Traditional and Domestic Cooking Dramatically Reduce Estrogenic Isoflavones in Soy Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Traditional Recipes
Soy Juice
Tofu
Tempeh
Miso
Hydrated Soy Proteins
Commercial Tofu and Tempeh
2.2.2. ELISA
Samples Preparation
Assay
Characteristics of the ELISA
2.3. Statistical Treatments
3. Results
3.1. Impact of Traditional Process on Isoflavones Content in Tempeh and Miso
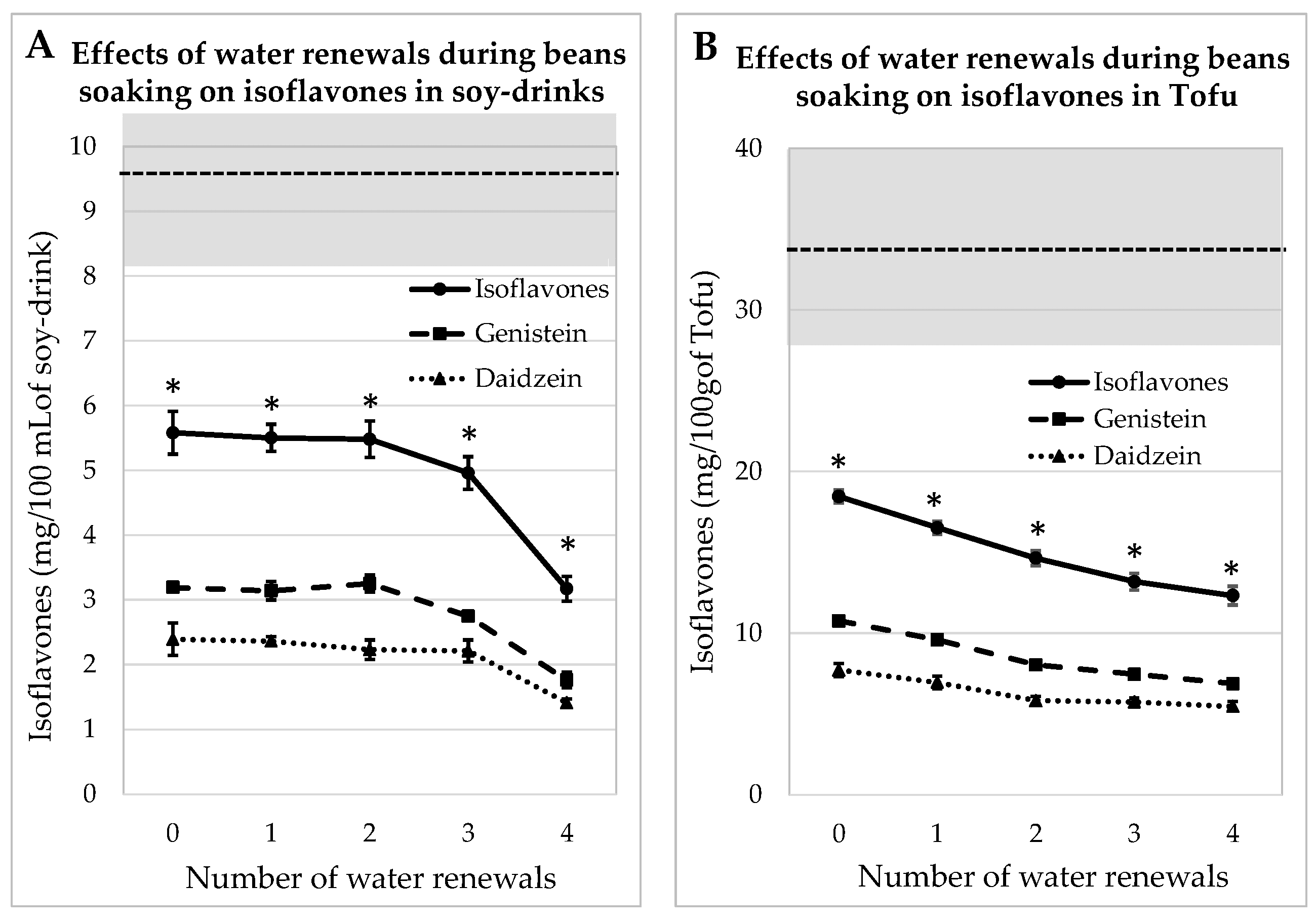
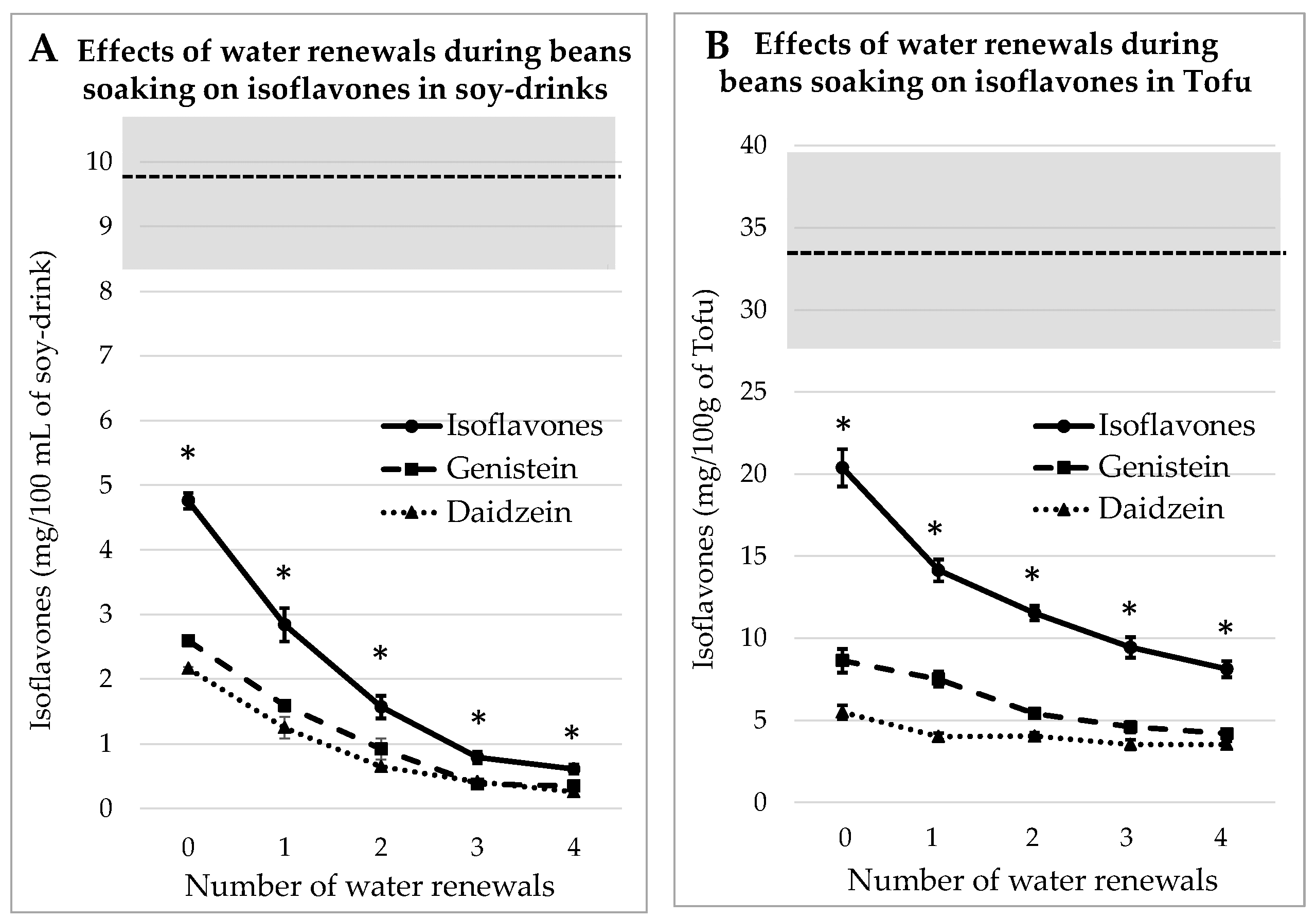
3.2. Impact of Water Renewals during Beans Soaking for Soy Juice and Tofu
3.2.1. Process Tested on Whole Soybeans
3.2.2. Process Tested on Dehulled Beans
3.3. Impact of Water Renewals on the Levels of Isoflavones in Textured Proteins
3.3.1. Treatment with Water at 22 °C
3.3.2. Treatment with Water at 65 °C
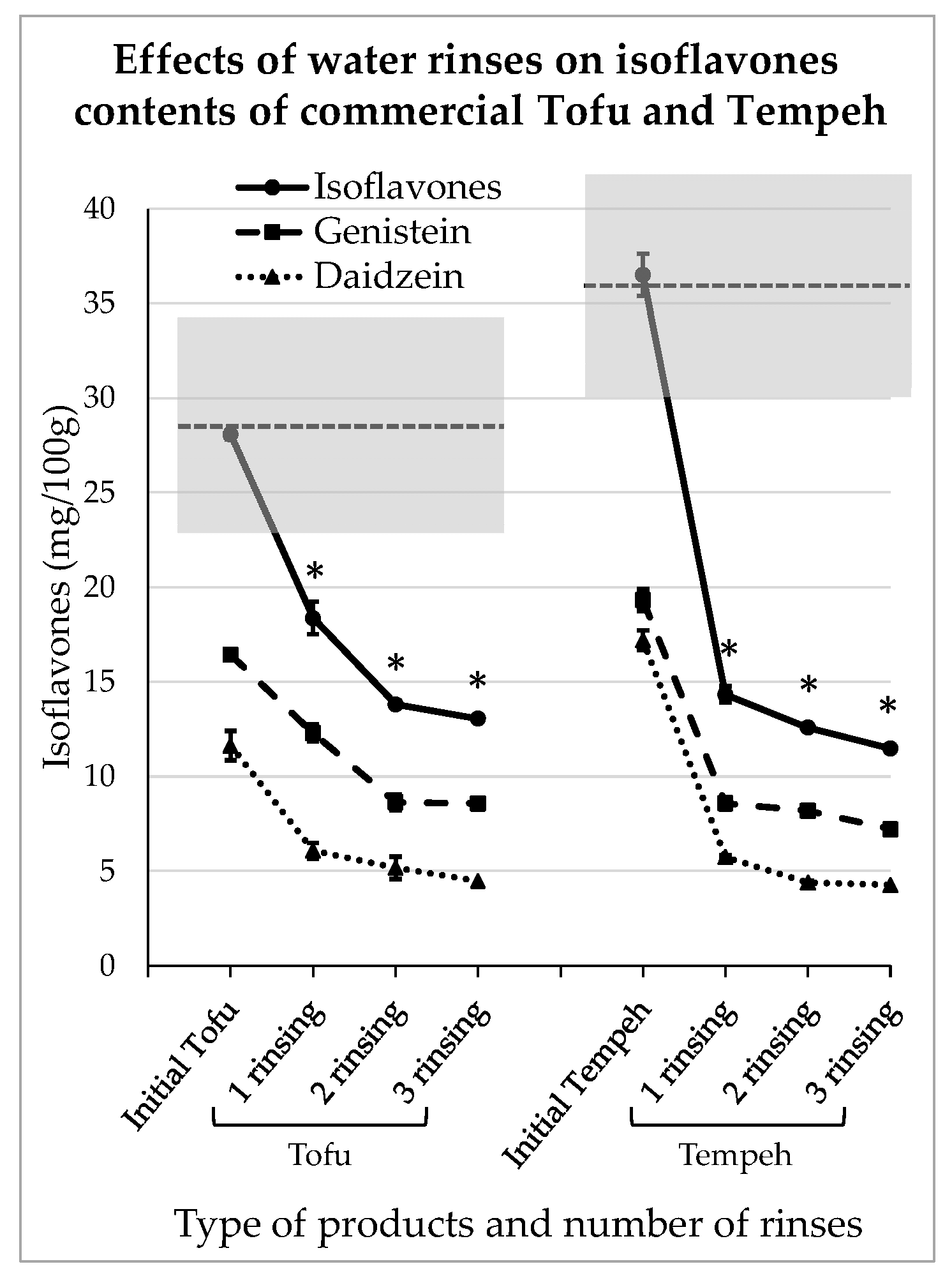
3.4. Impact of Domestic Rinsing on Isoflavones Content of Commercial Tofu and Tempeh
4. Discussion
4.1. Data Analysis
4.2. Traditional Treatments
4.3. Health Effects
4.3.1. Traditional Pharmacopeia
4.3.2. Toxic Effects and Reference Doses
4.3.3. Beneficial Health Effects for a Restricted Population
4.4. Consequences of this Finding on the Estimation of the Population’s Exposure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, A.; Bensaada, S.; Lamothe, V.; Lacoste, M.; Bennetau-Pelissero, C. Endocrine disruptors on and in fruits and vegetables: Estimation of the potential exposure of the French population. Food Chem. 2022, 373, 131513. [Google Scholar] [CrossRef] [PubMed]
- Gier, K.; Preininger, C.; Sauer, U. A Chip for Estrogen Receptor Action: Detection of Biomarkers Released by MCF-7 Cells through Estrogenic and Anti-Estrogenic Effects. Sensors 2017, 17, 1760. [Google Scholar] [CrossRef] [PubMed]
- Canivenc-Lavier, M.C.; Bennetau-Pelissero, C. Phytoestrogens and Health Effects. Nutrients 2023, 15, 317. [Google Scholar] [CrossRef]
- NTP. Multigenerational reproductive study of genistein (Cas No. 446-72-0) in Sprague-Dawley rats (feed study). Natl. Toxicol. Program. Tech. Rep. Ser. 2008, 539, 1–266. [Google Scholar]
- NTP. Toxicology and carcinogenesis studies of genistein (Cas No. 446-72-0) in Sprague-Dawley rats (feed study). Natl. Toxicol. Program. Tech. Rep. Ser. 2008, 545, 1–240. [Google Scholar]
- Cao, Z.H.; Green-Johnson, J.M.; Buckley, N.D.; Lin, Q.Y. Bioactivity of soy-based fermented foods: A review. Biotechnol. Adv. 2019, 37, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Baraibar Norberg, M.; Deutsh, L. The first soybean cycle (domestication to 900 CE). In The Soybean Through World History. Lessons for Sustainable Agrofood Systems, 1st ed.; Routledge Studies in Food, Society and the Environment Taylor & Francis: Abingdon, UK, 2023; pp. 22–56. [Google Scholar]
- Zhao, Z. New Archaeobotanic Data for the Study of the Origins of Agriculture in China. Curr. Anthropol. 2011, 52, 295–306. [Google Scholar] [CrossRef]
- Lee, C.-H.; Kim, M.L. History of Fermented Foods in Northeast Asia. In Ethnic Fermented Foods and Alcoholic Beverages of Asia; Tamang, J.P., Ed.; Spinger: New Delhi, India, 2016; pp. 1–16. [Google Scholar]
- Lander, B.; DuBois, T.D.A. History of Soy in China: From Weedy Bean to Global Commodity; Ethnic Fermented Foods and Alcoholic Beverages of Asia. In The Age of the Soybean: An Environmental History of Soy during the Great, Acceleration; da Silva, C.M., de Majo, C., Eds.; The White Horse Press: Cambridgeshire, UK, 2022; pp. 29–47. [Google Scholar]
- Traditional Tofu Making Experience in Debao Guangxi. Available online: https://twojadebowls.com/2013/08/16/traditional-tofu-making-experience-in-debao-guangxi/ (accessed on 27 February 2024).
- Bensaada, S.; Chabrier, F.; Ginisty, P.; Ferrand, C.; Peruzzi, G.; Valat, M.; Bennetau-Pelissero, C. Improved Food-Processing Techniques to Reduce Isoflavones in Soy-Based Foodstuffs. Foods 2023, 12, 1540. [Google Scholar] [CrossRef]
- McClure, T.; Cocuron, J.C.; Osmark, V.; McHale, L.K.; Alonso, A.P. Impact of Environment on the Biomass Composition of Soybean (Glycine max) seeds. J. Agric. Food Chem. 2017, 65, 6753–6761. [Google Scholar] [CrossRef]
- Vergne, S.; Sauvant, P.; Lamothe, V.; Chantre, P.; Asselineau, J.; Perez, P.; Durand, M.; Moore, N.; Bennetau-Pelissero, C. Influence of ethnic origin (Asian v. Caucasian) and background diet on the bioavailability of dietary isoflavones. Br. J. Nutr. 2009, 102, 1642–1653. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, Q.; Qiu, Y.; Zhang, Y.; Cui, S.; Yu, Y.; Chen, B.; Zhu, M.; Wang, N.; Liu, X.; et al. Soy Isoflavones Intake and Obesity in Chinese Adults: A Cross-Sectional Study in Shanghai, China. Nutrients 2021, 13, 2715. [Google Scholar] [CrossRef] [PubMed]
- Murai, U.; Sawada, N.; Charvat, H.; Inoue, M.; Yasuda, N.; Yamagishi, K.; Tsugane, S. Soy product intake and risk of incident disabling dementia: The JPHC Disabling Dementia Study. Eur. J. Nutr. 2022, 61, 4045–4057. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, A.; Lamothe, V.; Delample, M.; Denayrolles, M.; Bennetau-Pelissero, C. Removing isoflavones from modern soyfood: Why and how? Food Chem. 2016, 210, 286–294. [Google Scholar] [CrossRef] [PubMed]
- The Japanese Lab. How Miso Was Made Traditionally. Available online: https://thejapanesefoodlab.com/traditional-japanese-miso#how/ (accessed on 27 February 2024).
- Afssa, Afssaps. Sécurité et Bénéfices des Phyto-Estrogènes Apportés par L’alimentation—Recommandations Rapport D’expertise Mars 2005. Available online: https://www.anses.fr/fr/system/files/NUT-Ra-Phytoestrogenes.pdf (accessed on 23/03/24).
- Bennetau-Pelissero, C.; Arnal-Schnebelen, B.; Lamothe, V.; Sauvant, P.; Sagne, J.; Verbruggen, M.; Mathey, J.; Lavialle, O. ELISA as a new method to measure genistein and daidzein in food and human fluids. Food Chem. 2003, 82, 645–658. [Google Scholar] [CrossRef]
- Bensaada, S.; Raymond, I.; Breton, M.; Pellegrin, I.; Viallard, J.-F.; Bennetau-Pelissero, C. Development of an Assay for Soy Isoflavones in Women’s Hair. Nutrients 2022, 14, 3619. [Google Scholar] [CrossRef] [PubMed]
- Bensaada, S.; Raymond, I.; Pellegrin, I.; Viallard, J.F.; Bennetau-Pelissero, C. Validation of ELISAs for Isoflavones and Enterolactone for Phytoestrogen Intake Assessment in the French Population. Nutrients 2023, 15, 967. [Google Scholar] [CrossRef] [PubMed]
- Vergne, S.; Titier, K.; Bernard, V.; Asselineau, J.; Durand, M.; Lamothe, V.; Potier, M.; Perez, P.; Demotes-Mainard, J.; Chantre, P.; et al. Bioavailability and Urinary Excretion of Isoflavones in Humans: Effects of Soy-Based Supplements Formulation and Equol Production. J. Pharm. Biomed. Anal. 2007, 43, 1488–1494. [Google Scholar] [CrossRef]
- Shinkaruk, S.; Durand, M.; Lamothe, V.; Carpaye, A.; Martinet, A.; Chantre, P.; Vergne, S.; Nogues, X.; Moore, N.; Bennetau-Pelissero, C. Bioavailability of Glycitein Relatively to Other Soy Isoflavones in Healthy Young Caucasian Men. Food Chem. 2012, 135, 1104–1111. [Google Scholar] [CrossRef]
- Bennetau-Pelissero, C.; Le Houérou, C.; Lamothe, V.; Le Menn, F.; Babin, P.; Bennetau, B. Synthesis of Haptens and Conjugates for ELISAs of Phytoestrogens. Development of the Immunological Tests. J. Agric. Food Chem. 2000, 48, 305–311. [Google Scholar] [CrossRef]
- Le Houérou, C.; Bennetau-Pelissero, C.; Lamothe, V.; Le Menn, F.; Babin, P.; Bennetau, B. Syntheses of Novel Hapten-Protein Conjugates for Production of Highly Specific Antibodies to Formononetin, Daidzein and Genistein. Tetrahedron 2000, 56, 295–301. [Google Scholar] [CrossRef]
- Bennetau-Pelissero, C. Plant Proteins from Legumes. In Bioactive Molecules in Food Vol 1. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2018; pp. 223–266. [Google Scholar]
- Veremeichik, G.; Grigorchuk, V.; Butovets, E.; Lukyanchuk, L.; Brodovskaya, E.; Bulgakov, D.; Bulgakov, V. Isoflavonoid biosynthesis in cultivated and wild soybeans grown in the field under adverse climate conditions. Food Chem. 2021, 342, 128292–128300. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.H.; Lu, Y.F.; Hsieh, H.C.; Chen, B.H. Stability of isoflavone glucosides during processing of soymilk and tofu. Food Res. Int. 2004, 37, 891–900. [Google Scholar] [CrossRef]
- Chung, I.M.; Seo, S.H.; Ahn, J.K.; Kim, S.H. Effect of processing, fermentation, and aging treatment to content and profile of phenolic compounds in soybean seed, soy curd and soy paste. Food Chem. 2011, 127, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Mir, S.R.; Panda, B.P. Effect of sequential bio-processing conditions on the content and composition of vitamin K2 and isoflavones in fermented soy food. J. Food Sci. Technol. 2015, 52, 8228–8235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qiao, Y.; Zhang, K.; Zhang, Z.; Zhang, C.; Sun, Y.; Feng, Z. Fermented soybean foods: A review of their functional components, mechanism of action and factors influencing their health benefits. Food Res. Int. 2022, 158, 111575. [Google Scholar] [CrossRef] [PubMed]
- Kuligowski, M.; Sobkowiak, D.; Polanowska, K.; Jasinska-Kuligowska, I. Effect of different processing methods on isoflavone content in soybeans and soy products. J. Food Comp. Anal. 2022, 110, 104535–104541. [Google Scholar] [CrossRef]
- Shurtleff, W.; Aoyagi, A. Early History of Soybeans and Soyfoods Worldwide (1024 BCE to 1899): Extensively Annotated Bibliography and Sourcebook; Soyinfo Center: Lafayette, CA, USA, 2014. [Google Scholar]
- Wang, S.; Zhang, S.; Wang, S.; Gao, P.; Dai, L. A comprehensive review on Pueraria: Insights on its chemistry and medicinal value. Biomed. Pharmacother. 2020, 131, 110734. [Google Scholar] [CrossRef] [PubMed]
- Bennetts, H.W.; Underwood, E.J.; Shier, F.L. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust. Vet. J. 1946, 23, 2–13. [Google Scholar] [CrossRef]
- Findlay, J.K.; Buckmaster, J.M.; Chamley, W.A.; Cumming, I.A.; Hearnshaw, H.; Goding, J.R. Release of luteinising hormone by œstradiol 17β and a gonadotrophin-releasing hormone in ewes affected with clover disease. Neuroendocrinology 1973, 11, 57–66. [Google Scholar] [CrossRef]
- Cohen, B.L.; Katz, M. Further studies on pituitary and ovarian function in women receiving hormonal contraception. Contraception 1981, 24, 159–172. [Google Scholar] [CrossRef]
- Cassidy, A.; Bingham, S.; Setchell, K.D. Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am. J. Clin. Nutr. 1994, 60, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.A.; Schliep, K.C.; Wactawski-Wende, J.; Stanford, J.B.; Zarek, S.M.; Radin, R.G.; Sjaarda, L.A.; Perkins, N.J.; Kalwerisky, R.A.; Hammoud, A.O.; et al. Dietary factors and luteal phase deficiency in healthy eumenorrheic women. Hum. Reprod. 2015, 30, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.K.; Jaceldo-Siegl, K.; Knutsen, S.F.; Fan, J.; Oda, K.; Fraser, G.E. Soy isoflavone intake and the likelihood of ever becoming a mother: The Adventist Health Study-2. Int. J. Womens Health 2014, 6, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Strom, B.L.; Schinnar, R.; Barnhart, K.T.; Sammel, M.D.; Macones, G.A.; Stallings, V.A. Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA 2001, 286, 807–814. [Google Scholar] [CrossRef]
- Upson, K.; Harmon, Q.E.; Laughlin-Tommaso, S.K.; Umbach, D.M.; Baird, D.D. Soy-based Infant Formula Feeding and Heavy Menstrual Bleeding Among Young African American Women. Epidemiology 2016, 27, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Upson, K.; Adgent, M.A.; Wegienka, G.; Baird, D.D. Soy-based infant formula feeding and menstrual pain in a cohort of women aged 23–35 years. Hum. Reprod. 2019, 34, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Lin, Z.; Vásquez, E.; Luan, X.; Guo, F.; Xu, L. High soy isoflavone or soy-based food intake during infancy and in adulthood is associated with an increased risk of uterine fibroids in premenopausal women: A meta-analysis. Nutr. Res. 2019, 71, 30–42. [Google Scholar] [CrossRef]
- Chandrareddy, A.; Muneyyirci-Delale, O.; McFarlane, S.I.; Murad, O.M. Adverse effects of phytoestrogens on reproductive health: A report of three cases. Complement. Ther. Clin. Pract. 2008, 14, 132–135. [Google Scholar] [CrossRef]
- Imai, H.; Nishikawa, H.; Suzuki, A.; Kodama, E.; Iida, T.; Mikura, K.; Hashizume, M.; Kigawa, Y.; Tadokoro, R.; Sugisawa, C.; et al. Secondary Hypogonadism due to Excessive Ingestion of Isoflavone in a Man. Intern. Med. 2022, 61, 2899–2903. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Toth, T.L.; Sadio, S.M.; Hauser, R. Soy food and isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Hum. Reprod. 2008, 23, 2584–2590. [Google Scholar] [CrossRef]
- Toshima, H.; Suzuki, Y.; Imai, K.; Yoshinaga, J.; Shiraishi, H.; Mizumoto, Y.; Hatakeyama, S.; Onohara, C.; Tokuoka, S. Endocrine disrupting chemicals in urine of Japanese male partners of subfertile couples: A pilot study on exposure and semen quality. Int. J. Hyg. Environ. Health 2012, 215, 502–506. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, M.; Zhu, P.; Lu, C.; Fu, G.; Zhou, X.; Chen, D.; Wang, H.; Hang, B.; Wang, S.; et al. Urinary phytoestrogen levels related to idiopathic male infertility in Chinese men. Environ. Int. 2013, 59, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Mumford, S.L.; Kim, S.; Chen, Z.; Boyd Barr, D.; Buck Louis, G.M. Urinary Phytoestrogens Are Associated with Subtle Indicators of Semen Quality among Male Partners of Couples Desiring Pregnancy. J. Nutr. 2015, 145, 2535–2541. [Google Scholar] [CrossRef]
- Yuan, G.; Liu, Y.; Liu, G.; Wei, L.; Wen, Y.; Huang, S.; Guo, Y.; Zou, F.; Cheng, J. Associations between semen phytoestrogens concentrations and semen quality in Chinese men. Environ. Int. 2019, 129, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.; Dekant, W.; Stopper, H. Assaying the estrogenicity of phytoestrogens in cells of different estrogen sensitive tissues. Toxicol. Vitr. 2001, 15, 433–439. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, Y.; Yu, J.; Jin, N. Soy isoflavone extracts stimulate the growth of nude mouse xenografts bearing estrogen-dependent human breast cancer cells (MCF-7). J. Biomed. Res. 2012, 26, 44–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McMichael-Phillips, D.F.; Harding, C.; Morton, M.; A Roberts, S.; Howell, A.; Potten, C.S.; Bundred, N.J. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am. J. Clin. Nutr. 1998, 68 (Suppl. S6), 1431S–1435S. [Google Scholar] [CrossRef]
- Shike, M.; Doane, A.S.; Russo, L.; Cabal, R.; Reis-Filo, J.; Gerald, W.; Cody, H.; Khanin, R.; Bromberg, J.; Norton, L. The effects of soy supplementation on gene expression in breast cancer: A randomized placebo-controlled study. J. Natl. Cancer Inst. 2014, 106, dju189. [Google Scholar] [CrossRef]
- Van Die, M.D.; Bone, K.M.; Visvanathan, K.; Kyrø, C.; Aune, D.; Ee, C.; Paller, C.J. Phytonutrients and outcomes following breast cancer: A systematic review and meta-analysis of observational studies. JNCI Cancer Spectr. 2024, 8, pkad104. [Google Scholar] [CrossRef]
- Messina, M. Impact of Soy Foods on the Development of Breast Cancer and the Prognosis of Breast Cancer Patients. Forsch. Komplementmed. 2016, 23, 75–80. [Google Scholar] [CrossRef]
- Chen, M.; Rao, Y.; Zheng, Y.; Wei, S.; Li, Y.; Guo, T.; Yin, P. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: A meta-analysis of epidemiological studies. PLoS ONE 2014, 9, e89288. [Google Scholar] [CrossRef] [PubMed]
- Finkeldey, L.; Schmitz, E.; Ellinger, S. Effect of the Intake of Isoflavones on Risk Factors of Breast Cancer-A Systematic Review of Randomized Controlled Intervention Studies. Nutrients 2021, 13, 2309. [Google Scholar] [CrossRef] [PubMed]
- Caprio, A.M.; Umano, G.R.; Luongo, C.; Aiello, F.; Iacono, I.D.; Palumbo, S.; del Giudice, E.M.; Grandone, A. Case report: Goiter and overt hypothyroidism in an iodine-deficient toddler on soy milk and hypoallergenic diet. Front. Endocrinol. 2022, 13, 927726. [Google Scholar] [CrossRef]
- Fruzza, A.G.; Demeterco-Berggren, C.; Jones, K.L. Unawareness of the effects of soy intake on the management of congenital hypothyroidism. Pediatrics 2012, 130, e699–e702. [Google Scholar] [CrossRef]
- Conrad, S.C.; Chiu, H.; Silverman, B.L. Soy formula complicates management of congenital hypothyroidism. Arch. Dis. Child. 2004, 89, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Qian, H.; Wu, Z.; Li, Z.; Li, X.; Zhang, Y.; Xu, Q.; Lu, C.; Wang, X. Exploratory analysis of the associations between urinary phytoestrogens and thyroid hormones among adolescents and adults in the United States: National Health and Nutrition Examination Survey 2007–2010. Environ. Sci. Pollut. Res. 2022, 29, 2974–2984. [Google Scholar] [CrossRef] [PubMed]
- Sathyapalan, T.; Manuchehri, A.M.; Thatcher, N.J.; Rigby, A.S.; Chapman, T.; Kilpatrick, E.S.; Atkin, S.L. The effect of soy phytoestrogen supplementation on thyroid status and cardiovascular risk markers in patients with subclinical hypothyroidism: A randomized, double-blind, crossover study. J. Clin. Endocrinol. Metab. 2011, 96, 1442–1449. [Google Scholar] [CrossRef]
- Guo, P.-P.; Li, P.; Zhang, X.-H.; Liu, N.; Wang, J.; Chen, D.-D.; Sun, W.-J.; Zhang, W. Complementary and alternative medicine for natural and treatment-induced vasomotor symptoms: An overview of systematic reviews and meta-analyses. Complement. Ther. Clin. Pract. 2019, 36, 181–194. [Google Scholar] [CrossRef]
- Chen, M.N.; Lin, C.C.; Liu, C.F. Efficacy of phytoestrogens for menopausal symptoms: A meta-analysis and systematic review. Climacteric 2015, 18, 260–269. [Google Scholar] [CrossRef]
- Daily, J.W.; Ko, B.-S.; Ryuk, J.; Liu, M.; Zhang, W.; Park, S. Equol Decreases Hot Flashes in Postmenopausal Women: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Med. Food 2019, 22, 127–139. [Google Scholar] [CrossRef]
- Taku, K.; Melby, M.K.; Kronenberg, F.; Kurzer, M.S.; Messina, M. Extracted or synthesized soybean isoflavones reduce menopausal hot flash frequency and severity: Systematic review and meta-analysis of randomized controlled trials. Menopause 2012, 19, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.M.; Bell, R.J.; Rizvi, F.; Davis, S.R. Vasomotor symptoms in women in Asia appear comparable with women in Western countries: A systematic review. Menopause 2017, 24, 1313–1322. [Google Scholar] [CrossRef]
- Taku, K.; Melby, M.K.; Takebayashi, J.; Mizuno, S.; Ishimi, Y.; Omori, T.; Watanabe, S. Effect of soy isoflavone extract supplements on bone mineral density in menopausal women: Meta-analysis of randomized controlled trials. Asia Pac. J. Clin. Nutr. 2010, 19, 33–42. [Google Scholar] [PubMed]
- Corbi, G.; Nobile, V.; Conti, V.; Cannavo, A.; Sorrenti, V.; Medoro, A.; Scapagnini, G.; Davinelli, S. Equol and Resveratrol Improve Bone Turnover Biomarkers in Postmenopausal Women: A Clinical Trial. Int. J. Mol. Sci. 2023, 24, 12063. [Google Scholar] [CrossRef]
- Wu, A.H.; Yu, M.C.; Tseng, C.C.; Twaddle, N.C.; Doerge, D.R. Plasma isoflavone levels versus self-reported soy isoflavone levels in Asian-American women in Los Angeles County. Carcinogenesis 2004, 25, 77–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frankenfeld, C.L.; Lampe, J.W.; Shannon, J.; Gao, D.L.; Ray, R.M.; Prunty, J.; Kalhorn, T.F.; Wähälä, K.; E Patterson, R.; Thomas, D.B. Frequency of soy food consumption and serum isoflavone concentrations among Chinese women in Shanghai. Public Health Nutr. 2004, 7, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Kimira, M.; Arai, Y.; Shimoi, K.; Watanabe, S. Japanese intake of flavonoids and isoflavonoids from foods. J. Epidemiol. 1998, 8, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000, 130, 2243–2250. [Google Scholar] [CrossRef]
- Yamamoto, S.; Sobue, T.; Sasaki, S.; Kobayashi, M.; Tsugane, S.; Arai, Y.; Uehara, M.; Adlercreutz, H.; Watanabe, S.; Takahashi, T.; et al. Validity and reproducibility of a self-administered food-frequency questionnaire to assess isoflavone intake in a japanese population in comparison with dietary records and blood and urine isoflavones. J. Nutr. 2001, 131, 2741–2747. [Google Scholar] [CrossRef]
- Verkasalo, P.K.; Appleby, P.N.; Allen, N.E.; Davey, G.; Adlercreutz, H.; Key, T.J. Soya intake and plasma concentrations of daidzein and genistein: Validity of dietary assessment among eighty British women (Oxford arm of the European Prospective Investigation into Cancer and Nutrition). Br. J. Nutr. 2001, 86, 415–421. [Google Scholar] [CrossRef]
- Iwasaki, M.; Inoue, M.; Otani, T.; Sasazuki, S.; Kurahashi, N.; Miura, T.; Yamamoto, S.; Tsugane, S. Plasma isoflavone level and subsequent risk of breast cancer among Japanese women: A nested case-control study from the Japan Public Health Center-based prospective study group. J. Clin. Oncol. 2008, 26, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- van der Velpen, V.; Hollman, P.C.; van Nielen, M.; Schouten, E.G.; Mensink, M.; Veer, P.V.; Geelen, A. Large inter-individual variation in isoflavone plasma concentration limits use of isoflavone intake data for risk assessment. Eur. J. Clin. Nutr. 2014, 68, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Chatterjee, L.M.; Franke, A.A. Effects of isoflavone supplements vs. soy foods on blood concentrations of genistein and daidzein in adults. J. Nutr. Biochem. 2009, 20, 227–234. [Google Scholar] [CrossRef]
- Chan, S.G.; Murphy, P.A.; Ho, S.C.; Kreiger, N.; Darlington, G.; So, E.K.F.; Chong, P.Y.Y. Isoflavonoid content of Hong Kong soy foods. J. Agric. Food Chem. 2009, 57, 5386–5390. [Google Scholar] [CrossRef] [PubMed]


| Type of Product | Number of Measurements | Portion Size (g) | Mean GEN + DAI/ Portion (mg) | Standard Error of Mean/Portion | Range of Measurements * |
|---|---|---|---|---|---|
| Raw soybeans | 5 | 100 | 84.6 | 8.3 | 72.5 to 100.6 |
| Toasted soybeans | 5 | 100 | 134.0 | 37.9 | 61.9 to 247.7 |
| Edamame | 2 | 120 | 49.2 | 14.1 | 35.1 to 63.3 |
| Soybean flour | 2 | 100 | 38.5 | 5.5 | 32.9 to 43.9 |
| Protein isolate (sports) | 3 | 165 | 52.2 | 12.3 | 39.8 to 64.5 |
| Soy-based drinks | 9 | 100 | 9.9 | 1.7 | 2.8 to 17.7 |
| Soy-based dessert cream | 11 | 150 | 12.3 | 2.8 | 2.5 to 33.4 |
| Soy-based yogurt | 14 | 125 | 15.9 | 3.8 | 5.1 to 31.5 |
| Soy-based cream | 12 | 50 mL | 4.5 | 5.9 | 3.1 to 6.7 |
| Soy-based Vegan steak | 19 | 100 | 27.8 | 3.5 | 4.1 to 51.2 |
| Soy sausages | 4 | 90 | 24.8 | 7.3 | 11.1 to 44.1 |
| Soy-based raw pasta | 2 | 100 | 23.7 | 1.6 | 22.1 to 25.3 |
| Soy-based cooked pasta | 2 | 100 | 8.75 | 0.6 | 8.2 to 9.3 |
| Soy-based infant formulas | 6 | 4-month-old infants | 25.2 | 2.9 | 15.7 to 34.3 |
| Tempeh | 4 | 100 | 27.8 | 4.5 | 15.5 to 34.3 |
| Tofu | 13 | 100 | 34.8 | 5.9 | 9.4 to 79.8 |
| Soy-based cheese | 8 | 50 | 23.8 | 7.4 | 2.9 to 36.2 |
| Miso | 2 | 100 | 14.7 | 0.98 | 13.7 to 15.6 |
| Soy sauce | 9 | 10 mL | 0.16 | 0.004 | 0.16 to 0.17 |
| Location | Estimation Method | Estimated IFs Intake (mg) | IFs Plasma (nM) | Time of Plasma Collection | Study |
|---|---|---|---|---|---|
| Los Angeles | Hawaii Food Composition Database based on commercial items | 23.72 | 30.1 | No specific instruction | [73] |
| China (Shanghai) | USDA-ISU database based on commercial items | 23.5 | 106.3 | No specific instruction | [74] |
| 58.15 | 119.9 | ||||
| 84.5 | 146.3 | ||||
| 284.0 | 188.3 | ||||
| Japan | From [75] (commercial items) | 46.4 | 419.2 | Overnight fasting | [76] |
| Japan | From [75] (commercial items) | 40.8 | 411.0 | After fasting > 5h | [77] |
| England | USDA-ISU database based on commercial items | 33.7 | 510 | Not precise | [78] |
| Japan | From [75] (commercial items) | 32.5 | 553 * | No specific Instruction | [79] |
| 34.8 | 605 * | ||||
| The Netherlands | Strictly controlled soy-protein diet | 48.5 | 2160 | Fasting samples after chronic intake | [80] |
| 100.1 | 2530 | ||||
| 104.2 | 2900 | ||||
| 93.9 | 4800 | ||||
| Hawaii | USDA-ISU database based on commercial items | 96.0 | 5600 | 12h fasting and chronic intake | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bensaada, S.; Peruzzi, G.; Cubizolles, L.; Denayrolles, M.; Bennetau-Pelissero, C. Traditional and Domestic Cooking Dramatically Reduce Estrogenic Isoflavones in Soy Foods. Foods 2024, 13, 999. https://doi.org/10.3390/foods13070999
Bensaada S, Peruzzi G, Cubizolles L, Denayrolles M, Bennetau-Pelissero C. Traditional and Domestic Cooking Dramatically Reduce Estrogenic Isoflavones in Soy Foods. Foods. 2024; 13(7):999. https://doi.org/10.3390/foods13070999
Chicago/Turabian StyleBensaada, Souad, Gabriele Peruzzi, Laurent Cubizolles, Muriel Denayrolles, and Catherine Bennetau-Pelissero. 2024. "Traditional and Domestic Cooking Dramatically Reduce Estrogenic Isoflavones in Soy Foods" Foods 13, no. 7: 999. https://doi.org/10.3390/foods13070999
APA StyleBensaada, S., Peruzzi, G., Cubizolles, L., Denayrolles, M., & Bennetau-Pelissero, C. (2024). Traditional and Domestic Cooking Dramatically Reduce Estrogenic Isoflavones in Soy Foods. Foods, 13(7), 999. https://doi.org/10.3390/foods13070999