The Characterization of the Key Aroma Compounds in Non-Smoked Bacon by Instrumental and Sensory Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. HS–SPME–GC–MS–O Analysis
2.2.1. HS–SPME–GC–MS Analysis
2.2.2. Sensory Analysis by GC–O
2.2.3. Identification and Quantitation Analysis of Volatile Compounds
2.3. GC–IMS Analysis
2.4. GC × GC–TOFMS Analysis
2.5. Recombination and Omission Experiments
2.6. Statistical Analysis
3. Results and Discussion
3.1. Volatile Compounds in Non-Smoked Bacon Analyzed via GC–IMS and GC × GC–TOFMS
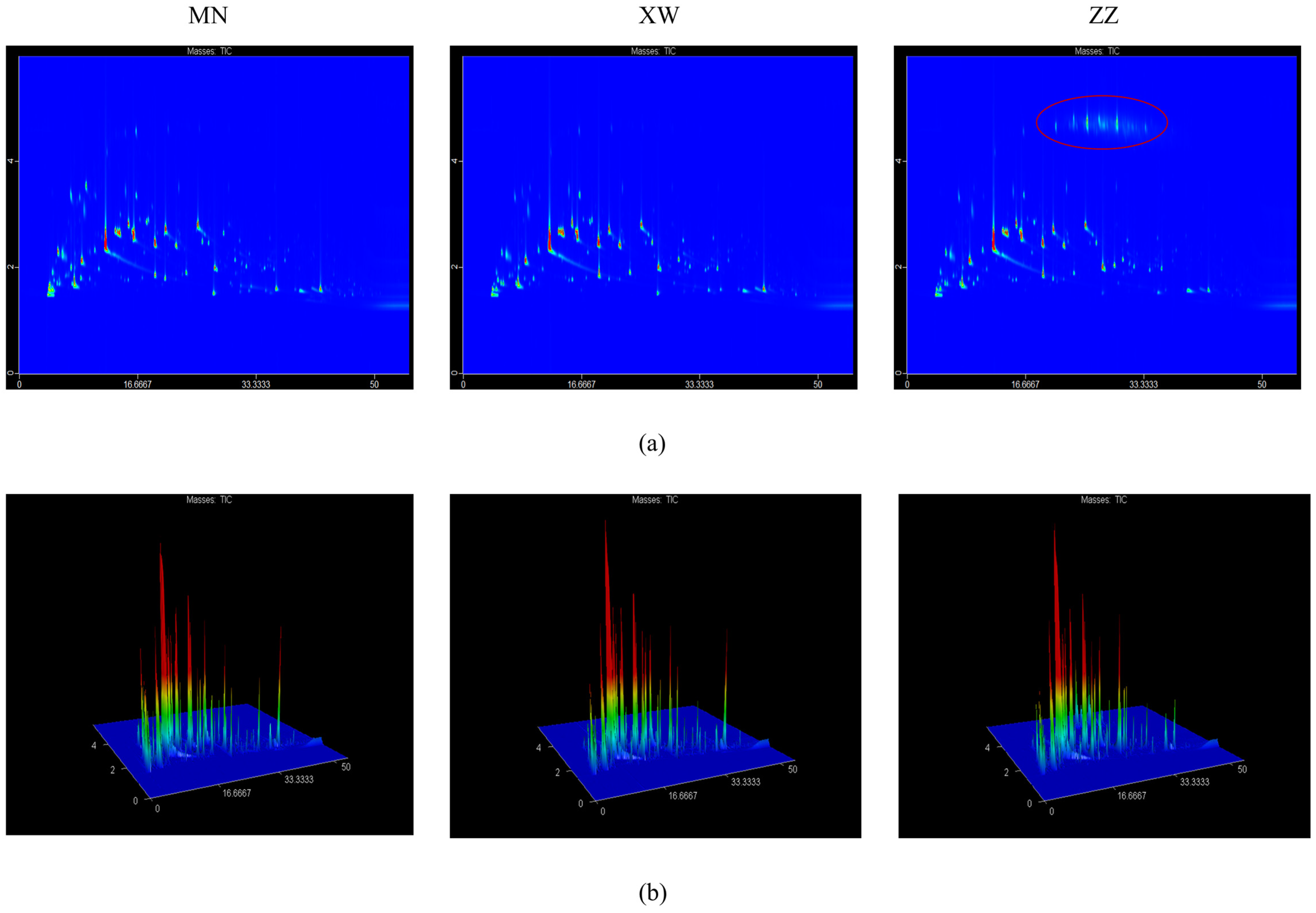
3.1.1. GC–IMS Topographic Plots in Different Non-Smoked Bacon
3.1.2. Volatile Compounds in Non-Smoked Bacon Identified via GC × GC–TOFMS
3.2. Key Aroma Compounds in Non-Smoked Bacon Identified via GC–O–MS
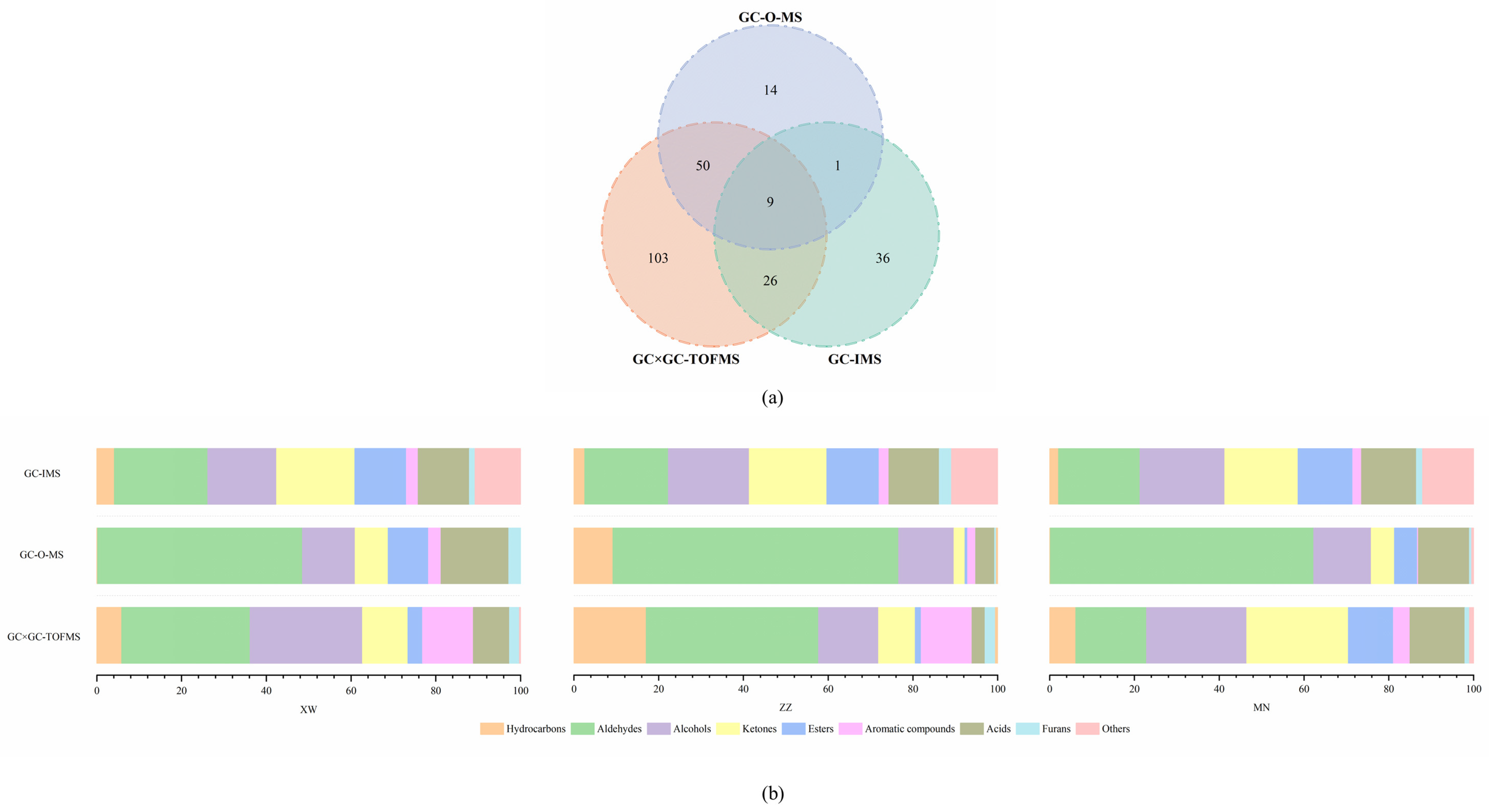
3.3. Comparison of Volatile Compounds in Non-Smoked Bacon via GC–O–MS, GC × GC–TOFMS, and GC–IMS
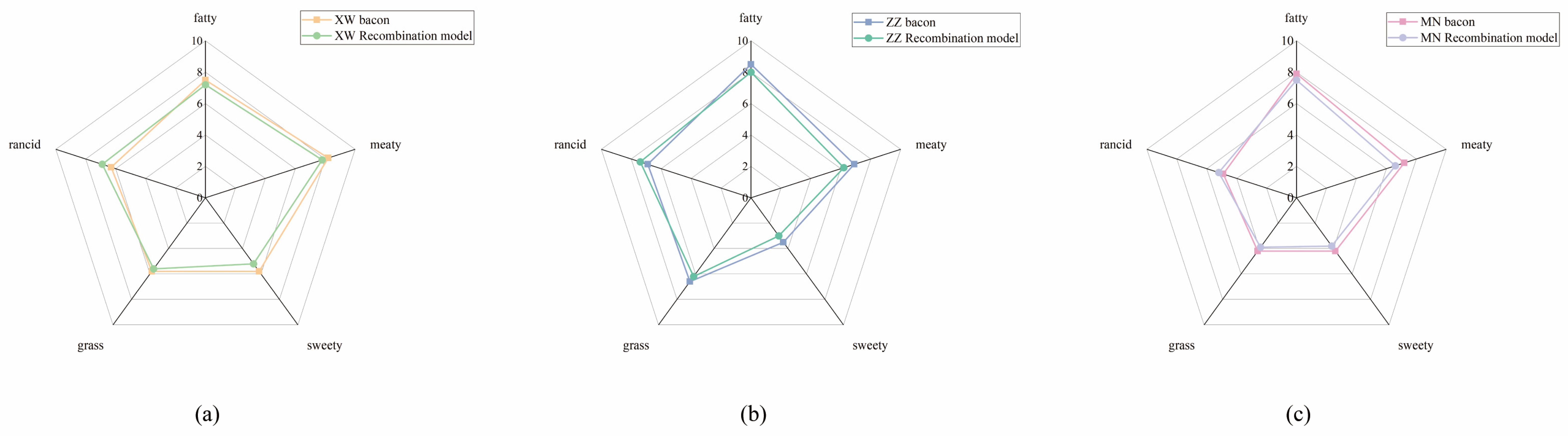
3.4. Aroma Recombination and Omission Studies of the Non-Smoked Bacon
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Zhang, W.; Zhou, L.; Zhang, R. Study on the influences of ultrasound on the flavor profile of unsmoked bacon and its underlying metabolic mechanism by using HS-GC-IMS. Ultrason. Sonochem. 2021, 80, 105807. [Google Scholar] [CrossRef]
- Zhang, M.; Qiao, H.; Zhang, W.; Zhang, Z.; Wen, P.; Zhu, Y. Tissue type: A crucial factor influencing the fungal diversity and communities in sichuan pork bacon. Front. Microbiol. 2021, 12, 655500. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Y.P.; Blank, I.; Li, F.; Li, C.; Liu, Y. GC× GC-ToF-MS and GC-IMS based volatile profile characterization of the Chinese dry-cured hams from different regions. Food Res. Int. 2021, 142, 110222. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Suo, H.; Zhao, X.; Kan, J. Aroma and flavor characteristics of commercial Chinese traditional bacon from different geographical regions. J. Sens. Stud. 2019, 34, e12475. [Google Scholar] [CrossRef]
- Pu, D.; Zhang, Y.; Zhang, H.; Sun, B.; Ren, F.; Chen, H.; Tang, Y. Characterization of the key aroma compounds in traditional hunan smoke-cured pork leg (Larou, THSL) by aroma extract dilution analysis (AEDA), odor activity value (OAV), and sensory evaluation experiments. Foods 2020, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Kosowska, M.; Majcher, M.A.; Jelen, H.H.; Fortuna, T. Key aroma compounds in smoked cooked loin. J. Agric. Food Chem. 2018, 66, 3683–3690. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Wang, Y.; Pan, D.; Sun, Y.; Cao, J. Study on the volatile compounds generated from lipid oxidation of Chinese bacon (unsmoked) during processing. Eur. J. Lipid Sci. Technol. 2017, 119, 1600512. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Cui, D.; Fang, X.; Gao, J.; Liu, Y. Fast and non-destructive profiling of commercial coffee aroma under three conditions (Beans, Powder, and Brews) Using GC-IMS. Molecules 2022, 27, 6262. [Google Scholar] [CrossRef]
- Feng, T.; Sun, J.; Song, S.; Wang, H.; Yao, L.; Sun, M.; Wang, K.; Chen, D. Geographical differentiation of Molixiang table grapes grown in China based on volatile compounds analysis by HS-GC-IMS coupled with PCA and sensory evaluation of the grapes. Food Chem. X 2022, 15, 100423. [Google Scholar] [CrossRef]
- Li, C.; Al-Dalali, S.; Wang, Z.; Xu, B.; Zhou, H. Investigation of volatile flavor compounds and characterization of aroma-active compounds of water-boiled salted duck using GC-MS-O, GC-IMS, and E-nose. Food Chem. 2022, 386, 132728. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Niu, Y.; Xiao, Z. Characterization of the key aroma compounds in Laoshan green teas by application of odour activity value (OAV), gas chromatography-mass spectrometry-olfactometry (GC-MS-O) and comprehensive two-dimensional gas chromatography mass spectrometry (GC× GC-qMS). Food Chem. 2021, 339, 128136. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qian, M.C.; Deng, Y.; Yuan, H.; Jiang, Y. Insight into aroma dynamic changes during the whole manufacturing process of chestnut-like aroma green tea by combining GC-E-Nose, GC-IMS, and GC× GC-TOFMS. Food Chem. 2022, 387, 132813. [Google Scholar] [CrossRef]
- Li, X.; Zeng, X.; Song, H.; Xi, Y.; Li, Y.; Hui, B.; Li, H.; Li, J. Characterization of the aroma profiles of cold and hot break tomato pastes by GC-O-MS, GC× GC-O-TOF-MS, and GC-IMS. Food Chem. 2023, 405, 134823. [Google Scholar] [CrossRef]
- Shen, D.-Y.; Song, H.-L.; Zou, T.-T.; Wan, S.-Y.; Li, M.-K. Characterization of odor-active compounds in moso bamboo (Phyllostachys pubescens Mazel) leaf via gas chromatography-ion mobility spectrometry, one- and two-dimensional gas chromatography-olfactory-mass spectrometry, and electronic nose. Food Res. Int. 2022, 152, 110916. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y.; Wang, B.; Song, H.; Zou, T. Screening of the volatile compounds in fresh and thermally treated watermelon juice via headspace-gas chromatography-ion mobility spectrometry and comprehensive two-dimensional gas chromatography-olfactory-mass spectrometry analysis. LWT-Food Sci. Technol. 2021, 137, 110478. [Google Scholar] [CrossRef]
- Xi, L.; Zhang, J.; Wu, R.; Wang, T.; Ding, W. Characterization of the volatile compounds of zhenba bacon at different process stages using GC-MS and GC-IMS. Foods 2021, 10, 2869. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cai, Y.; Sun-Waterhouse, D.; Cui, C.; Su, G.; Lin, L.; Zhao, M. Approaches of aroma extraction dilution analysis (AEDA) for headspace solid phase microextraction and gas chromatography-olfactometry (HS-SPME-GC-O): Altering sample amount, diluting the sample or adjusting split ratio? Food Chem. 2015, 187, 44–52. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Zhang, D.; Shen, Q.; Pan, T.; Hui, T.; Ma, J. Characterization of key aroma compounds in beijing roasted duck by gas chromatography-olfactometry-mass spectrometry, odor-activity values, and aroma-recombination experiments. J. Agric. Food Chem. 2019, 67, 5847–5856. [Google Scholar] [CrossRef]
- Chang, C.; Wu, G.; Zhang, H.; Jin, Q.; Wang, X. Deep-fried flavor: Characteristics, formation mechanisms, and influencing factors. Crit. Rev. Food Sci. Nutr. 2020, 60, 1496–1514. [Google Scholar] [CrossRef]
- Wu, N.; Wang, X.-C. Identification of important odorants derived from phosphatidylethanolamine species in steamed male Eriocheir sinensis hepatopancreas in model systems. Food Chem. 2019, 286, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Purrinos, L.; Perez-Santaescolastica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Bastianello Campagnol, P.C.; Lorenzo, J.M. Characterization of volatile compounds of dry-cured meat products using HS-SPME-GC/MS technique. Food Anal. Methods 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- Montanari, C.; Gatto, V.; Torriani, S.; Barbieri, F.; Bargossi, E.; Lanciotti, R.; Grazia, L.; Magnani, R.; Tabanelli, G.; Gardini, F. Effects of the diameter on physico-chemical, microbiological and volatile profile in dry fermented sausages produced with two different starter cultures. Food Biosci. 2018, 22, 9–18. [Google Scholar] [CrossRef]
- Benet, I.; Dolors Guardia, M.; Ibanez, C.; Sola, J.; Arnau, J.; Roura, E. Analysis of SPME or SBSE extracted volatile compounds from cooked cured pork ham differing in intramuscular fat profiles. LWT-Food Sci. Technol. 2015, 60, 393–399. [Google Scholar] [CrossRef]
- Bosse, R.; Wirth, M.; Konstanz, A.; Becker, T.; Weiss, J.; Gibis, M. Determination of volatile marker compounds in raw ham using headspace-trap gas chromatography. Food Chem. 2017, 219, 249–259. [Google Scholar] [CrossRef]
- Chen, X.; Luo, J.; Lou, A.; Wang, Y.; Yang, D.; Shen, Q.W. Duck breast muscle proteins, free fatty acids and volatile compounds as affected by curing methods. Food Chem. 2021, 338, 128138. [Google Scholar] [CrossRef]
- Ramirez, R.; Cava, R. Volatile profiles of dry-cured meat products from three different Iberian x Duroc genotypes. J. Agric. Food Chem. 2007, 55, 1923–1931. [Google Scholar] [CrossRef]
- Petricevic, S.; Radovcic, N.M.; Lukic, K.; Listes, E.; Medic, H. Differentiation of dry-cured hams from different processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018, 137, 217–227. [Google Scholar] [CrossRef]
- Jesus Andrade, M.; Jose Cordoba, J.; Ma Casado, E.; Cordoba, M.G.; Rodriguez, M. Effect of selected strains of Debaryomyces hansenii on the volatile compound production of dry fermented sausage “salchichon”. Meat Sci. 2010, 85, 256–264. [Google Scholar] [CrossRef]
- Perez-Santaescolastica, C.; Carballo, J.; Fulladosa, E.; Garcia-Perez, J.V.; Benedito, J.; Lorenzo, J.M. Effect of proteolysis index level on instrumental adhesiveness, free amino acids content and volatile compounds profile of dry-cured ham. Food Res. Int. 2018, 107, 559–566. [Google Scholar] [CrossRef]
- Kun, J.; Meng, Q.; Wei, C.-C.; Xie, G.; Yan, J.; Ho, C.-T.; Tong, H. Characterization of the key compounds responsible for the fermented soybean-like cup aroma of raw Pu-erh tea using instrumental and sensory methods. LWT-Food Sci. Technol. 2022, 162, 113458. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Carballo, J. Changes in physico-chemical properties and volatile compounds throughout the manufacturing process of dry-cured foal loin. Meat Sci. 2015, 99, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, H.; Wang, C.; Yang, X. Volatile component analysis in infant formula using SPME coupled with GCxGC-TOFMS. Anal. Methods 2019, 11, 5017–5022. [Google Scholar] [CrossRef]
- Shen, C.; Cai, Y.; Wu, X.; Gai, S.; Wang, B.; Liu, D. Characterization of selected commercially available grilled lamb shashliks based on flavor profiles using GC-MS, GC× GC-TOF-MS, GC-IMS, E-nose and E-tongue combined with chemometrics. Food Chem. 2023, 423, 136257. [Google Scholar] [CrossRef] [PubMed]
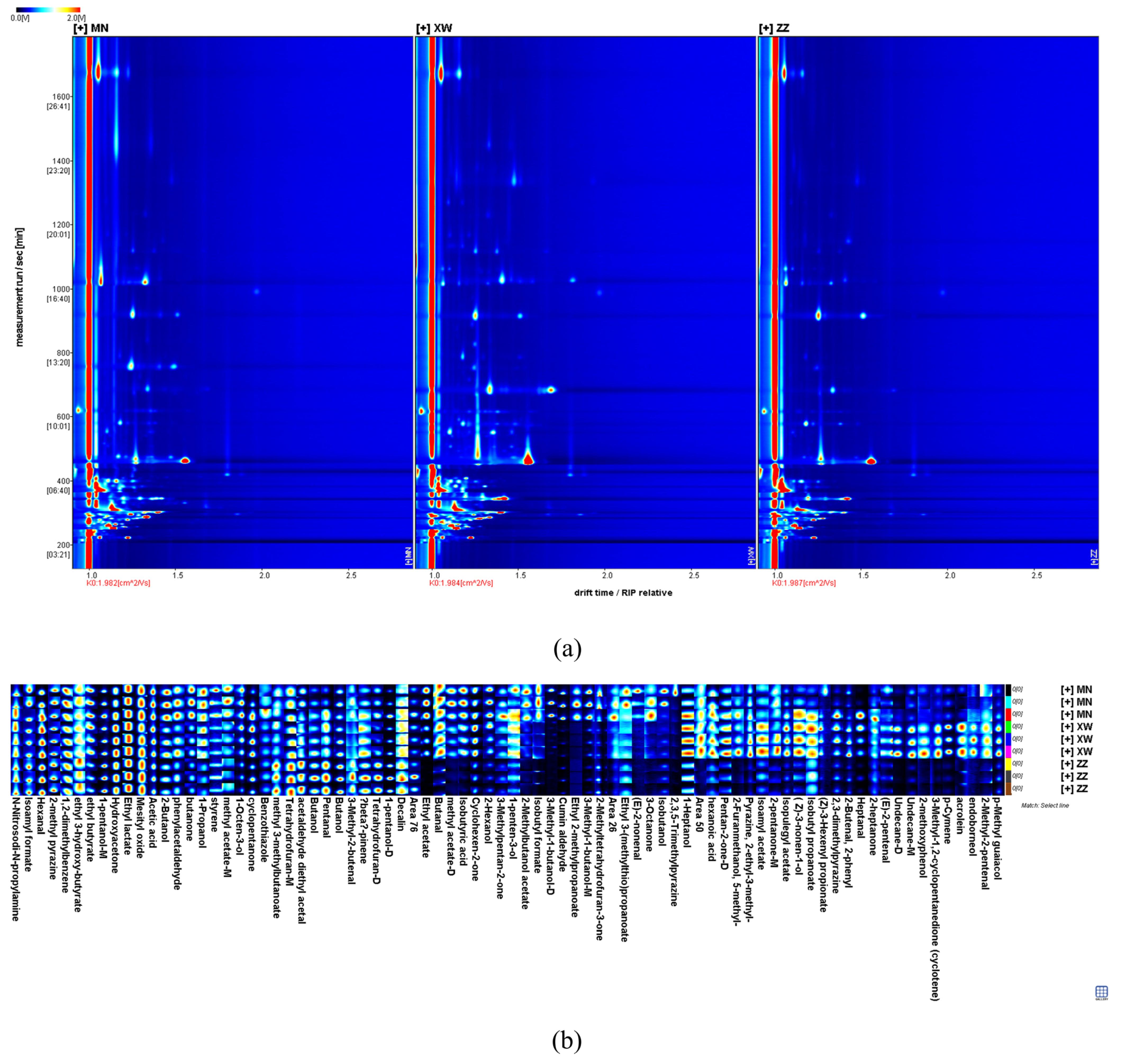
| No. | Compounds | CAS | Formula | MW | RI | Rt | Dt | Comment | Intensity | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MN | XW | ZZ | |||||||||
| Aldehydes | |||||||||||
| 1 | 2-Phenyl-2-butenal | 4411-89-6 | C10H10O | 146.2 | 1274 | 1120.272 | 1.25211 | null | 1032.07 ± 212.84 a | 2227.82 ± 247.86 b | 918.65 ± 165.77 a |
| 2 | Cumin aldehyde | 122-03-2 | C10H12O | 148.2 | 1248.5 | 1033.13 | 1.32881 | null | 7490.28 ± 528.96 b | 1996.99 ± 117.78 a | 1874.56 ± 280.68 a |
| 3 | (E)-2-nonenal | 18829-56-6 | C9H16O | 140.2 | 1178.1 | 758.443 | 1.40708 | null | 1203.76 ± 300.53 b | 512.86 ± 46.16 a | 403.28 ± 25.47 a |
| 4 | (E)-2-pentenal | 1576-87-0 | C5H8O | 84.1 | 1135.5 | 619.551 | 1.35549 | null | 567.27 ± 163.38 b | 879.12 ± 51.65c | 314.82 ± 38.23 a |
| 5 | Hexanal | 66-25-1 | C6H12O | 100.2 | 1073.2 | 463.893 | 1.56003 | null | 14,982.01 ± 7895.8 a | 19,343.69 ± 2755.72 a | 14,002.65 ± 1228.08 a |
| 6 | Phenylacetaldehyde | 122-78-1 | C8H8O | 120.2 | 1026.6 | 399.105 | 1.25636 | null | 1268.42 ± 176.14 b | 1030.65 ± 252.25 ab | 678.21 ± 100.88 a |
| 7 | Pentanal | 110-62-3 | C5H10O | 86.1 | 986.5 | 346.932 | 1.42372 | null | 2556.28 ± 2158.24 a | 4105.63 ± 717.16 a | 4489.65 ± 612.7 a |
| 8 | Acrolein | 107-02-8 | C3H4O | 56.1 | 841.5 | 272.365 | 1.05565 | null | 340.91 ± 101.35 a | 1521.76 ± 220.57 b | 338.19 ± 76.28 a |
| 9 | 3-Methyl-2-butenal | 107-86-8 | C5H8O | 84.1 | 770.2 | 243.259 | 1.08451 | null | 372.47 ± 60.14 ab | 295.76 ± 30.88 a | 426.43 ± 50.75 b |
| 10 | Butanal | 123-72-8 | C4H8O | 72.1 | 882.5 | 289.099 | 1.10631 | null | 792.55 ± 79.7 b | 689.4 ± 42.25 b | 161.43 ± 1.86 a |
| 11 | Heptanal | 111-71-7 | C7H14O | 114.2 | 1159.5 | 685.1 | 1.69677 | null | 2540.25 ± 3625.8 a | 3259.79 ± 1265.18 a | 641.64 ± 299.15 a |
| 12 | 2-Methyl-2-pentenal | 623-36-9 | C6H10O | 98.1 | 1124.3 | 591.599 | 1.16224 | null | 217.01 ± 77.48 b | 342.8 ± 12.04c | 101.6 ± 23.42 a |
| Alcohols | |||||||||||
| 13 | 1-Octen-3-ol | 3391-86-4 | C8H16O | 128.2 | 1436.4 | 1675.329 | 1.15976 | null | 5806.28 ± 402.67 a | 4127.67 ± 875.31 b | 2303.56 ± 278.74c |
| 14 | 1-Pentanol | 71-41-0 | C5H12O | 88.1 | 1219.3 | 921.36 | 1.25681 | M | 6676.16 ± 2342.53 a | 6574.05 ± 287.53 a | 7866.56 ± 717.7 a |
| 15 | 1-Pentanol | 71-41-0 | C5H12O | 88.1 | 1218.3 | 917.572 | 1.50882 | D | 2368.67 ± 1394.37 a | 2171.94 ± 167.02 a | 3668.55 ± 777.23 a |
| 16 | 3-Methyl-1-butanol | 123-51-3 | C5H12O | 88.1 | 1177.1 | 754.654 | 1.4916 | D | 2943.07 ± 318.44 b | 921.92 ± 60.54 a | 722.77 ± 80.81 a |
| 17 | 3-Methyl-1-butanol | 123-51-3 | C5H12O | 88.1 | 1178.1 | 758.443 | 1.24116 | M | 6600.43 ± 595.61c | 2273.48 ± 387.36 b | 1421.81 ± 199.14 a |
| 18 | Butanol | 71-36-3 | C4H10O | 74.1 | 1120.1 | 581.135 | 1.18272 | M | 1700.83 ± 135.33 a | 1904.22 ± 193.6 ab | 2189.02 ± 161.72 b |
| 19 | Butanol | 71-36-3 | C4H10O | 74.1 | 1120.9 | 582.965 | 1.37911 | D | 338.45 ± 107.93 a | 458.52 ± 71.9 ab | 560.22 ± 120.27 b |
| 20 | 1-Penten-3-ol | 616-25-1 | C5H10O | 86.1 | 1120.3 | 581.469 | 1.35116 | null | 401.68 ± 138.9 a | 439.1 ± 29.41 a | 290.78 ± 26.13 a |
| 21 | Isobutanol | 78-83-1 | C4H10O | 74.1 | 1078.7 | 477.547 | 1.16906 | null | 856.95 ± 523.29 a | 339.51 ± 76.92 a | 482.18 ± 19.13 a |
| 22 | 1-Propanol | 71-23-8 | C3H8O | 60.1 | 1029.1 | 402.379 | 1.1083 | null | 1826.39 ± 76.15c | 1659.05 ± 56.09 b | 869.12 ± 61.18 a |
| 23 | 2-Butanol | 78-92-2 | C4H10O | 74.1 | 1028.8 | 401.97 | 1.16018 | null | 2713.11 ± 51.24 b | 2139.17 ± 391.12 a | 1657.38 ± 172.27 a |
| 24 | 1-Heptanol | 111-70-6 | C7H16O | 116.2 | 985.2 | 345.35 | 1.3895 | null | 1335.95 ± 670.17 ab | 1987.96 ± 73.36 b | 1038.47 ± 149.55 a |
| 25 | 2-Hexanol | 626-93-7 | C6H14O | 102.2 | 803.9 | 257.034 | 1.28093 | null | 160.09 ± 4.09 b | 131.02 ± 27.25 b | 64.22 ± 4.68 a |
| 26 | 5-Methyl-2-furanmethanol | 3857-25-8 | C6H8O2 | 112.1 | 952 | 325.69 | 1.26008 | null | 286.23 ± 84.12 b | 299.82 ± 57.26 b | 72.9 ± 23.44 a |
| 27 | Endoborneol | 507-70-0 | C10H18O | 154.3 | 1167.5 | 716.542 | 1.21743 | null | 200.18 ± 55.23 a | 388.43 ± 47.52 b | 123.06 ± 9.38 a |
| 28 | (Z)-3-nonen-1-ol | 10340-23-5 | C9H18O | 142.2 | 1159.5 | 685.078 | 1.43546 | null | 517.18 ± 303.14 ab | 808.96 ± 28.35 b | 242.53 ± 44.49 a |
| Ketones | |||||||||||
| 29 | Cyclopentanone | 120-92-3 | C5H8O | 84.1 | 1161.2 | 691.808 | 1.34058 | null | 4223.43 ± 2787.21 a | 5886.26 ± 893.38 a | 3146.04 ± 705.27 a |
| 30 | 2-Heptanone | 110-43-0 | C7H14O | 114.2 | 1158.7 | 681.747 | 1.26103 | null | 1542.72 ± 792.29 a | 1826.45 ± 93.7 a | 1044.75 ± 157.83 a |
| 31 | 3-Methylpentan-2-one | 565-61-7 | C6H12O | 100.2 | 1077.5 | 474.513 | 1.47702 | null | 999.33 ± 190.33c | 716.47 ± 21.76 b | 467.12 ± 72.18 a |
| 32 | 3-Methyl-1,2-cyclopentanedione (cyclotene) | 80-71-7 | C6H8O2 | 112.1 | 1015.9 | 385.194 | 1.13993 | null | 291.49 ± 85.31 a | 1019.99 ± 99.44 b | 227.74 ± 26.71 a |
| 33 | 2-Pentanone | 107-87-9 | C5H10O | 86.1 | 989.7 | 351.179 | 1.12415 | M | 830.23 ± 46.12 b | 839.11 ± 326.82 b | 293.69 ± 8.87 a |
| 34 | 2-Methyltetrahydrofuran-3-one | 3188-00-9 | C5H8O2 | 100.1 | 1257.9 | 1065.334 | 1.07367 | null | 5105.7 ± 1042.13 b | 2613.35 ± 225.66 a | 3372.73 ± 158.06 a |
| 35 | Cyclohexen-2-one | 930-68-7 | C6H8O | 96.1 | 911.2 | 302.805 | 1.40278 | null | 3416.98 ± 352.54 b | 4084.01 ± 368.03c | 1841.85 ± 95.67 a |
| 36 | 3-Octanone | 106-68-3 | C8H16O | 128.2 | 964.2 | 332.578 | 1.31219 | null | 268.08 ± 67.69 b | 98.71 ± 19.71 a | 48.42 ± 11.3 a |
| 37 | Pentan-2-one | 107-87-9 | C5H10O | 86.1 | 984.7 | 344.697 | 1.36494 | D | 1467.15 ± 522.78 b | 2181.73 ± 75.49c | 799.35 ± 124.64 a |
| 38 | Butanone | 78-93-3 | C4H8O | 72.1 | 899.3 | 296.139 | 1.24164 | null | 2566.49 ± 38.88c | 1709.41 ± 136.73 b | 1488.81 ± 107.48 a |
| 39 | Hydroxyacetone | 116-09-6 | C3H6O2 | 74.1 | 725 | 224.818 | 1.22641 | null | 2598.7 ± 467.46 a | 3507.62 ± 29.96 b | 3153.34 ± 305.13 ab |
| 40 | Mesityl oxide | 141-79-7 | C6H10O | 98.1 | 813.9 | 261.078 | 1.11623 | null | 6729.15 ± 146.9 b | 5840.38 ± 233.57 a | 6691.56 ± 372.75 b |
| Esters | |||||||||||
| 41 | (Z)-3-Hexenyl propionate | 33467-74-2 | C9H16O2 | 156.2 | 1372.7 | 1457.474 | 1.35699 | null | 1629.29 ± 807.64 a | 1816.99 ± 240.06 a | 978.89 ± 39.23 a |
| 42 | Ethyl 3-(methylthio)propanoate | 13327-56-5 | C6H12O2S | 148.2 | 1078.1 | 476.03 | 1.21459 | null | 363.44 ± 213.61 a | 176.85 ± 48.87 a | 251.31 ± 11.8 a |
| 43 | Isoamyl formate | 110-45-2 | C6H12O2 | 116.2 | 1070.5 | 457.066 | 1.27082 | null | 1879.94 ± 566.87 a | 1564.48 ± 122.78 a | 2108.43 ± 177.37 a |
| 44 | Isobutyl formate | 542-55-2 | C5H10O2 | 102.1 | 964.7 | 332.851 | 1.20094 | null | 892.69 ± 133.04 b | 349.92 ± 82.16 a | 205.98 ± 48.05 a |
| 45 | Ethyl 2-methylpropanoate | 97-62-1 | C6H12O2 | 116.2 | 960.8 | 330.616 | 1.55549 | null | 603.01 ± 117.39 b | 215.44 ± 24.83 a | 203.9 ± 35.67 a |
| 46 | Methyl 3-methylbutanoate | 556-24-1 | C6H12O2 | 116.2 | 986.7 | 347.242 | 1.19675 | null | 965.62 ± 81.79 b | 759.79 ± 126.31 a | 1408.24 ± 19.67c |
| 47 | Ethyl acetate | 141-78-6 | C4H8O2 | 88.1 | 879.1 | 287.696 | 1.33464 | null | 5325.65 ± 20.87 b | 2615.38 ± 92.44c | 217.77 ± 43.87 a |
| 48 | 2-Methylbutanol acetate | 624-41-9 | C7H14O2 | 130.2 | 892.7 | 293.251 | 1.28894 | null | 642.69 ± 14.2c | 298.6 ± 22.32 b | 149.65 ± 11.16 a |
| 49 | Methyl acetate | 79-20-9 | C3H6O2 | 74.1 | 826.3 | 266.144 | 1.19194 | D | 414.05 ± 42.93c | 255.97 ± 28.37 b | 103.66 ± 6.91 a |
| 50 | Methyl acetate | 79-20-9 | C3H6O2 | 74.1 | 829 | 267.255 | 1.0308 | M | 380.4 ± 32.49 a | 267.37 ± 22.53 b | 278.27 ± 43.4 b |
| 51 | Isobutyl propanoate | 540-42-1 | C7H14O2 | 130.2 | 869.3 | 283.697 | 1.28012 | null | 683.28 ± 374.99 a | 1250.82 ± 32.77 b | 845.53 ± 110.56 ab |
| 52 | Ethyl 3-hydroxy-butyrate | 5405-41-4 | C6H12O3 | 132.2 | 906.7 | 300.307 | 1.17244 | null | 349.79 ± 50.55 a | 317.37 ± 20.64 a | 453.18 ± 54.04 b |
| 53 | Ethyl butyrate | 105-54-4 | C6H12O2 | 116.2 | 803.3 | 256.767 | 1.2075 | null | 778.07 ± 48.28 b | 874.46 ± 92.28 b | 502.39 ± 1.21 a |
| 54 | Isoamyl acetate | 123-92-2 | C7H14O2 | 130.2 | 1134.5 | 617.134 | 1.31495 | null | 204.49 ± 53.06 a | 415.03 ± 16.05 b | 168.47 ± 15.34 a |
| 55 | Isopulegyl acetate | 89-49-6 | C12H20O2 | 196.3 | 1274.2 | 1120.946 | 1.3833 | null | 572.72 ± 171.61 a | 1079.21 ± 42.98 b | 380.05 ± 67.17 a |
| 56 | Ethyl lactate | 97-64-3 | C5H10O3 | 118.1 | 791.1 | 251.81 | 1.1407 | null | 6738.69 ± 342.72 a | 7724.25 ± 62.49 b | 6978.09 ± 347.95 a |
| Acids | |||||||||||
| 57 | Acetic acid | 64-19-7 | C2H4O2 | 60.1 | 1440.3 | 1688.59 | 1.05645 | null | 19,402.19 ± 1032.21 a | 16,626.92 ± 906.72 b | 13,619.52 ± 623.04c |
| 58 | hexanoic acid | 142-62-1 | C6H12O2 | 116.2 | 987.1 | 347.826 | 1.29479 | null | 1455.95 ± 499.65 b | 1904.71 ± 138.14 b | 730.86 ± 101.78 a |
| 59 | Isobutyric acid | 79-31-2 | C4H8O2 | 88.1 | 822.5 | 264.589 | 1.15426 | null | 1677.81 ± 63.41c | 1361.61 ± 71.74 b | 323.87 ± 23.33 a |
| Aromatic compounds | |||||||||||
| 60 | Styrene | 100-42-5 | C8H8 | 104.2 | 1219.3 | 921.36 | 1.41021 | null | 1167.08 ± 58.58 b | 937.25 ± 62.2 a | 1107.18 ± 93.73 b |
| 61 | 2-Methoxyphenol | 90-05-1 | C7H8O2 | 124.1 | 1091.9 | 510.544 | 1.10837 | null | 101.31 ± 50.95 a | 318.66 ± 94.36 b | 51.67 ± 7.43 a |
| 62 | p-Cymene | 99-87-6 | C10H14 | 134.2 | 1014.1 | 382.918 | 1.30901 | null | 467.69 ± 14.37 b | 781.44 ± 92.72c | 192.11 ± 18.51 a |
| 63 | 1,2-Dimethylbenzene | 95-47-6 | C8H10 | 106.2 | 899.7 | 296.361 | 1.06367 | null | 933.37 ± 63.1 a | 859.72 ± 46.46 a | 845.01 ± 53.28 a |
| 64 | p-Methyl guaiacol | 93-51-6 | C8H10O2 | 138.2 | 1186.3 | 791.085 | 1.18367 | null | 887.62 ± 85.03 a | 1656.03 ± 168.95 b | 669.05 ± 71.28 a |
| Hydrocarbons | |||||||||||
| 65 | Undecane | 1120-21-4 | C11H24 | 156.3 | 1110.3 | 556.44 | 1.10442 | M | 491.55 ± 222.74 b | 1879.18 ± 371.65c | 176.03 ± 33.43 a |
| 66 | Undecane | 1120-21-4 | C11H24 | 156.3 | 1108.8 | 552.781 | 1.35425 | D | 681.7 ± 164.06 a | 1813.28 ± 136.3 b | 376.98 ± 22.6 a |
| 67 | β-pinene | 127-91-3 | C10H16 | 136.2 | 1120.1 | 581.135 | 1.22125 | null | 457.98 ± 31.59 a | 621.45 ± 51.86 b | 578.82 ± 105.57 ab |
| 68 | Decalin | 91-17-8 | C10H18 | 138.3 | 1071.6 | 459.848 | 1.34063 | null | 1978.78 ± 394.92 a | 2479.8 ± 56.82 a | 1993.26 ± 221.56 a |
| Others | |||||||||||
| 69 | 2,3,5-Trimethylpyrazine | 14667-55-1 | C7H10N2 | 122.2 | 1373.8 | 1461.263 | 1.15976 | null | 8770.03 ± 8081.31 a | 3253.72 ± 593.02 a | 2099.61 ± 261.55 a |
| 70 | 2,3-Dimethylpyrazine | 5910-89-4 | C6H8N2 | 108.1 | 1339.4 | 1343.81 | 1.48064 | null | 2281.48 ± 1902.79 a | 3182.45 ± 628.12 a | 1571 ± 233.44 a |
| 71 | N-Nitrosodi-N-propylamine | 621-64-7 | C6H14N2O | 130.2 | 1076 | 470.72 | 1.27082 | null | 6488.57 ± 1717.04 a | 7955.04 ± 546.09 a | 7247.1 ± 711.97 a |
| 72 | Pyrazine, 2-ethyl-3-methyl- | 15707-23-0 | C7H10N2 | 122.2 | 1002.1 | 367.272 | 1.17818 | null | 805.62 ± 174.83 b | 1120.47 ± 171.76c | 272.21 ± 34 a |
| 73 | Tetrahydrofuran | 109-99-9 | C4H8O | 72.1 | 859.5 | 279.697 | 1.22481 | D | 1710.09 ± 353.57 a | 1442.76 ± 253.38 a | 2693.38 ± 318.37 b |
| 74 | Tetrahydrofuran | 109-99-9 | C4H8O | 72.1 | 856.8 | 278.587 | 1.06447 | M | 773.17 ± 51.78 a | 726.02 ± 35.79 a | 863.56 ± 10.45 b |
| 75 | Acetaldehyde diethyl acetal | 105-57-7 | C6H14O2 | 118.2 | 866.6 | 282.586 | 1.13101 | null | 634.67 ± 56.13 a | 857.43 ± 36.96 b | 933.69 ± 43.44 b |
| 76 | 2-Methyl pyrazine | 109-08-0 | C5H6N2 | 94.1 | 787.1 | 250.147 | 1.07088 | null | 604.86 ± 29.1 b | 508.55 ± 22.23 a | 637.94 ± 13.92 b |
| 77 | Benzothiazole | 95-16-9 | C7H5NS | 135.2 | 1243.9 | 1017.518 | 1.16136 | null | 1347.67 ± 407.86 b | 756.5 ± 34.05 a | 715.51 ± 69.66 a |
| No. | Threshold | Compounds | SH-Rxi-5Sil MS | SH-Stabilwax-MS | Identification Methods | Odor | CAS | FD Factors | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Literature RI | Calculated RI | Literature RI | Calculated RI | XW | ZZ | MN | ||||||
| Aldehydes | ||||||||||||
| 1 | 4 | Hexanal | 800 | 798 | 1083 | 1080 | MS, RI, O, S | Green, grass | 66-25-1 | 81 | 243 | 27 |
| 2 | 0.7 | Octanal | 1003 | 987 | 1289 | 1279 | MS, RI, O, S | Fresh, fatty | 124-13-0 | 3 | 9 | 27 |
| 3 | − | (Z)-2-heptenal | 958 | 939 | 1322 | 1313 | MS, RI, O | Green | 57266-86-1 | − | 1 | 1 |
| 4 | 8 | Nonanal | 1104 | 1095 | 1391 | 1384 | MS, RI, O, S | Fatty, fresh | 124-19-6 | 1 | 1 | 81 |
| 5 | 4 | (E)-2-octenal | 1060 | 1046 | 1429 | 1419 | MS, RI, O, S | Leaf, herbal | 2548-87-0 | 9 | 9 | 9 |
| 6 | 3 | Decanal | 1206 | 1191 | 1498 | 1494 | MS, RI, O, S | Floral, orange | 112-31-2 | 3 | − | 27 |
| 7 | 0.25 | (E)-2-nonenal | 1162 | 1152 | 1534 | 1533 | MS, RI, O, S | Cucumber, green | 18829-56-6 | 27 | 81 | 9 |
| 8 | 0.1 | (E,E)-2,4-nonadienal | 1188 | 1198 | 1700 | − | MS, RI, S | − | 5910-87-2 | − | − | − |
| 9 | 0.2 | (E,E)-2,4-decadienal | 1317 | 1309 | 1811 | 1824 | MS, RI, O, S | Cucumber, oily | 25152-84-5 | 81 | 27 | 81 |
| 10 | 3 | Heptanal | 901 | 883 | 1184 | 1175 | MS, RI, O, S | Green, herbal | 111-71-7 | − | 9 | 27 |
| 11 | 0.3 | (E)-2-decenal | 1263 | 1249 | 1644 | 1648 | MS, RI, O, S | Mushroom, green | 3913-81-3 | − | 9 | 9 |
| 12 | 1.4 | 2-Undecenal | 1367 | 1352 | 1751 | 1760 | MS, RI, O, S | Peel, fresh | 2463-77-6 | − | 27 | 27 |
| 13 | 0.04 | (E,Z)-2,4-decadienal | 1295 | − | 1754 | 1773 | MS, RI, O | Fatty, green | 25152-83-4 | − | − | 3 |
| Alcohols | ||||||||||||
| 14 | 150.2 | 1-Pentanol | 765 | 774 | 1250 | 1247 | MS, RI, O | Fusel, balsam | 71-41-0 | 1 | 1 | − |
| 15 | 1 | 1-Octen-3-ol | 980 | 964 | 1450 | 1448 | MS, RI, O, S | Earthy, green | 3391-86-4 | 243 | 243 | 81 |
| 16 | 25,482 | 2-Ethyl-1-hexanol | 1030 | − | 1491 | 1486 | MS, RI | − | 104-76-7 | − | − | − |
| 17 | − | 2,3-Butanediol | 788 | − | 1543 | 1538 | MS, RI, O, S | Creamy, buttery | 513-85-9 | 27 | 1 | 81 |
| 18 | 110 | 1-Octanol | 1071 | − | 1557 | 1558 | MS, RI, O, S | Green, orange | 111-87-5 | 3 | 9 | 27 |
| 19 | 20 | (E)-2-octen-1-ol | 1067 | − | 1614 | 1616 | MS, RI, O | Green, fatty | 18409-17-1 | − | 1 | 1 |
| 20 | − | Isopinocarveol | 118 | − | 1646 | 1645 | MS, RI, O | Woody, balsam | 6712-79-4 | 1 | − | − |
| 21 | 5.6 | 1-Hexanol | 868 | − | 1355 | 1344 | MS, RI, O, S | Sweet, green | 111-27-3 | 27 | − | − |
| 22 | 3 | 1-Heptanol | 970 | 954 | 1453 | 1447 | MS, RI, O | Leafy, musty | 111-70-6 | − | − | 1 |
| 23 | 140 | Phenylethyl alcohol | 1116 | − | 1906 | 1924 | MS, RI, O | Floral, dried | 60-12-8 | − | − | 1 |
| Ketones | ||||||||||||
| 24 | 40 | 5-Nonanone | 1073 | − | 1334 | 1329 | MS, RI | − | 502-56-7 | − | − | − |
| 25 | 110 | 2,3-Octanedione | 984 | − | 1335 | 1326 | MS, RI, O, S | Asparagus, cortex | 585-25-1 | 27 | 27 | 1 |
| 26 | 100 | (E,E)-3,5-octadien-2-one | 1073 | 1068 | 1570 | 1571 | MS, RI, O | Fruity, green | 30086-02-3 | 1 | − | 9 |
| 27 | 65 | Acetophenone | 1065 | − | 1647 | 1657 | MS, RI, O | Almond, sweet | 98-86-2 | 3 | − | − |
| 28 | 14 | Acetoin | 713 | − | 1284 | 1278 | MS, RI, O | Milky, buttery | 513-86-0 | − | 3 | − |
| 29 | 50.2 | 2-Octanone | 990 | − | 1287 | 1274 | MS, RI, O | Earthy, weedy | 111-13-7 | − | − | 1 |
| 30 | − | (E)-3-octen-2-one | 1033 | 1025 | 1396 | 1390 | MS, RI | − | 18402-82-9 | − | − | − |
| 31 | − | 3,5-Octadien-2-one | 1063 | 1058 | 1522 | 1513 | MS, RI | − | 38284-27-4 | − | − | − |
| 32 | 260 | 5-Ethyldihydro-2(3H)-furanone | 1057 | 1039 | 1694 | 1701 | MS, RI, O, S | Sweet, coconut | 695-06-7 | 81 | − | 81 |
| 33 | 9.7 | Dihydro-5-propyl-2(3H)-furanone | 1159 | − | 1787 | 1799 | MS, RI, O, S | Nutty, sweet | 105-21-5 | 1 | − | − |
| 34 | 9.7 | Dihydro-5-pentyl-2(3H)-furanone | 1363 | 1349 | 2024 | 2032 | MS, RI, O, S | Buttery, creamy | 104-61-0 | 27 | 27 | 9 |
| 35 | 12 | 5-Butyldihydro-2(3H)-furanone | 1261 | 1247 | 1910 | 1921 | MS, RI, O, S | Sweet, coconut | 104-50-7 | 1 | 27 | |
| Ester | ||||||||||||
| 36 | 5 | Methyl isovalerate | 764 | 776 | 1018 | − | MS, RI | − | 556-24-1 | − | − | − |
| 37 | 70 | Hexanoic acid, methyl ester | 907 | 907 | 1184 | − | MS, RI | − | 106-70-7 | − | − | − |
| 38 | 5 | Hexanoic acid, ethyl ester | 1000 | 988 | 1233 | 1226 | MS, RI, O, S | Fruity, sweet | 123-66-0 | 3 | − | 1 |
| 39 | 200 | Octanoic acid, methyl ester | 1108 | 1111 | 1385 | − | MS, RI | − | 111-11-5 | − | − | − |
| 40 | 19 | Octanoic acid, ethyl ester | 1196 | − | 1435 | 1429 | MS, RI, O, S | Waxy, fruity | 106-32-1 | 9 | − | − |
| 41 | − | Nonanoic acid, methyl ester | 1208 | 1208 | 1491 | − | MS, RI | − | 1731-84-6 | − | − | − |
| 42 | 8 | Decanoic acid, methyl ester | 1308 | 1305 | 1593 | − | MS, RI | − | 110-42-9 | − | − | − |
| 43 | 5 | Decanoic acid, ethyl ester | 1379 | 1377 | 1638 | − | MS, RI | − | 110-38-3 | − | − | − |
| 44 | − | Valeric acid, 4-pentadecyl ester | − | − | − | 1505 | MS | − | 959021-71-7 | − | − | − |
| 45 | − | n-Caproic acid vinyl ester | − | − | − | 1665 | MS | − | 3050-69-9 | − | − | − |
| 46 | 781 | (E)-hexanoic acid, 2-hexenyl ester | 1391 | − | 1662 | 1663 | MS, RI, O | Natural, green | 53398-86-0 | − | 1 | − |
| 47 | − | Hexanoic acid, pentyl ester | 1270 | 1271 | 1501 | 1503 | MS, RI | − | 540-07-8 | − | − | − |
| 48 | − | Isoamyl lactate | 1047 | − | 1580 | 1573 | MS, RI, O | Fruity, nutty | 19329-89-6 | − | − | 1 |
| Aromatic compounds | ||||||||||||
| 49 | 1000 | p-Xylene | 860 | 845 | 1138 | − | MS, RI | − | 106-42-3 | − | − | − |
| 50 | 350 | Benzaldehyde | 962 | − | 1520 | 1518 | MS, RI, O | Almond, bitter | 100-52-7 | 9 | 1 | − |
| 51 | 5000 | Phenol | 980 | − | 2000 | 2014 | MS, RI, O | Plastic, rubber | 108-95-2 | 1 | 1 | 3 |
| 52 | 31 | 3-Methyl-phenol | 1075 | − | 2091 | 2102 | MS, RI, O | Medicinal, woody | 108-39-4 | 3 | − | − |
| 53 | 1000 | Butylated hydroxytoluene | 1513 | − | 1909 | 1925 | MS, RI, O | Mild, camphor | 128-37-0 | − | 9 | − |
| 54 | 30 | p-Cresol | 1077 | − | 2080 | 2092 | MS, RI, O | Animal, mimosa | 106-44-5 | − | − | 9 |
| Hydrocarbons | ||||||||||||
| 55 | 200 | Limonene | 1023 | 1011 | 1200 | − | MS, RI | − | 138-86-3 | − | − | − |
| 56 | 10,000 | Dodecane | 1200 | 1197 | 1200 | − | MS, RI, S | − | 112-40-3 | − | − | − |
| 57 | − | Tridecane | 1300 | 1289 | 1300 | − | MS, RI, S | − | 629-50-5 | − | − | − |
| 58 | − | 2,6,10-Trimethyl-dodecane | 1366 | − | 1354 | 1352 | MS, RI | − | 3891-98-3 | − | − | − |
| 59 | − | 3-Methyl-tridecane | 1371 | − | 1366 | 1359 | MS, RI | − | 6418-41-3 | − | − | − |
| 60 | 1000 | Tetradecane | 1400 | − | 1400 | 1395 | MS, RI, S | − | 629-59-4 | − | − | − |
| 61 | − | Pentadecane | 1500 | 1498 | 1500 | 1494 | MS, RI, S | − | 629-62-9 | − | − | − |
| 62 | − | Hexadecane | 1600 | 1594 | 1600 | 1597 | MS, RI, S | − | 544-76-3 | − | − | − |
| Acids | ||||||||||||
| 63 | 99,000 | Acetic acid | 610 | − | 1449 | 1451 | MS, RI, O | Sharp | 64-19-7 | 3 | − | − |
| 64 | 2400 | Butanoic acid | 805 | − | 1625 | 1633 | MS, RI, O, S | Cheese, sharp | 107-92-6 | 9 | 3 | 3 |
| 65 | 15.9 | 3-Methyl-butanoic acid | 863 | − | 1666 | 1675 | MS, RI, O, S | Feet, cheese | 503-74-2 | 243 | 81 | 81 |
| 66 | 1207 | Pentanoic acid | 904 | − | 1733 | 1744 | MS, RI, O, S | Rancid, putrid | 109-52-4 | 27 | 1 | − |
| 67 | 2517.6 | Hexanoic acid | 990 | 1000 | 1846 | 1852 | MS, RI, O, S | Sour, cheese | 142-62-1 | 27 | 3 | 27 |
| 68 | 640 | Heptanoic acid | 1078 | − | 1950 | 1961 | MS, RI, O, S | Sweat, sour | 111-14-8 | 1 | 27 | 27 |
| 69 | 3000 | Octanoic acid | 1180 | 1183 | 2060 | 2069 | MS, RI, O, S | Fatty, waxy | 124-07-2 | 1 | 3 | − |
| 70 | 4600 | Nonanoic acid | 1273 | − | 2171 | 2176 | MS, RI, O, S | Dirty, cheese | 112-05-0 | 3 | 1 | 1 |
| 71 | 130 | n-Decanoic acid | 1373 | 1365 | 2276 | 2282 | MS, RI, O | Unpleasant, fatty | 334-48-5 | 1 | 3 | 3 |
| 72 | − | (E)-2-octenoic acid | 1227 | − | 2182 | 2194 | MS, RI | − | 1871-67-6 | − | − | − |
| Others | ||||||||||||
| 73 | − | Glycerin | − | − | 2303 | 2305 | MS, RI | − | 56-81-5 | − | − | − |
| 74 | 5.8 | 2-Pentyl-furan | 993 | 979 | 1231 | 1225 | MS, RI, O | Fruity, beany | 3777-69-3 | − | 1 | − |
| 75 | − | Unknown | − | − | − | 1280 | O | Mushroom | − | 27 | 9 | 27 |
| 76 | − | Unknown | − | − | − | 1568 | O | Rice | − | − | − | 1 |
| No. | Threshold (µg/kg) | Compounds | Concentration (µg/kg) | OAV | ||||
|---|---|---|---|---|---|---|---|---|
| XW | MN | ZZ | XW | MN | ZZ | |||
| Aldehydes | ||||||||
| 1 | 4 | Hexanal | 868.55 ± 41.46 b | 628.88 ± 28.47 a | 1123.21 ± 66.27 c | 217.14 ± 10.37 b | 157.22 ± 7.12 a | 280.8 ± 16.57 c |
| 2 | 0.7 | Octanal | 65.21 ± 4.34 a | 199.05 ± 4.81 c | 79.25 ± 9.83 b | 93.16 ± 6.2 a | 284.36 ± 6.87 c | 113.22 ± 14.04 b |
| 3 | - | (Z)-2-heptenal | 21.77 ± 1.57 a | 140.53 ± 3.4 b | 146.91 ± 9.78 b | - | - | - |
| 4 | 8 | Nonanal | 218.24 ± 15.48 b | 1253.94 ± 27.8 c | 164.1 ± 11.92 a | 27.28 ± 1.94 b | 156.74 ± 3.47 c | 20.51 ± 1.49 a |
| 5 | 4 | (E)-2-octenal | 44.47 ± 4.29 a | 58.22 ± 2.9 b | 50.64 ± 7.49 b | 11.12 ± 1.07 a | 14.55 ± 0.73 b | 12.66 ± 1.87 b |
| 6 | 3 | Decanal | 17.94 ± 0.75 a | 53.62 ± 4.52 b | - | 5.98 ± 0.25 a | 17.87 ± 1.51 b | - |
| 7 | 0.25 | (E)-2-nonenal | 10.39 ± 0.56 b | 5.66 ± 0.89 a | 11.35 ± 1.6 b | 41.55 ± 2.25 b | 22.65 ± 3.55 a | 45.38 ± 6.41 b |
| 8 | 1 | (E,E)-2,4-nonadienal | 0.7 ± 0.2 | - | - | 0.7 ± 0.2 | - | - |
| 9 | 0.2 | (E,E)-2,4-decadienal | 16.45 ± 0.93 b | 18.84 ± 0.12 c | 7.84 ± 0.99 a | 82.25 ± 4.67 b | 94.18 ± 0.62 c | 39.2 ± 4.95 a |
| 10 | 3 | Heptanal | - | 423.63 ± 11.32 b | 146.91 ± 9.78 a | - | 141.21 ± 3.77 b | 48.97 ± 3.26 a |
| 11 | 0.3 | (E)-2-decenal | - | 23.48 ± 2.79 a | 31.74 ± 2.63 b | - | 78.26 ± 9.3 a | 105.8 ± 8.77 b |
| 12 | 1.4 | 2-Undecenal | - | 7.86 ± 0.97 a | 12.54 ± 1.19 b | - | 5.61 ± 0.7 a | 8.96 ± 0.85 b |
| 13 | 0.04 | (E,Z)-2,4-decadienal | - | 4.85 ± 0.45 | - | - | 121.28 ± 11.32 | - |
| Total | 1263.73 ± 14.86 a | 2818.54 ± 53.99 c | 1774.5 ± 42.45 b | 576.14 ± 13.18 a | 950.61 ± 22.68 c | 675.51 ± 2.32 b | ||
| Alcohols | ||||||||
| 14 | 150.2 | 1-Pentanol | 88.36 ± 9.83 c | 27.48 ± 3.58 a | 53.34 ± 5.24 b | 0.59 ± 0.07 c | 0.18 ± 0.02 a | 0.36 ± 0.03 b |
| 15 | 1 | 1-Octen-3-ol | 129.7 ± 5.96 c | 72.55 ± 1.98 a | 101.94 ± 3.28 b | 129.7 ± 5.96 c | 72.55 ± 1.98 a | 101.94 ± 3.28 b |
| 16 | 25,482 | 2-Ethyl-1-hexanol | 121.04 ± 19.38 | - | - | - | - | - |
| 17 | - | 2,3-Butanediol | 223.45 ± 0.79 b | 332.41 ± 2.89 c | 149.1 ± 33.13 a | - | - | - |
| 18 | 110 | 1-Octanol | 16.89 ± 2.33 a | 24.76 ± 2.14 b | 29.74 ± 1.88 v | 0.15 ± 0.02 a | 0.23 ± 0.02 v | 0.27 ± 0.02v |
| 19 | 20 | (E)-2-octen-1-ol | 8.6 ± 1.19 v | 3.36 ± 0.39 a | 10.14 ± 0.98 c | 0.43 ± 0.06 b | 0.17 ± 0.02 a | 0.51 ± 0.05 c |
| 20 | - | Isopinocarveol | 9.16 ± 2.71 | - | - | - | - | - |
| 21 | 5.6 | 1-Hexanol | 55.39 ± 1.27 b | 23.86 ± 2.38 a | - | 9.89 ± 0.23 b | 4.26 ± 0.42 a | - |
| 22 | 3 | 1-Heptanol | - | 89.62 ± 1.2 | - | - | 29.87 ± 0.4 | - |
| 23 | 140 | Phenylethyl alcohol | - | 3.71 ± 0.96 | - | - | 0.03 ± 0.01 | - |
| Total | 652.59 ± 26.12 c | 577.76 ± 2.6 b | 344.26 ± 34.04 a | 140.91 ± 5.65 b | 128.15 ± 1.86 a | 103.07 ± 3.31 c | ||
| Ketones | ||||||||
| 24 | 40 | 5-Nonanone | 80.76 ± 4.27 b | 16.51 ± 1.05 a | - | 2.02 ± 0.11 b | 0.41 ± 0.03 a | - |
| 25 | 110 | 2,3-Octanedione | 92.27 ± 7.8 c | 28.13 ± 2.25 a | 51.82 ± 3.95 b | 0.84 ± 0.07 c | 0.26 ± 0.02 a | 0.47 ± 0.04 b |
| 26 | 100 | (E,E)-3,5-octadien-2-one | 19.56 ± 1.68 a | 44.56 ± 5.39 b | - | 0.2 ± 0.02 a | 0.45 ± 0.05 b | - |
| 27 | 65 | Acetophenone | 12.88 ± 0.13 | - | - | 0.2 ± 0 | - | - |
| 28 | 14 | Acetoin | - | 5.32 ± 0.15 a | 10.32 ± 1.21 b | - | 0.38 ± 0.01 a | 0.74 ± 0.09 b |
| 29 | 50.2 | 2-Octanone | - | 5.28 ± 0.11 | - | - | 0.11 ± 0 | - |
| 30 | - | (E)-3-octen-2-one | 40.76 ± 5.97 a | 53.21 ± 5.13 b | - | - | - | - |
| 31 | - | 3,5-Octadien-2-one | - | 15.15 ± 0.7 | - | - | - | - |
| 32 | 260 | 5-Ethyldihydro-2(3H)-furanone | 24.65 ± 0.14 b | 17.94 ± 1.15 a | - | 0.09 ± 0 b | 0.07 ± 0 a | - |
| 33 | 9.7 | Dihydro-5-propyl-2(3H)-furanone | 2.75 ± 0.16 | - | - | 0.28 ± 0.02 | - | - |
| 34 | 9.7 | Dihydro-5-pentyl-2(3H)-furanone | 3.18 ± 0.1 b | 2.11 ± 0.89 a | 3.82 ± 0.07 c | 0.33 ± 0.01 b | 0.22 ± 0.09 a | 0.39 ± 0.01 c |
| 35 | 12 | 5-Butyldihydro-2(3H)-furanone | - | 8.95 ± 0.53 b | 2.38 ± 0.5 a | - | 0.75 ± 0.04 b | 0.2 ± 0.04 a |
| Total | 276.82 ± 4.36 c | 197.15 ± 1.33 b | 68.35 ± 3.3 a | 5.56 ± 0.06 c | 2.75 ± 0.07 b | 1.8 ± 0 a | ||
| Ester | ||||||||
| 36 | 5 | Methyl isovalerate | - | 4.38 ± 0.21 | - | - | 0.88 ± 0.04 | - |
| 37 | 70 | Hexanoic acid, methyl ester | 32.72 ± 0.93 b | 15.62 ± 3.15 a | - | 0.47 ± 0.01 b | 0.22 ± 0.05 a | - |
| 38 | 5 | Hexanoic acid, ethyl ester | 116.07 ± 10.83 b | 31.22 ± 3.03 a | - | 23.21 ± 2.17 b | 6.24 ± 0.61 a | - |
| 39 | 200 | Octanoic acid, methyl ester | 17.2 ± 0.05 b | 4.86 ± 0.52 a | - | 0.09 ± 0 b | 0.02 ± 0 a | - |
| 40 | 19 | Octanoic acid, ethyl ester | 12.52 ± 0.42 | - | - | 0.66 ± 0.02 | - | - |
| 41 | - | Nonanoic acid, methyl ester | - | 0.38 ± 0.01 | - | - | - | - |
| 42 | 8 | Decanoic acid, methyl ester | - | 0.45 ± 0.02 | - | - | 0.06 ± 0 | - |
| 43 | 5 | Decanoic acid, ethyl ester | - | 1.02 ± 0.01 | - | - | 0.2 ± 0 | - |
| 44 | - | Valeric acid, 4-pentadecyl ester | 98.9 ± 12.3 | - | - | - | - | - |
| 45 | - | n-Caproic acid vinyl ester | 81 ± 5.47 b | 62.29 ± 4.85 a | - | - | - | - |
| 46 | 781 | (E)-hexanoic acid, 2-hexenyl ester | - | - | 15.8 ± 1.53 | - | - | 0.02 ± 0 |
| 47 | - | Hexanoic acid, pentyl ester | - | 4.8 ± 0.52 | - | - | - | - |
| 48 | - | Isoamyl lactate | - | 31.1 ± 4.07 | - | - | - | - |
| Total | 358.4 ± 29.91 c | 156.11 ± 1.74 b | 15.8 ± 1.53 a | 24.43 ± 2.2 c | 7.63 ± 0.6 b | 0.02 ± 0 a | ||
| Aromatic compounds | ||||||||
| 49 | 1000 | p-Xylene | 11.3 ± 0.75 | - | - | 0.01 ± 0 | - | - |
| 50 | 350 | Benzaldehyde | 82.42 ± 13.27 b | - | 31.1 ± 2.23 a | 0.24 ± 0.04 b | - | 0.09 ± 0.01 a |
| 51 | 5000 | Phenol | 8.12 ± 1.11 b | 13.48 ± 0.09 c | 6.64 ± 0.86 a | - | - | - |
| 52 | 31 | 3-Methyl-phenol | 8.99 ± 1.02 | - | - | 0.29 ± 0.03 | - | - |
| 53 | 1000 | Butylated hydroxytoluene | - | - | 12.21 ± 1.91 | - | - | 0.01 ± 0 |
| 54 | 30 | p-Cresol | - | 3.86 ± 0.49 | - | - | 0.13 ± 0.02 | - |
| Total | 110.83 ± 14.66 c | 17.34 ± 0.58 a | 49.94 ± 1.18 b | 0.54 ± 0.07 b | 0.13 ± 0.02 a | 0.1 ± 0 a | ||
| Hydrocarbons | ||||||||
| 55 | 200 | Limonene | 7.19 ± 1.09 b | 0.38 ± 0.01 a | - | 0.04 ± 0.01 b | 0 ± 0 a | - |
| 56 | 10,000 | Dodecane | - | 3.37 ± 0.43 | - | - | - | - |
| 57 | - | Tridecane | - | 1.08 ± 0.05 | - | - | - | - |
| 58 | - | 2,6,10-Trimethyl-dodecane | - | - | 35.66 ± 1.02 | - | - | - |
| 59 | - | 3-Methyl-tridecane | - | - | 11.73 ± 1.46 | - | - | - |
| 60 | 1000 | Tetradecane | - | - | 112.5 ± 28.4 | - | - | 0.11 ± 0.03 |
| 61 | - | Pentadecane | - | - | 62.33 ± 10.48 | - | - | - |
| 62 | - | Hexadecane | - | - | 19.85 ± 1.51 | - | - | - |
| Total | 7.19 ± 1.09 b | 4.84 ± 0.37 a | 242.07 ± 42.87 c | 0.04 ± 0.01 a | - | 0.11 ± 0.03 b | ||
| Acids | ||||||||
| 63 | 99,000 | Acetic acid | 81.11 ± 4.98 | - | - | - | - | - |
| 64 | 2400 | Butanoic acid | 27.28 ± 0.17 c | 8.86 ± 0.39 a | 12.28 ± 0.84 b | 0.01 ± 0 | - | 0.01 ± 0 |
| 65 | 15.9 | 3-Methyl-butanoic acid | 58.97 ± 0.99 c | 12.03 ± 0.23 a | 16.36 ± 0.71 b | 3.71 ± 0.06 c | 0.76 ± 0.01 a | 1.03 ± 0.04 b |
| 66 | 1207 | Pentanoic acid | 15.3 ± 0.71 c | 1.4 ± 0.06 a | 4.1 ± 0.23 b | 0.01 ± 0 | - | - |
| 67 | 2517.6 | Hexanoic acid | 234.84 ± 5.36 c | 180.33 ± 11.5 b | 44.05 ± 2.71 a | 0.09 ± 0 c | 0.07 ± 0 b | 0.02 ± 0 a |
| 68 | 640 | Heptanoic acid | 1.49 ± 0.6 a | 3.28 ± 1.68 b | 3.79 ± 0.73 b | - | 0.01 ± 0 | 0.01 ± 0 |
| 69 | 3000 | Octanoic acid | 60.03 ± 9.37 c | 25.63 ± 1.63 b | 13.54 ± 0.28 a | 0.02 ± 0 b | 0.01 ± 0 a | - |
| 70 | 4600 | Nonanoic acid | 27.1 ± 0.2 c | 10.31 ± 0.81 b | 4.9 ± 0.08 a | 0.01 ± 0 | - | - |
| 71 | 130 | n-Decanoic acid | 12.27 ± 0.59 a | 22.79 ± 2.41 c | 19.27 ± 1.31 b | 0.09 ± 0 a | 0.18 ± 0.02 c | 0.15 ± 0.01 b |
| 72 | - | (E)-2-octenoic acid | - | 2.43 ± 0.2 | - | - | - | - |
| Total | 518.38 ± 16.47 c | 267.06 ± 7.57 b | 118.29 ± 0.28 a | 4.53 ± 0.2 b | 4.51 ± 0.41 b | 1.21 ± 0.05 a | ||
| Others | ||||||||
| 73 | - | Glycerin | 105.37 ± 22.25 c | 15.31 ± 0.34 b | 10.71 ± 0.19 a | - | - | - |
| 74 | 5.8 | 2-Pentyl-furan | - | 3.58 ± 2 a | 8.76 ± 0.76 b | - | 0.62 ± 0.34 a | 1.51 ± 0.13 b |
| Total | 105.37 ± 22.25 b | 18.89 ± 1.65 a | 19.46 ± 0.57 a | - | 2.34 ± 0.34 b | 1.51 ± 0.13 a | ||
| Compounds | Quantitative Ions a | Standard Curves b | R2 |
|---|---|---|---|
| Hexanal | 44, 56, 72, 27 | y = 0.2735x + 0.1596 | 0.9932 |
| Nonanal | 57, 41, 43, 56 | y = 0.4929x − 0.1045 | 0.9979 |
| Heptanal | 44, 27, 55, 70 | y = 0.2328x − 0.2125 | 0.9968 |
| Decanal | 56, 55, 41, 43 | y = 0.9334x − 0.2805 | 0.9861 |
| Octanal | 43, 44, 41, 56 | y = 0.1527x − 0.7610 | 0.9968 |
| (E)-2-Nonenal | 41, 43, 29, 55 | y = 0.3585x + 0.1831 | 0.9978 |
| (E,E)-2,4-Decadienal | 81, 41, 29, 39 | y = 0.3159x + 0.1903 | 0.9956 |
| 2-Undecenal | 70, 41, 57, 43 | y = 0.3336x + 0.1133 | 0.9975 |
| 1-Octen-3-ol | 57, 43, 72, 29 | y = 0.3077x + 0.2273 | 0.9974 |
| 1-Hexanol | 56, 43, 41, 39 | y = 0.3201x − 0.2449 | 0.9969 |
| 1-Octanol | 56, 55, 41, 43 | y = 0.6931x − 0.4813 | 0.9901 |
| 2,3-Butanediol | 45, 43, 27, 57 | y = 0.4102x − 0.1892 | 0.9924 |
| 2,3-Octanedione | 43, 30, 41, 27 | y = 0.1776x − 0.3727 | 0.9971 |
| Butyldihydro-2(3H)-furanone | 85, 29, 56, 41 | y = 0.5138x − 0.2752 | 0.9858 |
| Dihydro-5-pentyl-2(3H)-furanone | 85, 29, 41, 43 | y = 0.4636x + 0.1317 | 0.9988 |
| 5-Ethyldihydro-2(3H)-furanone | 85, 29, 56, 57 | y = 0.2432x − 0.1592 | 0.9986 |
| 3-Methyl-butanoic acid | 60, 43, 41, 45 | y = 0.1087x − 0.3425 | 0.9911 |
| Pentanoic acid | 60, 73, 41, 45 | y = 0.8277x − 0.2338 | 0.9878 |
| Hexanoic acid | 60, 73, 41, 43 | y = 0.6026x − 0.8420 | 0.9759 |
| Heptanoic acid | 60, 73, 41, 87 | y = 0.2074x + 0.6202 | 0.9972 |
| No. | Compounds a | Correct Number b | Significance c |
|---|---|---|---|
| Yunnan bacon | |||
| 1 | Hexanal | 12/12 | ** |
| 2 | (E)-2-Nonenal | 5/12 | – |
| 3 | (E,E)-2,4-Decadienal | 8/12 | * |
| 4 | 1-Octen-3-ol | 12/12 | ** |
| 5 | 2,3-Butanediol | 6/12 | – |
| 6 | 1-Hexanol | 8/12 | * |
| 7 | 2,3-Octanedione | 9/12 | ** |
| 8 | 5-Ethyldihydro-2(3H)-furanone | 3/12 | – |
| 9 | Dihydro-5-pentyl-2(3H)-furanone | 8/12 | * |
| 10 | 3-Methyl-butanoic acid | 10/12 | ** |
| 11 | Pentanoic acid | 4/12 | – |
| 12 | Hexanoic acid | 9/12 | ** |
| Chongqing bacon | |||
| 1 | Hexanal | 12/12 | ** |
| 2 | (E)-2-Nonenal | 9/12 | ** |
| 3 | (E,E)-2,4-Decadienal | 8/12 | * |
| 4 | 2-Undecenal | 9/12 | ** |
| 5 | 1-Octen-3-ol | 12/12 | ** |
| 6 | 2,3-Octanedione | 10/12 | ** |
| 7 | Dihydro-5-pentyl-2(3H)-furanone | 8/12 | * |
| 8 | 3-Methyl-butanoic acid | 8/12 | * |
| 9 | Heptanoic acid | 6/12 | – |
| Sichuan bacon | |||
| 1 | Hexanal | 12/12 | ** |
| 2 | Octanal | 11/12 | ** |
| 3 | Nonanal | 7/12 | – |
| 4 | Decanal | 6/12 | – |
| 5 | (E,E)-2,4-Decadienal | 8/12 | * |
| 6 | Heptanal | 5/12 | – |
| 7 | 2-Undecenal | 8/12 | * |
| 8 | 1-Octen-3-ol | 12/12 | ** |
| 9 | 2,3-Butanediol | 4/12 | – |
| 10 | 1-Octanol | 10/12 | ** |
| 11 | 5-Ethyldihydro-2(3H)-furanone | 3/12 | – |
| 12 | 5-Butyldihydro-2(3H)-furanone | 9/12 | ** |
| 13 | 3-Methyl-butanoic acid | 10/12 | ** |
| 14 | Hexanoic acid | 9/12 | ** |
| 15 | Heptanoic acid | 6/12 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; He, Z.; Yang, L.; Li, H. The Characterization of the Key Aroma Compounds in Non-Smoked Bacon by Instrumental and Sensory Methods. Foods 2024, 13, 1260. https://doi.org/10.3390/foods13081260
Wu H, He Z, Yang L, Li H. The Characterization of the Key Aroma Compounds in Non-Smoked Bacon by Instrumental and Sensory Methods. Foods. 2024; 13(8):1260. https://doi.org/10.3390/foods13081260
Chicago/Turabian StyleWu, Han, Zhifei He, Li Yang, and Hongjun Li. 2024. "The Characterization of the Key Aroma Compounds in Non-Smoked Bacon by Instrumental and Sensory Methods" Foods 13, no. 8: 1260. https://doi.org/10.3390/foods13081260
APA StyleWu, H., He, Z., Yang, L., & Li, H. (2024). The Characterization of the Key Aroma Compounds in Non-Smoked Bacon by Instrumental and Sensory Methods. Foods, 13(8), 1260. https://doi.org/10.3390/foods13081260




