Effect of Fucoidan on Structure and Bioactivity of Chinese Steamed Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Dough
2.3. Preparation of Chinese Steamed Bread (CSB)
2.4. Dough Properties
2.4.1. Rheological Properties
2.4.2. Determination of Free Sulfhydryl (SH) and Disulfide Bond (SS) Content
2.4.3. Determination of Expansion Volume of Dough
2.5. Textural Property Analysis (TPA)
2.6. Specific Volume of Chinese Steamed Bread (CSB)
2.7. Fourier Transform Infrared (FT-IR) Spectroscopy
2.8. X-ray Diffraction (XRD)
2.9. Scanning Electron Microscope (SEM)
2.10. In Vitro Starch Digestibility of CSB
2.11. Total Starch Test
2.12. Kinetics of Starch Hydrolysis
2.13. Fluorescence Quenching
2.14. Antioxidant Activity
2.14.1. DPPH· Radical Scavenging Activity
2.14.2. ABTS· Radical Scavenging Activity
2.15. Sensory Analysis
2.15.1. Electronic Tongue Analysis
2.15.2. Descriptive Sensory Analysis
2.16. Statistical Analysis
3. Results and Discussion
3.1. Effect of Fucoidan on the Rheological Properties of Dough
3.2. Analysis of Free Sulfhydryl (SH), Disulfide Bond (SS) Content and Expansion Volume
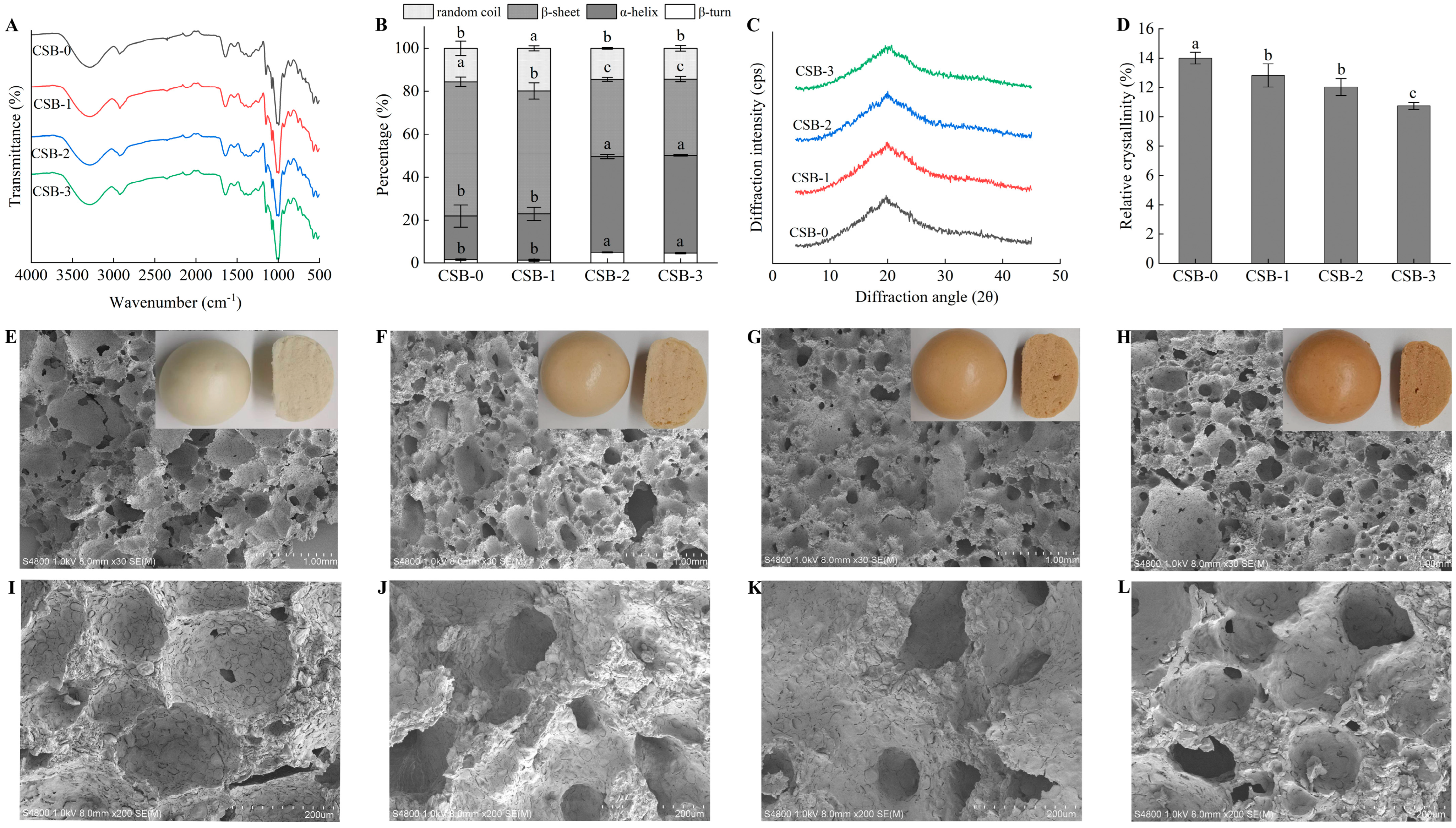
3.3. Effect of Fucoidan Addition on Structure Properties of CSB
3.3.1. Specific Volume and Textural Property Analysis (TPA) of CSB
3.3.2. Secondary Structure Analysis
3.3.3. Crystalline Structure Analysis
3.3.4. Microstructure Analysis
3.4. Effects of Fucoidan of on the Functional Properties and Sensory of CSB
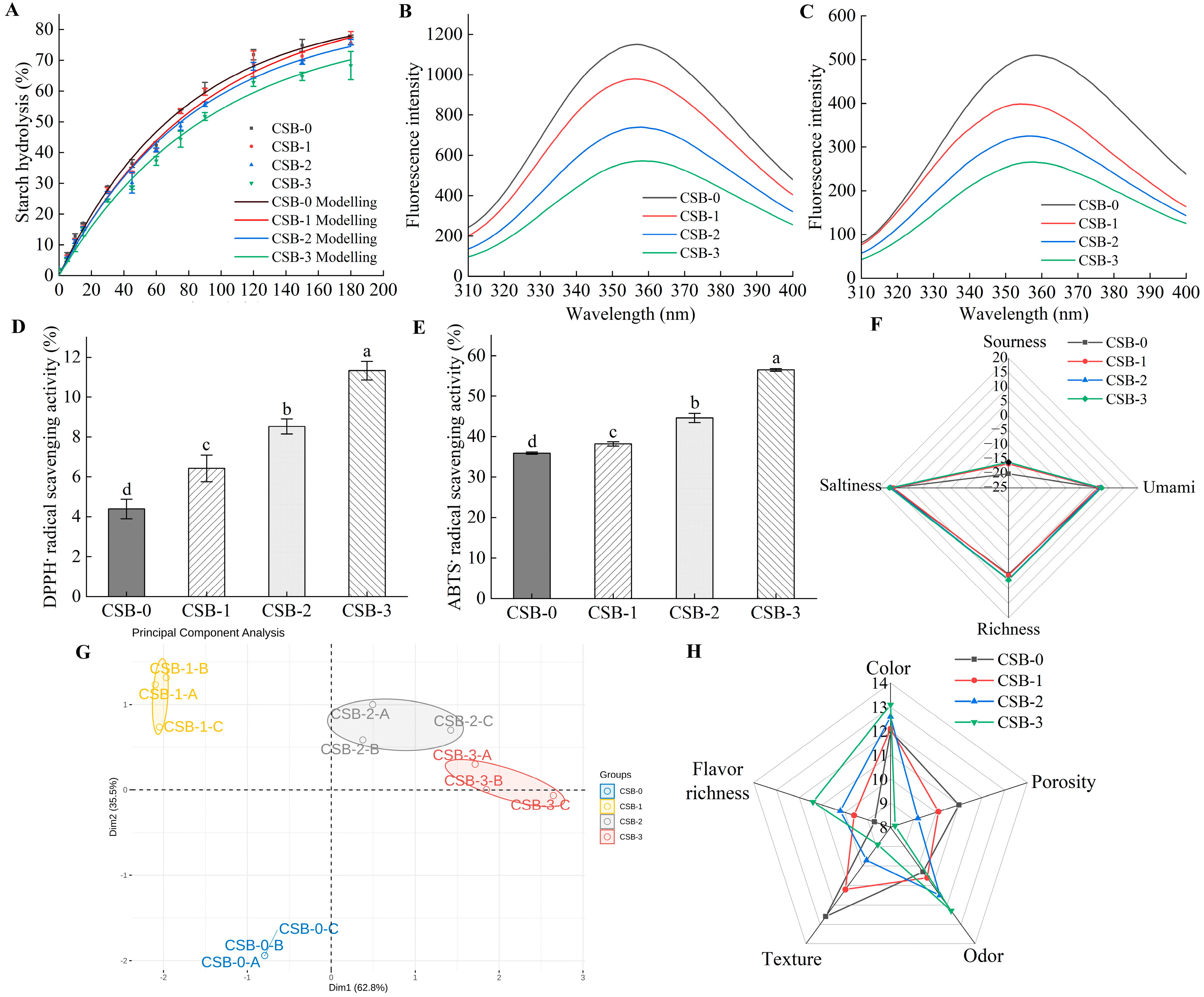
3.4.1. In Vitro Starch Digestibility of CSB
3.4.2. Antioxidant Activity of CSB Analysis
3.4.3. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, F. Staling of Chinese steamed bread: Quantification and control. Trends Food Sci. Technol. 2016, 55, 118–127. [Google Scholar] [CrossRef]
- Zhu, F. Frozen steamed breads and boiled noodles: Quality affected by ingredients and processing. Food Chem. 2021, 349, 129178. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xiong, W.; Wang, L.; Ju, X. Insight into the effect of gluten-starch ratio on the properties of Chinese steamed bread (Mantou). Int. J. Biol. Macromol. 2020, 163, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Glycemic control in Chinese steamed bread: Strategies and opportunities. Trends Food Sci. Technol. 2019, 86, 252–259. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Shi, Y. The Types, Regional Distribution, and Consumption Trend of Chinese Traditional Wheat-Based Foods. J. Food Qual. 2022, 2022, 9986119. [Google Scholar] [CrossRef]
- Syu, P.C.; Zhang, Q.F.; Lin, S.D. Physicochemical, Antioxidant, Sensory, and Starch Digestibility Properties of Steamed Bread Fortified with Tamarillo Powder. Foods 2023, 12, 2306. [Google Scholar] [CrossRef]
- Ma, R.C.W. Epidemiology of diabetes and diabetic complications in China. Diabetologia 2018, 61, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lau, E.S.H.; Wu, H.; Yang, A.; Chow, E.; Kong, A.P.S.; Ma, R.C.W.; Chan, J.C.N.; Luk, A.O.Y. Higher incidence of cardiovascular-kidney complications in Chinese with youth-onset type 2 diabetes versus youth-onset type 1 diabetes attenuated by control of cardio-metabolic risk factors: A population-based prospective cohort study in Hong Kong. Diabetes Res. Clin. Pract. 2023, 202, 110728. [Google Scholar] [CrossRef]
- Silva, M.M.C.L.; dos Santos Lisboa, L.; Paiva, W.S.; Batista, L.A.N.C.; Luchiari, A.C.; Rocha, H.A.O.; Camara, R.B.G. Comparison of in vitro and in vivo antioxidant activities of commercial fucoidans from Macrocystis pyrifera, Undaria pinnatifida, and Fucus vesiculosus. Int. J. Biol. Macromol. 2022, 216, 757–767. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lim, S.E.V.; Lu, J.; Zhou, W. Bioactivity enhancement of fucoidan through complexing with bread matrix and baking. LWT Food Sci. Technol. 2020, 130, 109646. [Google Scholar] [CrossRef]
- Wardani, G.; Nugraha, J.; Kurnijasanti, R.; Mustafa, M.R.; Sudjarwo, S.A. Molecular Mechanism of Fucoidan Nanoparticles as Protector on Endothelial Cell Dysfunction in Diabetic Rats’ Aortas. Nutrients 2023, 15, 568. [Google Scholar] [CrossRef]
- Koh, H.; Lu, J.; Zhou, W. Structural Dependence of Sulfated Polysaccharide for Diabetes Management: Fucoidan from Undaria pinnatifida Inhibiting α-Glucosidase More Strongly Than α-Amylase and Amyloglucosidase. Front. Pharmacol. 2020, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Ou, Y.; Lan, X.; Tang, J.; Zheng, B. Effects of laminarin on the structural properties and in vitro digestion of wheat starch and its application in noodles. LWT Food Sci. Technol. 2023, 178, 114543. [Google Scholar] [CrossRef]
- Nguyen, A.N.; Van Ngo, Q.; Quach, T.T.M.; Ueda, S.; Yuguchi, Y.; Matsumoto, Y.; Kitamura, S.; Ho, C.D.; Thanh, T.T.T. Fucoidan from brown seaweed Tubinaria decurrens: Structure and structure—Anticancer activity relationship. Int. J. Biol. Macromol. 2024, 259, 129326. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, A.; Nadeem, M.; Ahmad, R.; Ahmed, Z.; Khalid, N. Recent advances in applications of marine hydrocolloids for improving bread quality. Food Hydrocoll. 2024, 148, 109424. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Sun, B.; Ma, S. Interaction of wheat bran dietary fiber-gluten protein affects dough product: A critical review. Int. J. Biol. Macromol. 2024, 255, 128199. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.R.; Zhu, Z.Y.; Zhang, X.J.; Zhu, Y.M. Effects of Cordyceps polysaccharides on pasting properties and in vitro starch digestibility of wheat starch. Food Hydrocoll. 2020, 102, 105604. [Google Scholar] [CrossRef]
- Hong, T.; Wang, L.; Xu, Y.; Jin, Y.; Xu, D.; Wu, F.; Xu, X. Comparative study of soluble soybean polysaccharides on bread staling under acidic conditions. Food Chem. 2023, 400, 133950. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Liu, J.; Gan, S.; Xiong, G.; Qiao, F.; Mo, W.; Song, Y.; Fu, X.; Liu, C.; Lin, Q. Effects of soluble soybean polysaccharide on cooking and eating quality of dry rice noodles under single- and twin-screw extrusions. LWT Food Sci. Technol. 2023, 187, 115352. [Google Scholar] [CrossRef]
- Hamdani, A.M.; Wani, I.A.; Bhat, N.A.; Maqbool, K.; Mir, S.A. Effect of apricot, guar and locust bean gum hydrocolloids on pasting, antioxidant, rheology, thermal and sensory properties of gluten-free breads. Bioact. Carbohydr. Diet. Fibre 2024, 31, 100397. [Google Scholar] [CrossRef]
- Cao, Y.; Jiang, L.; Suo, W.; Deng, Y.; Zhang, M.; Dong, S.; Guo, P.; Chen, S.; Li, H. Influence of emulsifiers and enzymes on dough rheological properties and quality characteristics of steamed bread enriched with potato pulp. Food Chem. 2021, 360, 130015. [Google Scholar] [CrossRef]
- Beveridge, T.; Toma, S.J.; Nakai, S. Determination of SH- and SS-groups in some food proteins using Ellman’s Reagent. J. Food Sci. 1974, 39, 49–51. [Google Scholar] [CrossRef]
- Wang, P.; Lee, T.C.; Xu, X.; Jin, Z. The contribution of glutenin macropolymer depolymerization to the deterioration of frozen steamed bread dough quality. Food Chem. 2016, 211, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.J.; Guo, X.N.; Xing, J.J.; Zhu, K.X. Alleviative effects of chitooligosaccharides on the quality deterioration of frozen dough subjected to freeze–thaw cycles. Food Hydrocoll. 2023, 144, 109016. [Google Scholar] [CrossRef]
- Bouaziz, F.; Ben Abdeddayem, A.; Koubaa, M.A.O.; Ellouz Ghorbel, R.; Ellouz Chaabouni, S. Date Seeds as a Natural Source of Dietary Fibers to Improve Texture and Sensory Properties of Wheat Bread. Foods 2020, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yue, Y.; Liu, L.; Tong, L.; Wang, L.; Ashraf, J.; Li, N.; Zhou, X.; Zhou, S. Investigation of combined effects of xylanase and glucose oxidase in whole wheat buns making based on reconstituted model dough system. LWT Food Sci. Technol. 2021, 135, 110261. [Google Scholar] [CrossRef]
- Sheng, X.; Ma, Z.; Li, X.; Liu, L.; Hu, X. Effect of water migration on the thermal-vacuum packaged steamed buns under room temperature storage. J. Cereal Sci. 2016, 72, 117–123. [Google Scholar] [CrossRef]
- Gao, J.; Tan, E.Y.N.; Low, S.H.L.; Wang, Y.; Ying, J.; Dong, Z.; Zhou, W. From bolus to digesta: How structural disintegration affects starch hydrolysis during oral-gastro-intestinal digestion of bread. J. Food Eng. 2021, 289, 110161. [Google Scholar] [CrossRef]
- Koh, H.S.; Chong, J.E.; Lu, J.; Zhou, W. Fucoidan Regulates Starch Digestion: In Vitro and Mechanistic Study. Foods 2022, 11, 427. [Google Scholar] [CrossRef]
- Liu, X.; Lu, K.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. Effect of purple yam flour substitution for wheat flour on in vitro starch digestibility of wheat bread. Food Chem. 2019, 284, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Z.; Cheng, C.; Yao, L.; Wang, L.; Lu, W.; Yang, X.; Ma, F. In-Vitro free radical scavenging activities of anthocyanins from three berries. J. Med. Plants Res. 2012, 5, 7036–7042. [Google Scholar] [CrossRef]
- Feng, Y.; Xin, G.; Wei, Y.; Xu, H.; Sun, L.; Hou, Z.; Sun, B. Comparison of the umami taste and aroma of dried Suillus granulatus packed using four different packaging methods. Food Chem. 2022, 366, 130570. [Google Scholar] [CrossRef]
- Sasue, A.; Kasim, Z.M.; Zubairi, S.I. Evaluation of phytochemical, nutritional and sensory properties of high fibre bun developed by utilization of Kappaphycus alvarezii seaweed powder as a functional ingredient. Arab. J. Chem. 2023, 16, 104953. [Google Scholar] [CrossRef]
- Chang, X.; Liu, H.; Zhuang, K.; Chen, L.; Zhang, Q.; Chen, X.; Ding, W. Study on the Quality Variation and Internal Mechanisms of Frozen Oatmeal Cooked Noodles during Freeze–Thaw Cycles. Foods 2024, 13, 541. [Google Scholar] [CrossRef]
- Shao, T.; Ma, X.-y.; Zhang, Y.-y.; Wu, R.; Wang, X.; Gu, R.-X.; Chen, X. Physical and nutritional properties of Chinese Steamed Bun (mantou) made with fermented soy milk. LWT Food Sci. Technol. 2023, 183, 114849. [Google Scholar] [CrossRef]
- Zhao, Q.; She, Z.; Hou, D.; Wang, J.; Lan, T.; Lv, X.; Zhang, Y.; Sun, X.; Ma, T. Effect of partial substitution of wheat flour with kiwi starch on dough rheology, microstructure, the quality attributes and shelf life of Chinese steamed bread. Int. J. Biol. Macromol. 2024, 258, 128920. [Google Scholar] [CrossRef]
- Min, C.; Zhang, C.; Cao, Y.; Li, H.; Pu, H.; Huang, J.; Xiong, Y.L. Rheological, textural, and water-immobilizing properties of mung bean starch and flaxseed protein composite gels as potential dysphagia food: The effect of Astragalus polysaccharide. Int. J. Biol. Macromol. 2023, 239, 124236. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, P.; Deng, C.; Xu, L.; Yu, M.; Yang, W.; Zhao, R.; Li, B. Effects of Pleurotus eryngii (mushroom) powder and soluble polysaccharide addition on the rheological and microstructural properties of dough. Food Sci. Nutr. 2019, 7, 2113–2122. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, J.; Tu, J.; Yu, L.; Niu, L. The alleviative effect of sweet potato protein hydrolysates on the quality deterioration of frozen dough bread in comparison to trehalose. LWT Food Sci. Technol. 2023, 175, 114505. [Google Scholar] [CrossRef]
- Liu, Y.; Leng, Y.; Xiao, S.; Zhang, Y.; Ding, W.; Ding, B.; Wu, Y.; Wang, X.; Fu, Y. Effect of inulin with different degrees of polymerization on dough rheology, gelatinization, texture and protein composition properties of extruded flour products. LWT Food Sci. Technol. 2022, 159, 113225. [Google Scholar] [CrossRef]
- Du, Y.; Li, W.; Mariga, A.M.; Fang, Y.; Sun, X.; Hu, Q.; Pei, F. Effect of Auricularia auricula polysaccharide on characteristic structure, rheological properties, and tensile texture in whole wheat dough. J. Food Process. Preserv. 2022, 46, e17068. [Google Scholar] [CrossRef]
- Song, Y.; Zheng, Q. Dynamic rheological properties of wheat flour dough and proteins. Trends Food Sci. Technol. 2007, 18, 132–138. [Google Scholar] [CrossRef]
- Nirmala Prasadi, V.P.; Joye, I.J. Effect of soluble dietary fibre from barley on the rheology, water mobility and baking quality of wheat flour dough. J. Cereal Sci. 2023, 112, 103715. [Google Scholar] [CrossRef]
- Liu, J.; Luo, D.; Li, X.; Xu, B.; Zhang, X.; Liu, J. Effects of inulin on the structure and emulsifying properties of protein components in dough. Food Chem. 2016, 210, 235–241. [Google Scholar] [CrossRef]
- Al-Ansi, W.; Abdullah, A.; Alkawry, T.; Fadhl, J.; Abdulqader, A.A.; Mahdi, A.; AlMaqtari, Q.; Fan, M.; Li, Y.; Qian, H.; et al. The alterations in the thermomechanical, rheological, and microstructural properties of highland barley dough as affected by fermentation time. J. Food Meas. Charact. 2023, 17, 6065–6076. [Google Scholar] [CrossRef]
- Li, J.; Yadav, M.P.; Li, J. Effect of different hydrocolloids on gluten proteins, starch and dough microstructure. J. Cereal Sci. 2019, 87, 85–90. [Google Scholar] [CrossRef]
- Zeng, F.; Hu, Z.; Yang, Y.; Jin, Z.; Jiao, A. Regulation of baking quality and starch digestibility in whole wheat bread based on β-glucans and protein addition strategy: Significance of protein-starch-water interaction in dough. Int. J. Biol. Macromol. 2024, 256, 128021. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.; Yang, Q.; Huang, J.; Cao, Y.; Pu, H.; Ma, W.; Min, C.; Xiong, Y.L. Curdlan-induced rheological, thermal and structural property changes of wheat dough components during heat treatment. J. Cereal Sci. 2022, 107, 103528. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, Y.; Mu, M.; Hao, Z.; Zhang, J.; Wang, Q.; Liang, Y.; Wang, J. Trehalose-induced changes in the aggregation behavior and structural properties of wheat gluten. J. Cereal Sci. 2022, 108, 103568. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Wan, H.; Zhang, M. Inherent molecular characteristics and effect of garlic polysaccharides on dough micro- and mesoscopic properties. Food Chem. X 2023, 19, 100757. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, Z.; Zhang, H.; Guo, P.; Dong, S.; Li, H. Influence of potato pulp on gluten network structure in wheat dough and steamed bread. Cereal Chem. 2020, 97, 226–234. [Google Scholar] [CrossRef]
- Zhao, K.; Jia, Z.; Hou, L.; Xiao, S.; Yang, H.; Ding, W.; Wei, Y.; Wu, Y.; Wang, X. Study on physicochemical properties and anti-aging mechanism of wheat starch by anionic polysaccharides. Int. J. Biol. Macromol. 2023, 253, 127431. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Ou, Y.; Wang, J.; Zheng, B. Structure-digestibility relationships in the effect of fucoidan on A- and B-wheat starch. Int. J. Biol. Macromol. 2022, 215, 235–242. [Google Scholar] [CrossRef]
- Peng, P.; Wang, X.; Zou, X.; Zhang, X.; Hu, X. Dynamic behaviors of protein and starch and interactions associated with glutenin composition in wheat dough matrices during sequential thermo-mechanical treatments. Food Res. Int. 2022, 154, 110986. [Google Scholar] [CrossRef]
- Wu, X.; Ding, H.; Hu, X.; Pan, J.; Liao, Y.; Gong, D.; Zhang, G. Exploring inhibitory mechanism of gallocatechin gallate on a-amylase and a-glucosidase relevant to postprandial hyperglycemia. J. Funct. Foods 2018, 48, 200–209. [Google Scholar] [CrossRef]
- Zhao, M.; Bai, J.; Bu, X.; Yin, Y.; Wang, L.; Yang, Y.; Xu, Y. Characterization of selenized polysaccharides from Ribes nigrum L. and its inhibitory effects on α-amylase and α-glucosidase. Carbohydr. Polym. 2021, 259, 117729. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Han, J.H.; You, S. Inhibitory effects of fucan sulfates on enzymatic hydrolysis of starch. LWT Food Sci. Technol. 2011, 44, 1164–1171. [Google Scholar] [CrossRef]
- Lal, M.K.; Singh, B.; Sharma, S.; Singh, M.P.; Kumar, A. Glycemic index of starchy crops and factors affecting its digestibility: A review. Trends Food Sci. Technol. 2021, 111, 741–755. [Google Scholar] [CrossRef]
- Mohanta, B.; Sen, D.J.; Mahanti, B.; Nayak, A.K. Extraction, characterization, haematocompatibility and antioxidant activity of linseed polysaccharide. Carbohydr. Polym. Technol. Appl. 2023, 5, 100321. [Google Scholar] [CrossRef]
- Mirzadeh, M.; Arianejad, M.R.; Khedmat, L. Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohydr. Polym. 2020, 229, 115421. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Mu, T.; Zhou, L. Identification of saprophytic microorganisms and analysis of changes in sensory, physicochemical, and nutritional characteristics of potato and wheat steamed bread during different storage periods. Food Chem. 2021, 348, 128927. [Google Scholar] [CrossRef]
- Li, Z.; Song, K.; Li, H.; Ma, R.; Cui, M. Effect of mixed Saccharomyces cerevisiae Y10 and Torulaspora delbrueckii Y22 on dough fermentation for steamed bread making. Int. J. Food Microbiol. 2019, 303, 58–64. [Google Scholar] [CrossRef]

| Descriptors | Sensory Score |
|---|---|
| Color | Low vividness (0–4) More vividness (5–10) Strong vividness (11–15) |
| Porosity of the crumb (pore size) | Small (0–4) Comparatively small (5–10) Big (11–15) |
| Odor | CSB with weak odor (0–4) CSB with Comparatively weak odor (5–10) CSB with strong odor (11–15) |
| Texture (by feeling it with hands) (a) Softness-force required to compress sample between fingers; (b) Springiness-swiftness of returning to the initial shape after moderate pressure applied to the center of the CSB; (c) Crookedness-presence of cracks on the surface of the samples | Poor texture (0–4) Good texture (5–10) Better texture (11–15) |
| Flavor richness (Eating a variety of flavor in a CSB) | Weak (0–4) Weaker (5–10) Strong (11–15) |
| Sample | SH (μmol/g) | SS (μmol/g) | Expansion Volume (mL) |
|---|---|---|---|
| FWD-0 | 4.14 ± 0.06 d | 59.80 ± 1.09 a | 48.67 ± 4.16 a |
| FWD-1 | 4.26 ± 0.02 c | 59.28 ± 0.86 a | 46.00 ± 2.00 ab |
| FWD-2 | 4.50 ± 0.04 b | 51.23 ± 2.93 b | 42.67 ± 2.31 b |
| FWD-3 | 4.78 ± 0.06 a | 50.99 ± 3.22 b | 37.33 ± 1.15 c |
| Sample | Hardness (g) | Springiness | Gumminess | Chewiness | Specific Volume (cm3/g) |
|---|---|---|---|---|---|
| CSB-0 | 252.67 ± 11.12 c | 4.76 ± 0.15 a | 219.17 ± 14.31 c | 10.22 ± 0.69 c | 2.48 ± 0.26 a |
| CSB-1 | 439.00 ± 55.00 b | 3.86 ± 0.10 c | 395.00 ± 47.18 b | 15.13 ± 1.88 b | 1.95 ± 0.07 b |
| CSB-2 | 491.83 ± 69.03 ab | 4.08 ± 0.40 bc | 418.00 ± 33.00 b | 17.22 ± 1.53 b | 2.12 ± 0.12 b |
| CSB-3 | 549.00 ± 24.07 a | 4.31 ± 0.12 b | 487.07 ± 19.31 a | 20.59 ± 0.68 a | 2.01 ± 0.10 b |
| CSB-0 | CSB-1 | CSB-2 | CSB-3 | |
|---|---|---|---|---|
| C∞ (%) | 88.70 ± 0.92 a | 87.34 ± 2.31 a | 88.63± 0.91 a | 81.47 ± 5.31 b |
| K (10−2 cm−1) | 1.24 ± 0.04 a | 1.19 ± 0.06 ab | 1.08 ± 0.04 b | 1.08 ± 0.10 b |
| R2 | 0.9997 | 0.9992 | 0.9995 | 0.9993 |
| HI/% | 99.49 ± 0.52 a | 96.17 ± 0.46 b | 92.77 ± 0.85 c | 85.12 ± 1.10 d |
| pGI | 94.33 ± 0.29 a | 92.51 ± 0.25 b | 90.64 ± 0.47 c | 86.44 ± 0.60 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Li, M.; Gu, C.; Lu, A.; Dong, L.; Zhang, X.; Hu, X.; Liu, Y.; Lu, J. Effect of Fucoidan on Structure and Bioactivity of Chinese Steamed Bread. Foods 2024, 13, 1057. https://doi.org/10.3390/foods13071057
Yang Q, Li M, Gu C, Lu A, Dong L, Zhang X, Hu X, Liu Y, Lu J. Effect of Fucoidan on Structure and Bioactivity of Chinese Steamed Bread. Foods. 2024; 13(7):1057. https://doi.org/10.3390/foods13071057
Chicago/Turabian StyleYang, Qingyu, Man Li, Chenqi Gu, Anni Lu, Lijun Dong, Xiling Zhang, Xiufa Hu, Yao Liu, and Jun Lu. 2024. "Effect of Fucoidan on Structure and Bioactivity of Chinese Steamed Bread" Foods 13, no. 7: 1057. https://doi.org/10.3390/foods13071057
APA StyleYang, Q., Li, M., Gu, C., Lu, A., Dong, L., Zhang, X., Hu, X., Liu, Y., & Lu, J. (2024). Effect of Fucoidan on Structure and Bioactivity of Chinese Steamed Bread. Foods, 13(7), 1057. https://doi.org/10.3390/foods13071057







