Extension of Quality and Shelf Life of Tomatoes Using Chitosan Coating Incorporated with Cinnamon Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials, Chemicals, and Reagents
2.2. Preparation of Coating Solution: Application and Storage
2.3. Quality Determination
2.3.1. Weight Loss
2.3.2. Color Characteristics
2.3.3. Firmness
2.3.4. pH, Total Soluble Solids (TSS), and Titratable Acidity (TA), and TSS:TA Ratio
2.3.5. Chlorophyll Content
2.3.6. Lycopene Content and β-Carotene
2.3.7. Ascorbic Acid (AsA) Content
2.3.8. Total Phenolic Content (TPC) and Total Antioxidant Activity (TAC)
Sample Extraction
TPC
TAC
Microbiological Analysis
2.4. Statistical Analysis
3. Results and Discussion
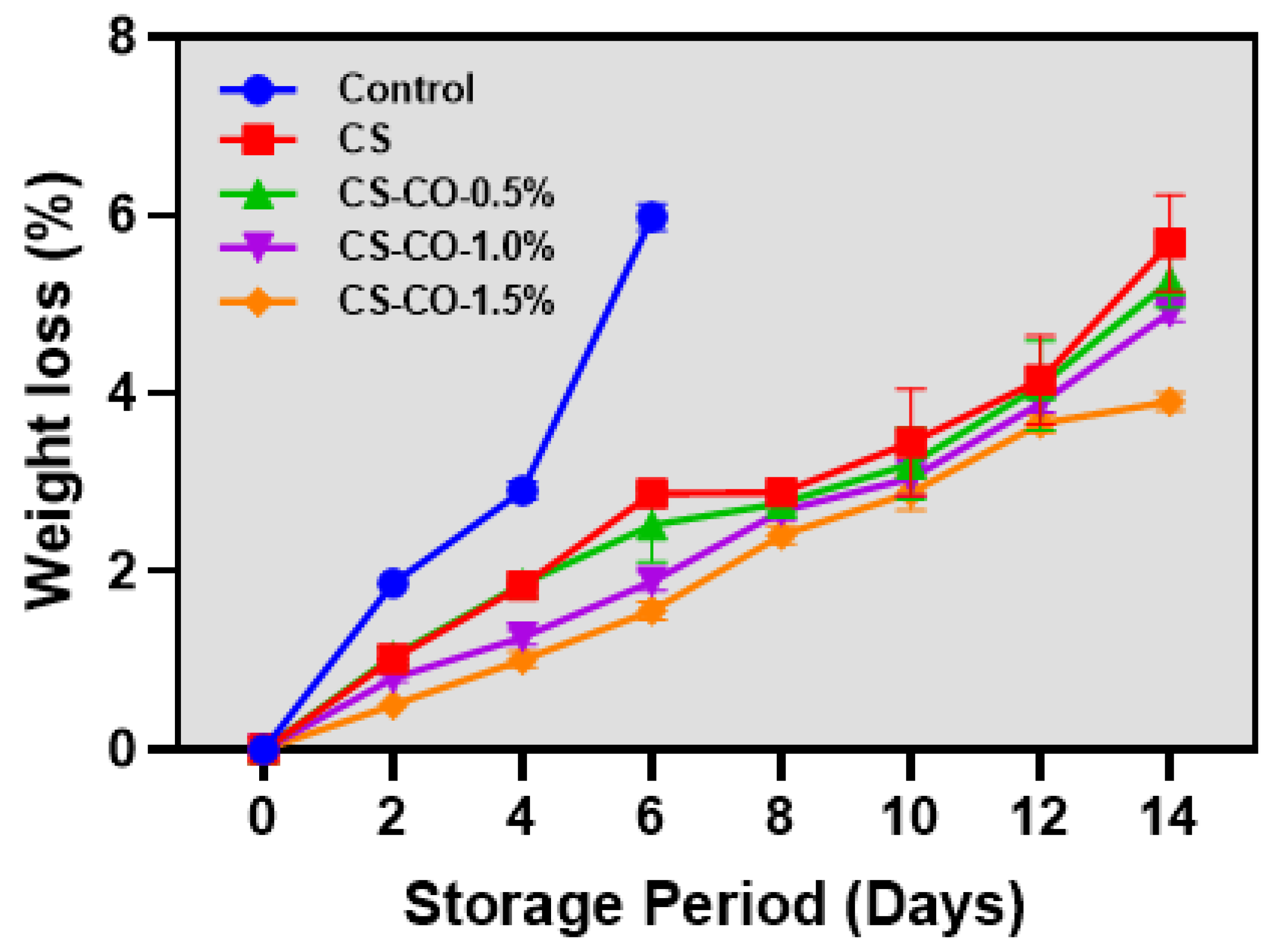
3.1. Weight Loss
3.2. Color Characteristics
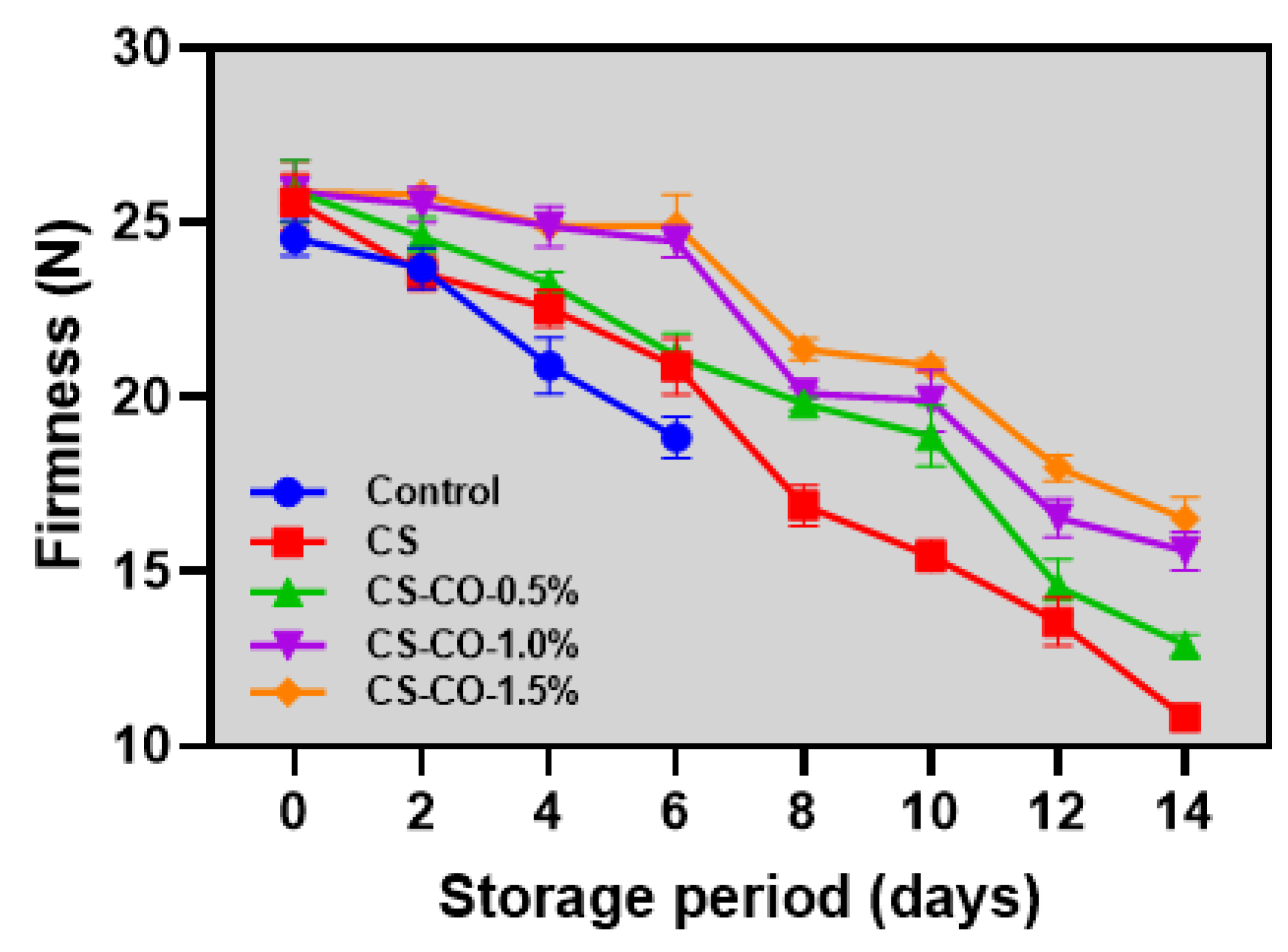
3.3. Firmness
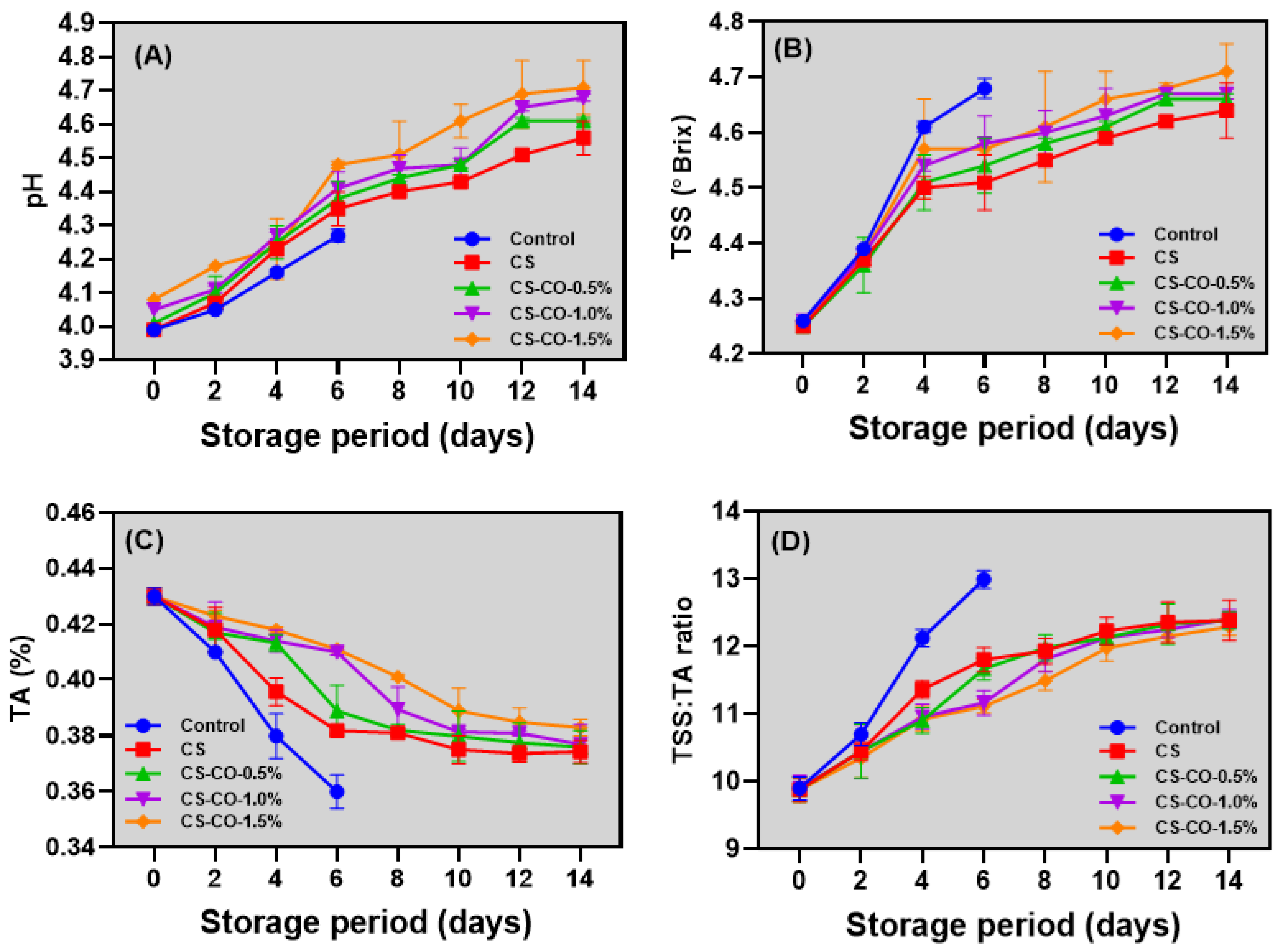
3.4. pH, TSS, TA, and TSS/TA Ratio
3.5. Chlorophyll, Lycopene Content, and β-Carotene Content
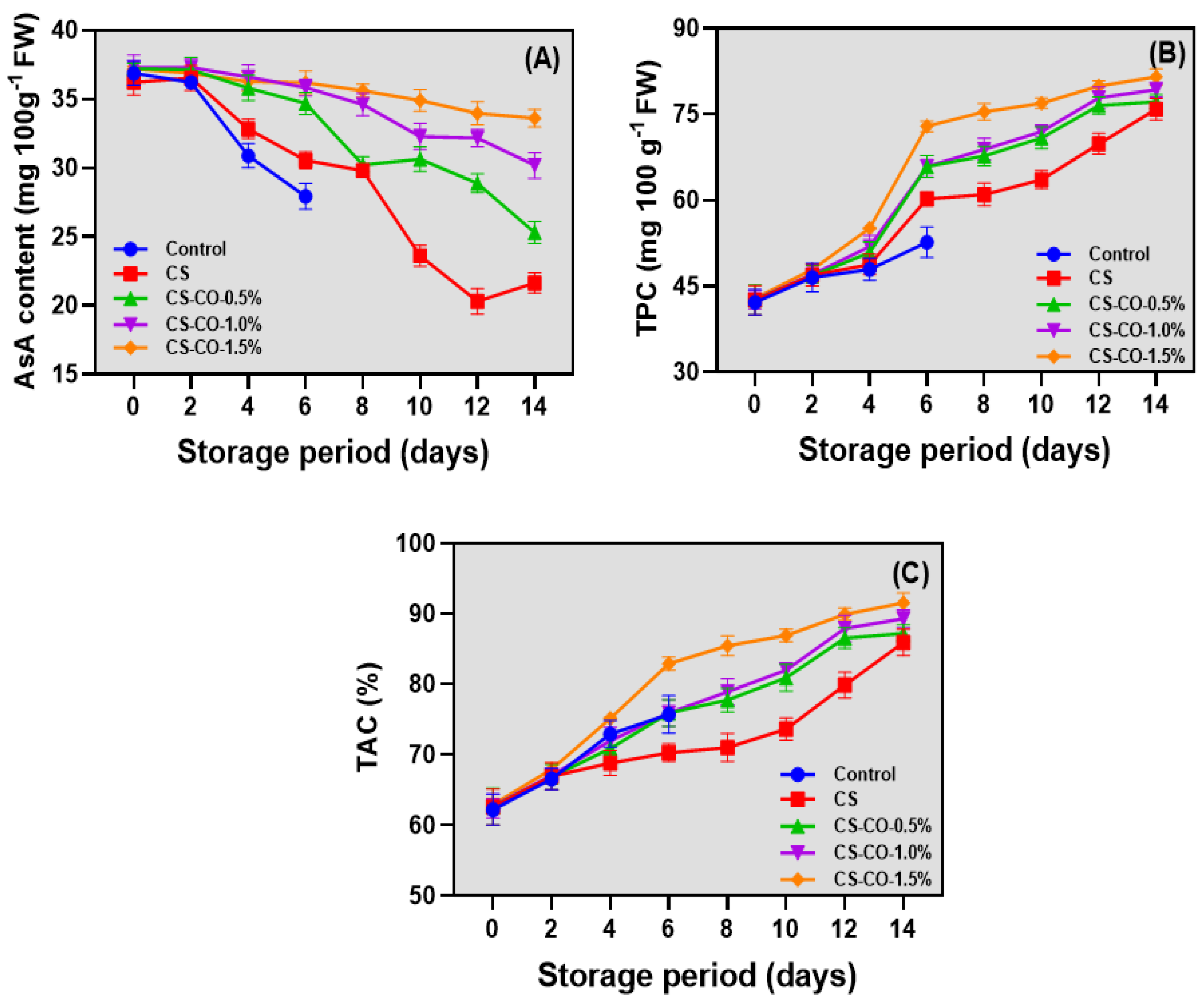
3.6. AsA, TPC, and TAC
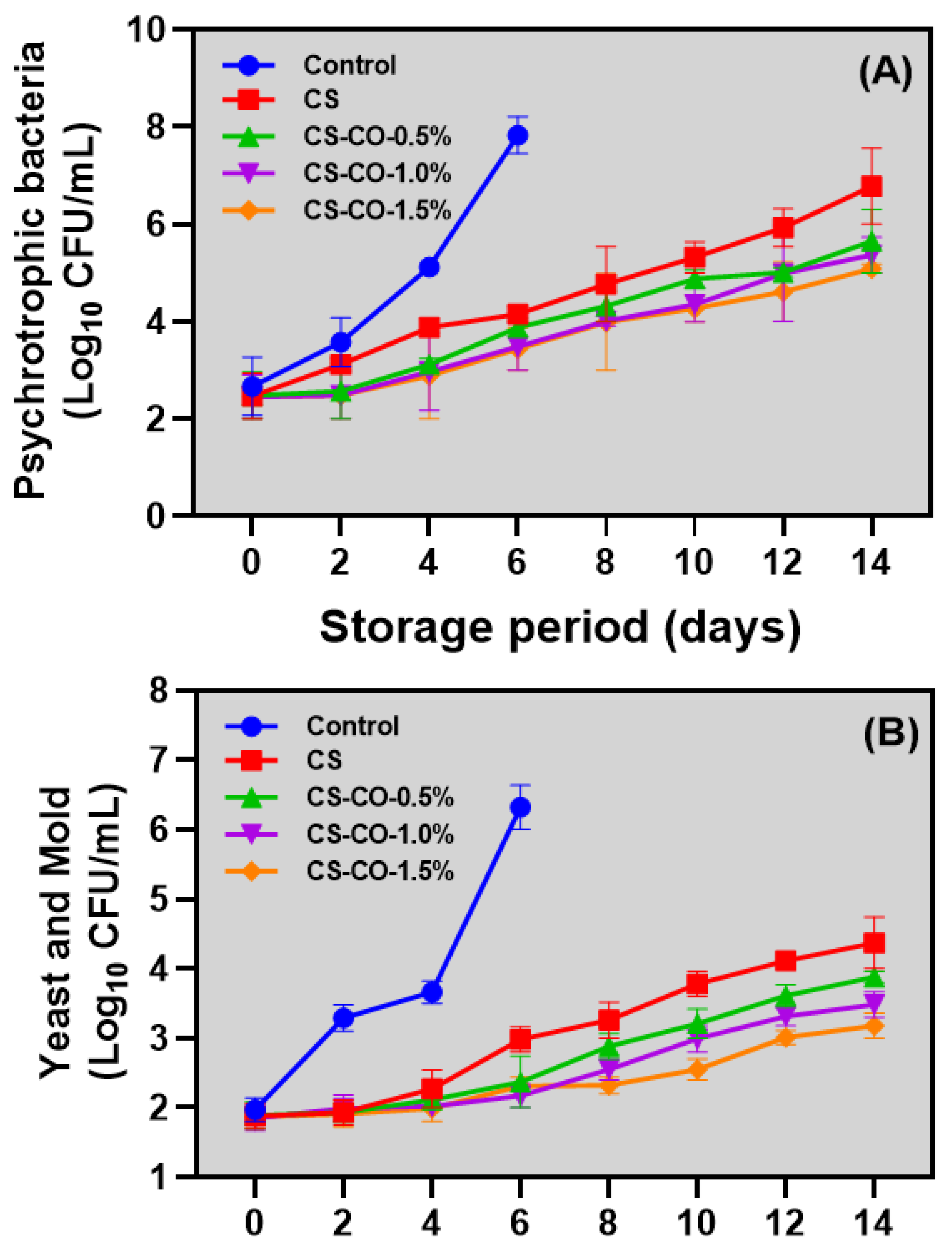
3.7. Microbial Growth
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coelho, M.C.; Rodrigues, A.S.; Texixeira, J.A.; Pintado, M.E. Integral valorization of tomato by-products towards bioactive compounds recovery: Human health benefits. Food Chem. 2023, 410, 135319. [Google Scholar] [CrossRef]
- Kumar, M.; Chandran, D.; Tomar, M.; Bhuyan, D.J.; Grasso, S.; Sa, A.G.A.; Carcifi, B.A.M.; Radha; Dhumal, S.; Singh, S.; et al. Valorization potential of tomato (Solanum lycopersicum L.) seed: Nutraceutical quality, food properties, safety aspects, and application as a health-promoting ingredient in foods. Horticulturae 2022, 8, 265. [Google Scholar] [CrossRef]
- Joshi, B.; Kar, S.K.; Yadav, P.K.; Yadav, S.; Shrestha, L.; Bera, T.K. Therapeutic and medicinal uses of lycopene: A systematic review. Int. J. Res. Med. Sci. 2020, 8, 1195. [Google Scholar] [CrossRef]
- Arah, I.K.; Ahorbo, G.K.; Anku, E.K.; Kumah, E.K.; Amaglo, H. Post-harvest handling practices and treatment methods for tomato handlers in developing countries: A mini review. Adv. Agric. 2016, 6436945. [Google Scholar]
- El-Ramady, H.R.; Domokos-Szabolcsy, E.; Abdalla, N.A.; Taha, H.S.; Fari, M. Post-harvest management of fruits and vegetables storage. Sustain. Agric. Res. 2015, 15, 65–152. [Google Scholar]
- Xi, Y.; Li, Q.; Yan, J.; Baldwin, E.; Plotto, A.; Rosskopf, E.; Hong, J.C.; Zuo, J.; Bai, J.; Li, J. Effects of harvest maturity, refrigeration and blanching treatments on the volatile profiles of ripe “Tasti-Lee” tomatoes. Foods 2021, 10, 1727. [Google Scholar] [CrossRef] [PubMed]
- Maul, F.; Sargent, S.A.; Sims, C.A.; Baldwin, E.A.; Balanban, M.O.; Huber, D.J. Tomato flavor and aroma quality as affected by storage temperature. J. Food Sci. 2000, 65, 1228–1237. [Google Scholar] [CrossRef]
- Peerzada Gh, J.; Sinclair, B.J.; Perinbarajan, G.K.; Dutta, R.; Shekhawat, R.; Saikai, N.; Chidambaram, R.; Mossa, A.T. An overview on smart and active edible coatings: Safety and regulations. Eur. Food Res. Technol. 2023, 249, 1935–1952. [Google Scholar] [CrossRef]
- Manojj, D.; Yasasve, M.; Hariharan, N.M.; Palaniven, R. Application of edible coatings and packaging materials for preservation of fruits-vegetables. In Coatings: Materials, Processes, Characterization and Optimization; Springer: Berlin/Heidelberg, Germany, 2021; pp. 59–79. [Google Scholar]
- Iber, B.T.; Kasan, N.A.; Torsabo, D.; Omuwa, J.W. A review of various sources of chitin and chitosan in nature. J. Renew. Mater. 2022, 10, 1097. [Google Scholar] [CrossRef]
- Mesa, A.; Mythatha, G.S.S.; Lodi, R.S.; Ravuri, S.; Balli, R. Chitosan nanoparticles: An overview on preparation, characterization and biomedical applications. In Nanotechnology for Advances in Medical Microbiology; Springer: Singapore, 2021; pp. 393–427. [Google Scholar]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, T.M.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- Zhang, W.; Goksen, G.; Zhou, Y.; Yang, J.; Khan, M.R.; Ahmad, N.; Fei, T. Application of a chitosan–cinnamon essential oil composite coating in inhibiting postharvest apple diseases. Foods 2023, 12, 3518. [Google Scholar] [CrossRef]
- Pinto, L.; Tapia-Rodriguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant antimicrobials for food quality and safety: Recent views and future challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef] [PubMed]
- Kacaniova, M.; Galovicova, L.; Valkova, V.; Tvrda, E.; Terentjeva, M.; Ziarovska, J.; Kunova, S.; Savitskaya, T.; Grinshpan, D.; Stefanikova, J.; et al. Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation. Open Chem. 2021, 19, 214–227. [Google Scholar] [CrossRef]
- Feng, K.; Wen, P.; Yang, H.; Li, N.; Lou, W.Y.; Zong, M.H.; Wu, H. Enhancement of the antimicrobial activity of cinnamon essential oil-loaded electrospun nanofilm by the incorporation of lysozyme. RSC Adv. 2017, 7, 1572–1580. [Google Scholar] [CrossRef]
- Golmohammadi, A.; Razavi, M.S.; Tahmasebi, M.; Carullo, D.; Farris, S. Cinnamon essential-oil-loaded fish gelatin–cellulose nanocrystal films prepared under acidic conditions. Coatings 2023, 13, 1360. [Google Scholar] [CrossRef]
- Ungureanu, C.; Tihan, G.; Zgarian, R.; Pandelea, G. Bio-coatings for preservation of fresh fruits and vegetables. Coatings 2023, 13, 1420. [Google Scholar] [CrossRef]
- Javanmardi, J.; Kubota, C. Variation of lycopene, antioxidant activity, total soluble solids and weight loss of tomato during post-harvest storage. Postharvest Biol. Technol. 2006, 41, 151–155. [Google Scholar] [CrossRef]
- Tadesse, T.N.; Ibrahim, A.M.; Abtew, W.G. Degradation and formation of fruit color in tomato (Solanum lycopersicum L.) in response to storage temperature. Am. J. Food Technol. 2015, 10, 147–157. [Google Scholar] [CrossRef]
- Alenazi, M.M.; Shafiq, M.; Alsadon, A.A.; Alhelal, I.M.; Alhamdan, A.M.; Solieman, T.H.I.; Ibrahim, A.A.; Shady, M.R.; Al-Selwey, W.A. Improved functional and nutritional properties of tomato fruit during cold storage. Saudi J. Biol. Sci. 2020, 27, 1467–1474. [Google Scholar] [CrossRef]
- Teka, T.A. Analysis of the effect of maturity stage on the post-harvest biochemical quality characteristics of tomato (Lycopersicon esculentum Mill.) fruit. Int. Res. J. Pharm. Appl. Sci. 2013, 3, 180–186. [Google Scholar]
- Tigist, M.; Workneh, T.S.; Woldetsadik, K. Effects of variety on the quality of tomato stored under ambient conditions. J. Food Sci. Technol. 2013, 50, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Ajdanian, L.; Aroiee, H.; Azizi, M.; Babaei, M. Changes in biochemical properties of tomato (cv. 240) affected by combination of blue/red optical spectra and Calfomyth spray (Ca and P). Int. J. Agric. Biol. Eng. 2020, 13, 79–84. [Google Scholar] [CrossRef]
- Navarro, J.M.; Flores, P.; Garrido, C.; Martinez, V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- Chebrolu, K.K.; Jayaprakasha, G.K.; Yoo, K.S.; Jifon, J.L.; Patil, B.S. An improved sample preparation method for quantification of ascorbic acid and dehydroascorbic acid by HPLC. LWT Food Sci. Technol. 2012, 47, 443–449. [Google Scholar] [CrossRef]
- Safari, Z.H.; Ding, P.; Sabir, A.A.; Atif, A.; Yaqubu, A.; Yusoff, S.F. Maintaining antioxidants in tomato fruit using chitosan and vanillin coating during ambient storage. Food Res. 2021, 5, 274–286. [Google Scholar] [CrossRef]
- Kaewseejan, N.; Siriamornpun, S. Bioactive components and properties of ethanolic extract and its fractions from Gynura procumbens leaves. Ind. Crop. Prod. 2015, 74, 271–278. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Giovagnoli-Vicuna, C.; Canas-Sarazua, R. Optimization of extraction yield, flavonoids and lycopene from tomato pulp by high hydrostatic pressure-assisted extraction. Food Chem. 2019, 278, 751–759. [Google Scholar] [CrossRef]
- Poubol, J.; Techavuthiporn, C.; Kanlayanarat, S. Guava fruit treated with hot water on microbiological quality of fresh-cut ‘Kimju’ and ‘Pan Srithong’ guava. Int. Food Res. J. 2018, 25, 903–907. [Google Scholar]
- Lufu, R.; Ambaw, A.; Opara, U.L. The contribution of transpiration and respiration processes in the mass loss of pomegranate fruit (cv. Wonderful). Post Harvest Biol. Technol. 2019, 157, 110982. [Google Scholar] [CrossRef]
- Zhang, D.F.; Xing, Y.G.; Xu, Q.L.; Che, Z.M.; Li, X.H.; Chen, Z.W.; Li, Z.G. Formula optimization and preservation effect of fresh-keeping agent coated with biological chitosan. J. Southeast Univ. Nat. Sci. Ed. 2012, 31, 68–72. [Google Scholar]
- Xing, Y.; Lin, H.; Cao, D.; Xu, Q.; Han, W.; Wang, R.; Che, Z.; Li, X. Effect of chitosan coating with cinnamon oil on the quality and physiological attributes of China jujube fruits. Biomed. Res. Int. 2015, 835151. [Google Scholar] [CrossRef]
- Singla, M.; Pareek, S.; Kumar, N.; Sagar, N.A.; Fawole, O.A. Chitosan-cinnamon oil coating maintains quality and extends shelf life of ready-to-use pomegranate arils under low-temperature storage. J. Food Qual. 2022, 3404691. [Google Scholar] [CrossRef]
- Gonzali, S.; Perata, P. Fruit colour and novel mechanisms of genetic regulation of pigment production in tomato fruits. Horticulturae 2021, 7, 259. [Google Scholar] [CrossRef]
- Viskelis, P.; Jankauskiene, J.; Bobinaite, R. Content of carotenoids and physical properties of tomatoes harvested at different ripening stages. Food Balt 2008, 166–170. [Google Scholar]
- Lopez Camelo, A.F.; Gomez, P.A. Comparison of color indexes for tomato ripening. Hortic. Bras. 2004, 22, 534–537. [Google Scholar] [CrossRef]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Rousos, C.; Xylia, P.; Tzortzakis, N. Vapour application of sage essential oil maintains tomato fruit quality in breaker and red ripening stages. Plants 2021, 10, 2645. [Google Scholar] [CrossRef]
- Thai, C.N.; Shewfelt, R.; Gamer, J. Tomato color changes under constant and variable storage temperatures: Empirical models. Trans. ASAE 1990, 33, 607–0614. [Google Scholar] [CrossRef]
- Zhao, Y.; McDaniel, M. Sensory quality of foods associated with edible film and coating systems and shelf-life extension. In Innovations in Food Packaging; Academic Press: Cambridge, MA, USA, 2005; pp. 434–453. [Google Scholar]
- Ruelas-Chacon, X.; Contreras-Esquivel, J.C.; Montanez, J.; Aguilera-Carbo, A.F.; Reyes-Vega, M.L.; Peralta-Rodriguez, R.D.; Sanchez-Brambila, G. Guar gum as an edible coating for enhancing shelf-life and improving post-harvest quality of Roma tomato (Solanum lycopersicum L.). J. Food Qual. 2017, 2017, 8608304. [Google Scholar] [CrossRef]
- Yang, Y.; Ge, L. Sensor coating employed to preliminarily evaluate the banana ripeness. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126057. [Google Scholar] [CrossRef]
- Alpos, M.A.; Bayogan, E.R.V. Effects of chitosan coating on the post-harvest quality and antioxidant properties of sweet pepper (Capsicum annuum L.). Philipp. J. Sci. 2023, 152, 919–929. [Google Scholar] [CrossRef]
- Choo, K.W.; Lin, M.; Mustapha, A. Chitosan/acetylated starch composite films incorporated with essential oils: Physiochemical and antimicrobial properties. Food Biosci. 2021, 43, 101287. [Google Scholar] [CrossRef]
- Acosta, S.; Chairalt, A.; Santamarina, P.; Rosello, J.; Gonzalez-martinez, C.; Chafer, M. Antifungal films based on starch-gelatin blend, containing essential oils. Food Hydrocoll. 2016, 61, 233–240. [Google Scholar] [CrossRef]
- Cofelice, M.; Cuomo, F.; Chiralt, A. Alginate films encapsulating lemongrass essential oil as affected by spray calcium application. Colloids Interfaces 2019, 3, 58. [Google Scholar] [CrossRef]
- Belay, Z.A.; Caleb, O.J.; Opara, U.L. Influence of initial gas modification on physicochemical quality attributes and molecular changes in fresh and fresh-cut fruit during modified atmosphere packaging. Food Packag. Shelf Life 2019, 21, 100359. [Google Scholar] [CrossRef]
- Dovale-Rosabal, G.; Casariego, A.; Forbes-Hernandez, T.; Garcia, M.A. Effect of chitosan-olive oil emulsion coating on quality of tomatoes during storage at ambient conditions. J. Berry Res. 2015, 5, 207–218. [Google Scholar] [CrossRef]
- Kostekli, M.; Ozdzikicerler, O.; Cortes, C.; Zulueta, A.; Esteve, M.J.; Frigola, A. Role of potassium permanganate ethylene on physicochemical properties, during storage of five different tomato cultivars. MOJ Food Process. Technol. 2016, 32, 81–289. [Google Scholar]
- Brizzolara, S.; Marganaris, G.A.; Fotopoulos, V.; Watkins, C.B.; Tonutti, P. Primary metabolism in fresh fruits during storage. Front. Plant Sci. 2020, 11, 80. [Google Scholar] [CrossRef]
- Anthon, G.E.; LeStranger, M.; Barrett, D.M. Changes in pH, acids, sugars and other quality parameters during extended vine holding of ripe processing tomatoes. J. Sci. Food Agric. 2011, 91, 1175–1181. [Google Scholar] [CrossRef]
- Amodio, M.L.; Rinaldi, R.; Colelli, G. Effects of controlled atmosphere and treatment with 1-methylcyclopropene (1-MCP) on ripening attributes of tomatoes. In V International Postharvest Symposium; International Society for Horticultural Science: Leuven, Belgium, 2004; Volume 682, pp. 737–742. [Google Scholar]
- Xing, Y.; Xu, Q.; Che, Z.; Li, X.; Li, W. Effects of chitosan-oil coating on blue mold disease and quality attributes of jujube fruits. Food Funct. 2011, 2, 466–474. [Google Scholar] [CrossRef]
- Amr, A.; Raie, W. Tomato components and quality parameters: A Review. Jordan J. Biol. Sci. 2022, 18, 199–220. [Google Scholar] [CrossRef]
- Sibomana, C.I.; Aguyoh, J.N.; Opiyo, A.M. Influence of soil moisture levels and packaging on post-harvest qualities of tomato (Solanum lycopersicum). Afr. J. Agric. Res. 2015, 10, 1392–1400. [Google Scholar]
- Munhuewyi, K. Post-Harvest Losses and Changes in Quality of Vegetables from Retail to Consumer: A Case Study of Tomato, Cabbage and Carrot. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2012. [Google Scholar]
- Park, M.H.; Sangwanangkul, P.; Baek, D.R. Changes in carotenoid and chlorophyll content of black tomatoes (Lycopersicone sculentum L.) during storage at various temperatures. Saudi J. Biol. Sci. 2018, 25, 57–65. [Google Scholar] [CrossRef]
- Mandal, D.; Lalhmingchawii, C.; Hazarika, T.K.; Shukla, A.C. Effect of chitosan, wax and particle film coating on shelf life and quality of tomato cv. Samrudhi at ambient storage. Res. J. Agric. Sci. 2018, 9, 111–116. [Google Scholar]
- Namitha, K.K.; Archana, S.N.; Negi, P.S. Expression of carotenoid biosynthetic pathway genes and changes in carotenoids during ripening in tomato (Lycopersicon esculentum). Food Funct. 2011, 2, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Hernandez, G.B.; Boluda-Aguilar, M.; Taboada-Rodriguez, A.; Soto-Jover, S.; Marin-Iniesta, F.; Lopez-Gomez, A. Processing, packaging, and storage of tomato products: Influence on the lycopene content. Food Eng. Rev. 2016, 8, 52–75. [Google Scholar] [CrossRef]
- Ronen, G.; Carmel-Goren, L.; Zamir, D.; Hirschberg, J. An alternative pathway to β-carotene formation in plant chromoplasts discovered by map-based cloning of Beta and old-gold color mutations in tomato. Proc. Natl. Acad. Sci. USA 2000, 97, 11102–11107. [Google Scholar] [CrossRef] [PubMed]
- Lopez Valenzuela, J.A.; Juarez, F.J.V.; Torres, S.L.M.; Angulo, G.L.; Garsia, M.O.V. Effect of controlled atmosphere storage on the post-harvest and nutritional quality of tomato fruit. Rev. Chapingo Ser. Hortic. 2011, 17, 115–128. [Google Scholar] [CrossRef]
- Steelheart, C.; Alegre, M.L.; Baldet, P.; Rothan, C.; Bres, C.; Just, D.; Okabe, Y.; Ezura, H.; Ganganelli, I.; Grozeff, G.E.G.; et al. The effect of low ascorbic acid content on tomato fruit ripening. Planta 2020, 252, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Moreno, C.; Ancos, L.P.B.D.; Cano, M.P. Impact of high-pressure and traditional thermal processing of tomato puree on carotenoids, vitamin C and antioxidant activity. J. Sci. Food Agric. 2006, 86, 171–179. [Google Scholar] [CrossRef]
- Sousa, B.; Rodrigues, F.; Soares, C.; Martins, M.; Azenha, M.; Lino-Neto, T.; Santos, C.; Cunha, A.; Fidalgo, F. Impact of combined heat and salt stresses on tomato plants—Insights into nutrient uptake and redox homeostasis. Antioxidants 2022, 11, 478. [Google Scholar] [CrossRef]
- Mellidou, I.; Koukounaras, A.; Kostas, S.; Patelou, E.; Kanellis, A.K. Regulation of vitamin C accumulation for improved tomato fruit quality and alleviation of abiotic stress. Genes 2021, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Cham, A.K.; Zacarias, M.D.C.O.; Saldana, H.L.; Alvarado, R.E.V.; Saenz, E.O.; Martinez-Avila, G.C.G.; Gomez, O.G.A. Potential elicitors on secondary metabolite production and antioxidant defence activity of two tomato (Solanum lycopersicum L.) varieties. Ital. J. Agron. 2021, 16, 1883. [Google Scholar] [CrossRef]
- Wang, C.; Wu, H.; Liu, Z.; Barrow, C.; Dunshea, F.; Suleria, H.A.R. Bioaccessibility and movement of phenolic compounds from tomato (Solanum lycopersicum) during in vitro gastrointestinal digestion and colonic fermentation. Food Funct. 2022, 13, 4954–4966. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.Q. Phenylalanine ammonia-lyase, a key component used for phenylpropanoids production by metabolic engineering. RSC Adv. 2015, 5, 62587–62603. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shang, S.; Yan, F.; Jiang, H.; Zhao, G.; Tian, S.; Chen, R.; Chen, D.; Dang, Y. Affiliations expand Antioxidant activities of essential oils and their major components in scavenging free radicals, inhibiting lipid oxidation and reducing cellular oxidative stress. Molecules 2023, 28, 4559. [Google Scholar] [CrossRef] [PubMed]
- Arah, I.K.; Amagle, H.; Kumah, E.K.; Ofori, H. Preharvest and post-harvest factors affecting the quality and shelf life of harvested tomatoes: A mini review. Int. J. Agron. 2015, 478041. [Google Scholar]
- Ayala-Zavala, J.F.; Oms-Oliu, G.; Odriozola-Serrano, I.; Gonzalez-Aguilar, G.A.; Alvarez-Parrilla, E.; Martin-Belloso, O. Bio-preservation of fresh-cut tomatoes using natural antimicrobials. Eur. Food Res. Technol. 2008, 226, 1047–1055. [Google Scholar] [CrossRef]
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized chitosan as an antimicrobial agent: Antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef]
- Goy, R.C.; Morias, S.T.B.; Assis, O.B.G. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sharifi-Rad, J.; Dey, A.; Koirala, N.; Shaheen, S.; Omari, N.E.; Salehi, B.; Goloshvili, T.; Silva, N.C.C.; Bouyahya, A.; et al. Cinnamomum species: Bridging phytochemistry knowledge, pharmacological properties and toxicological safety for health benefits. Front. Pharmacol. 2021, 12, 600139. [Google Scholar] [CrossRef] [PubMed]
- Sarengaowa; Wang, L.; Liu, Y.; Yang, C.; Feng, K.; Hu, W. Screening of essential oils and effect of a chitosan-based edible coating containing cinnamon oil on the quality and microbial safety of fresh-cut potatoes. Coating 2022, 12, 1492. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 12, 198–203. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatachalam, K.; Lekjing, S.; Noonim, P.; Charoenphun, N. Extension of Quality and Shelf Life of Tomatoes Using Chitosan Coating Incorporated with Cinnamon Oil. Foods 2024, 13, 1000. https://doi.org/10.3390/foods13071000
Venkatachalam K, Lekjing S, Noonim P, Charoenphun N. Extension of Quality and Shelf Life of Tomatoes Using Chitosan Coating Incorporated with Cinnamon Oil. Foods. 2024; 13(7):1000. https://doi.org/10.3390/foods13071000
Chicago/Turabian StyleVenkatachalam, Karthikeyan, Somwang Lekjing, Paramee Noonim, and Narin Charoenphun. 2024. "Extension of Quality and Shelf Life of Tomatoes Using Chitosan Coating Incorporated with Cinnamon Oil" Foods 13, no. 7: 1000. https://doi.org/10.3390/foods13071000
APA StyleVenkatachalam, K., Lekjing, S., Noonim, P., & Charoenphun, N. (2024). Extension of Quality and Shelf Life of Tomatoes Using Chitosan Coating Incorporated with Cinnamon Oil. Foods, 13(7), 1000. https://doi.org/10.3390/foods13071000








