Changes in Novel Biomarkers for Protein Oxidation in Pork Patties under Different Cooking Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
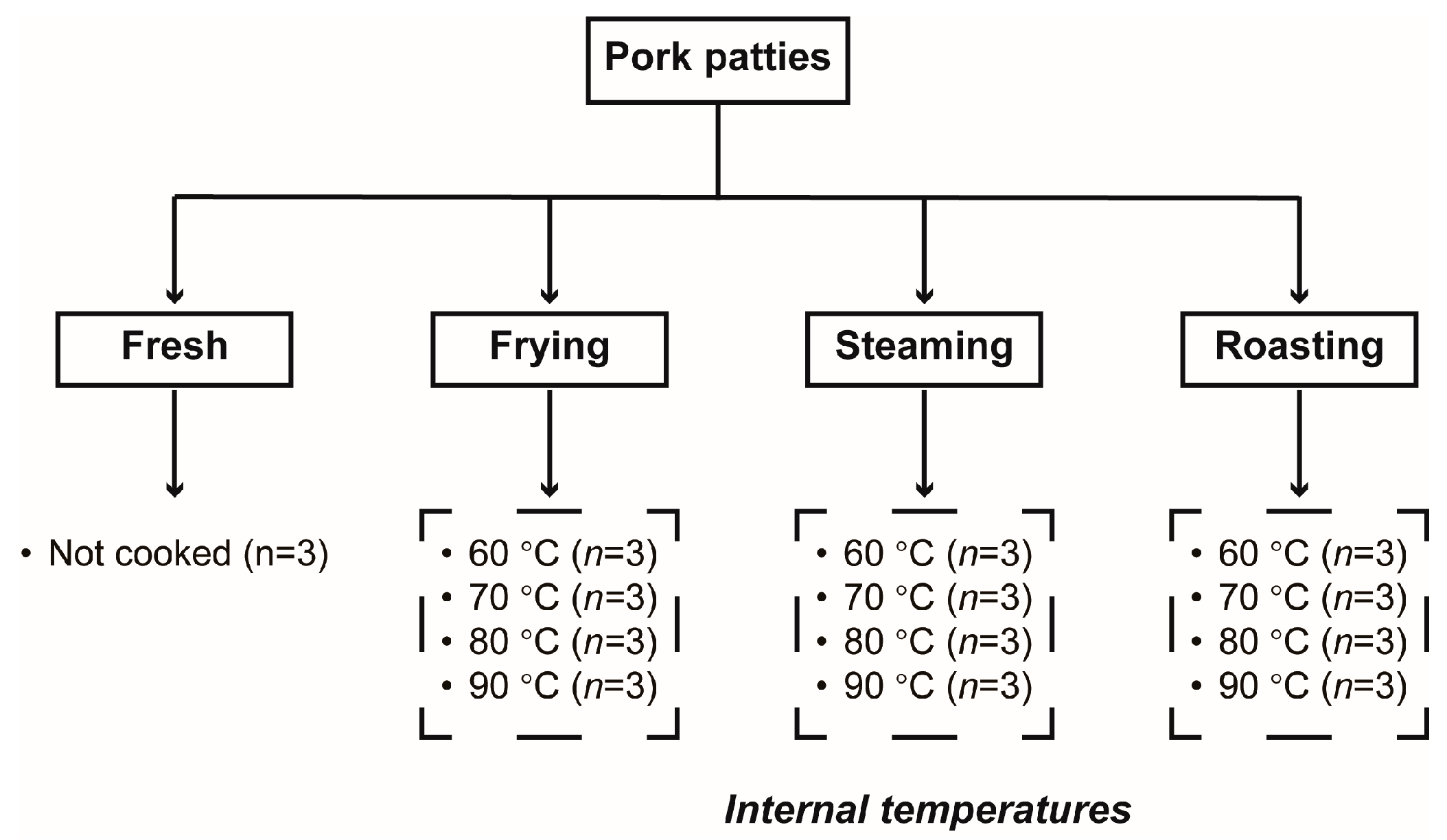
2.2. Sample Preparation
2.3. Determination of Total Carbonyl
2.4. Total Thiol Determination
2.5. AAS and LNL Determination
2.6. Statistical Analysis
3. Results and Discussion
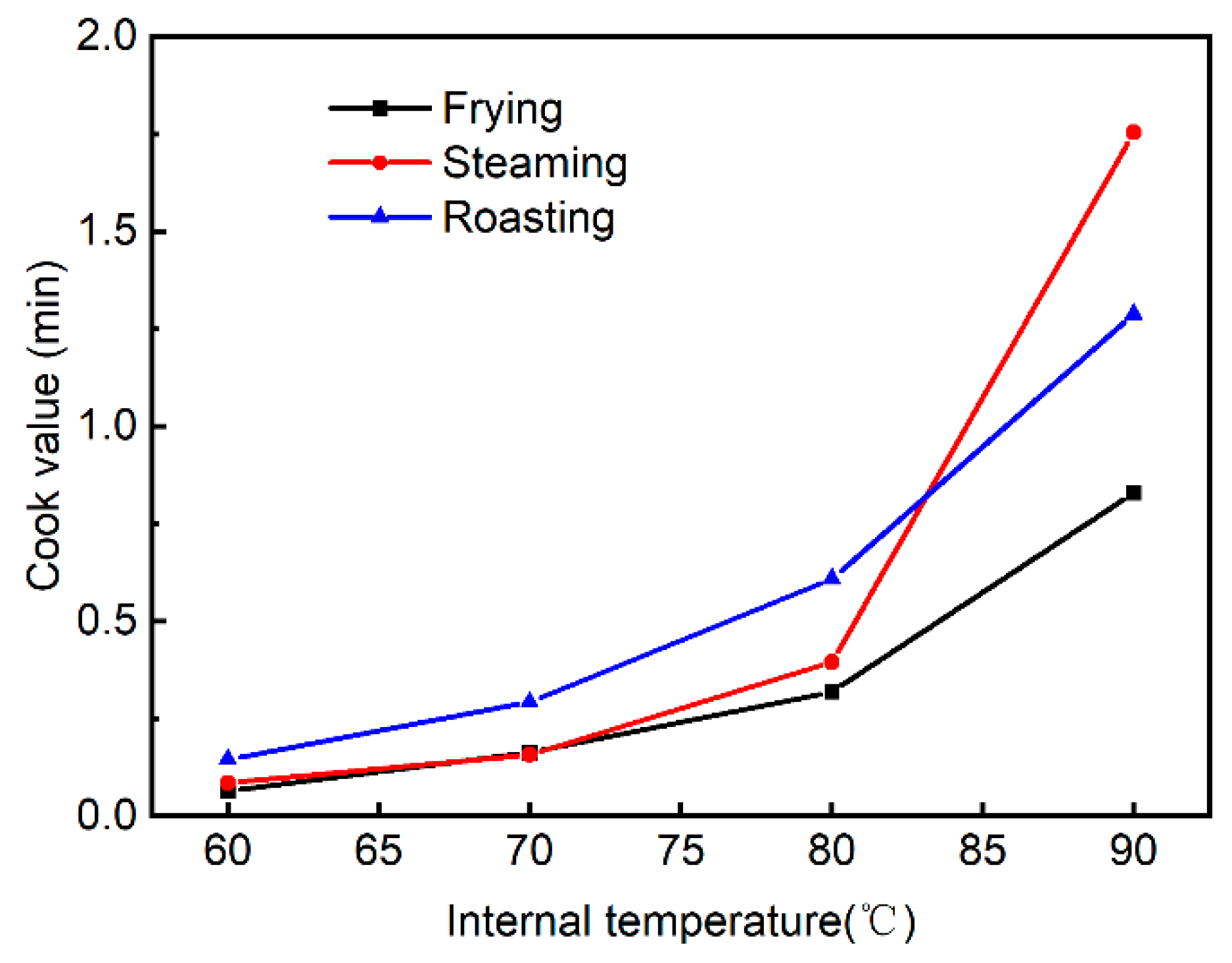
3.1. Differences in Heating Profile among Different Cooking Methods
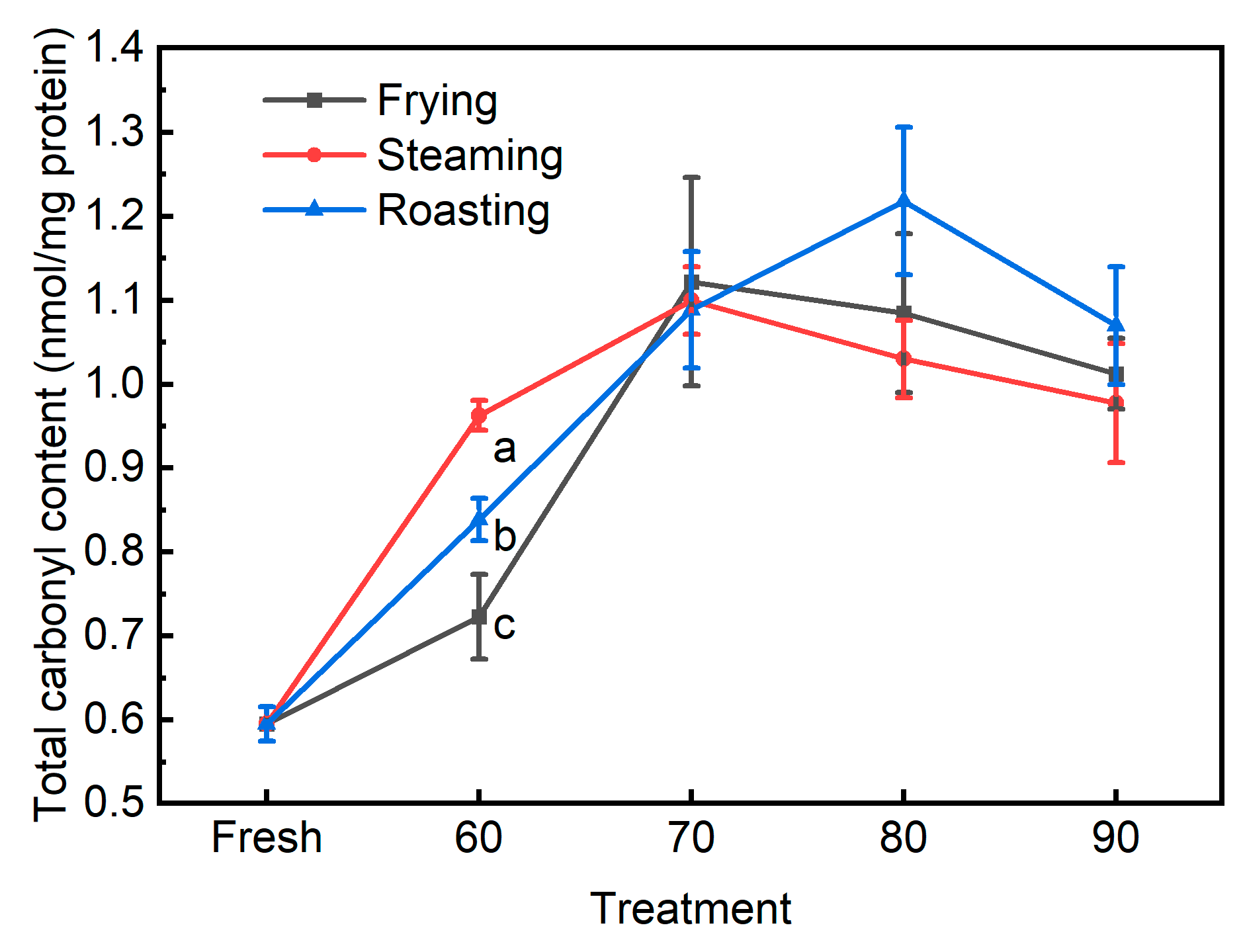
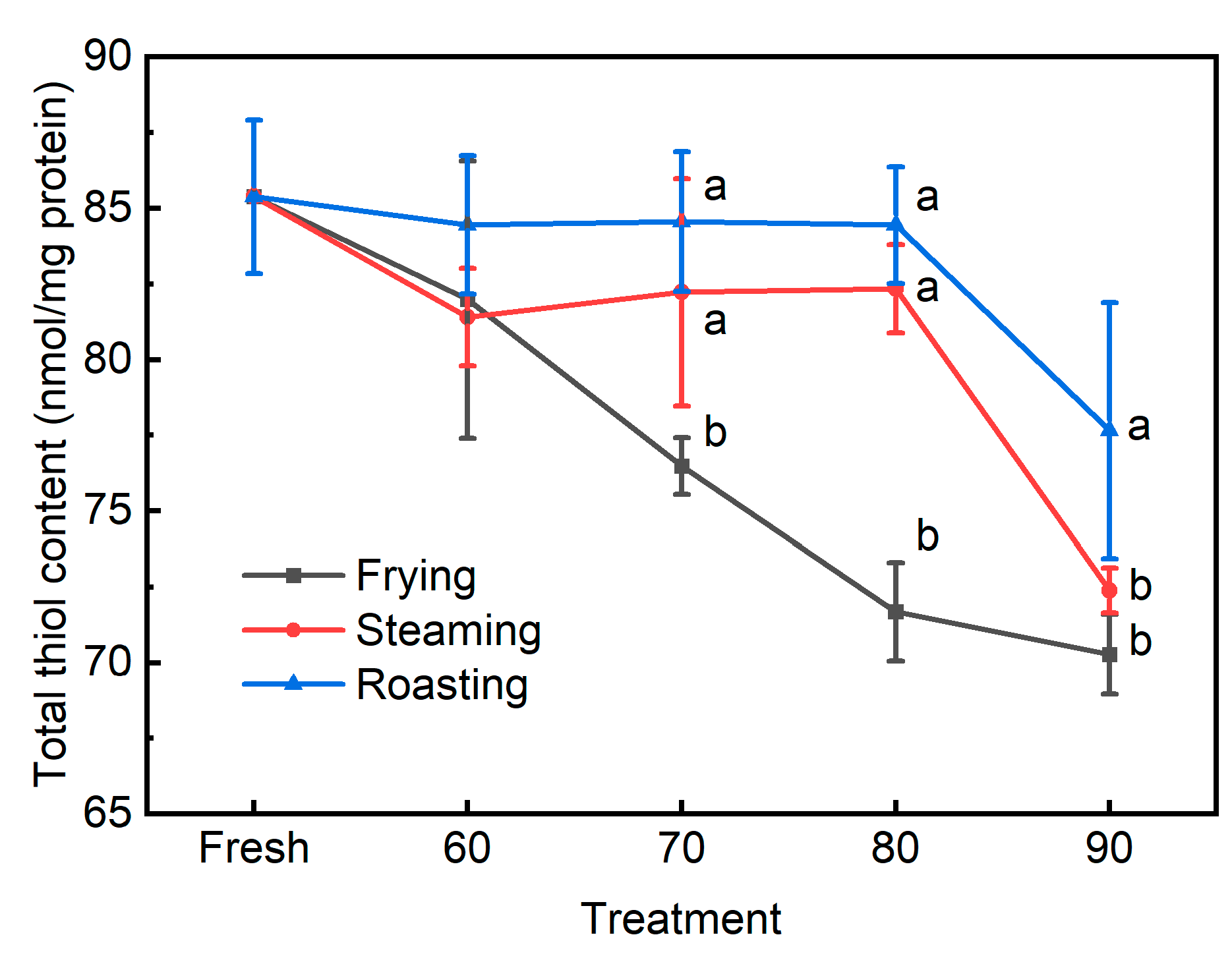
3.2. The Traditional Biomarkers for Protein Oxidation
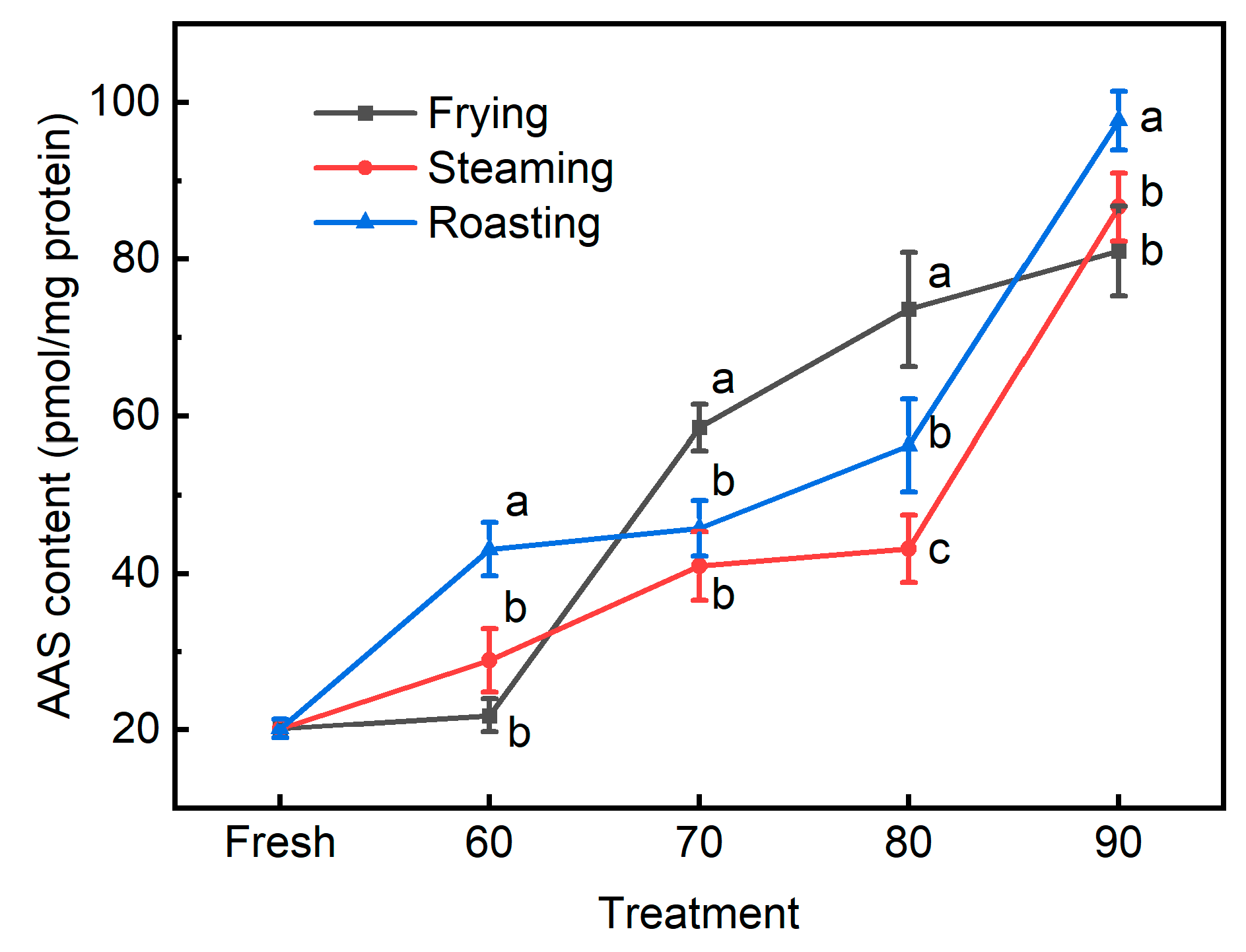
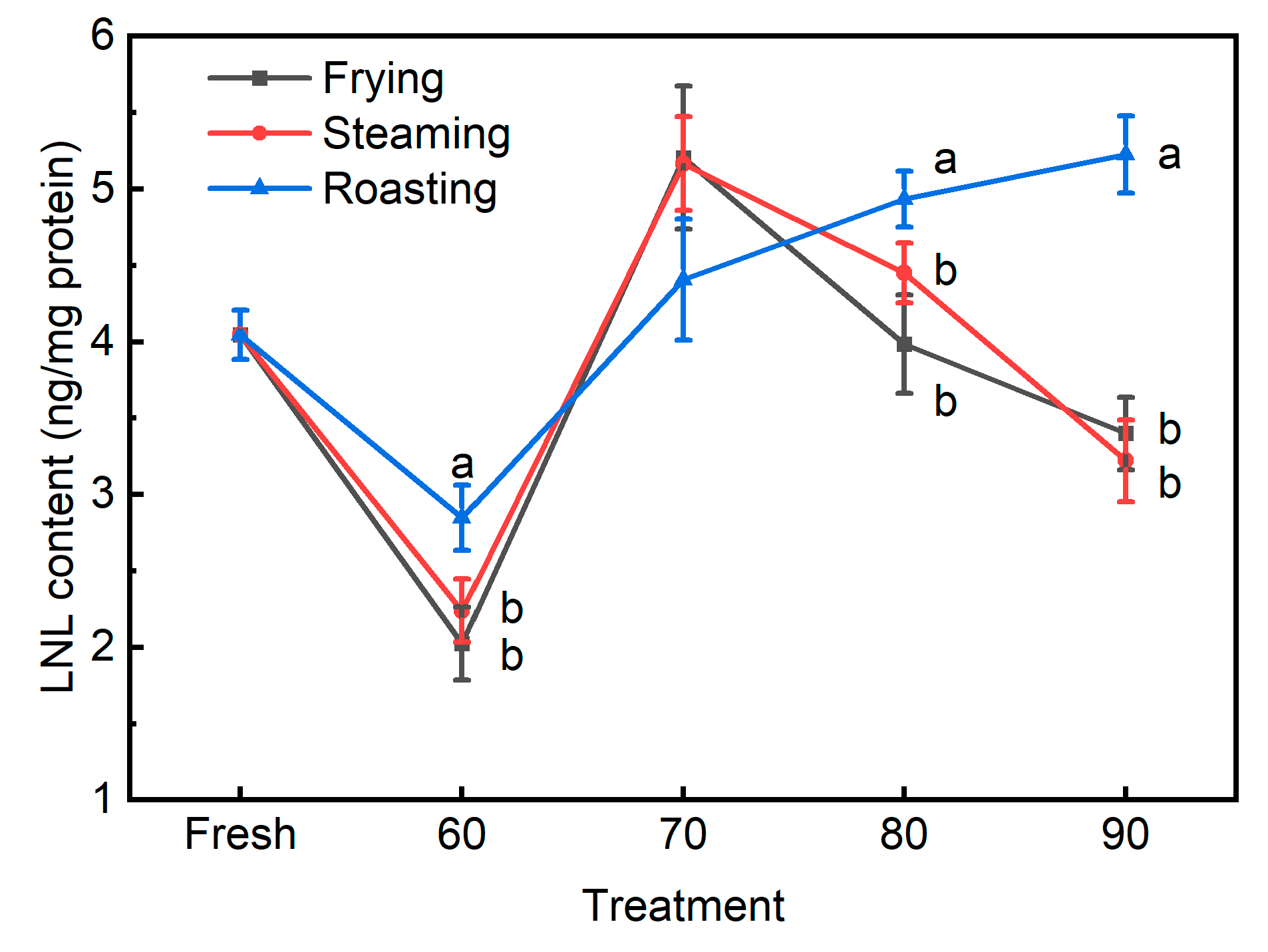
3.3. The Novel Biomarkers for Protein Oxidation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Suleman, R.; Wang, Z.; Aadil, R.M.; Hui, T.; Hopkins, D.L.; Zhang, D. Effect of Cooking on the Nutritive Quality, Sensory Properties and Safety of Lamb Meat: Current Challenges and Future Prospects. Meat Sci. 2020, 167, 108172. [Google Scholar] [CrossRef] [PubMed]
- Tornberg, E. Effects of Heat on Meat Proteins—Implications on Structure and Quality of Meat Products. Meat Sci. 2005, 70, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Guo, X.; Li, X.; Wang, W.; Wang, S.; Pan, J.; Dong, X.; Li, S. Effect of Cooking Temperatures on Meat Quality, Protein Carbonylation and Protein Cross-Linking of Beef Packed in High Oxygen Atmosphere. LWT-Food Sci. Technol. 2022, 154, 112633. [Google Scholar] [CrossRef]
- Traore, S.; Aubry, L.; Gatellier, P.; Przybylski, W.; Jaworska, D.; Kajak-Siemaszko, K.; Santé-Lhoutellier, V. Effect of Heat Treatment on Protein Oxidation in Pig Meat. Meat Sci. 2012, 91, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Luna, C. Dietary Protein Oxidation: A Silent Threat to Human Health? Crit. Rev. Food Sci. Nutr. 2017, 57, 3781–3793. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein Oxidation in Muscle Foods: A Review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef]
- Wang, H.H. The Perspective of Meat and Meat-Alternative Consumption in China. Meat Sci. 2022, 194, 108982. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.C.; Jo, C.; Lee, M. Meat Products and Consumption Culture in the East. Meat Sci. 2010, 86, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Mora, B.; Curti, E.; Vittadini, E.; Barbanti, D. Effect of Different Air/Steam Convection Cooking Methods on Turkey Breast Meat: Physical Characterization, Water Status and Sensory Properties. Meat Sci. 2011, 88, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Soladoye, O.P.; Juárez, M.L.; Aalhus, J.L.; Shand, P.; Estévez, M. Protein Oxidation in Processed Meat: Mechanisms and Potential Implications on Human Health. Compr. Rev. Food Sci. Food Saf. 2015, 14, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Roldan, M.; Antequera, T.; Armenteros, M.; Ruiz, J. Effect of Different Temperature-Time Combinations on Lipid and Protein Oxidation of Sous-Vide Cooked Lamb Loins. Food Chem. 2014, 149, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Padilla, P.; Carvalho, L.; Martín, L.; Carrapiso, A.; Delgado, J. Malondialdehyde Interferes with the Formation and Detection of Primary Carbonyls in Oxidized Proteins. Redox Biol. 2019, 26, 101277. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, M.; Sasaki, D.; Ishii, Y.; Kurota, Y.; Yotsu-Yamashita, M.; Uchida, K.; Suyama, K. New Method for the Quantitative Determination of Major Protein Carbonyls, α-Aminoadipic and γ-Glutamic Semialdehydes: Investigation of the Formation Mechanism and Chemical Nature In Vitro and In Vivo. Chem. Res. Toxicol. 2006, 19, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Ollilainen, V.; Heinonen, M. Analysis of Protein Oxidation Markers Alfa-Aminoadipic and Gamma-Glutamic Semialdehydes in Food Proteins Using Liquid Chromatography (LC)-Electrospray Ionization (ESI)-Multistage Tandem Mass Spectrometry (MS). J. Agric. Food Chem. 2009, 57, 3901–3910. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, S.; Wang, W.; Guo, X.; Pan, J.; Dong, X. HPLC-MS/MS Method for the Simultaneous Determination of Lysine-Derived Markers for Protein Carbonylation in Meat. J. Food Compos. Anal. 2023, 122, 105459. [Google Scholar] [CrossRef]
- van Roon, P.S.; Houben, J.H.; Koolmees, P.A.; van Vliet, T.; Krol, B. Mechanical and Microstructural Characteristics of Meat Doughs, Either Heated by a Continuous Process in a Radio-Frequency Field or Conventionally in a Waterbath. Meat Sci. 1994, 38, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N.; Lametsch, R.; Hviid, M.S.; Jensen, O.N.; Skibsted, L.H. High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Sci. 2007, 77, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Fellows, P.J. 10—Industrial Cooking. In Food Processing Technology, 4th ed.; Fellows, P.J., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2017; pp. 539–562. ISBN 978-0-08-101907-8. [Google Scholar]
- Carson, J.K.; Willix, J.; North, M.F. Measurements of Heat Transfer Coefficients within Convection Ovens. J. Food Eng. 2006, 72, 293–301. [Google Scholar] [CrossRef]
- Ikediala, J.N.; Correia, L.R.; Fenton, G.A.; Ben-Abdallah, N. Finite Element Modeling of Heat Transfer in Meat Patties during Single- Sided Pan-Frying. J. Food Sci. 1996, 61, 796–802. [Google Scholar] [CrossRef]
- Vittadini, E.; Rinaldi, M.; Chiavaro, E.; Barbanti, D.; Massini, R. The Effect of Different Convection Cooking Methods on the Instrumental Quality and Yield of Pork Longissimus Dorsi. Meat Sci. 2005, 69, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lanier, T.C. Rapid (Microwave) Heating Rate Effects on Texture, Fat/Water Holding, and Microstructure of Cooked Comminuted Meat Batters. Food Res. Int. 2016, 81, 108–113. [Google Scholar] [CrossRef]
- Pathare, P.B.; Roskilly, A.P. Quality and Energy Evaluation in Meat Cooking. Food Eng. Rev. 2016, 8, 435–447. [Google Scholar] [CrossRef]
- Schricker, B.R.; Miller, D.D.; Stouffer, J.R. Measurement and Content of Nonheme and Total Iron in Muscle. J. Food Sci. 1982, 47, 740–743. [Google Scholar] [CrossRef]
- Schricker, B.R.; Miller, D.D. Effects of Cooking and Chemical Treatment on Heme and Nonheme Iron in Meat. J. Food Sci. 1983, 48, 1340–1343. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein Oxidation: Basic Principles and Implications for Meat Quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef]
- Estévez, M. Protein Carbonyls in Meat Systems: A Review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Fagan, J.M.; Sleczka, B.G.; Sohar, I. Quantitation of Oxidative Damage to Tissue Proteins. Int. J. Biochem. Cell Biol. 1999, 31, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ren, S.; Shen, Q.; Chen, J.; Ye, X.; Ling, J. Proteomic Study of the Effect of Different Cooking Methods on Protein Oxidation in Fish Fillets. RSC Adv. 2017, 7, 27496–27505. [Google Scholar] [CrossRef]
- Estévez, M.; Geraert, P.A.; Liu, R.; Delgado, J.; Mercier, Y.; Zhang, W. Sulphur Amino Acids, Muscle Redox Status and Meat Quality: More than Building Blocks—Invited Review. Meat Sci. 2020, 163, 108087. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Díaz-Velasco, S.; Martínez, R. Protein Carbonylation in Food and Nutrition: A Concise Update. Amino Acids 2022, 54, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jia, X.; Guo, C.; Pan, J.; Dong, X.; Li, S. Protein Carbonylation and Structural Changes in Porcine Myofibrillar Protein Exposed to Metal Ion-H2O2-Ascorbate and Linoleic Acid-Lipoxidase Oxidizing Systems. Food Res. Int. 2023, 173, 113420. [Google Scholar] [CrossRef] [PubMed]
- Mitra, B.; Lametsch, R.; Akcan, T.; Ruiz-Carrascal, J. Pork Proteins Oxidative Modifications under the Influence of Varied Time-Temperature Thermal Treatments: A Chemical and Redox Proteomics Assessment. Meat Sci. 2018, 140, 134–144. [Google Scholar] [CrossRef] [PubMed]

| Cooking Method | Internal Temperature | Interaction | |
|---|---|---|---|
| Total thiol | <0.001 | <0.001 | 0.016 |
| Total carbonyl | 0.187 | <0.001 | 0.028 |
| AAS | 0.113 | <0.001 | <0.006 |
| LNL | <0.001 | <0.001 | <0.001 |
| TBARS | <0.001 | <0.001 | <0.001 |
| Cooking Time | Heating Rate | Cook Value | Total Thiol | Total Carbonyl | AAS | LNL | |
|---|---|---|---|---|---|---|---|
| Internal temperature | 0.71 *** | −0.36 * | 0.83 *** | −0.59 *** | 0.40 * | 0.80 *** | 0.22 |
| Cooking time | −0.86 *** | 0.81 *** | −0.08 | 0.41 * | 0.68 *** | 0.21 | |
| Heating rate | −0.52 ** | −0.23 | −0.34 * | −0.45 ** | −0.19 | ||
| Cook value | −0.48 ** | 0.27 | 0.77 *** | 0.05 | |||
| Total thiol | −0.11 | −0.60 *** | 0.04 | ||||
| Total carbonyl | 0.40 * | 0.51 ** | |||||
| AAS | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Wang, S.; Jia, X.; Pan, J.; Dong, X.; Li, S. Changes in Novel Biomarkers for Protein Oxidation in Pork Patties under Different Cooking Methods. Foods 2024, 13, 1034. https://doi.org/10.3390/foods13071034
Guo C, Wang S, Jia X, Pan J, Dong X, Li S. Changes in Novel Biomarkers for Protein Oxidation in Pork Patties under Different Cooking Methods. Foods. 2024; 13(7):1034. https://doi.org/10.3390/foods13071034
Chicago/Turabian StyleGuo, Chuanyu, Shouyin Wang, Xiaolei Jia, Jinfeng Pan, Xiuping Dong, and Shengjie Li. 2024. "Changes in Novel Biomarkers for Protein Oxidation in Pork Patties under Different Cooking Methods" Foods 13, no. 7: 1034. https://doi.org/10.3390/foods13071034
APA StyleGuo, C., Wang, S., Jia, X., Pan, J., Dong, X., & Li, S. (2024). Changes in Novel Biomarkers for Protein Oxidation in Pork Patties under Different Cooking Methods. Foods, 13(7), 1034. https://doi.org/10.3390/foods13071034





