Old Apple Cultivars as a Natural Source of Phenolics and Triterpenoids with Cytoprotective Activity on Caco-2 and HepG2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Samples
2.3. Extraction Process
2.4. Antioxidant Properties
2.5. Total Phenolic Content
2.6. GC–MS Analysis of Phenolic Acids
2.7. GC-–MS Analysis of Triterpenoids
2.8. HPLC Analysis of Polyphenols
2.9. Cell Culture
2.10. Metabolic Activity of Cells
2.11. Detection of Intracellular Oxygen Free Radicals
2.12. ATP Level Measurement
2.13. Externalization of Phosphatidylserine (PS)
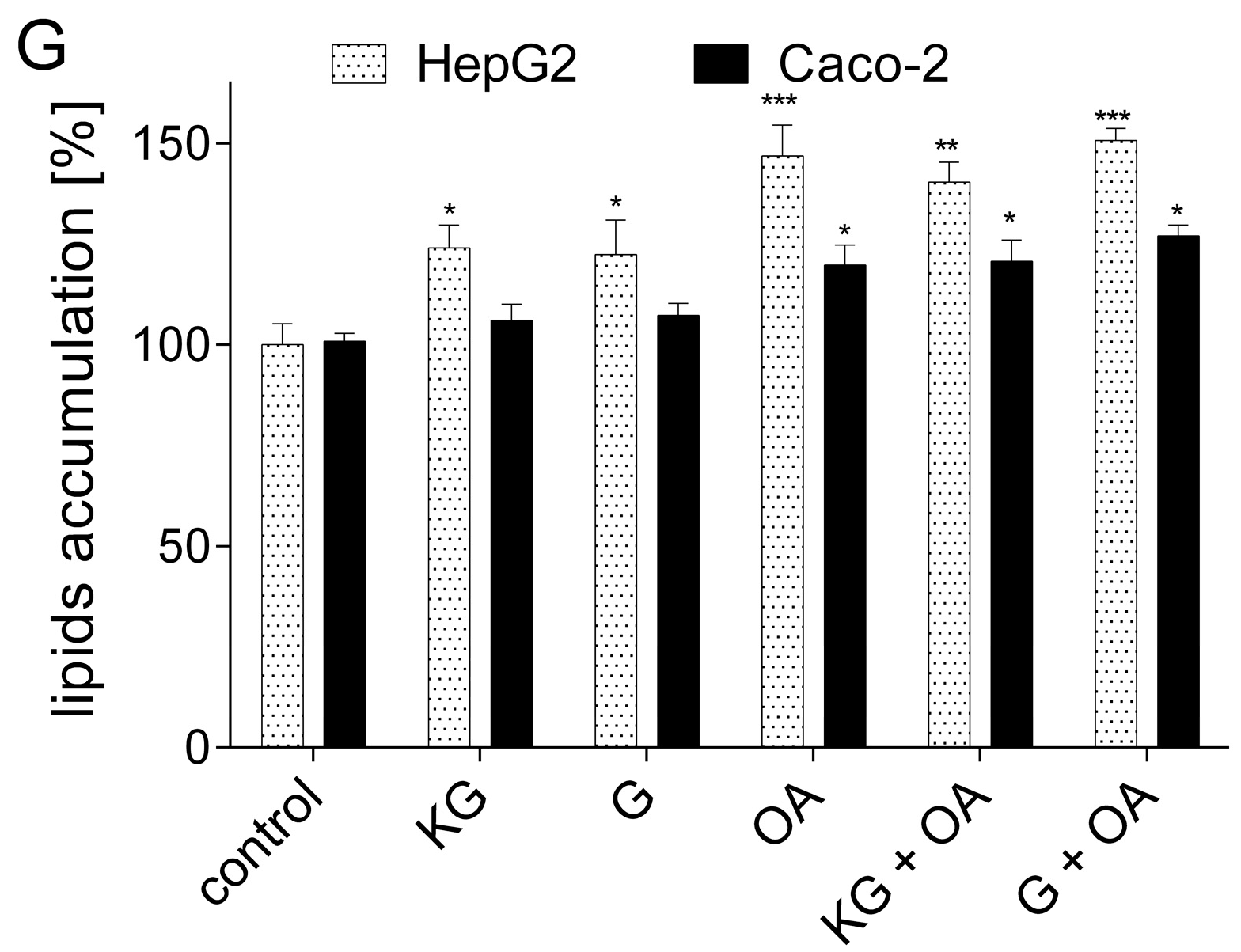
2.14. The Level of Lipid Accumulation
2.15. Glutathione Peroxidase (GPx) and Superoxide Dismutase (SOD) Activity
2.16. Statistical Analysis
3. Results and Discussion
3.1. Content of Health-Promoting Compounds in Old Apple Cultivars
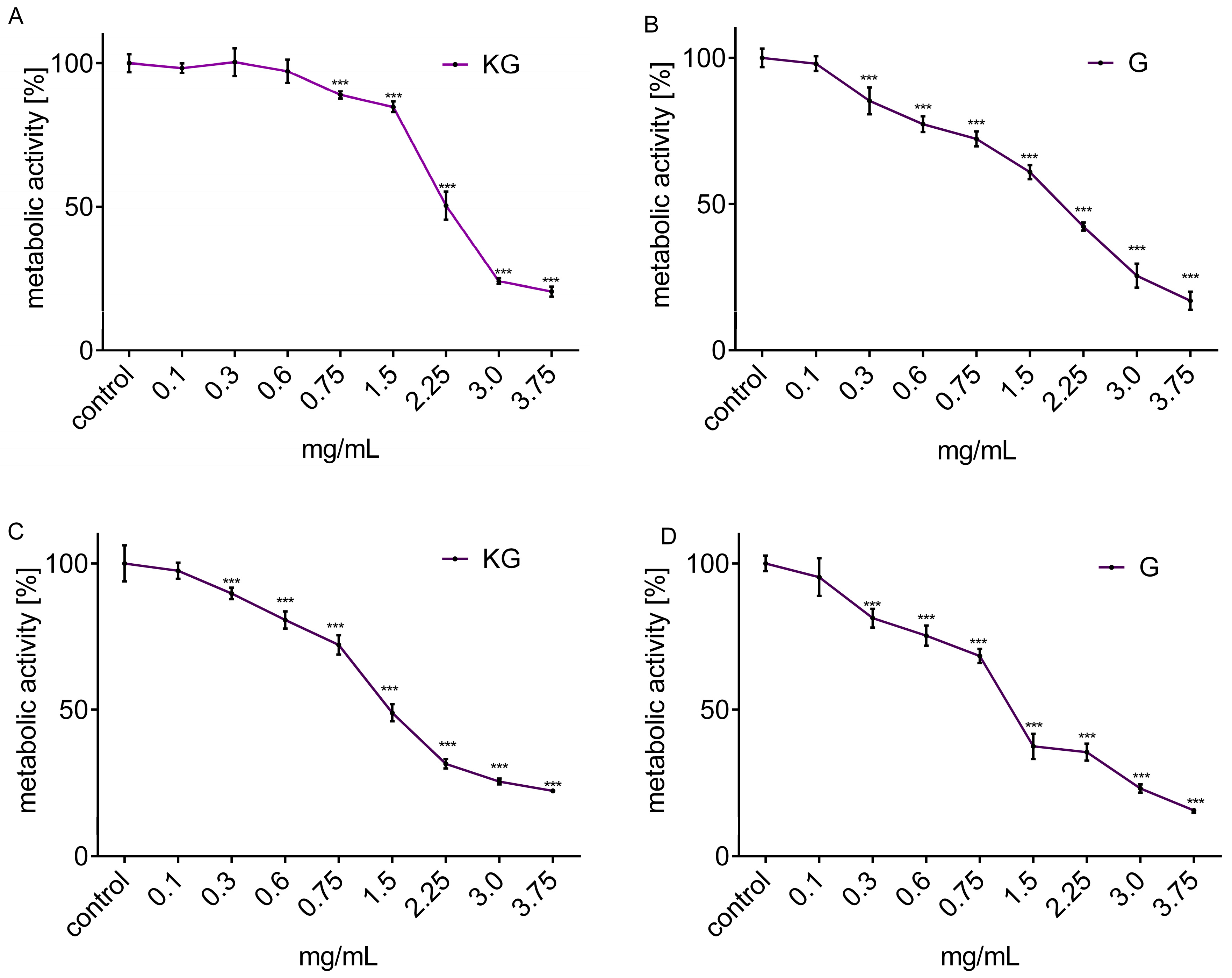
3.2. Cytotoxic Activity of Extracts Obtained from KG and G Apple Peel
3.3. Cytoprotective Effect of Extracts Obtained from KG and G Apple Peel Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slováčková, B.; Mišíková, O. Observing the Structure Diversity of Historic Heirloom Apple Tree (Malus domestica Borkh.) Wood in Central Slovakia. Diversity 2023, 15, 15. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Apple Peels as a Value-Added Food Ingredient. J. Agric. Food Chem. 2003, 51, 1676–1683. [Google Scholar] [CrossRef]
- Chinnici, F.; Bendini, A.; Gaiani, A.; Riponi, C. Radical Scavenging Activities of Peels and Pulps from Cv. Golden Delicious Apples as Related to Their Phenolic Composition. J. Agric. Food Chem. 2004, 52, 4684–4689. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid Intake and Risk of Chronic Diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant Activity of Fresh Apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Kim, Y.J.; Kim, D.-O.; Lee, H.J.; Lee, C.Y. Major Phenolics in Apple and Their Contribution to the Total Antioxidant Capacity. J. Agric. Food Chem. 2003, 51, 6516–6520. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Ichwan, M.; Walker, T.L.; Nicola, Z.; Ludwig-Müller, J.; Böttcher, C.; Overall, R.W.; Adusumilli, V.S.; Bulut, M.; Sykes, A.M.; Hübner, N.; et al. Apple Peel and Flesh Contain Pro-Neurogenic Compounds. Stem Cell Rep. 2021, 16, 548–565. [Google Scholar] [CrossRef] [PubMed]
- Oei, S.; Millar, C.L.; Nguyen Lily, T.N.; Mukamal, K.J.; Kiel, D.P.; Lipsitz, L.A.; Hannan, M.T.; Sahni, S. Higher Intake of Dietary Flavonols, Specifically Dietary Quercetin, Is Associated with Lower Odds of Frailty Onset over 12 Years of Follow-up among Adults in the Framingham Heart Study. Am. J. Clin. Nutr. 2023, 118, 27–33. [Google Scholar] [CrossRef]
- Paraiso, I.L.; Revel, J.S.; Stevens, J.F. Potential Use of Polyphenols in the Battle against COVID-19. Curr. Opin. Food Sci. 2020, 32, 149–155. [Google Scholar] [CrossRef]
- Russo, M.; Moccia, S.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Roles of Flavonoids against Coronavirus Infection. Chem.-Biol. Interact. 2020, 328, 109211. [Google Scholar] [CrossRef]
- Poirier, B.C.; Buchanan, D.A.; Rudell, D.R.; Mattheis, J.P. Differential Partitioning of Triterpenes and Triterpene Esters in Apple Peel. J. Agric. Food Chem. 2018, 66, 1800–1806. [Google Scholar] [CrossRef]
- Jemmali, Z.; Chartier, A.; Dufresne, C.; Elfakir, C. Optimization of the Derivatization Protocol of Pentacyclic Triterpenes Prior to Their Gas Chromatography–Mass Spectrometry Analysis in Plant Extracts. Talanta 2016, 147, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Butkevičiūtė, A.; Janulis, V.; Kviklys, D. Triterpene Content in Flesh and Peel of Apples Grown on Different Rootstocks. Plants 2022, 11, 1247. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Szakiel, A.; Głowacka, A.; Rozpara, E.; Marszałek, K.; Skąpska, S. Triterpenoids of Three Apple Cultivars—Biosynthesis, Antioxidative and Anti-Inflammatory Properties, and Fate during Processing. Molecules 2023, 28, 2584. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Nile, A.; Liu, J.; Kim, D.H.; Kai, G. Exploitation of Apple Pomace towards Extraction of Triterpenic Acids, Antioxidant Potential, Cytotoxic Effects, and Inhibition of Clinically Important Enzymes. Food Chem. Toxicol. 2019, 131, 110563. [Google Scholar] [CrossRef]
- Andre, C.M.; Greenwood, J.M.; Walker, E.G.; Rassam, M.; Sullivan, M.; Evers, D.; Perry, N.B.; Laing, W.A. Anti-Inflammatory Procyanidins and Triterpenes in 109 Apple Varieties. J. Agric. Food Chem. 2012, 60, 10546–10554. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A Reproducible, Rapid and Inexpensive Folin–Ciocalteu Micro-Method in Determining Phenolics of Plant Methanol Extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Cyran, M.R.; Dynkowska, W.M.; Ceglińska, A.; Bonikowski, R. Improving Rye Bread Antioxidant Capacity by Bread-Making Methodology: Contribution of Phosphate-Buffered Saline- and Methanol-Soluble Phenolic Phytochemicals with Different Molecular Profiles. J. Cereal Sci. 2021, 100, 103262. [Google Scholar] [CrossRef]
- Podsędek, A.; Frąszczak, B.; Sosnowska, D.; Kajszczak, D.; Szymczak, K.; Bonikowski, R. LED Light Quality Affected Bioactive Compounds, Antioxidant Potential, and Nutritional Value of Red and White Cabbage Microgreens. Appl. Sci. 2023, 13, 5435. [Google Scholar] [CrossRef]
- Caligiani, A.; Malavasi, G.; Palla, G.; Marseglia, A.; Tognolini, M.; Bruni, R. A Simple GC-MS Method for the Screening of Betulinic, Corosolic, Maslinic, Oleanolic and Ursolic Acid Contents in Commercial Botanicals Used as Food Supplement Ingredients. Food Chem. 2013, 136, 735–741. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Nešović, M.; Ćirić, I.; Tešić, Ž.; Pezo, L.; Tosti, T.; Gašić, U.; Dojčinović, B.; Lončar, B.; Meland, M. Polyphenolics and Chemical Profiles of Domestic Norwegian Apple (Malus × Domestica Borkh.) Cultivars. Front. Nutr. 2022, 9, 941487. [Google Scholar] [CrossRef]
- Butkevičiūtė-Bračiulienė, A.; Mindaugas, L.; Kviklys, D.; Gelvonauskiene, D.; Janulis, V. Qualitative and Quantitative Composition of Triterpenic Compounds in the Fruit of Apple Old Cultivars Grown in Lithuania. Zemdirb. Agric. 2021, 108, 63–70. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gławdel, E.; Cebulak, T.; Ochmian, I. Determination of Phytochemical Composition and Antioxidant Capacity of 22 Old Apple Cultivars Grown in Poland. Eur. Food Res. Technol. 2018, 244, 647–662. [Google Scholar] [CrossRef]
- Geană, E.-I.; Ciucure, C.T.; Ionete, R.E.; Ciocârlan, A.; Aricu, A.; Ficai, A.; Andronescu, E. Profiling of Phenolic Compounds and Triterpene Acids of Twelve Apple (Malus domestica Borkh.) Cultivars. Foods 2021, 10, 267. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Laskowski, P. Polyphenolic Compounds and Antioxidant Activity of New and Old Apple Varieties. J. Agric. Food Chem. 2008, 56, 6520–6530. [Google Scholar] [CrossRef]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of Phenolic Compounds vs. Variety, Part of Apple and Cultivation Model, Extraction of Phenolic Compounds, Biological Properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant Properties of Phenolic Compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Francini, A.; Sebastiani, L. Phenolic Compounds in Apple (Malus x Domestica Borkh.): Compounds Characterization and Stability during Postharvest and after Processing. Antioxidants 2013, 2, 181–193. [Google Scholar] [CrossRef]
- Hu, W.; Sarengaowa; Guan, Y.; Feng, K. Biosynthesis of Phenolic Compounds and Antioxidant Activity in Fresh-Cut Fruits and Vegetables. Front. Microbiol. 2022, 13, 906069. [Google Scholar]
- Sambuy, Y.; Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 Cell Line as a Model of the Intestinal Barrier: Influence of Cell and Culture-Related Factors on Caco-2 Cell Functional Characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Arzumanian, V.A.; Kiseleva, O.I.; Poverennaya, E.V. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int. J. Mol. Sci. 2021, 22, 13135. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Pawlik, N.; Polka, D.; Nowak, A.; Koziołkiewicz, M.; Podsędek, A. Viburnum Opulus Fruit Phenolic Compounds as Cytoprotective Agents Able to Decrease Free Fatty Acids and Glucose Uptake by Caco-2 Cells. Antioxidants 2019, 8, 262. [Google Scholar] [CrossRef]
- Grzelczyk, J.; Szwajgier, D.; Baranowska-Wójcik, E.; Budryn, G.; Zakłos-Szyda, M.; Sosnowska, B. Bioaccessibility of Coffee Bean Hydroxycinnamic Acids during in Vitro Digestion Influenced by the Degree of Roasting and Activity of Intestinal Probiotic Bacteria, and Their Activity in Caco-2 and HT29 Cells. Food Chem. 2022, 392, 133328. [Google Scholar] [CrossRef]
- Pietrzyk, N.; Zakłos-Szyda, M.; Koziołkiewicz, M.; Podsędek, A. Viburnum Opulus L. Fruit Phenolic Compounds Protect against FFA-Induced Steatosis of HepG2 Cells via AMPK Pathway. J. Funct. Foods 2021, 80, 104437. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, G.; Zhang, P.; Fan, J. Programmed Cell Death and Its Role in Inflammation. Mil. Med. Res. 2015, 2, 12. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Brereton, M.F.; Rohm, M.; Ashcroft, F.M. β-Cell Dysfunction in Diabetes: A Crisis of Identity? Diabetes Obes. Metab. 2016, 18, 102–109. [Google Scholar] [CrossRef]
- Rachek, L.I. Chapter Eight—Free Fatty Acids and Skeletal Muscle Insulin Resistance. In Progress in Molecular Biology and Translational Science; Tao, Y.-X., Ed.; Glucose Homeostatis and the Pathogenesis of Diabetes Mellitus; Academic Press: Cambridge, MA, USA, 2014; Volume 121, pp. 267–292. [Google Scholar]
- Gajęcka, M.; Przybylska-Gornowicz, B.; Zakłos-Szyda, M.; Dąbrowski, M.; Michalczuk, L.; Koziołkiewicz, M.; Babuchowski, A.; Zielonka, Ł.; Lewczuk, B.; Gajęcki, M.T. The Influence of a Natural Triterpene Preparation on the Gastrointestinal Tract of Gilts with Streptozocin-Induced Diabetes and on Cell Metabolic Activity. J. Funct. Foods 2017, 33, 11–20. [Google Scholar] [CrossRef]

| Antioxidant Properties (DPPH Method) | Total Phenolic Content (FC Method) | Total Phenolic Acids Content (GC–MS Method) | Total Triterpenoic Content (GC–MS Method) | Total Polyphenol Content (HPLC–DAD Method) | |
|---|---|---|---|---|---|
| Antioxidant properties (DPPH method) | X | 0.65 | 0.13 | −0.01 | 0.30 |
| Total phenolic content (FC method) | 0.65 | X | 0.32 | 0.38 | 0.61 |
| Total phenolic acids content (GC-MS method) | 0.13 | 0.32 | X | 0.28 | 0.64 |
| Total triterpenoic content (GC-MS method) | −0.01 | 0.38 | 0.28 | X | 0.55 |
| Total polyphenol content (HPLC-DAD method) | 0.30 | 0.61 | 0.64 | 0.55 | X |
| Cultivar | Antioxidant Properties [mgTE/100 g of DAP] | Total Phenolic Content [mg GAE/100 g of DAP] | Total Phenolic Acids Content [mg/100 g of DAP] | Total Triterpenoic Content [mg/100 g of DAP] | Total Polyphenols Content [mg/100 g of DAP] |
|---|---|---|---|---|---|
| Boskoop | 852.1 ± 45.5 c | 164.6 ± 6.8 a | 870.1 ± 25.4 ghij | 1494.1 ± 50.7 fgh | 2225.3 ± 79.2 ef |
| Grochówka | 1105.5 ± 51.8 fghij | 217.0 ± 10.4 ghij | 872.1 ± 26.1 hij | 1976.8 ± 48.5 k | 4721.0 ± 99.3 k |
| Jakub Lebel | 1129.8 ± 53.2 ijk | 203.7 ± 10.6 defg | 401.3 ± 18.4 d | 1491.8 ± 49.6 fgh | 1464.5 ± 46.6 b |
| James Grieve | 1090.2 ± 46.7 ghij | 246.6 ± 12.4 k | 685.4 ± 29.9 f | 1759.7 ± 40.2 j | 3898.6 ± 87.1 i |
| Kantówka Gdańska | 951.0 ± 21.3 de | 214.2 ± 2.5 ghi | 1661.0 ± 35.6 k | 1617.7 ± 42.9 i | 4327.3 ± 110.2 j |
| Kronselska | 536.9 ± 32.2 a | 199.4 ± 5.3 cdef | 627.4 ± 10.7 e | 1518.5 ± 38.3 fgh | 2976.0 ± 67.5 gh |
| Książę Albert | 1058.8 ± 71.0 efg | 204.1 ± 15.7 efgh | 210.4 ± 14.3 a | 983.0 ± 22.0 a | 854.6 ± 23.9 a |
| Książę Albrecht Pruski | 1136.5 ± 46.4 ijk | 199.1 ± 7.3 cde | 269.4 ± 5.8 b | 1297.6 ± 29.1 cde | 3039.3 ± 67.4 gh |
| Niezrównane Peasgooda | 647.5 ± 39.6 b | 176.7 ± 5.6 b | 853.6 ± 30.3 ghi | 1304.7 ± 36.8 cde | 1846.8 ± 56.0 cd |
| Schieblers Taubenapfel | 1076.6 ± 36.8 fgh | 193.7 ± 27.1 c | 310.0 ± 17.1 c | 1063.1 ± 34.5 b | 2275.0 ± 47.1 ef |
| Złota Reneta | 1006.5 ± 61.8 ef | 195.5 ± 8.1 cd | 801.3 ± 30.8 gh | 1274.3 ± 31.9 cde | 1858.5 ± 21.9 cd |
| Cell Line | IC50 [mg/mL] | IC0 [mg/mL] | ||
|---|---|---|---|---|
| KG | G | KG | G | |
| HepG2 | 2.25 | 2.00 | 0.60 | 0.10 |
| Caco-2 | 1.50 | 1.25 | 0.10 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczak, K.; Zakłos-Szyda, M.; Mietlińska, K.; Eliašová, A.; Jodłowska, I.; Gruľová, D.; Hodun, G.; Bonikowski, R. Old Apple Cultivars as a Natural Source of Phenolics and Triterpenoids with Cytoprotective Activity on Caco-2 and HepG2 Cells. Foods 2024, 13, 1014. https://doi.org/10.3390/foods13071014
Szymczak K, Zakłos-Szyda M, Mietlińska K, Eliašová A, Jodłowska I, Gruľová D, Hodun G, Bonikowski R. Old Apple Cultivars as a Natural Source of Phenolics and Triterpenoids with Cytoprotective Activity on Caco-2 and HepG2 Cells. Foods. 2024; 13(7):1014. https://doi.org/10.3390/foods13071014
Chicago/Turabian StyleSzymczak, Kamil, Małgorzata Zakłos-Szyda, Katarzyna Mietlińska, Adriana Eliašová, Iga Jodłowska, Daniela Gruľová, Grzegorz Hodun, and Radosław Bonikowski. 2024. "Old Apple Cultivars as a Natural Source of Phenolics and Triterpenoids with Cytoprotective Activity on Caco-2 and HepG2 Cells" Foods 13, no. 7: 1014. https://doi.org/10.3390/foods13071014
APA StyleSzymczak, K., Zakłos-Szyda, M., Mietlińska, K., Eliašová, A., Jodłowska, I., Gruľová, D., Hodun, G., & Bonikowski, R. (2024). Old Apple Cultivars as a Natural Source of Phenolics and Triterpenoids with Cytoprotective Activity on Caco-2 and HepG2 Cells. Foods, 13(7), 1014. https://doi.org/10.3390/foods13071014






