gašić, U.M.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Bioaccessibility of phenolic compounds and antioxidant properties of goat-milk powder fortified with grape-pomace-seed extract after in vitro gastrointestinal digestion. Antioxidants 2022, 11, 2164. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Kostić, A.Ž.; Gašić, U.M.; Lević, S.; Stanojević, S.P.; Barać, M.B.; Tešić, Ž.L.; Nedović, V.; Pešić, M.B. Skimmed goat’s milk powder enriched with grape pomace seed extract: Phenolics and protein characterization and antioxidant properties. Biomolecules 2021, 11, 965. [Google Scholar] [CrossRef]
- Minić, D.A.P.; Milinčić, D.D.; Kolašinac, S.; Rac, V.; Petrović, J.; Soković, M.; Banjac, N.; Lađarević, J.; Vidović, B.B.; Kostić, A.; et al. Goat milk proteins enriched with Agaricus blazei Murrill ss. Heinem extracts: Electrophoretic, FTIR, DLS and microstructure characterization. Food Chem. 2023, 402, 134299. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Vidović, B.B.; Gašić, U.M.; Milenković, M.; Kostić, A.Ž.; Stanojević, S.P.; Ilić, T.; Pešić, M.B. A systematic UHPLC Q-ToF MS approach for the characterization of bioactive compounds from freeze-dried red goji berries (L. barbarum L.) grown in Serbia: Phenolic compounds and phenylamides. Food Chem. 2024, 456, 140044. [Google Scholar] [CrossRef] [PubMed]
- Ilić, T.; Krgović, N.; Božić, D.; Samardžić, S.; Marcetic, M.; Zdunić, G.; Vidović, B. Polyphenols profile and in vitro biological activities of black goji berries (Lycium ruthenicum Murr.). J. Berry Res. 2024, 14, 15–28. [Google Scholar] [CrossRef]
- Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Djuriš, J.D.; Ilić, T.D.; Kostić, A.; Pešić, M.B. Health benefits and applications of goji berries in functional food products development: A review. Antioxidants 2022, 11, 248. [Google Scholar] [CrossRef]
- Rotar, A.; Vodnar, D.; Bunghez, F.; Catunescu, G.; Pop, C.; Jimborean, M.; Semeniuc, C. Effect of goji berries and honey on lactic acid bacteria viability and shelf life stability of yoghurt. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 196–203. [Google Scholar] [CrossRef]
- Skenderidis, P.; Mitsagga, C.; Lampakis, D.; Petrotos, K.; Giavasis, I. The Effect of encapsulated powder of goji berry (Lycium barbarum) on growth and survival of probiotic bacteria. Microorganisms 2019, 8, 57. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Peng, W.; Chen, C.; Dan, C.; Cao, Y. Prebiotic effects in vitro of anthocyanins from the fruits of Lycium ruthenicum Murray on gut microbiota compositions of feces from healthy human and patients with inflammatory bowel disease. LWT 2021, 149, 111829. [Google Scholar] [CrossRef]
- Maurya, V.; Aggarwal, M. Impact of aqueous/ethanolic goji berry (Lycium barbarum) fruit extract supplementation on vitamin D stability in yoghurt. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2016–2029. [Google Scholar] [CrossRef]
- Taneva, I.; Zlatev, Z. Total phenolic content and antioxidant activity of yoghurt with goji berries (Lycium barbarum). Sci. Stud. Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2020, 21, 125–131. [Google Scholar]
- Wu, K.; Huang, J.; Bu, P.; Gao, H.; Yang, T.; Shi, M.; Han, J.; Fan, Y. Quality characteristics and antioxidant activity of goat milk yoghurt fortified with Lycium ruthenicum Murr. fruit. Czech J. Food Sci. 2023, 41, 382–392. [Google Scholar] [CrossRef]
- Pesic, M.B.; Barac, M.B.; Stanojevic, S.P.; Ristic, N.M.; Macej, O.D.; Vrvic, M.M. Heat induced casein–whey protein interactions at natural pH of milk: A comparison between caprine and bovine milk. Small Rumin. Res. 2012, 108, 77–86. [Google Scholar] [CrossRef]
- AOAC. Official Methods of AOAC International, 17th ed.; Horwitz, W., Ed.; AOAC: Arlington, VA, USA, 2000. [Google Scholar]
- Ljubobratović, U.; Fazekas, G.; Koljukaj, A.; Ristović, T.; Vass, V.; Ardó, L.; Stanisavljević, N.; Vukotić, G.; Pešić, M.; Milinčić, D.; et al. Pike-perch larvae growth in response to administration of lactobacilli-enriched inert feed during first feeding. Aquaculture 2021, 542, 736901. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.Ž.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A.; et al. In vitro digestion of meat- and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Grewal, M.K.; Chandrapala, J.; Donkor, O.; Apostolopoulos, V.; Stojanovska, L.; Vasiljevic, T. Fourier transform infrared spectroscopy analysis of physicochemical changes in UHT milk during accelerated storage. Int. Dairy J. 2017, 66, 99–107. [Google Scholar] [CrossRef]
- Thakur, M.; Pant, K.; Naik, R.R.; Nanda, V. Optimization of spray drying operating conditions for production of functional milk powder encapsulating bee pollen. Dry. Technol. 2021, 39, 777–790. [Google Scholar] [CrossRef]
- Du, L. New insights into raw milk adulterated with milk powder identification: ATR-FTIR spectroscopic fingerprints combined with machine learning and feature selection approaches. J. Food Compost. Anal. 2024, 133, 106443. [Google Scholar] [CrossRef]
- Saji, R.; Ramani, A.; Gandhi, K.; Seth, R.; Sharma, R. Application of FTIR spectroscopy in dairy products: A systematic review. Food Humanit. 2024, 2, 100239. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Tao, W.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J.; Zhang, L.; Li, Y.; Duan, J.A. Lycium ruthenicum studies: Molecular biology, phytochemistry and pharmacology. Food Chem. 2018, 240, 759–766. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Lu, H.; Kao, T.H.; Chen, B.H. Simultaneous determination of phenolic acids and flavonoids in Lycium barbarum Linnaeus by HPLC–DAD–ESI-MS. J. Pharm. Biomed. Anal. 2010, 51, 549–556. [Google Scholar] [CrossRef]
- Yossa Nzeuwa, I.B.; Nea, F.; Maemteu, J.; Neubi, G.; Florence Déclaire, M.; Noumedem, J.; Djeussi, D.; Sun, G. Comparative study of polyphenols quantification, total phenolic content, and antioxidant activities of the fruits of three plants of the family of Solanaceae: Lycium ruthenicum, Lycium barbarum, and Lycium chinense. Investig. Med. Chem. Pharmacol. 2022, 5, 67. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic compounds profile, nutritional compounds and bioactive properties of Lycium barbarum L.: A comparative study with stems and fruits. Ind. Crops Prod. 2018, 122, 574–581. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Bąbelewski, P. Phenolic and carotenoid profile of new goji cultivars and their anti-hyperglycemic, anti-aging and antioxidant properties. J. Funct. Foods 2018, 48, 632–642. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, S.; Zhou, W.; Meng, J.; Deng, K.; Zhou, H.; Hu, N.; Suo, Y. Rapid qualitative and quantitative analyses of eighteen phenolic compounds from Lycium ruthenicum Murray by UPLC-Q-Orbitrap MS and their antioxidant activity. Food Chem. 2018, 269, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Hao, F.; Fei, P.; Chen, D.; Fan, H.; Zhao, S.; Wang, Y.; Li, B.; Ma, Y.; Zhao, X.; et al. Advance on traditional uses, phytochemistry and pharmacology of Lycium ruthenicum Murr. Pharm. Chem. J. 2022, 56, 844–861. [Google Scholar] [CrossRef]
- Huang, J.; Fan, X.; Li, X.; Dai, D.; Huang, W.; Ruan, D.; Yan, S.; Huang, X. Phenolic profile, antioxidant properties, and beneficial gut microbiota associated with freeze dried Lycium ruthenicum. Food Sci. Hum. Wellness 2024, 14. [Google Scholar] [CrossRef]
- Vidana Gamage, G.C.; Choo, W.S. Effect of hot water, ultrasound, microwave, and pectinase-assisted extraction of anthocyanins from black goji berry for food application. Heliyon 2023, 9, e14426. [Google Scholar] [CrossRef]
- Jin, H.; Liu, Y.; Guo, Z.; Yang, F.; Wang, J.; Li, X.; Peng, X.; Liang, X. High-performance liquid chromatography separation of cis-trans anthocyanin isomers from wild Lycium ruthenicum Murr. employing a mixed-mode reversed-phase/strong anion-exchange stationary phase. J. Agric. Food Chem. 2015, 63, 500–508. [Google Scholar] [CrossRef]
- Jin, H.; Liu, Y.; Yang, F.; Wang, J.; Fu, D.; Peng, X.; Liang, X. Characterization of anthocyanins in wild Lycium ruthenicum Murray by HPLC-DAD/QTOF-MS/MS. Anal. Methods 2015, 7, 4947–4956. [Google Scholar] [CrossRef]
- Zheng, J.; Ding, C.; Wang, L.; Li, G.; Shi, J.; Li, H.; Wang, H.; Suo, Y. Anthocyanins composition and antioxidant activity of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau. Food Chem. 2011, 126, 859–865. [Google Scholar] [CrossRef]
- Ahad, H.; Jin, H.; Liu, Y.; Wang, J.; Sun, G.; Liang, X.; Akber Aisa, H. Chemical profiling of spermidines in goji berry by strong cation exchange solid-phase extraction (SCX-SPE) combined with ultrahigh-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF/MS/MS). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1137, 121923. [Google Scholar] [CrossRef]
- dos Santos, G.S.; de Almeida Veiga, A.; Carlotto, J.; Mello, R.G.; Serrato, R.V.; de Souza, L.M. Identification and fingerprint analysis of novel multi-isomeric Lycibarbarspermidines and Lycibarbarspermines from Lycium barbarum L. by liquid chromatography with high-resolution mass spectrometry (UHPLC-Orbitrap). J. Food Compost. Anal. 2022, 105, 104194. [Google Scholar] [CrossRef]
- Han, C.; Zhang, Z.; Feng, Z.; Zhai, C.; Li, X.; Shi, Y.; Li, X.; Li, M.; Wang, Y.; Luo, G.; et al. The “depict” strategy for discovering new compounds in complex matrices: Lycibarbarspermidines as a case. J. Pharm. Anal. 2023, 14, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Yossa Nzeuwa, I.B.; Xia, Y.; Qiao, Z.; Feng, F.; Bian, J.; Liu, W.; Qu, W. Comparison of the origin and phenolic contents of Lycium ruthenicum Murr. by high-performance liquid chromatography fingerprinting combined with quadrupole time-of-flight mass spectrometry and chemometrics. J. Sep. Sci. 2017, 40, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhao, J.; Zhou, W.; Shen, A.; Yang, F.; Liu, Y.; Guo, Z.; Tao, Y.; Peng, X.; Liang, X. Preparative separation of a challenging anthocyanin from Lycium ruthenicum Murr. by two-dimensional reversed-phase liquid chromatography/hydrophilic interaction chromatography. RSC Adv. 2015, 5, 62134–62141. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem 2020, 44, e13394. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Roumani, M.; Duval, R.E.; Ropars, A.; Risler, A.; Robin, C.; Larbat, R. Phenolamides: Plant specialized metabolites with a wide range of promising pharmacological and health-promoting interests. Biomed. Pharmacother. 2020, 131, 110762. [Google Scholar] [CrossRef]
- Sakanaka, S.; Tachibana, Y.; Ishihara, N.; Juneja, L. Antioxidant properties of casein calcium peptides and their effects on lipid oxidation in beef homogenates. J. Agric. Food Chem. 2005, 53, 464–468. [Google Scholar] [CrossRef]
- Naito, K.; Iio, T.; Katagi, M.; Yoshikawa, Y.; Ohtsuka, H.; Orino, K. Binding analysis of bovine milk proteins, especially casein interactions and the interaction between α-casein and lactoferrin, using beads immobilised with zinc ion, poly-l-lysine or α-casein. Int. Dairy J. 2020, 105, 104690. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Ozdal, T.; Yalcinkaya, İ.; Toydemir, G.; Capanoglu, E. Polyphenol-protein interactions and changes in functional properties and digestibility. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 566–577. [Google Scholar]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut microbiota interrelationship: A transition to a new generation of prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Pap, N.; Fidelis, M.; Azevedo, L.; do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B. Berry polyphenols and human health: Evidence of antioxidant, anti-inflammatory, microbiota modulation, and cell-protecting effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Al-Yami, A.M.; Al-Mousa, A.T.; Al-Otaibi, S.A.; Khalifa, A.Y. Lactobacillus species as probiotic: Isolation, sources and health benefits. J. Pure Appl. Microbiol. 2022, 16, 2270–2291. [Google Scholar] [CrossRef]
- Shah, B.R.; Li, B.; Al Sabbah, H.; Xu, W.; Mráz, J. Effects of prebiotic dietary fibers and probiotics on human health: With special focus on recent advancement in their encapsulated formulations. Trends Food Sci. Technol. 2020, 102, 178–192. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, J.; Pan, L.; Zhang, Y. Roles and applications of probiotic Lactobacillus strains. Appl. Microbiol. Biotechnol. 2018, 102, 8135–8143. [Google Scholar] [CrossRef]
- Antonelli, M.; Donelli, D. Health-promoting effects of goji berries (Lycium barbarum): A Literature Overview. Biol. Life Sci. Forum 2024, 40, 1. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, S.; Liu, J.; McLean, R.J.C.; Chu, W. Prebiotic, immuno-stimulating and gut microbiota-modulating effects of Lycium barbarum polysaccharide. Biomed. Pharmacother. 2020, 121, 109591. [Google Scholar] [CrossRef]
- Ilić, T.; Đuričić, I.; Kodranov, I.; Ušjak, L.; Kolašinac, S.; Milenković, M.; Marčetić, M.; Božić, D.D.; Vidović, B. Nutritional value, phytochemical composition and biological activities of Lycium barbarum L. fruits from Serbia. Plant Foods Hum. Nutr. 2024, 79, 662–668. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.; Zhang, J.; Lang, Y.-Z.; Lu, L.; Mi, J.; Cao, Y.-L.; Yan, Y.-M.; Ran, L.-W. Preventive effects of anthocyanins from Lycium ruthenicum Murray in high-fat diet-induced obese mice are related to the regulation of intestinal microbiota and inhibition of pancreatic lipase activity. Molecules 2022, 27, 2141. [Google Scholar] [CrossRef]

| Parameter | GMLR | GMLB | GM |
|---|---|---|---|
| Moisture | 6.10 ± 0.12 b | 8.99 ± 0.15 a | 4.52 ± 0.11 c |
| Ash | 9.04 ± 0.45 a | 8.2 ± 0.48 b | 6.85 ± 0.55 c |
| Protein | 32.16 ± 0.35 a | 31.71 ± 0.41 a | 31.71 ± 0.43 a |
| Fat | 4.02 ± 0.11 a | 4.61 ± 0.12 a | 3.63 ± 0.10 b |
| Carbohydrate | 48.68 ± 0.36 b | 46.49 ± 0.45 b | 53.29 ± 0.40 a |
| No | RT | Compounds | Formulas | Calculated Mass | m/z Exact Mass | mDa | MS Fragments (Main Fragment) | Samples | ||
|---|---|---|---|---|---|---|---|---|---|---|
| GMLR | GMLB | GM | ||||||||
| Phenolic acid and derivatives | ||||||||||
| 1 | 4.29 | Hydroxybenzoic acid | C7H5O3– | 137.0239 | 137.0249 | 1.03 | 108.0218(100) | + | + | − |
| 2 | 5.64 | Dihydroxybenzoic acid (like gentisic acid) | C7H5O4– | 153.0188 | 153.0206 | 1.82 | 109.0293(100), 135.0069 | + | + | + |
| 3 | 7.97 | Ferulic acid | C10H9O4– | 193.0501 | 193.0512 | 1.12 | 134.0357(100), 133.0342, 148.9154, 178.0351 | − | + | − |
| 4 | 2.61 | Hydroxybenzoic acid hexoside is. I | C13H15O8– | 299.0767 | 299.0794 | 2.71 | 137.0241(100) | + | − | − |
| 5 | 3.24 | Hydroxybenzoic acid hexoside is. II | C13H15O8– | 299.0767 | 299.0794 | 2.71 | 137.0246(100) | + | − | − |
| 6 | 6.40 | Coumaric acid hexoside | C15H17O8– | 325.0923 | 325.0947 | 2.36 | 145.031(100), 119.0501, 163.0328 | + | − | − |
| 7 | 5.41 | Caffeic acid hexoside | C15H17O9– | 341.0873 | 341.0925 | 5.24 | 161.0241(100), 133.0291, 135.0443, 179.0358 | + | − | − |
| 8 | 6.31 | Caffeoylquinic acid (like chlorogenic acid) | C16H17O9– | 353.0873 | 353.0900 | 2.74 | 191.0558(100), 127.0401, 161.0242, 173.0455, 135.0445 | + | − | − |
| 9 | 7.28 | Coumaric acid rhamnosyl hexoside | C21H27O12– | 471.1503 | 471.1562 | 5.95 | 163.0401(100), 145.0295, 351.1093, 309.0989, 119.0498 | + | − | − |
| 10 | 8.43 | Di-caffeoylquinic acid | C25H23O12– | 515.119 | 515.1226 | 3.65 | 173.0458(100), 179.035, 191.0557, 353.0885, 135.0449, 155.0343, 161.0242 | + | − | − |
| 11 | 5.26 | Caffeoylquinic acid hexoside is. I | C22H27O14– | 515.1401 | 515.1414 | 1.32 | 191.0560(100), 179.035, 173.0458, 353.088, 175.0604, 161.0248, 135.0446 | + | + | − |
| 12 | 6.14 | Caffeoylquinic acid hexoside is. II | C22H27O14– | 515.1401 | 515.1414 | 1.32 | 191.0563(100), 179.0349, 161.0246, 323.0777, 353.0874, 135.0451 | + | + | − |
| 13 | 6.47 | Caffeoylquinic acid hexoside is. III | C22H27O14– | 515.1401 | 515.1412 | 1.12 | 191.0566(100), 515.1442, 179.0355, 161.0251, 173.0452, 135.0448, 353.0880, 323.0763, 395.0993 | − | + | − |
| 14 | 6.84 | Caffeoylquinic acid hexoside is. IV | C22H27O14– | 515.1401 | 515.143 | 2.92 | 191.0556(100), 323.0776, 161.0255, 134.0382, 341.0850 | − | + | − |
| 15 | 7.03 | Ferulic acid dihexoside | C22H29O14– | 517.1557 | 517.1588 | 3.07 | 175.0401(100), 193.0510, 160.0165, 149.0672, 134.0364 | − | + | − |
| 16 | 4.45 | Coumaric acid dihexoside + HCOOH | C22H29O15– | 533.1506 | 533.1539 | 3.25 | 163.0401(100), 145.0292, 325.0931, 119.05 | + | + | − |
| Flavonoids (Flavanon and flavonol glycosides) | ||||||||||
| 17 | 8.85 | Naringenin-7-O-hexoside (like Prunin) | C21H21O10– | 433.1135 | 433.1180 | 4.53 | 271.0620(100), 151.0041, 119.0498, 313.0636, 253.0238, 227.0728, 187.0399 | + | − | − |
| 18 | 8.39 | Naringenin-7-O-(2”-O-rhamnosyl)-hexoside (like Naringin) | C27H31O14– | 579.1714 | 579.1708 | −0.58 | 271.0598(100), 151.0034, 119.0560, 579.1765, 459.1145, 417.1513 | + | − | − |
| 19 | 8.12 | Kaempferol-3-O-(6”-O-rhamnosyl)-hexoside | C27H29O15– | 593.1506 | 593.1518 | 1.15 | 285.0401(100), 593.1545, 284.0305, 227.0384, 255.0524 | − | + | − |
| 20 | 7.75 | Quercetin-3-O-(6”-O-rhamnosyl)-hexoside (like Rutin) | C27H29O16– | 609.1456 | 609.1481 | 2.54 | 300.0286(100), 609.1499, 301.0349, 271.0247, 255.0328, 178.9997, 151.0049, 343.0454 | − | + | − |
| 21 | 8.17 | Isorhamnetin-3-O-(6”-O-rhamnosyl) -hexoside | C28H31O16– | 623.1612 | 623.163 | 1.79 | 315.0504(100), 623.165, 314.0433, 300.0271, 299.0232, 357.0625 | − | + | − |
| 22 | 9.00 | Laricitrin-3-O-[6-O-(4-O-(p-coumaroyl) -rhamnosyl)-hexoside] | C37H37O19– | 785.1929 | 785.1956 | 2.7 | 639.1579(100), 785.1993, 331.0464, 315.0148, 316.0170, 287.0246, 621.1381, 179.0054, 151.0032 | + | − | − |
| Anthocyanins | ||||||||||
| 23 | 8.02 | Petunidin-3-O-(6-O-p-coumaroyl) -rhamnoside-5-O-hexoside | C37H39O18+ | 771.2136 | 771.2167 | 3.06 | 317.0664(100), 771.2166, 479.1191 | + | − | − |
| 24 | 7.62 | Petunidin-3-O-[6-O-(4-O-(p-coumaroyl) -rhamnosyl)-hexoside]-5-O-hexoside | C43H49O23+ | 933.2665 | 933.269 | 2.54 | 317.0652(100), 771.2156, 479.1207, 318.0699, 933.2715 | + | − | − |
| 25 | 6.86 | Petunidin-3-O-[6-O-(4-O-(4-O-hexosyl) -p-coumaroyl)-rhamnosyl)-hexoside]-5-O-hexoside | C49H59O28+ | 1095.3193 | 1095.3201 | 0.81 | 317.0651(100), 1095.3234, 479.1185, 455.1599, 933.2677 | + | − | − |
| No | RT | Compounds | Formulas | Calculated Mass | m/z Exact Mass | mDa | MS Fragments (Main Fragment) | Samples | ||
|---|---|---|---|---|---|---|---|---|---|---|
| GM LR | GM LB | GM | ||||||||
| Putrescine derivatives | ||||||||||
| 26 | 3.32 | N-caffeoyl putrescine | C13H19N2O3+ | 251.13960 | 251.14 | 0.43 | 163.038(100), 145.0279, 117.0336 | + | − | − |
| 27 | 3.57 | N-caffeoyl putrescine-hexoside | C19H29N2O8+ | 413.19240 | 413.1932 | 0.81 | 163.0389(100), 145.0285, 234.1108, 117.0332, 251.1375 | − | + | − |
| Non-glycosylated and glycosylated spermidine derivatives | ||||||||||
| 28 | 7.31 | N-dihydrocaffeoyl-N′-coumaroyl spermidine | C25H34N3O5+ | 456.24980 | 456.2490 | −0.85 | 222.114(100), 147.0445, 456.2521, 293.1856, 310.2120, 439.2281, 204.1021, 165.0540, 123.0443, 218.1164 | + | − | − |
| 29 | 7.15 | N,N′-bis-caffeoyl spermidine | C25H32N3O6+ | 470.22910 | 470.2307 | 1.59 | 220.0969(100), 163.0390, 308.1964, 470.2341, 453.2082, 291.1721, 234.1133, 251.1386, 145.0286 | + | − | − |
| 30 | 6.88 | N-caffeoyl-N′-dihydrocaffeoyl spermidine | C25H34N3O6+ | 472.24480 | 472.2453 | 0.54 | 220.0988(100), 163.0390, 222.1118, 472.2456, 310.2136, 293.1866, 234.1122, 145.0289, 123.0449, 165.0551, 455.2234 | + | + | − |
| 31 | 6.74 | N,N′-bis-dihydrocaffeoyl spermidine | C25H36N3O6+ | 474.26040 | 474.262 | 1.59 | 222.1164(100), 474.2772, 236.1279, 165.0548, 123.0445, 293.1874, 253.1542, 310.2133, 457.2377 | + | + | − |
| 32 | 7.46 | N-feruloyl-N′-dihydrocaffeoyl spermidine | C26H36N3O6+ | 486.26040 | 486.2602 | −0.21 | 177.054(100), 222.1118, 234.1117, 310.2117, 486.2609, 145.0283, 163.0384, 165.054, 248.1266, 293.1854, 236.126, 469.2378 | + | − | − |
| 33 | 6.54 | N-caffeoyl-N′-dihydrocaffeoyl spermidine-monohexoside | C31H44N3O11+ | 634.29760 | 634.2988 | 1.22 | 634.3022(100), 222.1118, 472.249, 163.0398, 310.2119, 384.1647, 455.2294, 293.1859, 236.1274, 123.0443, 398.1803, 617.2744 | + | + | − |
| 34 | 6.51 | N,N′-bis-dihydrocaffeoyl spermidine-monohexoside | C31H46N3O11+ | 636.31320 | 636.3132 | −0.03 | 636.3222(100), 222.1120, 474.2612, 165.0535, 236.1271, 293.1859, 384.1647, 398.1802, 457.2364, 619.2884 | + | − | − |
| 35 | 6.72 | N,N′-bis-caffeoyl spermidine-dihexoside | C37H52N3O16+ | 794.33480 | 794.3370 | 2.24 | 796.3488(100), 794.3370, 632.2817, 470.2479, 382.1479, 220.0950, 163.0375 | − | + | − |
| 36 | 6.41 | N-caffeoyl-N′-dihydrocaffeoyl spermidine-dihexoside | C37H54N3O16+ | 796.35040 | 796.3523 | 1.89 | 796.3527(100), 634.2976, 472.2497, 384.1640, 310.2118, 222.1113, 163.0385, 220.0959, 382.1500 | + | + | − |
| 37 | 6.34 | N,N′-bis-dihydrocaffeoyl spermidine-dihexoside | C37H56N3O16+ | 798.36610 | 798.3661 | 0.04 | 798.3681(100), 636.3119, 474.2593, 384.164, 222.1115 | + | + | − |
| 38 | 6.52 | N-caffeoyl-N′-dihydrocaffeoyl spermidine-trihexoside | C43H64N3O21+ | 958.40320 | 958.4039 | 0.67 | 958.4058(100), 796.3462, 634.2952, 472.2510, 544.1995, 310.2113, 220.0961, 163.0386 | − | + | − |
| Non-glycosylated and glycosylated spermine derivatives | ||||||||||
| 39 | 6.25 | N-caffeoyl-N′-dihydrocaffeoyl spermine | C28H41N4O6+ | 529.30260 | 529.3036 | 0.99 | 220.0957(100), 293.1855, 291.1705, 222.1116, 367.2694, 163.0389, 129.1388, 511.2911, 165.0538, 512.2833, 529.3044 | + | − | − |
| 40 | 5.99 | N,N′-bis-dihydrocaffeoyl spermine (kukoamines) | C28H43N4O6+ | 531.31830 | 531.3185 | 0.24 | 293.1863(100), 222.1124, 531.3253, 165.0549, 123.0449, 310.2116, 367.2700, 514.2978 | + | − | − |
| 41 | 5.91 | N-caffeoyl-N′-dihydrocaffeoyl spermine-monohexoside | C34H51N4O11+ | 691.35540 | 691.3558 | 0.37 | 693.3715(100), 293.1868, 455.2387, 222.1120, 220.096, 384.1635, 531.3168, 529.3107, 291.1698, 163.0391, 675.3593 | + | − | − |
| 42 | 5.89 | N,N′-bis-dihydrocaffeoyl spermine-monohexoside | C34H53N4O11+ | 693.37110 | 693.3718 | 0.72 | 693.3734(100), 293.1866, 455.2386, 222.1124, 531.3169, 676.3508, 384.1649, 165.0537 | + | − | − |
| 43 | 5.84 | N,N′-bis-dihydrocaffeoyl spermine-dihexoside | C40H63N4O16+ | 855.42390 | 855.4248 | 0.89 | 855.4283(100), 455.2404, 693.3706, 384.1647, 293.1850, 222.1120 | − | + | − |
| Parameter | Unit | GMLR | GMLB | GM |
|---|---|---|---|---|
| TPC | mg GAE/100 mL | 6.63 ± 0.16 a | 4.77 ± 0.0001 c | 5.41 ± 0.09 b |
| TPrC | mg BSA/100 mL | 27.85 ± 3.20 b | 26.00 ± 1.44 b | 35.44 ± 2.74 a |
| FRP | µg AA/mL | 13.98 ± 1.37 | / | / |
| ABTS | µg TE/mL | 81.97 ± 4.54 a | 24.017 ± 2.42 b | 8.33 ± 1.66 c |
| FCC | µg EDTA/mL | 331.10 ± 2.34 b | 321.51 ± 5.38 b | 396.92 ± 1.10 a |
| Goat′s Milk Powder and Goat′s Milk/Goji Extract Powders | Concentration | ||||
|---|---|---|---|---|---|
| 5 mg/mL | 2.5 mg/mL | 1.25 mg/mL | 0.625 mg/mL | 0.312 mg/mL | |
| GMLB | |||||
| Lactobacillus plantarum | 157.9 ± 18.9 ab* | 141.4 ± 17.0 ab* | 132.9 ± 15.9 ab* | 122.0 ± 14.6 ab* | 109.5 ± 8.8 ab* |
| Lactobacillus reuteri | 132.1 ± 15.8 a* | 127.7 ± 15.3 ab* | 120.8 ± 14.5 ab* | 116.4 ± 14.0 ab* | 107.5 ± 8.6 ab* |
| Lactobacillus rhamnosus | 141.4 ± 17.0 ab* | 130.9 ± 15.7 ab* | 114.8 ± 13.8 ab* | 110.5 ± 13.3 ab* | 107.9 ± 8.6 ab |
| Streptococcus thermophilus | 151.6 ± 18.2 ab* | 129.7 ± 15.6 ab* | 119.4 ± 14.3 ab* | 112.9 ± 13.5 ab* | 105.2 ± 8.4 ab |
| Saccharomyces boulardii | 169.9 ± 20.4 ab* | 157.1 ± 18.8 ab* | 141.0 ± 16.9 ab* | 128.8 ± 15.5 ab | 115.4 ± 9.2 ab |
| Lactobacilli/Bifidobacterium mixture | 168.2 ± 20.2 ab* | 161.2 ± 19.3 ab* | 146.3 ± 17.6 ab* | 133.2 ± 16.0 ab | 118.9 ± 9.5 ab* |
| GMLR | |||||
| Lactobacillus plantarum | 184.5 ± 22.1 ac* | 172.0 ± 20.6 ac | 163.8 ± 19.7 ac* | 148.4 ± 17.8 ac | 125.3 ± 10.0 ac* |
| Lactobacillus reuteri | 162.9 ± 19.5 ac* | 147.8 ± 17.7 ac | 136.2 ± 16.3 ac* | 128.9 ± 15.5 ac | 113.2 ± 9.1 ac* |
| Lactobacillus rhamnosus | 180.9 ± 21.7 ac* | 159.2 ± 19.1 ac* | 130.9 ± 15.7 ac* | 121.7 ± 14.6 ac* | 114.5 ± 9.2 ac |
| Streptococcus thermophilus | 170.3 ± 20.4 ac* | 152.9 ± 18.3 ac* | 137.4 ± 16.5 ac | 125.8 ± 15.1 ac | 113.5 ± 9.1 ac* |
| Saccharomyces boulardii | 211.5 ± 25.4 ac* | 179.5 ± 21.5 ac* | 156.1 ± 18.7 ac* | 144.6 ± 17.3 ac* | 130.8 ± 10.5 ac* |
| Lactobacilli/Bifidobacterium mixture | 193.9 ± 23.3 ac* | 181.8 ± 21.8 ac* | 165.9 ± 19.9 ac* | 152.3 ± 18.3 ac | 133.2 ± 10.7 ac* |
| GM | |||||
| Lactobacillus plantarum | 122.4 ± 14.7 bc* | 109.2 ± 13.1 bc* | 104.6 ± 12.6 bc* | 102.6 ± 12.3 bc* | 100.7 ± 8.1 bc |
| Lactobacillus reuteri | 117.0 ± 14.0 c* | 110.7 ± 13.3 bc* | 105.0 ± 12.5 bc* | 100.6 ± 12.1 bc* | 100.0 ± 8.0 bc |
| Lactobacillus rhamnosus | 119.7 ± 14.4 bc* | 109.2 ± 13.1 bc* | 104.6 ± 12.6 bc | 102.0 ± 12.2 bc | 101.3 ± 8.1 bc |
| Streptococcus thermophilus | 121.3 ± 14.6 bc* | 111.6 ± 13.4 bc* | 105.2 ± 12.4 bc | 101.9 ± 12.2 bc | 103.2 ± 8.3 bc |
| Saccharomyces boulardii | 123.1 ± 14.8 bc* | 113.5 ± 13.6 bc* | 107.1 ± 12.8 bc* | 103.2 ± 12.4 bc | 101.9 ± 8.2 bc |
| Lactobacilli/Bifidobacterium mixture | 136.0 ± 16.3 bc* | 126.6 ± 15.2 bc* | 118.7 ± 14.2 bc | 112.6 ± 13.5 bc | 106.5 ± 8.5 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milinčić, D.D.; Kostić, A.Ž.; Lević, S.; Gašić, U.M.; Božić, D.D.; Suručić, R.; Ilić, T.D.; Nedović, V.A.; Vidović, B.B.; Pešić, M.B. Goat’s Milk Powder Enriched with Red (Lycium barbarum L.) and Black (Lycium ruthenicum Murray) Goji Berry Extracts: Chemical Characterization, Antioxidant Properties, and Prebiotic Activity. Foods 2025, 14, 62. https://doi.org/10.3390/foods14010062
Milinčić DD, Kostić AŽ, Lević S, Gašić UM, Božić DD, Suručić R, Ilić TD, Nedović VA, Vidović BB, Pešić MB. Goat’s Milk Powder Enriched with Red (Lycium barbarum L.) and Black (Lycium ruthenicum Murray) Goji Berry Extracts: Chemical Characterization, Antioxidant Properties, and Prebiotic Activity. Foods. 2025; 14(1):62. https://doi.org/10.3390/foods14010062
Chicago/Turabian StyleMilinčić, Danijel D., Aleksandar Ž. Kostić, Steva Lević, Uroš M. Gašić, Dragana D. Božić, Relja Suručić, Tijana D. Ilić, Viktor A. Nedović, Bojana B. Vidović, and Mirjana B. Pešić. 2025. "Goat’s Milk Powder Enriched with Red (Lycium barbarum L.) and Black (Lycium ruthenicum Murray) Goji Berry Extracts: Chemical Characterization, Antioxidant Properties, and Prebiotic Activity" Foods 14, no. 1: 62. https://doi.org/10.3390/foods14010062
APA StyleMilinčić, D. D., Kostić, A. Ž., Lević, S., Gašić, U. M., Božić, D. D., Suručić, R., Ilić, T. D., Nedović, V. A., Vidović, B. B., & Pešić, M. B. (2025). Goat’s Milk Powder Enriched with Red (Lycium barbarum L.) and Black (Lycium ruthenicum Murray) Goji Berry Extracts: Chemical Characterization, Antioxidant Properties, and Prebiotic Activity. Foods, 14(1), 62. https://doi.org/10.3390/foods14010062











