The Effects of Food Nutrients and Bioactive Compounds on the Gut Microbiota: A Comprehensive Review
Abstract
1. Introduction
2. The Effects of Nutrients on Microbiota and Diseases
2.1. The Effects of Probiotic Microorganisms and Mineral Ions
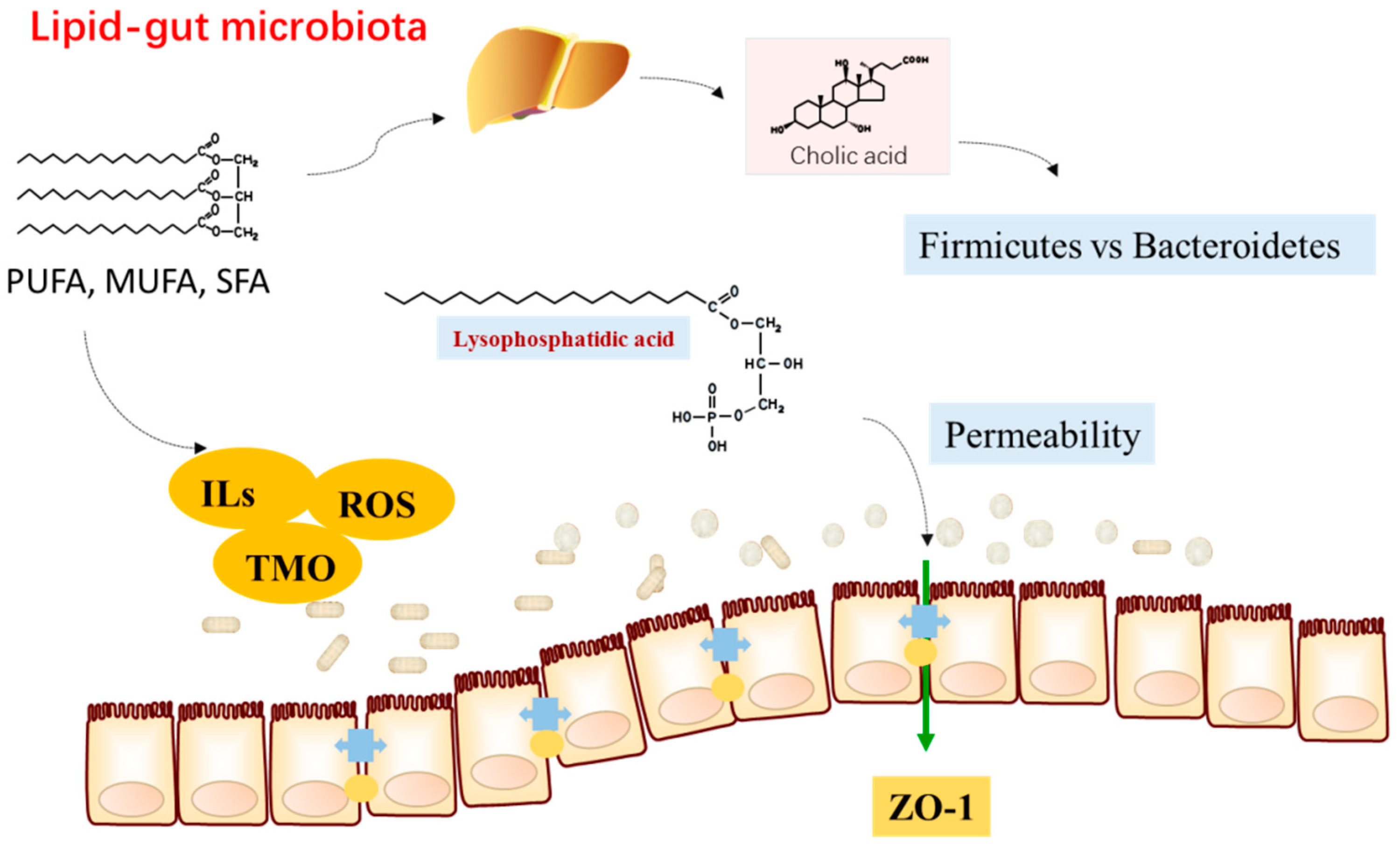
2.2. Fatty Acids and Microbiota
2.2.1. High-Fat Induced Model
2.2.2. Polyunsaturated Fatty Acids
2.2.3. The Comparison of Different Fatty Acids
2.3. Carbohydrate and Gut Microbiota
2.3.1. Dietary Fiber
2.3.2. The Polysaccharides
2.4. Studies on the Potential Prebiotic Effects of Plant Compounds on the Intestinal Microbiota
3. Plant Compounds: Prebiotic Effects and Gut Health
3.1. Tea and Plant Extracts
3.2. Polyphenol Compounds
3.3. Plant Polysaccharides
4. Clinical Trails
5. Conclusions and Perspectives
5.1. Conclusions
5.2. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Quintanar, L.; Ortuño-Sahagún, D.; Franco-Arroyo, N.N.; Viveros-Paredes, J.M.; Zepeda-Morales, A.S.; Lopez-Roa, R.I. The Human Microbiota and Obesity: A Literature Systematic Review of In Vivo Models and Technical Approaches. Int. J. Mol. Sci. 2018, 19, 3827. [Google Scholar] [CrossRef]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110–119. [Google Scholar] [CrossRef]
- Mirmohammadali, S.N.; Rosenkranz, S.K. Dietary phytochemicals, gut microbiota composition, and health outcomes in human and animal models. Biosci. Microbiota Food Health 2023, 42, 152–171. [Google Scholar] [CrossRef]
- Zsálig, D.; Berta, A.; Tóth, V.; Szabó, Z.; Simon, K.; Figler, M.; Pusztafalvi, H.; Polyák, É. A Review of the Relationship between Gut Microbiome and Obesity. Appl. Sci. 2023, 13, 610. [Google Scholar] [CrossRef]
- Puljiz, Z.; Kumric, M.; Vrdoljak, J.; Martinovic, D.; Ticinovic Kurir, T.; Krnic, M.O.; Urlic, H.; Puljiz, Z.; Zucko, J.; Dumanic, P.; et al. Obesity, Gut Microbiota, and Metabolome: From Pathophysiology to Nutritional Interventions. Nutrients 2023, 15, 2236. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Schillinger, U. Introduction to pre- and probiotics. Food Res. Int. 2002, 35, 109–116. [Google Scholar] [CrossRef]
- Fuller, R. Probiotics in man and animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar] [PubMed]
- Havenaar, R.; Brink, B.T.; Huis In ’t Veld, J.H.J. Selection of strains for probiotic use. In Probiotics: The Scientific Basis; Fuller, R., Ed.; Springer: Dordrecht, The Netherlands, 1992; pp. 209–224. [Google Scholar]
- Lilly, D.M.; Stillwell, R.H. Probiotics: Growth-Promoting Factors Produced by Microorganisms. Science 1965, 147, 747–748. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. The Gut Microbiota Influenced by the Intake of Probiotics and Functional Foods with Prebiotics Can Sustain Wellness and Alleviate Certain Ailments like Gut-Inflammation and Colon-Cancer. Microorganisms 2022, 10, 665. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: A systematic review of randomized controlled trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Hadadi, N.; Berweiler, V.; Wang, H.; Trajkovski, M. Intestinal microbiota as a route for micronutrient bioavailability. Curr. Opin. Endocr. Metab. Res. 2021, 20, 100285. [Google Scholar] [CrossRef] [PubMed]
- National Research Council; Division on Earth; Life Studies; Commission on Life Sciences; Committee on Diet. Diet and Health: Implications for Reducing Chronic Disease Risk; National Academies Press: Washington, DC, USA, 1989. [Google Scholar]
- Su, Q.; Liu, Q. Factors Affecting Gut Microbiome in Daily Diet. Front. Nutr. 2021, 8, 644138. [Google Scholar] [CrossRef]
- Bielik, V.; Kolisek, M. Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 6803. [Google Scholar] [CrossRef] [PubMed]
- Skrypnik, K.; Suliburska, J. Association between the gut microbiota and mineral metabolism. J. Sci. Food Agric. 2018, 98, 2449–2460. [Google Scholar] [CrossRef] [PubMed]
- Pajarillo, E.A.B.; Lee, E.; Kang, D.K. Trace metals and animal health: Interplay of the gut microbiota with iron, manganese, zinc, and copper. Anim. Nutr. 2021, 7, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Yokota, A.; Fukiya, S.; Islam, K.B.; Ooka, T.; Ogura, Y.; Hayashi, T.; Hagio, M.; Ishizuka, S. Is bile acid a determinant of the gut microbiota on a high-fat diet? Gut Microbes 2012, 3, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.B.; Fukiya, S.; Hagio, M.; Fujii, N.; Ishizuka, S.; Ooka, T.; Ogura, Y.; Hayashi, T.; Yokota, A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011, 141, 1773–1781. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J. The association of diet, gut microbiota and colorectal cancer: What we eat may imply what we get. Protein Cell 2018, 9, 474–487. [Google Scholar] [CrossRef]
- Schulz, M.D.; Atay, C.; Heringer, J.; Romrig, F.K.; Schwitalla, S.; Aydin, B.; Ziegler, P.K.; Varga, J.; Reindl, W.; Pommerenke, C.; et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 2014, 514, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Kim, M.Y.; Seo, Y.R.; Kim, J.S.; Sung, M.K. High-fat Diet Accelerates Intestinal Tumorigenesis Through Disrupting Intestinal Cell Membrane Integrity. J. Cancer Prev. 2016, 21, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; Qiu, Y.; Mu, Y.; Zhang, X.J.; Liu, L.; Hou, X.H.; Zhang, L.; Xu, X.N.; Ji, A.L.; Cao, R.; et al. A high ratio of dietary n-3/n-6 polyunsaturated fatty acids improves obesity-linked inflammation and insulin resistance through suppressing activation of TLR4 in SD rats. Nutr. Res. 2013, 33, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut 2014, 63, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Kishino, S.; Ogawa, J.; Omura, Y.; Matsumura, K.; Shimizu, S. Conjugated linoleic acid production from linoleic acid by lactic acid bacteria. J. Am. Oil Chem. Soc. 2002, 79, 159–163. [Google Scholar] [CrossRef]
- van Kruijsdijk, R.C.; van der Wall, E.; Visseren, F.L. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.D.; Mølbak, L.; Michaelsen, K.F.; Lauritzen, L. Molecular fingerprints of the human fecal microbiota from 9 to 18 months old and the effect of fish oil supplementation. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Broytman, O.; Curran-Everett, D.; Sorkness, R.L.; Crisafi, G.; Bleecker, E.R.; Erzurum, S.; Gaston, B.M.; Wenzel, S.E.; Jarjour, N.N. Obstructive Sleep Apnea Risk, Asthma Burden, and Lower Airway Inflammation in Adults in the Severe Asthma Research Program (SARP) II. J. Allergy Clin. Immunol. Pract. 2015, 3, 566–575.e561. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, H.; Yuan, F.; Li, N.; Huang, Q.; He, L.; Wang, L.; Liu, Z. Perilla Oil Has Similar Protective Effects of Fish Oil on High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease and Gut Dysbiosis. Biomed. Res. Int. 2016, 2016, 9462571. [Google Scholar] [CrossRef]
- Ruan, M.; Zhang, Z.; Yuan, X.; Zhou, R.; Zhang, S.; Tian, Y.; Li, X.; Li, N.; Liu, Z.; Zhu, R.; et al. Effects of deep frying vegetable oils rich in PUFAs on gut microbiota in rats. Int. J. Food Sci. Technol. 2023, 58, 37–44. [Google Scholar] [CrossRef]
- Zapata, J.; Gallardo, A.; Romero, C.; Valenzuela, R.; Garcia-Diaz, D.F.; Duarte, L.; Bustamante, A.; Gasaly, N.; Gotteland, M.; Echeverria, F. n-3 polyunsaturated fatty acids in the regulation of adipose tissue browning and thermogenesis in obesity: Potential relationship with gut microbiota. Prostaglandins Leukot. Essent. Fat. Acids 2022, 177, 102388. [Google Scholar] [CrossRef]
- Cândido, F.G.; Valente, F.X.; Grześkowiak, Ł.M.; Moreira, A.P.B.; Rocha, D.; Alfenas, R.C.G. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: Mechanisms and clinical implications on obesity. Int. J. Food Sci. Nutr. 2018, 69, 125–143. [Google Scholar] [CrossRef]
- Patterson, E.; O’Doherty, R.M.; Murphy, E.F.; Wall, R.; O’Sullivan, O.; Nilaweera, K.; Fitzgerald, G.F.; Cotter, P.D.; Ross, R.P.; Stanton, C. Impact of dietary fatty acids on metabolic activity and host intestinal microbiota composition in C57BL/6J mice. Br. J. Nutr. 2014, 111, 1905–1917. [Google Scholar] [CrossRef]
- Chaplin, A.; Parra, P.; Serra, F.; Palou, A. Conjugated Linoleic Acid Supplementation under a High-Fat Diet Modulates Stomach Protein Expression and Intestinal Microbiota in Adult Mice. PLoS ONE 2015, 10, e0125091. [Google Scholar] [CrossRef]
- Yu, H.N.; Zhu, J.; Pan, W.S.; Shen, S.R.; Shan, W.G.; Das, U.N. Effects of fish oil with a high content of n-3 polyunsaturated fatty acids on mouse gut microbiota. Arch. Med. Res. 2014, 45, 195–202. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, X.; Zhang, Q.; Yu, M.; Xu, J.; Qi, C.; Wang, T. The programming effects of nutrition-induced catch-up growth on gut microbiota and metabolic diseases in adult mice. MicrobiologyOpen 2016, 5, 296–306. [Google Scholar] [CrossRef]
- Woting, A.; Pfeiffer, N.; Hanske, L.; Loh, G.; Klaus, S.; Blaut, M. Alleviation of high fat diet-induced obesity by oligofructose in gnotobiotic mice is independent of presence of Bifidobacterium longum. Mol. Nutr. Food Res. 2015, 59, 2267–2278. [Google Scholar] [CrossRef]
- Vincent, M.; Philippe, E.; Everard, A.; Kassis, N.; Rouch, C.; Denom, J.; Takeda, Y.; Uchiyama, S.; Delzenne, N.M.; Cani, P.D.; et al. Dietary supplementation with Agaricus blazei murill extract prevents diet-induced obesity and insulin resistance in rats. Obesity 2013, 21, 553–561. [Google Scholar] [CrossRef]
- Marungruang, N.; Fåk, F.; Tareke, E. Heat-treated high-fat diet modifies gut microbiota and metabolic markers in apoe-/- mice. Nutr. Metab. 2016, 13, 22. [Google Scholar] [CrossRef]
- Li, M.; Shu, X.; Xu, H.; Zhang, C.; Yang, L.; Zhang, L.; Ji, G. Integrative analysis of metabolome and gut microbiota in diet-induced hyperlipidemic rats treated with berberine compounds. J. Transl. Med. 2016, 14, 237. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, A.; Sieber, J.R.; Schmidt, A.W.; Waldron, C.; Theis, K.R.; Schmidt, T.M. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome 2016, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Snauwaert, E.; Paglialonga, F.; Vande Walle, J.; Wan, M.; Desloovere, A.; Polderman, N.; Renken-Terhaerdt, J.; Shaw, V.; Shroff, R. The benefits of dietary fiber: The gastrointestinal tract and beyond. Pediatr. Nephrol. 2023, 38, 2929–2938. [Google Scholar] [CrossRef] [PubMed]
- Murga-Garrido, S.M.; Hong, Q.; Cross, T.-W.L.; Hutchison, E.R.; Han, J.; Thomas, S.P.; Vivas, E.I.; Denu, J.; Ceschin, D.G.; Tang, Z.-Z.; et al. Gut microbiome variation modulates the effects of dietary fiber on host metabolism. Microbiome 2021, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Chan, D.S.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, Y.M.; McSorley, E.M.; Allsopp, P.J. Effect of soluble dietary fibre on postprandial blood glucose response and its potential as a functional food ingredient. J. Funct. Food. 2018, 46, 423–439. [Google Scholar] [CrossRef]
- Hajipour, A.; Afsharfar, M.; Jonoush, M.; Ahmadzadeh, M.; Gholamalizadeh, M.; Hassanpour Ardekanizadeh, N.; Doaei, S.; Mohammadi-Nasrabadi, F. The effects of dietary fiber on common complications in critically ill patients; with a special focus on viral infections; a systematic reveiw. Immun. Inflamm. Dis. 2022, 10, e613. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Meng, Y.; Li, L.; Wu, Y.; Liu, C.; Dong, W.; Chen, C. Association of Dietary Fiber, Composite Dietary Antioxidant Index and Risk of Death in Tumor Survivors: National Health and Nutrition Examination Survey 2001–2018. Nutrients 2023, 15, 2968. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, D.; Wang, Y.; Liu, L.; Li, J.; Yuan, J.; Jiang, Z.; Jiang, Z.; Hsiao, W.W.; Liu, H.; et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 2022, 71, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Marcobal, A.; Sonnenburg, J.L. Human milk oligosaccharide consumption by intestinal microbiota. Clin. Microbiol. Infect. 2012, 18 (Suppl. S4), 12–15. [Google Scholar] [CrossRef]
- Laursen, M.F.; Pekmez, C.T.; Larsson, M.W.; Lind, M.V.; Yonemitsu, C.; Larnkjær, A.; Mølgaard, C.; Bode, L.; Dragsted, L.O.; Michaelsen, K.F.; et al. Maternal milk microbiota and oligosaccharides contribute to the infant gut microbiota assembly. ISME Commun. 2021, 1, 21. [Google Scholar] [CrossRef] [PubMed]
- Haenen, D.; Zhang, J.; Souza da Silva, C.; Bosch, G.; van der Meer, I.M.; van Arkel, J.; van den Borne, J.J.; Pérez Gutiérrez, O.; Smidt, H.; Kemp, B.; et al. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J. Nutr. 2013, 143, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.; Falck, P.; Linninge, C.; Nilsson, U.; Axling, U.; Grey, C.; Stålbrand, H.; Nordberg Karlsson, E.; Nyman, M.; Holm, C.; et al. Cereal byproducts have prebiotic potential in mice fed a high-fat diet. J. Agric. Food Chem. 2014, 62, 8169–8178. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, A.; van Zanten, G.C.; Jensen, S.L.; Forssten, S.D.; Saarinen, M.; Lahtinen, S.J.; Bandsholm, O.; Svensson, B.; Jespersen, L.; Blennow, A. Comparative fermentation of insoluble carbohydrates in an in vitro human feces model spiked with Lactobacillus acidophilus NCFM. Starch-Stärke 2013, 65, 346–353. [Google Scholar] [CrossRef]
- Lange, K.; Hugenholtz, F.; Jonathan, M.C.; Schols, H.A.; Kleerebezem, M.; Smidt, H.; Müller, M.; Hooiveld, G.J. Comparison of the effects of five dietary fibers on mucosal transcriptional profiles, and luminal microbiota composition and SCFA concentrations in murine colon. Mol. Nutr. Food Res. 2015, 59, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Magistrelli, D.; Zanchi, R.; Malagutti, L.; Galassi, G.; Canzi, E.; Rosi, F. Effects of Cocoa Husk Feeding on the Composition of Swine Intestinal Microbiota. J. Agric. Food Chem. 2016, 64, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Barry, K.A.; Middelbos, I.S.; Vester Boler, B.M.; Dowd, S.E.; Suchodolski, J.S.; Henrissat, B.; Coutinho, P.M.; White, B.A.; Fahey, G.C., Jr.; Swanson, K.S. Effects of dietary fiber on the feline gastrointestinal metagenome. J. Proteome Res. 2012, 11, 5924–5933. [Google Scholar] [CrossRef]
- Peng, X.; Li, S.; Luo, J.; Wu, X.; Liu, L. Effects of dietary fibers and their mixtures on short chain fatty acids and microbiota in mice guts. Food Funct. 2013, 4, 932–938. [Google Scholar] [CrossRef]
- Majid, H.A.; Emery, P.W.; Whelan, K. Faecal microbiota and short-chain fatty acids in patients receiving enteral nutrition with standard or fructo-oligosaccharides and fibre-enriched formulas. J. Hum. Nutr. Diet. 2011, 24, 260–268. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Lamichhane, S.; Yde, C.C.; Forssten, S.; Ouwehand, A.C.; Saarinen, M.; Jensen, H.M.; Gibson, G.R.; Rastall, R.; Fava, F.; Bertram, H.C. Impact of dietary polydextrose fiber on the human gut metabolome. J. Agric. Food Chem. 2014, 62, 9944–9951. [Google Scholar] [CrossRef]
- Yang, J.; Martínez, I.; Walter, J.; Keshavarzian, A.; Rose, D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 2013, 23, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, H.; Kim, H.J.; Yum, M.Y.; Campbell, M.R.; Jane, J.L.; White, P.J.; Hendrich, S. Increased Butyrate Production During Long-Term Fermentation of In Vitro-Digested High Amylose Cornstarch Residues with Human Feces. J. Food Sci. 2015, 80, M1997–M2004. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Nyman, M.; Fåk, F. Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol. Nutr. Food Res. 2015, 59, 2066–2076. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.C.; Reimer, R.A. Long-term intake of a high prebiotic fiber diet but not high protein reduces metabolic risk after a high fat challenge and uniquely alters gut microbiota and hepatic gene expression. Nutr. Res. 2014, 34, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Bhat, Z.F.; Gounder, R.S.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Ding, Y.; Bekhit, A.E.A. Effect of Dietary Protein and Processing on Gut Microbiota-A Systematic Review. Nutrients 2022, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Ntemiri, A.; Ribière, C.; Stanton, C.; Ross, R.P.; O’Connor, E.M.; O’Toole, P.W. Retention of Microbiota Diversity by Lactose-Free Milk in a Mouse Model of Elderly Gut Microbiota. J. Agric. Food Chem. 2019, 67, 2098–2112. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.E.S.; Roquetto, A.R.; de Pace, F.; Moura, C.S.; Santos, A.D.; Yamada, A.T.; Saad, M.J.A.; Amaya-Farfan, J. Dietary whey proteins shield murine cecal microbiota from extensive disarray caused by a high-fat diet. Food Res. Int. 2016, 85, 121–130. [Google Scholar] [CrossRef]
- Ye, S.; Shah, B.R.; Li, J.; Liang, H.; Zhan, F.; Geng, F.; Li, B. A critical review on interplay between dietary fibers and gut microbiota. Trends Food Sci. Technol. 2022, 124, 237–249. [Google Scholar] [CrossRef]
- Foster, M.T.; Gentile, C.L.; Cox-York, K.; Wei, Y.; Wang, D.; Estrada, A.L.; Reese, L.; Miller, T.; Pagliassotti, M.J.; Weir, T.L. Fuzhuan tea consumption imparts hepatoprotective effects and alters intestinal microbiota in high saturated fat diet-fed rats. Mol. Nutr. Food Res. 2016, 60, 1213–1220. [Google Scholar] [CrossRef]
- Chao, J.; Huo, T.I.; Cheng, H.Y.; Tsai, J.C.; Liao, J.W.; Lee, M.S.; Qin, X.M.; Hsieh, M.T.; Pao, L.H.; Peng, W.H. Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice. PLoS ONE 2014, 9, e96969. [Google Scholar] [CrossRef] [PubMed]
- Axling, U.; Olsson, C.; Xu, J.; Fernandez, C.; Larsson, S.; Ström, K.; Ahrné, S.; Holm, C.; Molin, G.; Berger, K. Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr. Metab. 2012, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Cowan, T.E.; Palmnäs, M.S.; Yang, J.; Bomhof, M.R.; Ardell, K.L.; Reimer, R.A.; Vogel, H.J.; Shearer, J. Chronic coffee consumption in the diet-induced obese rat: Impact on gut microbiota and serum metabolomics. J. Nutr. Biochem. 2014, 25, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Taira, T.; Yamaguchi, S.; Takahashi, A.; Okazaki, Y.; Yamaguchi, A.; Sakaguchi, H.; Chiji, H. Dietary polyphenols increase fecal mucin and immunoglobulin A and ameliorate the disturbance in gut microbiota caused by a high fat diet. J. Clin. Biochem. Nutr. 2015, 57, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bai, J.; Zhang, Y.; Xiao, X.; Dong, Y. Effects of bitter melon (Momordica charantia L.) on the gut microbiota in high fat diet and low dose streptozocin-induced rats. Int. J. Food Sci. Nutr. 2016, 67, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Bose, S.; Kim, H.G.; Han, K.S.; Kim, H. Fermented Rhizoma Atractylodis Macrocephalae alleviates high fat diet-induced obesity in association with regulation of intestinal permeability and microbiota in rats. Sci. Rep. 2015, 5, 8391. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.; Hoffman, J.; Martinez, K.; Grace, M.; Lila, M.A.; Cockrell, C.; Nadimpalli, A.; Chang, E.; Chuang, C.-C.; Zhong, W.; et al. A polyphenol-rich fraction obtained from table grapes decreases adiposity, insulin resistance and markers of inflammation and impacts gut microbiota in high-fat-fed mice. J. Nutr. Biochem. 2016, 31, 150–165. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Q. Roles of the Polyphenol-Gut Microbiota Interaction in Alleviating Colitis and Preventing Colitis-Associated Colorectal Cancer. Adv. Nutr. 2021, 12, 546–565. [Google Scholar] [CrossRef]
- Dong, L.; Qin, C.; Li, Y.; Wu, Z.; Liu, L. Oat phenolic compounds regulate metabolic syndrome in high fat diet-fed mice via gut microbiota. Food Biosci. 2022, 50, 101946. [Google Scholar] [CrossRef]
- Song, H.; Han, W.; Yan, F.; Xu, D.; Chu, Q.; Zheng, X. Dietary Phaseolus vulgaris extract alleviated diet-induced obesity, insulin resistance and hepatic steatosis and alters gut microbiota composition in mice. J. Funct. Food. 2016, 20, 236–244. [Google Scholar] [CrossRef]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Tian, D.; Hu, C.Y.; Meng, Y.H. Chlorogenic Acid Ameliorates High-Fat and High-Fructose Diet-Induced Cognitive Impairment via Mediating the Microbiota-Gut-Brain Axis. J. Agric. Food Chem. 2022, 70, 2600–2615. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-K.; Panyod, S.; Ho, C.-T.; Kuo, C.-H.; Wu, M.-S.; Sheen, L.-Y. Dietary allicin reduces transformation of L-carnitine to TMAO through impact on gut microbiota. J. Func. Food. 2015, 15, 408–417. [Google Scholar] [CrossRef]
- Ahrén, I.L.; Xu, J.; Önning, G.; Olsson, C.; Ahrné, S.; Molin, G. Antihypertensive activity of blueberries fermented by Lactobacillus plantarum DSM 15313 and effects on the gut microbiota in healthy rats. Clin. Nutr. 2015, 34, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Topping, D.L. Dietary polysaccharides and polyphenols can promote health by influencing gut microbiota populations. Food Funct. 2016, 7, 1730. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tai, W.C.S.; Hsiao, W.L.W. Dietary saponins from four popular herbal tea exert prebiotic-like effects on gut microbiota in C57BL/6 mice. J. Funct. Food. 2015, 17, 892–902. [Google Scholar] [CrossRef]
- Yang, J.; Bindels, L.B.; Segura Munoz, R.R.; Martínez, I.; Walter, J.; Ramer-Tait, A.E.; Rose, D.J. Disparate Metabolic Responses in Mice Fed a High-Fat Diet Supplemented with Maize-Derived Non-Digestible Feruloylated Oligo- and Polysaccharides Are Linked to Changes in the Gut Microbiota. PLoS ONE 2016, 11, e0146144. [Google Scholar] [CrossRef]
- Mitsuoka, T.; Hidaka, H.; Eida, T. Effect of fructo-oligosaccharides on intestinal microflora. Nahrung 1987, 31, 427–436. [Google Scholar] [CrossRef]
- Tap, J.; Furet, J.-P.; Bensaada, M.; Philippe, C.; Roth, H.; Rabot, S.; Lakhdari, O.; Lombard, V.; Henrissat, B.; Corthier, G.; et al. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ. Microbiol. 2015, 17, 4954–4964. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Salonen, A.; Lahti, L.; Salojärvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014, 8, 2218–2230. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.S.; Comelli, E.M. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef] [PubMed]
- Armougom, F.; Henry, M.; Vialettes, B.; Raccah, D.; Raoult, D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS ONE 2009, 4, e7125. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Fei, N.; Zhao, L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013, 7, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Murphy, E.F.; Cotter, P.D.; Hogan, A.; O’Sullivan, O.; Joyce, A.; Fouhy, F.; Clarke, S.F.; Marques, T.M.; O’Toole, P.W.; Stanton, C.; et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut 2013, 62, 220–226. [Google Scholar] [CrossRef]
- Greenblum, S.; Turnbaugh, P.J.; Borenstein, E. Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2012, 109, 594–599. [Google Scholar] [CrossRef]
- Verdam, F.J.; Fuentes, S.; de Jonge, C.; Zoetendal, E.G.; Erbil, R.; Greve, J.W.; Buurman, W.A.; de Vos, W.M.; Rensen, S.S. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity 2013, 21, E607–E615. [Google Scholar] [CrossRef]
- Wang, T.; Brown, N.M.; McCoy, A.N.; Sandler, R.S.; Keku, T.O. Omega-3 Polyunsaturated Fatty Acids, Gut Microbiota, Microbial Metabolites, and Risk of Colorectal Adenomas. Cancers 2022, 14, 4443. [Google Scholar] [CrossRef]
- Chen, H.-M.; Yu, Y.-N.; Wang, J.-L.; Lin, Y.-W.; Kong, X.; Yang, C.-Q.; Yang, L.; Liu, Z.-J.; Yuan, Y.-Z.; Liu, F.; et al. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma1234. Am. J. Clin. Nutr. 2013, 97, 1044–1052. [Google Scholar] [CrossRef]
- Ou, J.; Carbonero, F.; Zoetendal, E.G.; DeLany, J.P.; Wang, M.; Newton, K.; Gaskins, H.R.; O’Keefe, S.J.D. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans1234. Am. J. Clin. Nutr. 2013, 98, 111–120. [Google Scholar] [CrossRef] [PubMed]
| Lactobacillus Species | Impact on Health/Disease |
|---|---|
| L. acidophilus | Vaginal health |
| L. amylovorus | Lowers cholesterol and improves bowel function |
| L. casei | Enhances immune response and improves digestive problems |
| L. crispatus | Prevention of urinary tract infections |
| L. delbrueckii subsp. bulgaricus | lactose digestion |
| L. gallinarum | Chicken Gut Health |
| L. gasseri | Weight loss and improved metabolism |
| L. johnsonii | Inhibits intestinal pathogenic bacteria |
| L. paracasei | Reducing inflammation |
| L. plantarum | Helps digestion |
| L. reuteri | Improve dental health |
| L. rhamnosus | Prevention and treatment of diarrhea |
| Bifidobacterium species | |
| B. adolescentis | Intestinal health |
| B. animalis | Fighting infections of the digestive system |
| B. bifidum | Improve allergy symptoms |
| B. breve | Good for the intestinal health of infants and young children |
| B. infantis | Relief of Irritable Bowel Syndrome |
| B. lactis | improve digestion |
| B. longum | Lower cholesterol, fight inflammation and stress |
| Other lactic acid bacteria | |
| Enterococcus faecalis | May become pathogenic |
| Enterococcus faecium | cause infection |
| Lactococcus lactis | Fermentation of dairy products |
| Leuconostoc mesenteroides | Food fermentation |
| Pediococcus acidilactici | Maintaining intestinal balance |
| Sporolactobacillus inulinus | Involved in intestinal fermentation |
| Streptococcus thermophilus | Making yoghurt and cheese |
| Nonlactic acid bacteria | |
| Bacillus cereus var. toyoi | Feed for animals |
| Escherichia coli strain nissle | Probiotics for certain conditions |
| Propionibacterium freudenreichii | Cheese production |
| Saccharomyces cerevisiae | Brewer’s yeast |
| Saccharomyces boulardii | Treatment and prevention of various gastrointestinal diseases |
| Subject | HF Diet Composition | Increased | Decreased | References |
|---|---|---|---|---|
| C57/BL6 mice | 58% fat, 25.6% carbohydrates, 16.4% protein | Verrucomicrobias, Proteobacteria, Akkermansia, Parabacteroides, Lactobacillus genus genera | Bacteroidetes, Erysipelotrichaceae_Incertae_Sedis | [38] |
| SIHUMI mice | Lard and corn oil | Anaerostipes caccae, L. plantarum, C. butyricum, B. producta | [39] | |
| Wistar rats | 45% HF | Lactobacillus spp. | [40] | |
| Male apoe−/− mice | heat-treated high-fat | Firmicutes, Clostridiales, Allobaculum, Allobaculum | Bacteroidetes, Rikenellaceae | [41] |
| Wistar rats | (10% lard, 20% sucrose, 2% cholesterol, 1% bile salt and 67% standard chow) | Akkermansia | Bacteroides, Prevotella, Escherichia, Sutterella, Parabacteroides, Clostridium, Blautia | [42] |
| Wistar rats | 45% of energy as fat (31.4% saturated fats, | [43] |
| Fiber Source | Subjects | Up-Graded Microbiota | Down-Graded Microbiota | Increased SCFAs | In Vivo or In Vitro | References |
|---|---|---|---|---|---|---|
| RS type 3 vs. Pregelatinized potato starch (DS) | Pig | Actinobacteria, Weissella-like group, Clostridium cluster IV, IX, XV, XVI, and XVII, Mollicutes, Fusobacteria | Bacilli (i.e., Allofustis, Lactobacillus acidophilus–like group, Lactobacillus plantarum–like group), Clostridium cluster XI and XIVa, Deltaproteobacteria, Gammaproteobacteria | Total SCFAs, Acetate, propionate, butyrate, valerate | In vivo | [54] |
| Oat fiber | Mouse | Bifidobacterium, Lactobacillus | Total SCFAs, Propionic, iso-butyric, butyric, iso-valeric, valeric | In vivo | [55] | |
| Barley husks | ||||||
| Rye bran | Bifidobacterium | Butyric, iso-valeric | ||||
| Guar | Bifidobacterium, Akkermansia muciniphila-like bacteria | Total SCFAs, Propionic, iso-butyric, butyric, iso-valeric, valeric | ||||
| Waxy maize | Human fecal microbiota | Bacteroidetes/Firmicutes ratio | Lactic acid | In vitro | [56] | |
| Potato fiber | Bacteroidetes/Firmicutes ratio | Lactic acid | ||||
| Potato lintner | Bacteroidetes/Firmicutes ratio | Lactic acid | ||||
| inulin | C57BL/6J mice | Total SCFA, Acetate, propionate | In vivo | [57] | ||
| oligofructose | Acetate, propionate | |||||
| arabinoxylan | Total SCFA, Acetate, propionate | |||||
| guar gum | Total SCFA, Acetate, propionate | |||||
| RS | propionate | |||||
| Cocoa Husk | Pig | Bacteroides-Prevotella, Faecalibacterium prausnitzii, Bacteroidetes, Bacteroidetes/Firmicutes ratio | Firmicutes, Lactobacillus-Enterococcus, Clostridium histolyticum | In vivo | [58] | |
| Pectin vs. cellulose | Cat | Firmicutes, Chlorobi, Elusimicrobia, Proteobacteria | Archaea | In vivo | [59] | |
| FOS VS. cellulose | Actinobacteria | |||||
| Pectin | Mouse | Not measured | Not measured | Acetate, propionate | In vivo | [60] |
| Resistant starch (RS) | Total SCFAs | |||||
| Fructo-oligosaccharide | ||||||
| Cellulose | Lactic acid | |||||
| Fructo-oligosaccharides (FOS)—six different dietary, sources of nondigestible carbohydrate (soy polysaccharides, resistant starch, arabic gum, cellulose, inulin and oligofructose) | Human | Bifidobacteria (n.s.d) | Butyrate | In vivo | [61] | |
| Pectin | Mouse | Bacteroidetes | Firmicutes/Bacteroidetes | Acetate, propionate | In vivo | [62] |
| Polydextrose Fiber | Human | No changes | In vivo | [63] | ||
| Pectin | Actinobacteria, Bifidobacterium, Proteobacteria | Bacteroides, Firmicutes | Total SCFAs, Acetate | In vitro | [64] | |
| Guar gum | Bacteroidetes, Proteobacteria | Bacteroides, Firmicutes | Total SCFAs, Acetate, Propionate | |||
| Inulin | Actinobacteria, Proteobacteria | Bacteroidetes, Bacteroides, Firmicutes | Total SCFAs, Acetate | |||
| Arabinoxylan | Bacteroidetes, Proteobacteria Bacteroides | Firmicutes | Total SCFAs, Acetate, Propionate | |||
| β-Glucan | Bacteroides, Proteobacteria | Firmicutes | Total SCFAs, Acetate, Propionate | |||
| Resistant starch | Bifidobacterium, adolescentis, Proteobacteria | Bacteroides, Firmicutes | Total SCFAs, Acetate, Propionate | |||
| Resistant starch | Human | Ruminococcus bromii, Bifiodbacterium adolescentis | Butyrate | In vivo | [60] | |
| Arabinoxylan | Prevotella | Total SCFAs | ||||
| Inulin | Faecalibacterium prausnitzii | Not measured | ||||
| Amylose Cornstarch | Human | Butyrate, propionate | In vitro | [65] | ||
| Whole-grain barley | Rat | Verrucomicrobia, Deferribacteres, Bacteroides, Blautia, Clostridium (Lachnospiraceae), Ruminococcus (Lachnospiraceae), Akkermansia, Adlercreutzia | Firmicutes Actinobacteria, Firmicutes/Bacteroidetes, Parabacteroides, Clostridiaceae, Dehalobacterium, Oscillopira, Ruminococcus, Mucispirillum | Acetic, propionic acid | In vivo | [66] |
| Barley malt | Bacteroides, Roseburia, Coprococcus, Lactobacillus, Blautia, Alphaproteobacteria | Parabacteroides, Akkermansia | Butyric acid | |||
| Inulin (Orafti HP) and oligofructose | Rat | Bacteroidetes | Firmicutes, Firmicutes/Bacteroidetes | In vivo | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Qin, C.; Wen, M.; Zhang, L.; Wang, W. The Effects of Food Nutrients and Bioactive Compounds on the Gut Microbiota: A Comprehensive Review. Foods 2024, 13, 1345. https://doi.org/10.3390/foods13091345
Zheng Y, Qin C, Wen M, Zhang L, Wang W. The Effects of Food Nutrients and Bioactive Compounds on the Gut Microbiota: A Comprehensive Review. Foods. 2024; 13(9):1345. https://doi.org/10.3390/foods13091345
Chicago/Turabian StyleZheng, Yijun, Chunyin Qin, Mingchun Wen, Liang Zhang, and Weinan Wang. 2024. "The Effects of Food Nutrients and Bioactive Compounds on the Gut Microbiota: A Comprehensive Review" Foods 13, no. 9: 1345. https://doi.org/10.3390/foods13091345
APA StyleZheng, Y., Qin, C., Wen, M., Zhang, L., & Wang, W. (2024). The Effects of Food Nutrients and Bioactive Compounds on the Gut Microbiota: A Comprehensive Review. Foods, 13(9), 1345. https://doi.org/10.3390/foods13091345







