UV-C Treatment Impact on the Availability of Water-Soluble Carbohydrates, Polyphenols, and Antioxidant Capacity of an Algerian Underutilized Date Fruit (Phoenix dactylifera L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. UV-C Light Treatment
2.4. Techno-Functional Properties
2.5. Characterization of Dietary Fiber
2.6. Characterization of Soluble Carbohydrate Composition by HPLC-RID
2.7. Characterization of Phenolic Compounds by HPLC-QTOF
2.8. Multifunctional Antioxidant Capacity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Techno-Functional Properties
3.2. Characterization of Dietary Fiber Composition
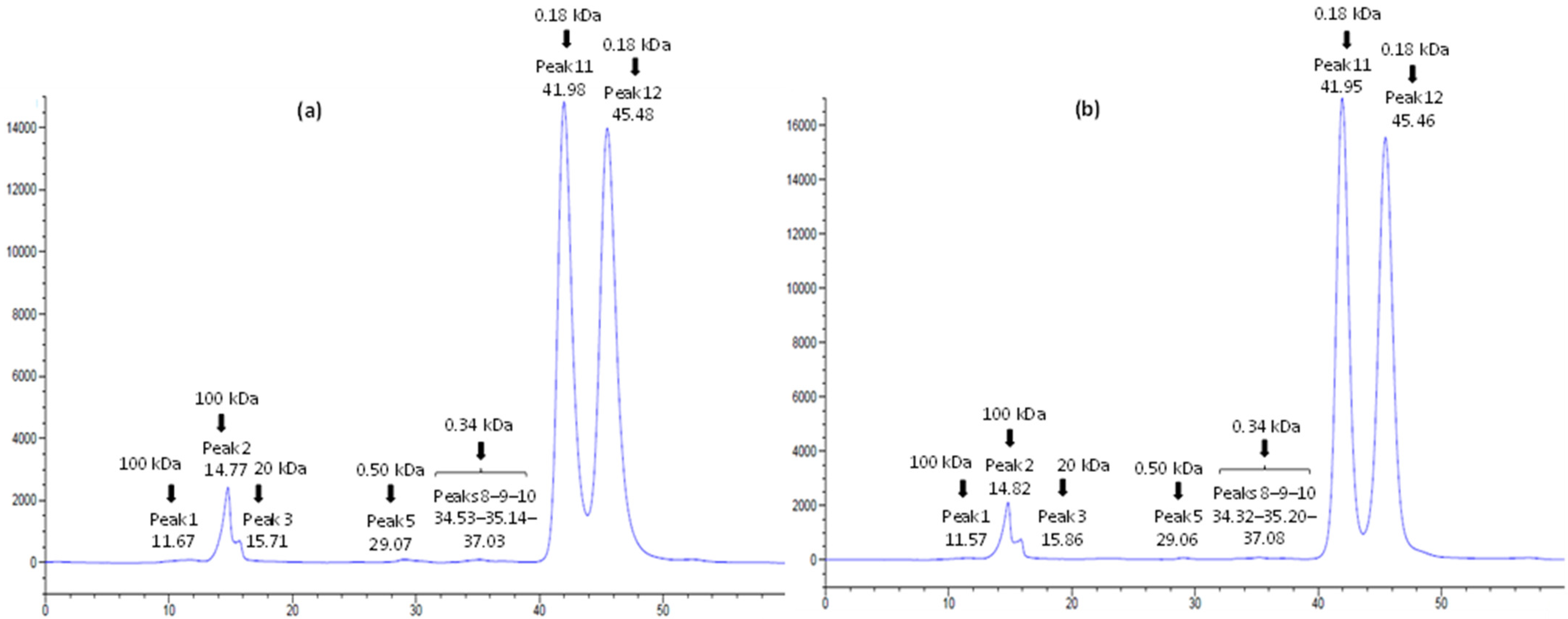
3.3. Characterization of Carbohydrate Content by HPLC-RID
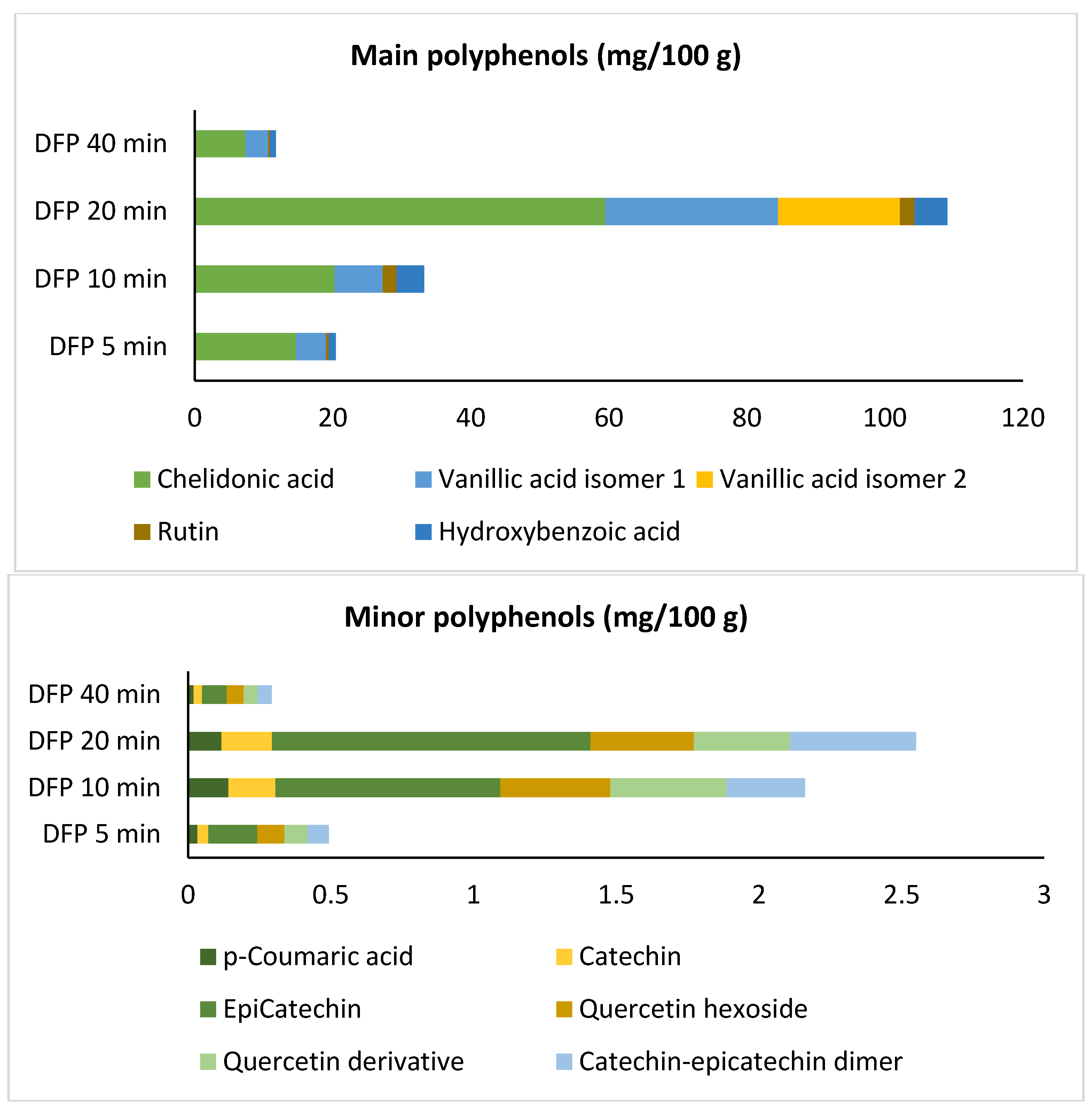
3.4. Characterization of Phenolic Compounds by HPLC-QTOF
3.5. Antioxidant Capacity In Vitro
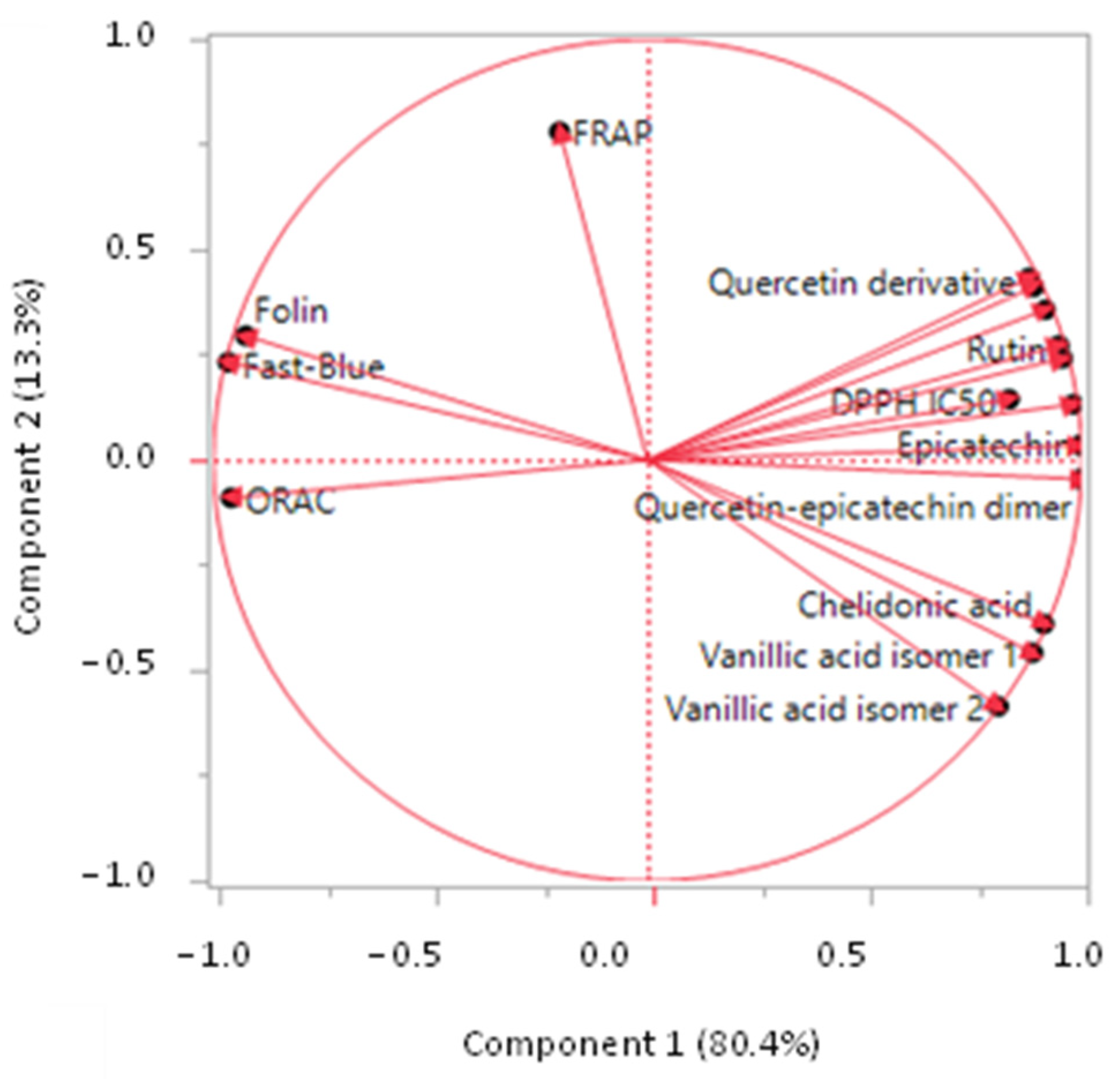
3.6. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallo, F.; Manzardo, A.; Camana, D.; Fedele, A.; Scipioni, A. Integration of a circular economy metric with life cycle assessment: Methodological proposal of compared agri-food products. Int. J. Life Cycle Assess. 2023. [Google Scholar] [CrossRef]
- Yontar, E. Critical success factor analysis of blockchain technology in agri-food supply chain management: A circular economy perspective. J. Environ. Manag. 2023, 330, 117173. [Google Scholar] [CrossRef]
- Bettaieb, I.; Kilani, A.; Ben Othman, K.; Benabderrahim, M.A.; Elfalleh, W. Phenolic Profile, Sugar Composition, and Antioxidant Capacities of Some Common Date Palm (Phoenix dactylifera L.) Cultivars as a Potential Nutraceutical and Functional Food Ingredients. J. Food Qual. 2023, 2023, 2474900. [Google Scholar] [CrossRef]
- Oladzad, S.; Fallah, N.; Mahboubi, A.; Afsham, N.; Taherzadeh, M.J. Date fruit processing waste and approaches to its valorization: A review. Bioresour. Technol. 2021, 340, 125625. [Google Scholar] [CrossRef]
- Taghian Dinani, S.; van der Goot, A.J. Challenges and solutions of extracting value-added ingredients from fruit and vegetable by-products: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 7749–7771. [Google Scholar] [CrossRef]
- Mandal, D.D.; Singh, G.; Majumdar, S.; Chanda, P. Challenges in developing strategies for the valorization of lignin—A major pollutant of the paper mill industry. Environ. Sci. Pollut. Res. 2023, 30, 11119–11140. [Google Scholar] [CrossRef]
- Fernández-López, J.; Viuda-Martos, M.; Sayas-Barberá, E.; Navarro-Rodríguez de Vera, C.; Pérez-Álvarez, J.Á. Biological, nutritive, functional and healthy potential of date palm fruit (Phoenix dactylifera L.): Current research and future prospects. Agronomy 2022, 12, 876. [Google Scholar] [CrossRef]
- El-Far, A.H.; Oyinloye, B.E.; Sepehrimanesh, M.; Allah, M.A.G.; Abu-Reidah, I.; Shaheen, H.M.; Razeghian-Jahromi, I.; Noreldin, A.E.; Al Jaouni, S.K.; Mousa, S.A. Date palm (Phoenix dactylifera): Novel findings and future directions for food and drug discovery. Curr. Drug Discov. Technol. 2019, 16, 2–10. [Google Scholar] [CrossRef]
- Echegaray, N.; Gullón, B.; Pateiro, M.; Amarowicz, R.; Misihairabgwi, J.M.; Lorenzo, J.M. Date fruit and its by-products as promising source of bioactive components: A review. Food Rev. Int. 2023, 39, 1411–1432. [Google Scholar] [CrossRef]
- Hussain, M.I.; Farooq, M.; Syed, Q.A. Nutritional and biological characteristics of the date palm fruit (Phoenix dactylifera L.)–A review. Food Biosci. 2020, 34, 100509. [Google Scholar] [CrossRef]
- Al-Qarni, S.S.M.; Bazzi, M.D. Date fruit ripening with degradation of chlorophylls, carotenes, and other pigments. Int. J. Fruit Sci. 2020, 20 (Suppl. S2), S827–S839. [Google Scholar] [CrossRef]
- Muñoz-Bas, C.; Muñoz-Tebar, N.; Candela-Salvador, L.; Pérez-Alvarez, J.A.; Lorenzo, J.M.; Viuda-Martos, M.; Fernández-López, J. Quality characteristics of fresh date palm fruits of “Medjoul” and “Confitera” cv. from the Southeast of Spain (Elche Palm Grove). Foods 2023, 12, 2659. [Google Scholar] [CrossRef]
- Muñoz-Tebar, N.; Viuda-Martos, M.; Lorenzo, J.M.; Fernandez-Lopez, J.; Perez-Alvarez, J.A. Strategies for the Valorization of Date Fruit and Its Co-Products: A New Ingredient in the Development of Value-Added Foods. Foods 2023, 12, 1456. [Google Scholar] [CrossRef]
- Abdul-Hamid, N.A.; Mustaffer, N.H.; Maulidiani, M.; Mediani, A.; Ismail, I.S.; Tham, C.L.; Shadid, K.; Abas, F. Quality evaluation of the physical properties, phytochemicals, biological activities and proximate analysis of nine Saudi date palm fruit varieties. J. Saudi Soc. Agric. Sci. 2020, 19, 151–160. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Lante, A. Environmentally friendly techniques for the recovery of polyphenols from food by-products and their impact on polyphenol oxidase: A critical review. Appl. Sci. 2022, 12, 1923. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Shokramraji, Z.; Tavakkoli, S.; Mihaylova, D.; Lante, A. Chlorophylls as Natural Bioactive Compounds Existing in Food By-Products: A Critical Review. Plants 2023, 12, 1533. [Google Scholar] [CrossRef]
- Koutchma, T.; Bissonnette, S.; Popović, V. An Update on Research, Development and Implementation of UV and Pulsed Light Technologies for Nonthermal Preservation of Milk and Dairy Products; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Singh, H.; Bhardwaj, S.K.; Khatri, M.; Kim, K.-H.; Bhardwaj, N. UVC radiation for food safety: An emerging technology for the microbial disinfection of food products. Chem. Eng. J. 2021, 417, 128084. [Google Scholar] [CrossRef]
- EFSA on Dietetic Products, Nutrition and Allergies. Scientific opinion on the safety of UV-treated milk as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2016, 14, 4370. [Google Scholar]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol. Biochem. 2016, 105, 1–11. [Google Scholar] [CrossRef]
- Gil, M.I.; Selma, M.V.; López-Gálvez, F.; Allende, A. Fresh-cut product sanitation and wash water disinfection: Problems and solutions. Int. J. Food Microbiol. 2009, 134, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, S.; Xu, J.; Liu, S.; Li, G. Effects of combined aqueous chlorine dioxide and UV-C on shelf-life quality of blueberries. Postharvest Biol. Technol. 2016, 117, 125–131. [Google Scholar] [CrossRef]
- Delorme, M.M.; Guimarães, J.T.; Coutinho, N.M.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; Margalho, L.P.; Pimentel, T.C.; Silva, M.C.; Freitas, M.Q. Ultraviolet radiation: An interesting technology to preserve quality and safety of milk and dairy foods. Trends Food Sci. Technol. 2020, 102, 146–154. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Z.; Guo, C.; Wu, Y.; Liu, W.; Yu, J.; Menghe, B.; Yang, R.; Zhang, H. Genetic diversity and population structure of Lactobacillus delbrueckii subspecies bulgaricus isolated from naturally fermented dairy foods. Sci. Rep. 2016, 6, 22704. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Cao, J.; Jiang, W. UV-C treatment controls brown rot in postharvest nectarine by regulating ROS metabolism and anthocyanin synthesis. Postharvest Biol. Technol. 2021, 180, 111613. [Google Scholar] [CrossRef]
- Günter, E.A.; Kapustina, O.M.; Popeyko, O.V.; Ovodov, Y.S. Influence of ultraviolet-C on the compositions of cell-wall polysaccharides and carbohydrase activities of Silene vulgaris callus. Carbohydr. Res. 2007, 342, 182–189. [Google Scholar] [CrossRef]
- 28 Meléndez-Pizarro, C.O.; Calva-Quintana, A.; Espinoza-Hicks, J.C.; Sánchez-Madrigal, M.Á.; Quintero-Ramos, A. Continuous Flow UV-C Irradiation Effects on the Physicochemical Properties of Aloe vera Gel and Pitaya (S tenocereus spp.) Blend. Foods 2020, 9, 1068. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Robles, P.A.; Gómez, P.A.; Tomás-Callejas, A.; Artés, F.; Martínez-Hernández, G.B. Quality changes of fresh-cut watermelon during storage as affected by cut intensity and UV-C pre-treatment. Food Bioprocess Technol. 2021, 14, 505–517. [Google Scholar] [CrossRef]
- Rabelo, M.C.; Bang, W.Y.; Nair, V.; Alves, R.E.; Jacobo-Velázquez, D.A.; Sreedharan, S.; de Miranda, M.R.A.; Cisneros-Zevallos, L. UVC light modulates vitamin C and phenolic biosynthesis in acerola fruit: Role of increased mitochondria activity and ROS production. Sci. Rep. 2020, 10, 21972. [Google Scholar] [CrossRef]
- Djaoud, K.; Boulekbache-Makhlouf, L.; Yahia, M.; Mansouri, H.; Mansouri, N.; Madani, K.; Romero, A. Dairy dessert processing: Effect of sugar substitution by date syrup and powder on its quality characteristics. J. Food Process. Preserv. 2020, 44, e14414. [Google Scholar] [CrossRef]
- Delgado, A.; Unamuno, V.; Muñiz, J.L.; Correcher, V.; Gomez-Ros, J.M. A simple UV irradiator for low dose reassessment with LiF TLD-100. Radiat. Prot. Dosim. 1996, 67, 303–306. [Google Scholar] [CrossRef]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andréu, F.J.; Esteban, R.M. Effect of sterilisation on dietary fibre and physicochemical properties of onion by-products. Food Chem. 2011, 127, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Aparicio, I.; Mateos-Peinado, C.; Rupérez, P. High hydrostatic pressure improves the functionality of dietary fibre in okara by-product from soybean. Innov. Food Sci. Emerg. Technol. 2010, 11, 445–450. [Google Scholar] [CrossRef]
- De la Peña-Armada, R.; Villanueva-Suárez, M.J.; Rupérez, P.; Mateos-Aparicio, I. High hydrostatic pressure assisted by celluclast® releases oligosaccharides from apple by-product. Foods 2020, 9, 1058. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Medina, M.B. Simple and rapid method for the analysis of phenolic compounds in beverages and grains. J. Agric. Food Chem. 2011, 59, 1565–1571. [Google Scholar] [CrossRef]
- Chen, Z.; Bertin, R.; Froldi, G. EC50 estimation of antioxidant activity in DPPH assay using several statistical programs. Food Chem. 2013, 138, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.T.; Matias, A.A.; Almeida, A.P.; Bronze, M.; Alves, P.M.; de Sousa, H.C.; Duarte, C.M. Processing cherries (Prunus avium) using supercritical fluid technology. Part 2. Evaluation of SCF extracts as promising natural chemotherapeutical agents. J. Supercrit. Fluids 2011, 55, 1007–1013. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Godswill, C.; Somtochukwu, V.; Kate, C. The functional properties of foods and flours. Int. J. Adv. Acad. Res. 2019, 5, 2488–9849. [Google Scholar]
- Yousf, N.; Nazir, F.; Salim, R.; Ahsan, H.; Sirwal, A. Water solubility index and water absorption index of extruded product from rice and carrot blend. J. Pharmacogn. Phytochem. 2017, 6, 2165–2168. [Google Scholar]
- Laaroussi, H.; Ferreira-Santos, P.; Genisheva, Z.; Bakour, M.; Ousaaid, D.; El Ghouizi, A.; Teixeira, J.A.; Lyoussi, B. Unveiling the techno-functional and bioactive properties of bee pollen as an added-value food ingredient. Food Chem. 2023, 405, 134958. [Google Scholar] [CrossRef] [PubMed]
- Teye, E.; Agbemafle, R.; Lamptey, F.P. Development and examination of sweet potato flour fortified with indigenous underutilized seasonal vegetables. Beverages 2018, 4, 5. [Google Scholar] [CrossRef]
- Jose, M.; Himashree, P.; Sengar, A.S.; Sunil, C. Valorization of food industry by-product (Pineapple Pomace): A study to evaluate its effect on physicochemical and textural properties of developed cookies. Meas. Food 2022, 6, 100031. [Google Scholar] [CrossRef]
- Lorente-Mento, J.M.; Lucas-González, R.; Sayas-Barbera, E.; Pérez-Álvarez, J.Á.; Fernández-López, J.; Viuda-Martos, M. Turrón coproducts as source of bioactive compounds: Assessment of chemical, physico-chemical, techno-functional and antioxidant properties. Foods 2020, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martí, J.; Panušková, K.; Larrea, V.; Bleha, R.; Quiles, A.; Hernando, I. Using different physical treatments to modify the structure and improve the technofunctional properties of clementine by-products. Food Struct. 2023, 38, 100346. [Google Scholar] [CrossRef]
- Megías-Pérez, R.; Ferreira-Lazarte, A.; Villamiel, M. Valorization of Grape Pomace as a Renewable Source of Techno-Functional and Antioxidant Pectins. Antioxidants 2023, 12, 957. [Google Scholar] [CrossRef]
- Alvarez-Ossorio, C.; Orive, M.; Sanmartín, E.; Alvarez-Sabatel, S.; Labidi, J.; Zufia, J.; Bald, C. Composition and Techno-functional Properties of Grape Seed Flour Protein Extracts. ACS Food Sci. Technol. 2022, 2, 125–135. [Google Scholar] [CrossRef]
- Grasso, N.; Lynch, N.L.; Arendt, E.K.; O’Mahony, J.A. Chickpea protein ingredients: A review of composition, functionality, and applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Chukwu, M.; Kabuo, N.; Nwokocha, N. Effects of fermentation time on the functional properties of ogiri-ahuekere (Arachis hypogaea Linn) seed condiment. Int. J. Biotechnol. Food Sci. 2018, 6, 77–85. [Google Scholar] [CrossRef]
- Oladele, A.; Aina, J. Chemical composition and properties of flour produced from two varieties of tigernut (Cyperns esculentus). Afr. J. Biotechnol. 2009, 6, 2473–2476. [Google Scholar] [CrossRef]
- Aguilera, Y.; Benítez, V.; Mollá, E.; Esteban, R.M.; Martín-Cabrejas, M.A. Influence of dehydration process in castellano chickpea: Changes in bioactive carbohydrates and functional properties. Plant Foods Hum. Nutr. 2011, 66, 391–400. [Google Scholar] [CrossRef]
- Hashim, I.; Khalil, A. Composition and functional properties of the date fruit residue a byproduct of date syrup/Debis production. Nutr. Food Tech 2015, 1, 1–5. [Google Scholar]
- Garcia-Amezquita, L.E.; Tejada-Ortigoza, V.; Heredia-Olea, E.; Serna-Saldívar, S.O.; Welti-Chanes, J. Differences in the dietary fiber content of fruits and their by-products quantified by conventional and integrated AOAC official methodologies. J. Food Compos. Anal. 2018, 67, 77–85. [Google Scholar] [CrossRef]
- Ma, J.; Adler, L.; Srzednicki, G.; Arcot, J. Quantitative determination of non-starch polysaccharides in foods using Gas Chromatography with flame ionization detection. Food Chem. 2017, 220, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.E.B. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 2014, 93, 2380–2393. [Google Scholar] [CrossRef] [PubMed]
- Noorbakhsh, H.; Khorasgani, M.R. Date (Phoenix dactylifera L.) polysaccharides: A review on Chemical structure and nutritional properties. J. Food Meas. Charact. 2022, 16, 3240–3250. [Google Scholar] [CrossRef]
- Birkett, A.; Cho, S. Cereal fiber and health: Current knowledge. Cereal Food World 2013, 58, 309–313. [Google Scholar] [CrossRef]
- Mrabet, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Hamza, H.; Rodríguez-Arcos, R.; Guillén-Bejarano, R.; Sindic, M.; Jiménez-Araujo, A. Enzymatic conversion of date fruit fiber concentrates into a new product enriched in antioxidant soluble fiber. LWT 2017, 75, 727–734. [Google Scholar] [CrossRef]
- Bano, Y.; Rakha, A.; Khan, M.I.; Asgher, M. Chemical composition and antioxidant activity of date (Phoenix dactylifera L.) varieties at various maturity stages. Food Sci. Technol. 2022, 42, e29022. [Google Scholar] [CrossRef]
- Ötles, S.; Ozgoz, S. Health effects of dietary fiber. Acta Sci. Pol. Technol. Aliment. 2014, 13, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Djaoudene, O.; Bey, M.B.; Louaileche, H. Physicochemical Characteristics and Nutritional Compositions of Some Date (Phoenix dactylifera L.) Fruit Cultivars. Acta Univ. Cibiniensis. Ser. E Food Technol. 2019, 23, 129–138. [Google Scholar] [CrossRef]
- Hariri, A.; Ouis, N.; Bouhadi, D. Effect of substitution of sugars by date powders variety H’lowa on the quality of the soft drinks. J. Appl. Biotechnol. Bioeng. 2017, 3, 450–457. [Google Scholar]
- Assirey, E.A.R. Nutritional composition of fruit of 10 date palm (Phoenix dactylifera L.) cultivars grown in Saudi Arabia. J. Taibah Univ. Sci. 2015, 9, 75–79. [Google Scholar] [CrossRef]
- Aslam, A.; Leghari, S.; Asrar, M.; Saeed, S.; Shafi, M.; Siddiqi, M.; Sumalani, M.; Maham, F.; Merri, A. Physico-Chemical Diversity and Microbial Burden in Four Dates Palm (Phoenix Dactylifera L.) Fruit Varieties Grown in Agro-Climatic Condition of Turbat, Balochistan-Pakistan. Appl. Ecol. Environ. Res. 2019, 17, 6625–6642. [Google Scholar] [CrossRef]
- Taha, R.; Sihem, B.M.; Marianne, S.; Ali, S.; Ahmed, N.; Mars, M. Variability of physicochemical properties of ‘Deglet Nour’date fruits collected from different oases in Djerid Region, Tunisia. J. Hortic. Postharvest Res. 2020, 3, 85–100. [Google Scholar]
- Hussain, I.; Ahmad, S.; Amjad, M.; Ahmed, R. Execution of strands thinning improves the phytochemicals and sugars profiling in date palm (Phoenix dactylifera L.) fruit. Pak. J. Pharm. Sci. 2016, 29, 1209–1215. [Google Scholar]
- Haider, M.S.; Khan, I.A.; Naqvi, S.A.; Jaskani, M.; Khan, R.W.; Nafees, M.; Pasha, I. Fruit developmental stages effects on biochemical attributes in date palm. Pak. J. Agric. Sci. 2013, 50, 577–583. [Google Scholar]
- Airouyuwa, J.O.; Mostafa, H.; Riaz, A.; Stathopoulos, C.; Maqsood, S. Natural Deep Eutectic Solvents and Microwave-Assisted Green Extraction for Efficient Recovery of Bioactive Compounds from By-Products of Date Fruit (Phoenix dactylifera L.) Processing: Modeling, Optimization, and Phenolic Characterization. Food Bioprocess Technol. 2023, 16, 824–843. [Google Scholar] [CrossRef]
- Bouhlali, E.d.T.; Hmidani, A.; Bourkhis, B.; Khouya, T.; Ramchoun, M.; Filali-Zegzouti, Y.; Alem, C. Phenolic profile and anti-inflammatory activity of four Moroccan date (Phoenix dactylifera L.) seed varieties. Heliyon 2020, 6, e03436. [Google Scholar] [CrossRef]
- Benmeddour, Z.; Mehinagic, E.; Le Meurlay, D.; Louaileche, H. Phenolic composition and antioxidant capacities of ten Algerian date (Phoenix dactylifera L.) cultivars: A comparative study. J. Funct. Foods 2013, 5, 346–354. [Google Scholar] [CrossRef]
- Khallouki, F.; Ricarte, I.; Breuer, A.; Owen, R.W. Characterization of phenolic compounds in mature Moroccan Medjool date palm fruits (Phoenix dactylifera) by HPLC-DAD-ESI-MS. J. Food Compos. Anal. 2018, 70, 63–71. [Google Scholar] [CrossRef]
- Hilary, S.; Tomás-Barberán, F.A.; Martinez-Blazquez, J.A.; Kizhakkayil, J.; Souka, U.; Al-Hammadi, S.; Habib, H.; Ibrahim, W.; Platat, C. Polyphenol characterisation of Phoenix dactylifera L. (date) seeds using HPLC-mass spectrometry and its bioaccessibility using simulated in-vitro digestion/Caco-2 culture model. Food Chem. 2020, 311, 125969. [Google Scholar] [CrossRef]
- Bayrak, Ç.; Birinci, C.; Kemal, M.; Kolayli, S. The Phenolic Composition and Antioxidant Properties of Figs (Ficus carica L.) Grown in the Black Sea Region. Plant Foods Hum. Nutr. 2023, 78, 539–545. [Google Scholar] [CrossRef]
- Echegaray, N.; Pateiro, M.; Gullon, B.; Amarowicz, R.; Misihairabgwi, J.M.; Lorenzo, J.M. Phoenix dactylifera products in human health—A review. Trends Food Sci. Technol. 2020, 105, 238–250. [Google Scholar] [CrossRef]
- Maqsood, S.; Adiamo, O.; Ahmad, M.; Mudgil, P. Bioactive compounds from date fruit and seed as potential nutraceutical and functional food ingredients. Food Chem. 2020, 308, 125522. [Google Scholar] [CrossRef]
- Costa-Pérez, A.; Ferrer, M.A.; Calderón, A.A. Combined Effects of Cytokinin and UV-C Light on Phenolic Pattern in Ceratonia siliqua Shoot Cultures. Agronomy 2023, 13, 621. [Google Scholar] [CrossRef]
- Valerga, L.; González, R.E.; Pérez, M.B.; Concellón, A.; Cavagnaro, P.F. Differential and Cultivar-Dependent Antioxidant Response of Whole and Fresh-Cut Carrots of Different Root Colors to Postharvest UV-C Radiation. Plants 2023, 12, 1297. [Google Scholar] [CrossRef] [PubMed]
- Xuejiao, Z.; Xiaoyuan, Z.; Ye, H.; Ruirui, Y.; Qihui, W.; Di, G.; Yongcai, L.; Dov, P.; Yang, B. UV-C irradiation maintains cell membrane integrity at wounds of potato tubers during healing by regulating ROS homeostasis and increasing antioxidant activity. Postharvest Biol. Technol. 2023, 199, 112308. [Google Scholar]
- Harrison, K.; Were, L. Effect of gamma irradiation on total phenolic content yield and antioxidant capacity of almond skin extracts. Food Chem. 2007, 102, 932–937. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; Gallo, C.R.; Shahidi, F. Gamma-irradiation induced changes in microbiological status, phenolic profile and antioxidant activity of peanut skin. J. Funct. Foods 2015, 12, 129–143. [Google Scholar] [CrossRef]
- Taheri, S.; Abdullah, T.L.; Karimi, E.; Oskoueian, E.; Ebrahimi, M. Antioxidant capacities and total phenolic contents enhancement with acute gamma irradiation in Curcuma alismatifolia (Zingiberaceae) leaves. Int. J. Mol. Sci. 2014, 15, 13077–13090. [Google Scholar] [CrossRef]
- Gerolis, L.G.L.; Lameiras, F.S.; Krambrock, K.; Neves, M.J. Effect of gamma radiation on antioxidant capacity of green tea, yerba mate, and chamomile tea as evaluated by different methods. Radiat. Phys. Chem. 2017, 130, 177–185. [Google Scholar] [CrossRef]
- Corrales, M.; de Souza, P.M.; Stahl, M.R.; Fernández, A. Effects of the decontamination of a fresh tiger nuts’ milk beverage (horchata) with short wave ultraviolet treatments (UV-C) on quality attributes. Innovative Food Sci. Emerg. Technol. 2012, 13, 163–168. [Google Scholar] [CrossRef]
- Crupi, P.; Pichierri, A.; Basile, T.; Antonacci, D. Postharvest stilbenes and flavonoids enrichment of table grape cv Redglobe (Vitis vinifera L.) as affected by interactive UV-C exposure and storage conditions. Food Chem. 2013, 141, 802–808. [Google Scholar] [CrossRef]
- Freitas, A.; Moldão-Martins, M.; Costa, H.S.; Albuquerque, T.G.; Valente, A.; Sanches-Silva, A. Effect of UV-C radiation on bioactive compounds of pineapple (Ananas comosus L. Merr.) by-products. J. Sci. Food Agric. 2015, 95, 44–52. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Villegas-Ochoa, M.A.; Martínez-Téllez, M.; Gardea, A.; Ayala-Zavala, J.F. Improving antioxidant capacity of fresh-cut mangoes treated with UV-C. J. Food Sci. 2007, 72, S197–S202. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Zhang, X.; Yong, H.; Wang, X.; Liu, Y.; Liu, J. Development and characterization of antioxidant active packaging and intelligent Al3+-sensing films based on carboxymethyl chitosan and quercetin. Int. J. Biol. Macromol. 2019, 126, 1074–1084. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef]
- Zhuang, X.-C.; Chen, G.-L.; Liu, Y.; Zhang, Y.-L.; Guo, M.-Q. New lignanamides with antioxidant and anti-inflammatory activities screened out and identified from Warburgia ugandensis combining affinity ultrafiltration LC-MS with SOD and XOD enzymes. Antioxidants 2021, 10, 370. [Google Scholar] [CrossRef]
- Tang, Y.; Zuo, C.; Li, J.; Li, L.; Zhang, H. Synergism of antioxidant and antibacterial activities between Hanzhongxianhao tea polyphenols and vitamin C. J. Food Sci. Technol. 2017, 35, 55–60. [Google Scholar]
| Property | Value |
|---|---|
| Swelling capacity (mL/g) | 3.94 ± 0.003 |
| Oil holding capacity (g/g) | 7.38 ± 0.30 |
| Water holding capacity (g/g) | 9.30 ± 0.25 |
| Bulk density (g/mL) | 1.81 ± 0.01 |
| Monomers | Date Fruit Powder (DFP) | ||
|---|---|---|---|
| IDF (g/100 g) | SDF (g/100 g) | TDF (g/100 g) | |
| Rhamnose | 0.60 ± 0.05 de | 0.71 ± 0.07 bc | 1.31 ± 0.03 d |
| Fucose | 0.55 ± 0.03 e | 0.67 ± 0.03 c | 1.22 ± 0.03 e |
| Arabinose | 4.28 ± 0.10 a | 0.79 ± 0.11 b | 5.07 ± 0.13 a |
| Xylose | 3.17 ± 0.14 b | 0.56 ± 0.10 d | 3.73 ± 0.13 b |
| Mannose | 0.64 ± 0.05 de | 0.51 ± 0.14 de | 1.15 ± 0.13 ab |
| Galactose | 1.03 ± 0.03 c | 0.36 ± 0.11 e | 1.38 ± 0.10 d |
| Glucose | 0.98 ± 0.06 cd | 0.17 ± 0.03 f | 1.02 ± 0.09 f |
| Uronic acids | 0.78 ± 0.05 d | 1.39 ± 0.09 a | 2.19 ± 0.10 c |
| Total | 11.89 ± 0.25 | 5.15 ± 0.33 | 17.06 ± 0.25 |
| Peak N° | Standards | Untreated (g/100 g) | 5 min (g/100 g) | 10 min (g/100 g) | 20 min (g/100 g) | 40 min (g/100 g) | |
|---|---|---|---|---|---|---|---|
| Mw (kDa) | RT (min) | ||||||
| Peak 1 | 100 | 11.90 | 0.38 ± 0.03 b | 0.22 ± 0.03 c | 0.28 ± 0.01 c | 0.53 ± 0.05 a | 0.53 ± 0.02 a |
| Peak 2 | 100 | 14.55 | 4.43 ± 0.08 b | 4.52 ± 0.02 a | 4.52 ± 0.02 a | 4.56 ± 0.03 a | 4.48 ± 0.04 ab |
| Peak 3 | 20 | 15.20 | nd | 0.84 ± 0.03 c | 0.94 ± 0.03 b | 1.08 ± 0.03 a | nd |
| Peak 4 | 20 | 15.85 | nd | nd | nd | nd | 1.32 ± 0.01 b |
| Peak 5 | 0.50 | 29.00 | 0.17 ± 0.01 c | 0.42 ± 0.02 a | 0.37 ± 0.03 a | 0.33 ± 0.07 b | 0.16 ± 0.02 c |
| Peak 6 | 0.50 | 30.00 | nd | 0.23 ± 0.04 a | 0.16 ± 0.02 b | nd | nd |
| Peak 7 | 0.34 | 33.80 | nd | 0.05 ± 0.02 a | nd | nd | nd |
| Peak 8 | 0.34 | 34.30 | 0.06 ± 0.01 a | nd | 0.03 ± 0.01 b | 0.07 ± 0.03 a | 0.04 ± 0.005 b |
| Peak 9 | 0.34 | 35.10 | 0.17 ± 0.02 b | 0.25 ± 0.05 a | 0.21 ± 0.05 a | 0.21 ± 0.02 a | 0.23 ± 0.02 a |
| Peak 10 | 0.34 | 37.00 | 0.09 ± 0.01 b | 0.15 ± 0.03 a | 0.16 ± 0.01 a | 0.12 ± 0.01 b | 0.14 ± 0.02 a |
| Peak 11 | 0.18 | 41.90 | 34.90 ± 0.10 a | 33.58 ± 0.05 c | 33.59 ± 0.13 c | 33.84 ± 0.10 b | 33.29 ± 0.01 d |
| Peak 12 | 0.18 | 45.30 | 39.83 ± 0.14 b | 39.77 ± 0.03 b | 39.79 ± 0.02 b | 40.36 ± 0.02 a | 39.26 ± 0.10 c |
| Total | 80.02 ± 0.01 b | 80.03 ± 0.01 b | 80.05 ± 0.04 b | 81.10 ± 0.01 a | 78.45 ± 0.01 c | ||
| Sample | Fast-Blue (mg GAE/g dw) | Folin (mg GAE/g dw) | FRAP (mg TE/g dw) | ORAC (µmol TE/g dw) | DPPH EC50 (mg TE/mL) |
|---|---|---|---|---|---|
| Untreated | 106.17 ± 2.66 a | 16.12 ± 0.62 a | 146.54 ± 1.84 a | 75.60 ± 8.24 a | 21.30 ± 0.27 b |
| 5 min | 99.14 ± 0.52 b | 14.12 ± 0.08 b | 88.50 ± 0.12 b | 62.74 ± 12.55 ab | 58.24 ± 1.11 a |
| 10 min | 92.10 ± 1.40 c | 13.82 ± 1.03 bc | 86.35 ± 0.30 c | 61.65 ± 6.88 b | 58.27 ± 0.47 a |
| 20 min | 73.46 ± 0.17 d | 11.01 ± 2.03 c | 77.60 ± 0.24 d | 60.32 ± 2.05 b | 58.73 ± 0.97 a |
| 40 min | 104.81 ± 3.32 a | 15.28 ± 0.65 a | 77.70 ± 0.94 d | 64.57 ± 14.45 ab | 57.14 ± 0.40 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djaoud, K.; De la Peña-Armada, R.; García-Alonso, A.; Correcher, V.; Boulekbache-Makhlouf, L.; Mateos-Aparicio, I. UV-C Treatment Impact on the Availability of Water-Soluble Carbohydrates, Polyphenols, and Antioxidant Capacity of an Algerian Underutilized Date Fruit (Phoenix dactylifera L.). Foods 2024, 13, 893. https://doi.org/10.3390/foods13060893
Djaoud K, De la Peña-Armada R, García-Alonso A, Correcher V, Boulekbache-Makhlouf L, Mateos-Aparicio I. UV-C Treatment Impact on the Availability of Water-Soluble Carbohydrates, Polyphenols, and Antioxidant Capacity of an Algerian Underutilized Date Fruit (Phoenix dactylifera L.). Foods. 2024; 13(6):893. https://doi.org/10.3390/foods13060893
Chicago/Turabian StyleDjaoud, Kahina, Rocío De la Peña-Armada, Alejandra García-Alonso, Virgilio Correcher, Lila Boulekbache-Makhlouf, and Inmaculada Mateos-Aparicio. 2024. "UV-C Treatment Impact on the Availability of Water-Soluble Carbohydrates, Polyphenols, and Antioxidant Capacity of an Algerian Underutilized Date Fruit (Phoenix dactylifera L.)" Foods 13, no. 6: 893. https://doi.org/10.3390/foods13060893
APA StyleDjaoud, K., De la Peña-Armada, R., García-Alonso, A., Correcher, V., Boulekbache-Makhlouf, L., & Mateos-Aparicio, I. (2024). UV-C Treatment Impact on the Availability of Water-Soluble Carbohydrates, Polyphenols, and Antioxidant Capacity of an Algerian Underutilized Date Fruit (Phoenix dactylifera L.). Foods, 13(6), 893. https://doi.org/10.3390/foods13060893






