Extraction of Protein and Bioactive Compounds from Mediterranean Red Algae (Sphaerococcus coronopifolius and Gelidium spinosum) Using Various Innovative Pretreatment Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Collection and Preparation of Algal Materials
2.3. Determination of Crude Protein
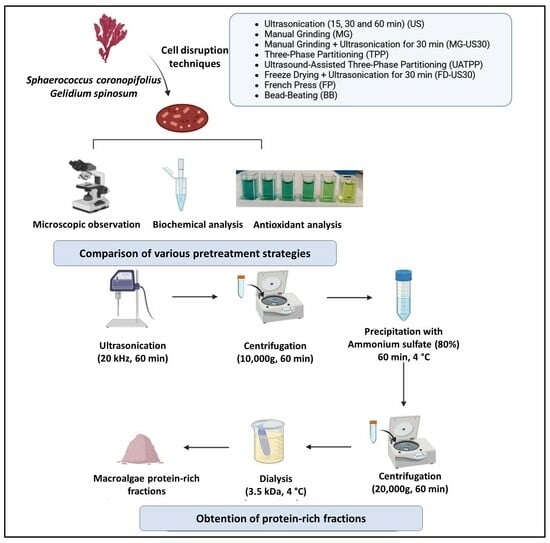
2.4. Pretreatment Methods for Red Macroalgae Cell Disruption and Protein Extraction
2.4.1. Control
2.4.2. Ultrasonication (US)
2.4.3. Manual Grinding (MG)
2.4.4. Combination of Manual Grinding and Ultrasonication for 30 min (MG-US30)
2.4.5. Three-Phase Partitioning (TPP)
2.4.6. Ultrasound-Assisted Three-Phase Partitioning (UATPP)
2.4.7. Combination of Freeze Drying with Ultrasonication for 30 Min (FD-US30)
2.4.8. French Press (FP)
2.4.9. Bead-Beating (BB)
2.4.10. Mass Extraction Yield Calculation
2.5. Optical Microscopic Observation
2.6. Estimation of Total Soluble Protein
2.7. Protein Profile by SDS-PAGE
2.8. Quantification of Co-Extracted Compounds
2.8.1. Total Carbohydrate Analysis
2.8.2. Pigment Analysis
2.8.3. Quantitative Polyphenol Analysis
2.9. Antioxidant Assays
2.9.1. DPPH Free Radical Scavenging Assay
2.9.2. Ferrous Ion-Chelating Ability Assay
2.9.3. Reducing Power Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Total Protein Contents of S. coronopifolius and G. spinosum
3.2. Optical Scanning Microscopy Morphology Observation of Macroalgae Cells
3.3. Mass Extraction Yield
3.4. Release of Soluble Protein
3.5. Protein Molecular Weights Profile
3.6. Release of Soluble Carbohydrates
3.7. Release of Pigments
3.8. Release of Phenolic Compounds
3.9. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grossmann, L.; Weiss, J. Alternative Protein Sources as Technofunctional Food Ingredients. Annu. Rev. Food Sci. Technol. 2021, 12, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Gamero-Vega, G.; Palacios-Palacios, M.; Quitral, V. Nutritional Composition and Bioactive Compounds of Red Seaweed: A Mini-Review. J. Food Nutr. Res. 2020, 8, 431–440. [Google Scholar] [CrossRef]
- Qiu, S.M.; Aweya, J.J.; Liu, X.; Liu, Y.; Tang, S.; Zhang, W.; Cheong, K.L. Bioactive polysaccharides from red seaweed as potent food supplements: A systematic review of their extraction, purification, and biological activities. Carbohydr. Polym. 2022, 275, 118696. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Bharathiraja, S.; Santha Moorthy, M.; Mondal, S.; Seo, H.; Dae Lee, K.; Oh, J. Marine natural pigments as potential sources for therapeutic applications. Crit. Rev. Biotechnol. 2018, 38, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Kitade, Y.; Kobayashi, M.; Watanabe, K.; Kurita, H.; Takeda, H.; Yasui, H.; Kishimura, H. Identification of ACE inhibitory peptides from red alga Mazzaella japonica. Eur. Food Res. Technol. 2020, 246, 2225–2231. [Google Scholar] [CrossRef]
- Ismail, M.M.; Alotaibi, B.S.; El-Sheekh, M.M. Therapeutic Uses of Red Macroalgae. Molecules 2020, 25, 4411. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Lee, W.; Jeon, Y.-J. Nutrients and bioactive potentials of edible green and red seaweed in Korea. Fish. Aquat. Sci. 2018, 21, 19. [Google Scholar] [CrossRef]
- Rawiwan, P.; Peng, Y.; Paramayuda, I.G.P.B.; Quek, S.Y. Red seaweed: A promising alternative protein source for global food sustainability. Trends Food Sci. Technol. 2022, 123, 37–56. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Markets, R.A. Seaweed Protein Market by Source (Red Seaweed, Green Seaweed & Brown Seaweed), Extraction Process (Conventional Method & Current Method), Mode of Application (Food, Animal Feed & Additives, Personal Care & Cosmetics) & Region—Global Forecast to 2027. Research and Markets. 2022. Available online: https://www.giiresearch.com/report/mama1083115-seaweed-protein-market-by-source-red-seaweed-green.html (accessed on 25 March 2024).
- Pliego-Cortés, H.; Wijesekara, I.; Lang, M.; Bourgougnon, N.; Bedoux, G. Current knowledge and challenges in extraction, characterization and bioactivity of seaweed protein and seaweed-derived proteins. Adv. Bot. Res. 2019, 95, 289–326. [Google Scholar]
- Sarker, A. A review on the application of bioactive peptides as preservatives and functional ingredients in food model systems. J. Food Process. Preserv. 2022, 46, e16800. [Google Scholar] [CrossRef]
- Reboleira, J.; Silva, S.; Chatzifragkou, A.; Niranjan, K.; Lemos, M.F.L. Seaweed fermentation within the fields of food and natural products. Trends Food Sci. Technol. 2021, 116, 1056–1073. [Google Scholar] [CrossRef]
- Filote, C.; Santos, S.C.R.; Popa, V.I.; Botelho, C.M.S.; Volf, I. Biorefinery of marine macroalgae into high-tech bioproducts: A review. Environ. Chem. Lett. 2020, 19, 969–1000. [Google Scholar] [CrossRef]
- Deniaud, E.; Quemener, B.; Fleurence, J.; Lahaye, M. Structural studies of the mix-linked β-(1→3)/β-(1→4)-d-xylans from the cell wall of Palmaria palmata (Rhodophyta). Int. J. Biol. Macromol. 2003, 33, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Domozych, D. Algal Cell Walls; Wiley: Hoboken, NJ, USA, 2019; pp. 1–11. [Google Scholar]
- Wijesinghe, W.A.; Jeon, Y.J. Enzyme-assistant extraction (EAE) of bioactive components: A useful approach for recovery of industrially important metabolites from seaweeds: A review. Fitoterapia 2012, 83, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Cermeno, M.; Kleekayai, T.; Amigo-Benavent, M.; Harnedy-Rothwell, P.; FitzGerald, R.J. Current knowledge on the extraction, purification, identification, and validation of bioactive peptides from seaweed. Electrophoresis 2020, 41, 1694–1717. [Google Scholar] [CrossRef] [PubMed]
- Conde, C.; Osswald, M.; Sunkel, C.E. All together now: Polo joins the kinase network controlling the spindle assembly checkpoint in Drosophila. Fly 2013, 7, 224–228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiménez-González, C.; Agrasar, A.M.T.; Mallo, F.; Rúa, M.L.; Fuciños, C. Red seaweed proteins: Valuable marine-origin compounds with encouraging applications. Algal Res. 2023, 75, 103262. [Google Scholar] [CrossRef]
- Doucha, J.; Livansky, K. Influence of processing parameters on disintegration of Chlorella cells in various types of homogenizers. Appl. Microbiol. Biotechnol. 2008, 81, 431–440. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yoo, C.; Jun, S.Y.; Ahn, C.Y.; Oh, H.M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101 (Suppl. S1), S75–S77. [Google Scholar] [CrossRef]
- Le Guillard, C.; Bergé, J.-P.; Donnay-Moreno, C.; Bruzac, S.; Ragon, J.-Y.; Baron, R.; Fleurence, J.; Dumay, J. Soft liquefaction of the red seaweed Grateloupia turuturu Yamada by ultrasound-assisted enzymatic hydrolysis process. J. Appl. Phycol. 2016, 28, 2575–2585. [Google Scholar] [CrossRef]
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K. Ultrasound assisted methods for enhanced extraction of phycobiliproteins from marine macro-algae, Gelidium pusillum (Rhodophyta). Ultrason. Sonochem. 2017, 38, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.J.; Kim, H.T.; Cho, K.M.; Yu, S.; Kim, S.; Choi, I.G.; Kim, K.H. Pretreatment and saccharification of red macroalgae to produce fermentable sugars. Bioresour. Technol. 2016, 199, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; Tiwari, B.K.; Smyth, T.J.; O’Donnell, C.P. Optimization of ultrasound assisted extraction of bioactive components from brown seaweed Ascophyllum nodosum using response surface methodology. Ultrason. Sonochem. 2015, 23, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Pinto, M.M.; Raposo, M.F.J.; Bowen, J.; Young, A.J.; Morais, R. Evaluation of different cell disruption processes on encysted cells of Haematococcus pluvialis: Effects on astaxanthin recovery and implications for bio-availability. J. Appl. Phycol. 2001, 13, 19–24. [Google Scholar] [CrossRef]
- O’ Connor, J.; Meaney, S.; Williams, G.A.; Hayes, M. Extraction of Protein from Four Different Seaweeds Using Three Different Physical Pre-Treatment Strategies. Molecules 2020, 25, 2005. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Kazir, M.; Abuhassira, Y.; Robin, A.; Nahor, O.; Luo, J.; Israel, A.; Golberg, A.; Livney, Y.D. Extraction of proteins from two marine macroalgae, Ulva sp. and Gracilaria sp., for food application, and evaluating digestibility, amino acid composition and antioxidant properties of the protein concentrates. Food Hydrocoll. 2019, 87, 194–203. [Google Scholar] [CrossRef]
- Sanz-Pintos, N.; Perez-Jimenez, J.; Buschmann, A.H.; Vergara-Salinas, J.R.; Perez-Correa, J.R.; Saura-Calixto, F. Macromolecular Antioxidants and Dietary Fiber in Edible Seaweeds. J. Food Sci. 2017, 82, 289–295. [Google Scholar] [CrossRef]
- Zehlila, A.; Schaumann, A.; Mlouka, A.B.; Bourguiba, I.; Hardouin, J.; Masmoudi, O.; Cosette, P.; Amri, M.; Jouenne, T. Glioprotective effect of Ulva rigida extract against UVB cellular damages. Algal Res. 2017, 23, 203–215. [Google Scholar] [CrossRef]
- AOAC. Protein (Crude) Determination in Animal Feed: Copper Catalyst Kjeldahl Method, 15th ed.; Official Methods of Analysis of AOAC International: Gaithersburg, MD, USA, 1990; Volume 984.13. [Google Scholar]
- Lorenzo, J.M.; Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F.J. Proximate Composition and Nutritional Value of Three Macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef] [PubMed]
- Safi, C.; Ursu, A.V.; Laroche, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Aqueous extraction of proteins from microalgae: Effect of different cell disruption methods. Algal Res. 2014, 3, 61–65. [Google Scholar] [CrossRef]
- Malik, M.A.; Sharma, H.K.; Saini, C.S. High intensity ultrasound treatment of protein isolate extracted from dephenolized sunflower meal: Effect on physicochemical and functional properties. Ultrason. Sonochem. 2017, 39, 511–519. [Google Scholar] [CrossRef]
- Chia, S.R.; Chew, K.W.; Zaid, H.F.M.; Chu, D.T.; Tao, Y.; Show, P.L. Microalgal Protein Extraction from Chlorella vulgaris FSP-E Using Triphasic Partitioning Technique with Sonication. Front. Bioeng. Biotechnol. 2019, 7, 396. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Cho, J.M.; Chang, Y.K.; Oh, Y.K. Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresour. Technol. 2017, 244, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Suarez Garcia, E.; Miranda, C.F.; Cesario, M.T.; Wijffels, R.H.; van den Berg, C.; Eppink, M.H.M. Ionic Liquid-Assisted Selective Extraction and Partitioning of Biomolecules from Macroalgae. ACS Sustain. Chem. Eng. 2023, 11, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- He, F. Laemmli-SDS-PAGE. Bio-Protocol 2011, 1, e80. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Kirk, J.T.O.; Allen, R.L. Dependence of chloroplast pigment synthesis on protein synthesis: Effect of actidione. Biochem. Biophys. Res. Commun. 1965, 21, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, K.; Wong, C.; Fan, K.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Dhifallah, A.; Selmi, H.; Ouerghui, A.; Sammeri, H.; Aouini, D.; Rouissi, H. Comparative Study of Phenolic Compounds and Antiradical Activities of Four Extracts of Tunisian Artemisia herba alba. Pharm. Chem. J. 2022, 56, 226–232. [Google Scholar] [CrossRef]
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from a heated histidine-glucose model system. I: Investigation of the antioxidant role of histidine and isolation of antioxidants by high-performance liquid chromatography. J. Am. Oil Chem. Soc. 1998, 75, 181–187. [Google Scholar] [CrossRef]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Yildirim, A.; Mavi, A.; Oktay, M.; Kara, A.A.; Algur, O.F.; Bilaloglu, V. Comparison of antioxidant and antimicrobial activities of tilia (Tilia argentea Desf ex DC), sage (Salvia triloba L.), and black tea (Camellia sinensis) extracts. J. Agric. Food Chem. 2000, 48, 5030–5034. [Google Scholar] [CrossRef] [PubMed]
- Patarra, R.F.; Paiva, L.; Neto, A.I.; Lima, E.; Baptista, J. Nutritional value of selected macroalgae. J. Appl. Phycol. 2010, 23, 205–208. [Google Scholar] [CrossRef]
- Said, R.; Romdhane, M.S.; Abed, A.; M’Rabet, R. Temporal variation of some biometric parameters, agar-yield and quality of Gelidium spinosum (S.G. Gmelin) P.C. Silva (Rhodophyta: Rhodophyceae: Gelidiales) from Monastir coasts (Tunisia). Cah. Biol. Mar. 2011, 52, 71–78. [Google Scholar]
- Kadam, S.U.; Alvarez, C.; Tiwari, B.K.; O’Donnell, C.P. Extraction and characterization of protein from Irish brown seaweed Ascophyllum nodosum. Food Res. Int. 2017, 99, 1021–1027. [Google Scholar] [CrossRef]
- Jung, S.M.; Kang, S.G.; Son, J.S.; Jeon, J.H.; Lee, H.J.; Shin, H.W. Temporal and spatial variations in the proximate composition, amino acid, and mineral content of Pyropia yezoensis. J. Appl. Phycol. 2016, 28, 3459–3467. [Google Scholar] [CrossRef]
- Madden, M.; Mitra, M.; Ruby, D.; Schwarz, J. Seasonality of Selected Nutritional Constituents of Edible Delmarva Seaweeds. J. Phycol. 2012, 48, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Rosemary, T.; Arulkumar, A.; Paramasivam, S.; Mondragon-Portocarrero, A.; Miranda, J.M. Biochemical, Micronutrient and Physicochemical Properties of the Dried Red Seaweeds Gracilaria edulis and Gracilaria corticata. Molecules 2019, 24, 2225. [Google Scholar] [CrossRef] [PubMed]
- Stévant, P.; Ólafsdóttir, A.; Déléris, P.; Dumay, J.; Fleurence, J.; Ingadóttir, B.; Jónsdóttir, R.; Ragueneau, É.; Rebours, C.; Rustad, T. Semi-dry storage as a maturation process for improving the sensory characteristics of the edible red seaweed dulse (Palmaria palmata). Algal Res. 2020, 51, 102048. [Google Scholar] [CrossRef]
- Vásquez, V.; Martínez, R.; Bernal, C. Enzyme-assisted extraction of proteins from the seaweeds Macrocystis pyrifera and Chondracanthus chamissoi: Characterization of the extracts and their bioactive potential. J. Appl. Phycol. 2019, 31, 1999–2010. [Google Scholar] [CrossRef]
- Uribe, E.; Vega-Gálvez, A.; García, V.; Pastén, A.; Rodríguez, K.; López, J.; Scala, K.D. Evaluation of physicochemical composition and bioactivity of a red seaweed (Pyropia orbicularis) as affected by different drying technologies. Dry. Technol. 2019, 38, 1218–1230. [Google Scholar] [CrossRef]
- Jong Lee, T.; Nakano, K.; Matsumura, M. A new method for the rapid evaluation of gas vacuoles regeneration and viability of cyanobacteria by flow cytometry. Biotechnol. Lett. 2000, 22, 1833–1838. [Google Scholar] [CrossRef]
- Lee, T.J.; Nakano, K.; Matsumara, M. Ultrasonic irradiation for blue-green algae bloom control. Environ. Technol. 2001, 22, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Yang, J.L.; Shi, Y.P. Optimization of ultrasonic cell grinder extraction of anthocyanins from blueberry using response surface methodology. Ultrason. Sonochem. 2017, 34, 325–331. [Google Scholar] [CrossRef]
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish Brown Seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound Assisted Extraction, Characterization and Bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef]
- Kołodziejska, I.; Skierka, E.; Sadowska, M.; Kołodziejski, W.; Niecikowska, C. Effect of extracting time and temperature on yield of gelatin from different fish offal. Food Chem. 2008, 107, 700–706. [Google Scholar] [CrossRef]
- Barba, F.J.; Grimi, N.; Vorobiev, E. New Approaches for the Use of Non-Conventional Cell Disruption Technologies to Extract Potential Food Additives and Nutraceuticals from Microalgae. Food Eng. Rev. 2014, 7, 45–62. [Google Scholar] [CrossRef]
- Safi, C.; Charton, M.; Pignolet, O.; Silvestre, F.; Vaca-Garcia, C.; Pontalier, P.-Y. Influence of microalgae cell wall characteristics on protein extractability and determination of nitrogen-to-protein conversion factors. J. Appl. Phycol. 2012, 25, 523–529. [Google Scholar] [CrossRef]
- Cordero, B.; Voltolina, D. Viability of mass algal cultures preserved by freezing and freeze-drying. Aquac. Eng. 1997, 16, 205–211. [Google Scholar] [CrossRef]
- Rossignol, N.; Vandanjon, L.; Jaouen, P.; Quéméneur, F. Membrane technology for the continuous separation microalgae/culture medium: Compared performances of cross-flow microfiltration and ultrafiltration. Aquac. Eng. 1999, 20, 191–208. [Google Scholar] [CrossRef]
- Barbarino, E.; Lourenço, S.O. An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- Bermano, G.; Stoyanova, T.; Hennequart, F.; Wainwright, C.L. Seaweed-derived bioactives as potential energy regulators in obesity and type 2 diabetes. Adv. Pharmacol. 2020, 87, 205–256. [Google Scholar] [PubMed]
- Lakka, A.; Grigorakis, S.; Kaltsa, O.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. The Effect of Ultrasonication Pretreatment on the Production of Polyphenol-Enriched Extracts from Moringa oleifera L. (Drumstick Tree) Using a Novel Bio-Based Deep Eutectic Solvent. Appl. Sci. 2019, 10, 220. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A Green Extraction Process for Polyphenols from Elderberry (Sambucus nigra) Flowers Using Deep Eutectic Solvent and Ultrasound-Assisted Pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Ashraf, S.; Hisaindee, S.; Darmaki, N.A.; Battah, S.; Svistunenko, D.; Reeder, B.; Stanway, G.; Chaudhary, A. Enzymatic pre-treatment of microalgae cells for enhanced extraction of proteins. Eng. Life Sci. 2017, 17, 175–185. [Google Scholar] [CrossRef]
- Pernet, F.; Tremblay, R. Effect of ultrasonication and grinding on the determination of lipid class content of microalgae harvested on filters. Lipids 2003, 38, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Suwal, S.; Perreault, V.; Marciniak, A.; Tamigneaux, E.; Deslandes, É.; Bazinet, L.; Jacques, H.; Beaulieu, L.; Doyen, A. Effects of high hydrostatic pressure and polysaccharidases on the extraction of antioxidant compounds from red macroalgae, Palmaria palmata and Solieria chordalis. J. Food Eng. 2019, 252, 53–59. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Zhou, W.; Yuan, W.; Wang, D. Algal cell lysis by bacteria: A review and comparison to conventional methods. Algal Res. 2020, 46, 101794. [Google Scholar] [CrossRef]
- Passos, F.; Ferrer, I. Microalgae conversion to biogas: Thermal pretreatment contribution on net energy production. Environ. Sci. Technol. 2014, 48, 7171–7178. [Google Scholar] [CrossRef] [PubMed]
- Ortigueira, J.; Pinto, T.; Gouveia, L.; Moura, P. Production and storage of biohydrogen during sequential batch fermentation of Spirogyra hydrolyzate by Clostridium butyricum. Energy 2015, 88, 528–536. [Google Scholar] [CrossRef]
- Ometto, F.; Quiroga, G.; Psenicka, P.; Whitton, R.; Jefferson, B.; Villa, R. Impacts of microalgae pre-treatments for improved anaerobic digestion: Thermal treatment, thermal hydrolysis, ultrasound and enzymatic hydrolysis. Water Res. 2014, 65, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Duong-Ly, K.C.; Gabelli, S.B. Salting out of proteins using ammonium sulfate precipitation. Methods Enzymol. 2014, 541, 85–94. [Google Scholar] [PubMed]
- Burgess, R.R. Protein precipitation techniques. Methods Enzymol. 2009, 463, 331–342. [Google Scholar]
- Harnedy, P.A.; FitzGerald, R.J. Extraction of protein from the macroalga Palmaria palmata. LWT—Food Sci. Technol. 2013, 51, 375–382. [Google Scholar] [CrossRef]
- Fleurence, J.; Le Coeur, C.; Mabeau, S.; Maurice, M.; Landrein, A. Comparison of different extractive procedures for proteins from the edible seaweeds Ulva rigida and Ulva rotundata. J. Appl. Phycol. 1995, 7, 577–582. [Google Scholar] [CrossRef]
- Macias-Sanchez, M.D.; Mantell, C.; Rodriguez, M.; Martinez de la Ossa, E.; Lubian, L.M.; Montero, O. Comparison of supercritical fluid and ultrasound-assisted extraction of carotenoids and chlorophyll a from Dunaliella salina. Talanta 2009, 77, 948–952. [Google Scholar] [CrossRef]
- Simon, D.; Helliwell, S. Extraction and quantification of chlorophyll a from freshwater green algae. Water Res. 1998, 32, 2220–2223. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chem. 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Ben Khaled, H.; Ktari, N.; Ghorbel-Bellaaj, O.; Jridi, M.; Lassoued, I.; Nasri, M. Composition, functional properties and in vitro antioxidant activity of protein hydrolysates prepared from sardinelle (Sardinella aurita) muscle. J. Food Sci. Technol. 2014, 51, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Khantaphant, S.; Benjakul, S. Comparative study on the proteases from fish pyloric caeca and the use for production of gelatin hydrolysate with antioxidative activity. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 410–419. [Google Scholar] [CrossRef]
- Seneviratne, R.W.; Young, M.G.; Beltranena, E.; Goonewardene, L.A.; Newkirk, R.W.; Zijlstra, R.T. The nutritional value of expeller-pressed canola meal for grower-finisher pigs. J. Anim. Sci. 2010, 88, 2073–2083. [Google Scholar] [CrossRef]
- Guerard, F.; Sumaya-Martinez, M.T.; Laroque, D.; Chabeaud, A.; Dufossé, L. Optimization of free radical scavenging activity by response surface methodology in the hydrolysis of shrimp processing discards. Process Biochem. 2007, 42, 1486–1491. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Lee, K.W.; Jeon, Y.J.; Kim, S.H.; Haw, J.W. Antioxidant Activity of Hizikia fusiformis on Reactive Oxygen Species Scavenging and Lipid Peroxidation Inhibition. Food Sci. Technol. Int. 2016, 9, 339–346. [Google Scholar] [CrossRef]
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Antioxidant activities and polyphenolics of various solvent extracts of red seaweed, Gracilaria changii. J. Appl. Phycol. 2014, 27, 2377–2386. [Google Scholar] [CrossRef]
- Subhashini, N.; Nagarajan, G.; Kaviman, S. In vitro antioxidant and anticholinesterase activities of Garcinia combogia. Int. J. Pharm. Pharm. Sci. 2011, 3, 129–132. [Google Scholar]
- Bak, S.B.; Song, Y.R.; Bae, S.J.; Lee, W.Y.; Kim, Y.W. Integrative approach to uncover antioxidant properties of Bupleuri Radix and its active compounds: Multiscale interactome-level analysis with experimental validation. Free Radic. Biol. Med. 2023, 199, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Landete, J.M. Dietary intake of natural antioxidants: Vitamins and polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Safafar, H.; van Wagenen, J.; Moller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [PubMed]
- Okuzumi, J.; Takahashi, T.; Yamane, T.; Kitao, Y.; Inagake, M.; Ohya, K.; Nishino, H.; Tanaka, Y. Inhibitory effects of fucoxanthin, a natural carotenoid, on N-ethyl-N′-nitro-N-nitrosoguanidine-induced mouse duodenal carcinogenesis. Cancer Lett. 1993, 68, 159–168. [Google Scholar] [CrossRef]
- Goksen, G. Elucidation and quantification health-promoting phenolic compounds, antioxidant properties and sugar levels of ultrasound assisted extraction, aroma compositions and amino acids profiles of macroalgae, Laurencia papillosa. Ultrason. Sonochem. 2023, 98, 106527. [Google Scholar] [CrossRef]








| S. coronopifolius | G. spinosum | |||
|---|---|---|---|---|
| TPC (mg GAE/g of DW) | TFC (mg QE/g of DW) | TPC (mg GAE/g of DW) | TFC (mg QE/g of DW) | |
| Control (untreated) | 5.35 ± 0.30 a | 0.71 ± 0.09 a | 24.62 ± 0.36 a | 0.38 ± 0.01 a |
| US 60 min | 21.00 ± 1.61 b | 1.30 ± 1.11 b | 32.79 ± 0.68 b | 1.56 ± 0.05 b |
| US 30 min | 9.78 ± 1.11 c | 1.23 ± 1.97 b | 28.02 ± 0.74 c | 1.00 ± 0.02 c |
| US 15 min | 8.35 ± 0.70 d | 0.98 ± 0.01 c | 25.61 ± 0.21 ac | 0.73 ± 0.02 d |
| MG | 7.21 ± 0.10 d | 0.70 ± 0.09 a | 22.79 ± 2.28 a | 0.69 ± 0.03 d |
| MG-US30 | 9.50 ± 1.31 cd | 1.03 ± 0.47 cd | 30.57 ± 1.83 bc | 0.95 ± 0.02 e |
| TPP | 4.85 ± 0.80 a | 0.84 ± 0.62 e | 14.66 ± 0.21 d | 0.48 ± 0.02 e |
| UATPP | 11.14 ± 0.00 e | 0.88 ± 0.41 e | 16.68 ± 3.02 d | 1.03 ± 0.02 c |
| FD-US30 | 10.35 ± 0.30 ce | 1.01 ± 1.03 cd | 28.66 ± 0.21 c | 1.00 ± 0.07 ce |
| FP | 9.14 ± 0.20 cd | 1.06 ± 0.60 d | 32.42 ± 1.04 b | 0.86 ± 0.02 f |
| BB | 7.57 ± 0.20 d | 0.35 ± 0.64 f | 22.03 ± 2.13 a | 0.69 ± 0.02 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhaouafi, J.; Nedjar, N.; Jridi, M.; Romdhani, M.; Balti, R. Extraction of Protein and Bioactive Compounds from Mediterranean Red Algae (Sphaerococcus coronopifolius and Gelidium spinosum) Using Various Innovative Pretreatment Strategies. Foods 2024, 13, 1362. https://doi.org/10.3390/foods13091362
Dhaouafi J, Nedjar N, Jridi M, Romdhani M, Balti R. Extraction of Protein and Bioactive Compounds from Mediterranean Red Algae (Sphaerococcus coronopifolius and Gelidium spinosum) Using Various Innovative Pretreatment Strategies. Foods. 2024; 13(9):1362. https://doi.org/10.3390/foods13091362
Chicago/Turabian StyleDhaouafi, Jihen, Naima Nedjar, Mourad Jridi, Montassar Romdhani, and Rafik Balti. 2024. "Extraction of Protein and Bioactive Compounds from Mediterranean Red Algae (Sphaerococcus coronopifolius and Gelidium spinosum) Using Various Innovative Pretreatment Strategies" Foods 13, no. 9: 1362. https://doi.org/10.3390/foods13091362
APA StyleDhaouafi, J., Nedjar, N., Jridi, M., Romdhani, M., & Balti, R. (2024). Extraction of Protein and Bioactive Compounds from Mediterranean Red Algae (Sphaerococcus coronopifolius and Gelidium spinosum) Using Various Innovative Pretreatment Strategies. Foods, 13(9), 1362. https://doi.org/10.3390/foods13091362