Volatilomics-Based Discovery of Key Volatiles Affecting Flavor Quality in Tomato
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Materials and Sampling
2.2. Reagents and Standards
2.3. Sensory Analysis
2.4. Sample Preparation and Extraction
2.5. HS-SPME/GC–MS Analysis
2.6. Identification and Quantification of Volatiles of Tomato Fruits
2.7. Metabolome Data Analysis
3. Results
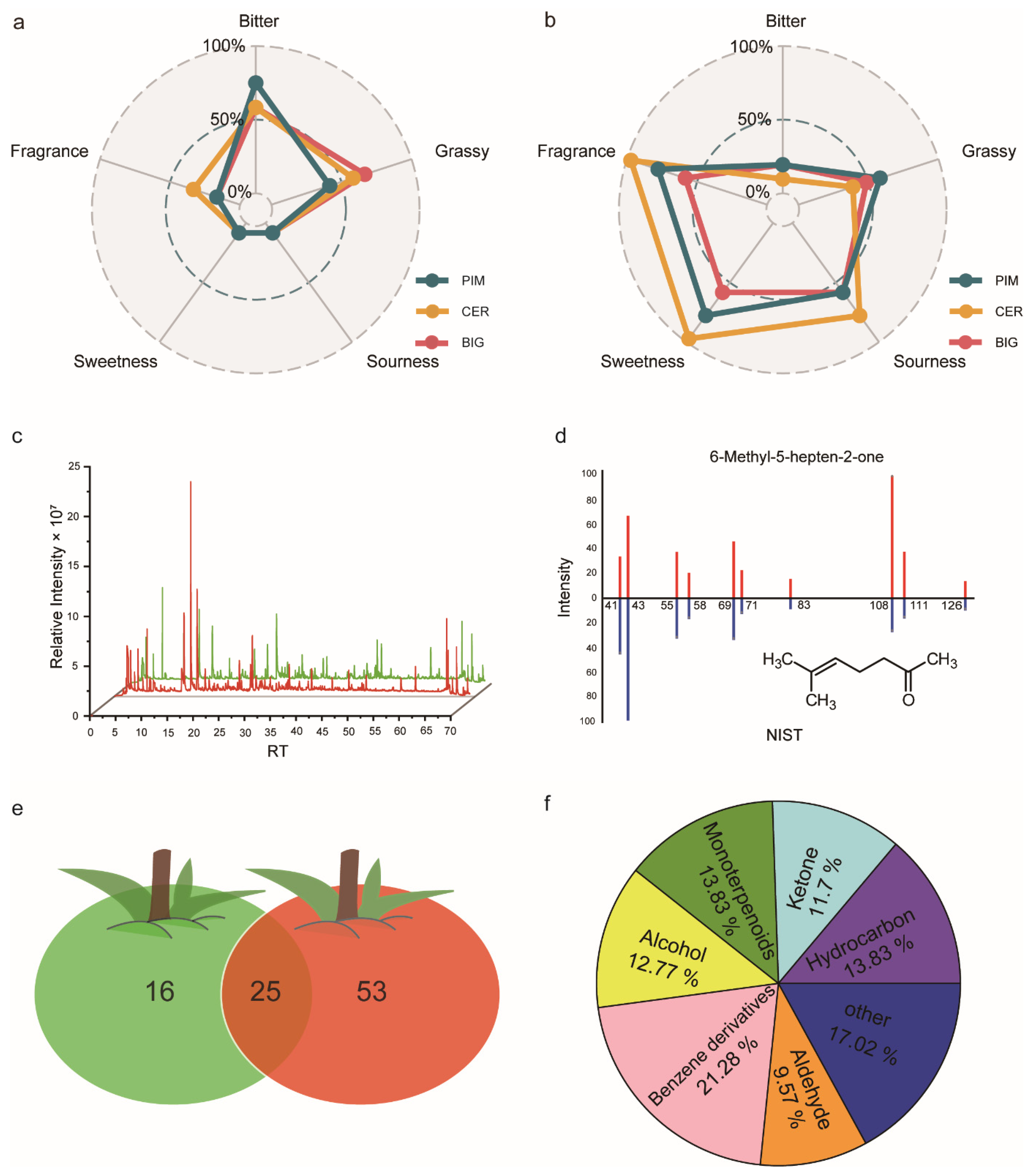
3.1. Construction of a Tomato-Fruit Volatiles Database
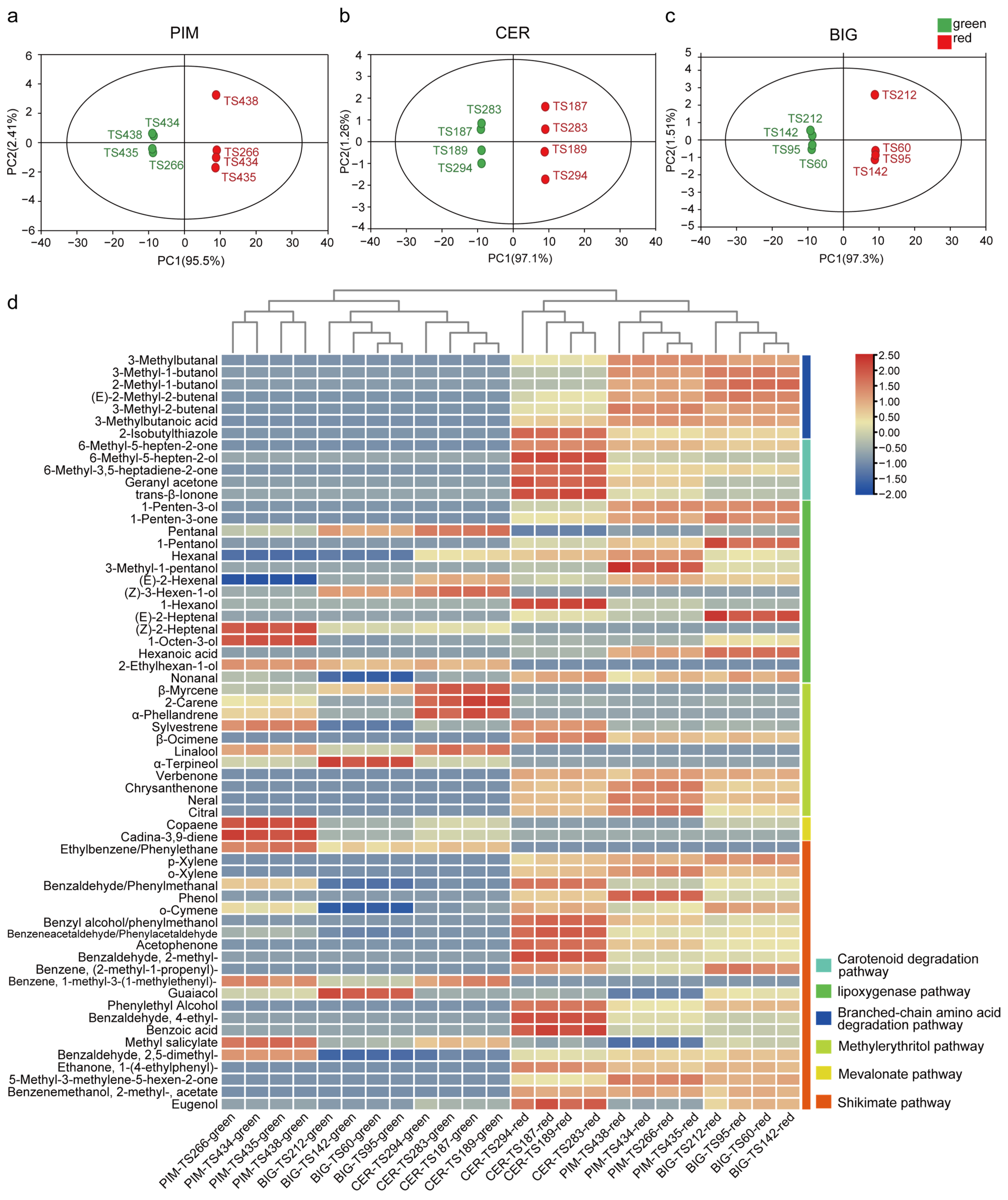
3.2. Volatile Accumulation during Tomato-Fruit Ripening
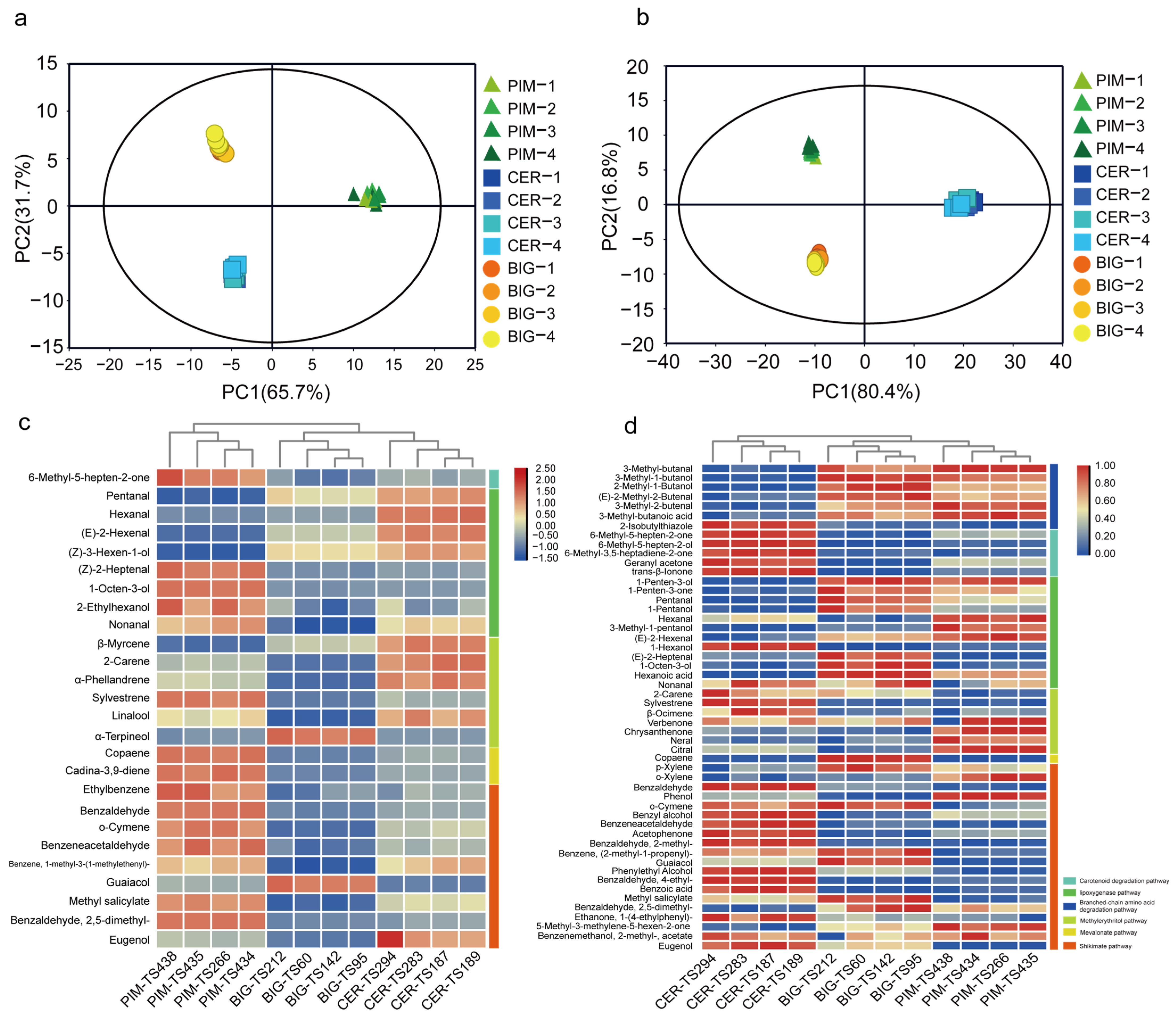
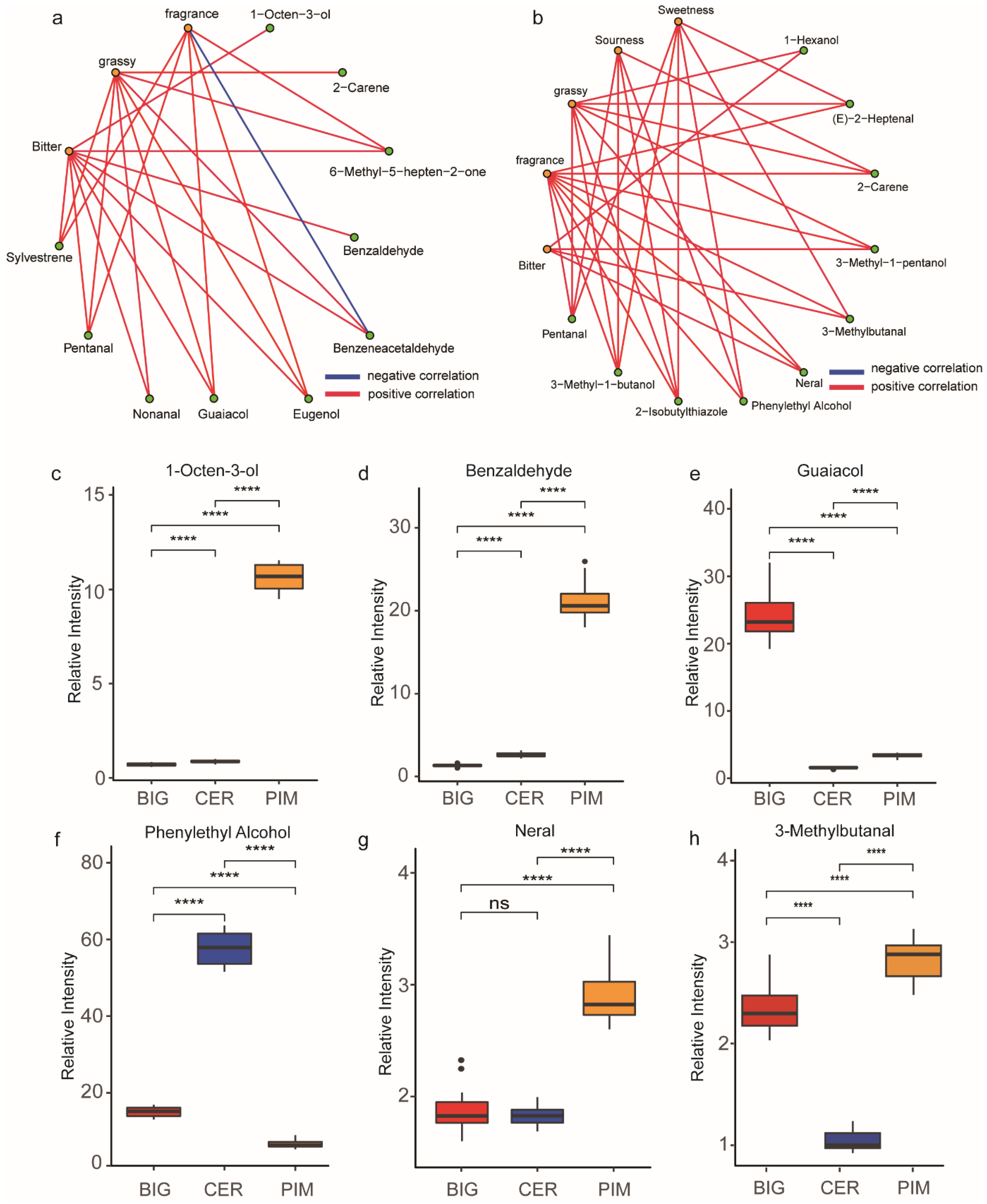
3.3. Relationship between Volatile Accumulation and Flavor Quality during Tomato Domestication and Improvement
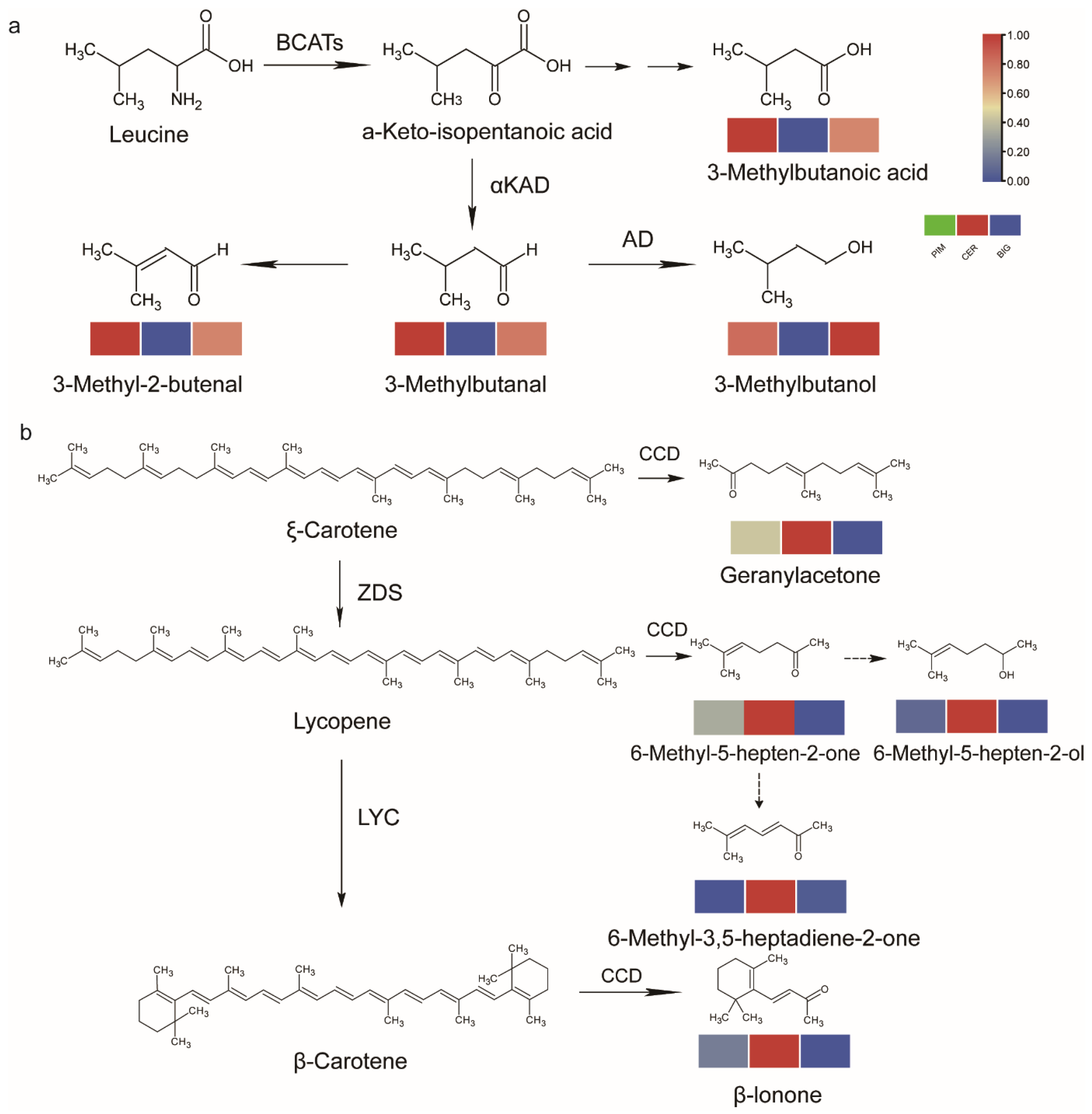
3.4. Volatile Metabolic Pathways Affect Tomato Flavor Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.P.; Lutts, S. Tomato Fruit Development and Metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Viljanen, K.; Lille, M.; Heiniö, R.L.; Buchert, J. Effect of high-pressure processing on volatile composition and odour of cherry tomato puree. Food Chem. 2011, 129, 1759–1765. [Google Scholar] [CrossRef]
- El Hadi, M.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in Fruit Aroma Volatile Research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, B. Biogenesis of aroma compounds: Flavour formation in fruits and vegetables. In Flavour Development; Woodhead Publishing: Sawston, UK, 2015; pp. 127–149. [Google Scholar]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Abugu, M.; Tieman, D. The dissection of tomato flavor: Biochemistry, genetics, and omics. Front. Plant Sci. 2023, 14, 1144113. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Baldwin, E.A.; Plotto, A.; Luo, W.; Raithore, S.; Yu, Z.; Bai, J. Effect of methyl salicylate and methyl jasmonate pre-treatment on the volatile profile in tomato fruit subjected to chilling temperature. Postharvest Biol. Technol. 2015, 108, 28–38. [Google Scholar] [CrossRef]
- Colantonio, V.; Ferra, L.F.V.; Tieman, D.M.; Bliznyuk, N.; Sims, C.; Klee, H.J.; Munoz, P.; Resende, M.F.R. Metabolomic selection for enhanced fruit flavor. Proc. Natl. Acad. Sci. USA 2022, 119, e2115865119. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Zhang, Q.R.; Zhu, H.S.; Li, J.M.; Wen, Q.F. Transcriptome and Metabolome Provide Insights into Fruit Ripening of Cherry Tomato (Solanum lycopersicum var. cerasiforme). Plants 2023, 12, 3505. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, E.A.; Goodner, K.; Plotto, A. Interaction of volatiles, sugars, and acids on perception of tomato aroma and flavor descriptors. J. Food Sci. 2008, 73, 294–307. [Google Scholar] [CrossRef]
- Wang, L.; Baldwin, E.A.; Bai, J. Recent Advance in Aromatic Volatile Research in Tomato Fruit: The Metabolisms and Regulations. Food Bioprocess Technol. 2015, 9, 203–216. [Google Scholar] [CrossRef]
- Kovács, K.; Fray, R.G.; Tikunov, Y.; Graham, N.; Bradley, G.; Seymour, G.B.; Bovy, A.G.; Grierson, D. Effect of tomato pleiotropic ripening mutations on flavour volatile biosynthesis. Phytochemistry 2009, 70, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Boukobza, F.T.; Andrew, J.T. Effect of postharvest treatment on flavour volatiles of tomatoes. Postharvest Biol. Technol. 2002, 25, 321–331. [Google Scholar] [CrossRef]
- Valentina, B.; Giovanni, C.; Spadafora, N.D.; Alessio, A.; Müller, C.T.; Rogers, H.J.; Antonio, F. Wounding tomato fruit elicits ripening-stage specific changes in gene expression and production of volatile compounds. J. Exp. Bot. 2015, 66, 1511–1526. [Google Scholar] [CrossRef]
- Birtic, S.; Ginies, C.; Causse, M.; Renard, C.M.; Page, D. Changes in Volatiles and Glycosides during Fruit Maturation of Two Contrasted Tomato (Solanum lycopersicum) Lines. J. Agric. Food Chem 2009, 57, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Tieman, D.; Zhu, G.; Resende, M.F.; Lin, T.; Nguyen, C.; Bies, D.; Rambla, J.L.; Beltran, K.S.; Taylor, M.; Zhang, B.; et al. A chemical genetic roadmap to improved tomato flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef]
- Gao, Y.; Lin, Y.J.; Xu, M.; Bian, H.X.; Zhang, C.; Wang, J.Y.; Wang, H.Q.; Xu, Y.P.; Niu, Q.F.; Zuo, J.H.; et al. The role and interaction between transcription factor NAC-NOR and DNA demethylase SlDML2 in the biosynthesis of tomato fruit flavor volatiles. New Phytol. 2022, 235, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Sapkota, M.; Alonge, M.; Zheng, Y.; Zhang, Y.J.; Razifard, H.; Taitano, N.K.; Schatz, M.C.; Fernie, A.R.; Wang, Y.; et al. Natural Genetic Diversity in Tomato Flavor Genes. Front. Plant Sci. 2021, 12, 642828. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.T.; Xu, Y.; Liang, J.; Chang, P.P.; Yan, F.; Li, M.J.; Liang, Y.; Zou, Z.R. Genome-Wide Association Mapping for Tomato Volatiles Positively Contributing to Tomato Flavor. Front. Plant Sci. 2015, 6, 1042. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Zhou, L.; You, S.; Deng, H.; Chen, Y.; Alseekh, S.; Yuan, Y.; Fu, R.; Zhang, Z.; et al. MicroTom Metabolic Network: Rewiring Tomato Metabolic Regulatory Network throughout the Growth Cycle. Mol. Plant. 2020, 13, 1203–1218. [Google Scholar] [CrossRef]
- Yuan, H.; Cao, G.; Hou, X.; Huang, M.; Du, P.; Tan, T.; Zhang, Y.; Zhou, H.; Liu, X.; Liu, L.; et al. Development of a widely targeted volatilomics method for profiling volatilomes in plants. Mol. Plant 2022, 15, 189–202. [Google Scholar] [CrossRef]
- Du, P.; Yuan, H.; Chen, Y.; Zhou, H.; Zhang, Y.; Huang, M.; Jiangfang, Y.; Su, R.; Chen, Q.; Lai, J.; et al. Identification of Key Aromatic Compounds in Basil (Ocimum L.) Using Sensory Evaluation, Metabolomics and Volatilomics Analysis. Metabolites 2023, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.B.; Luo, W.Q.; Sun, X.X.; Qian, C.L. Changes in flavor-relevant compounds during vine ripening of tomato fruit and their relationship with ethylene production. Hortic. Environ. Biotechnol. 2018, 59, 787–804. [Google Scholar] [CrossRef]
- Tieman, D.M.; Zeigler, M.; Schmelz, E.A.; Taylor, M.G.; Bliss, P.; Kirst, M.; Klee, H.J. Identification of loci affecting flavour volatile emissions in tomato fruits. J. Exp. Bot. 2006, 57, 887–896. [Google Scholar] [CrossRef]
- Rowan, D.D.; Hunt, M.B.; Alspach, P.A.; Whitworth, C.J.; Oraguzie, N.C. Heritability and genetic and phenotypic correlations of apple (Malus × domestica) fruit volatiles in a genetically diverse breeding population. J. Agric. Food Chem. 2009, 57, 7944–7952. [Google Scholar] [CrossRef] [PubMed]
- Bineau, E.; Rambla, J.L.; Priego-Cubero, S.; Hereil, A.; Bitton, F.; Plissonneau, C.; Granell, A.; Causse, M. Breeding Tomato Hybrids for Flavour: Comparison of GWAS Results Obtained on Lines and F1 Hybrids. Genes 2021, 12, 1443. [Google Scholar] [CrossRef] [PubMed]
- Cortina, P.R.; Asis, R.; Peralta, I.E.; Asprelli, P.D.; Santiago, A.N. Determination of Volatile Organic Compounds in Andean Tomato Landraces by Headspace Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry. J. Braz. Chem. Soc. 2017, 28, 30–41. [Google Scholar] [CrossRef]
- Olbricht, K.; Ulrich, D.; Weiss, K.; Grafe, C. Variation in the Amounts of Selected Volatiles in a Model Population of Fragaria × ananassa Duch. As Influenced by Harvest Year. J. Agric. Food Chem. 2011, 59, 944–952. [Google Scholar] [CrossRef]
- Rambla, J.L.; Medina, A.; Fernández-del-Carmen, A.; Barrantes, W.; Grandillo, S.; Cammareri, M.; López-Casado, G.; Rodrigo, G.; Alonso, A.; García-Martínez, S.; et al. Identification, introgression, and validation of fruit volatile QTLs from a red-fruited wild tomato species. J. Exp. Bot. 2017, 68, 429–442. [Google Scholar] [CrossRef]
- Raffo, A.; Baiamonte, I.; De Benedetti, L.; Lupotto, E.; Marchioni, I.; Nardo, N.; Cervelli, C. Exploring volatile aroma and non-volatile bioactive compounds diversity in wild populations of rosemary (Salvia rosmarinus Schleid.). Food Chem. 2023, 404, 134532. [Google Scholar] [CrossRef]
- Koyama, K.; Kono, A.; Ban, Y.; Bahena-Garrido, S.M.; Ohama, T.; Iwashita, K.; Fukuda, H.; Goto-Yamamoto, N. Genetic architecture of berry aroma compounds in a QTL (quantitative trait loci) mapping population of interspecific hybrid grapes (Vitis labruscana × Vitis vinifera). BMC Plant Biol. 2022, 22, 458. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, J.S.; Zlatkovic, B.K.; Jovanovic, S.C.; Stojanovic, G.S.; Marin, P.D.; Mitic, Z.S. Needle volatiles as chemophenetic markers in differentiation of natural populations of Abies alba, A. × borisii-regis, and A. cephalonica. Phytochemistry 2021, 183, 112612. [Google Scholar] [CrossRef]
- Barnett, J.R.; Tieman, D.M.; Caicedo, A.L. Variation in ripe fruit volatiles across the tomato clade: An evolutionary framework for studying fruit scent diversity in a crop wild relative. Am. J. Bot. 2023, 110, 16223. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Plotto, A.; Bai, J.; Whitaker, V.M. Volatiles Influencing Sensory Attributes and Bayesian Modeling of the Soluble Solids–Sweetness Relationship in Strawberry. Front. Plant Sci. 2021, 12, 640704. [Google Scholar] [CrossRef]
- Fan, Z.; Hasing, T.; Johnson, T.S.; Garner, D.M.; Schwieterman, M.L.; Barbey, C.R.; Colquhoun, T.A.; Sims, C.A.; Resende, M.F.R.; Whitaker, V.M. Strawberry sweetness and consumer preference are enhanced by specific volatile compounds. Hortic. Res. 2021, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Barabási, A.L.; Menichetti, G.; Loscalzo, J. The unmapped chemical complexity of our diet. Nat. Food 2020, 1, 33–37. [Google Scholar] [CrossRef]
- Hooton, F.; Menichetti, G.; Barabási, A.L. Exploring food contents in scientific literature with FoodMine. Sci. Rep.-UK 2020, 10, 16191. [Google Scholar] [CrossRef]
- Wang, S.C.; Qiang, Q.; Xiang, L.J.; Fernie, A.R.; Yang, J. Targeted approaches to improve tomato fruit taste. Hortic. Res. 2023, 10, 229. [Google Scholar] [CrossRef]
- Wang, L.B.; Qian, C.L.; Bai, J.H.; Luo, W.Q.; Jin, C.H.; Yu, Z.F. Difference in volatile composition between the pericarp tissue and inner tissue of tomato (Solanum lycopersicum) fruit. J. Food Process. Preserv. 2018, 42, 13387. [Google Scholar] [CrossRef]
- Vogel, J.T.; Tieman, D.M.; Sims, C.A.; Odabasi, A.Z.; Clark, D.G.; Klee, H.J. Carotenoid content impacts flavor acceptability in tomato (Solanum lycopersicum). J. Sci. Food Agric. 2010, 90, 2233–2240. [Google Scholar] [CrossRef]
- Rambla, J.L.; Tikunov, Y.M.; Monforte, A.J.; Bovy, A.G.; Granell, A. The expanded tomato fruit volatile landscape. J. Exp. Bot. 2014, 65, 4613–4623. [Google Scholar] [CrossRef] [PubMed]
- Bizzio, L.N.; Tieman, D.; Munoz, P.R. Branched-Chain Volatiles in Fruit: A Molecular Perspective. Front. Plant Sci. 2022, 12, 814138. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Bi, Y.; Zong, Y.; Li, Y.; Sionov, E.; Prusky, D. Penicillium expansum—Induced release of branched-chain volatile compounds in apple fruit by increasing amino acids accumulation. Postharvest Biol. Technol. 2021, 173, 111432–111441. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, W.; Huang, J.; Chen, H.H.; Nie, R.Z.; Li, C.M. Comparison of the nutritional as well as the volatile composition of in-season and off-season Hezuo 903 tomato at red stage. Eur. Food Res. Technol. 2017, 243, 203–214. [Google Scholar] [CrossRef]
- Goulet, C.; Kamiyoshihara, Y.; Lam, N.B.; Richard, T.; Taylor, M.G.; Tieman, D.M.; Klee, H.J. Divergence in the Enzymatic Activities of a Tomato and Solanum pennellii Alcohol Acyltransferase Impacts Fruit Volatile Ester Composition. Mol. Plant 2015, 8, 153–162. [Google Scholar] [CrossRef]
- Gorman, Z.; Tolley, J.P.; Koiwa, H.; Kolomiets, M.V. The Synthesis of Pentyl Leaf Volatiles and Their Role in Resistance to Anthracnose Leaf Blight. Front. Plant Sci. 2021, 12, 719587. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Ye, W.; Li, C.; Zhou, H.; Wang, C.; Liu, P.; Zhou, B.; Zhao, H.; Wang, S.; Yang, J. Volatilomics-Based Discovery of Key Volatiles Affecting Flavor Quality in Tomato. Foods 2024, 13, 879. https://doi.org/10.3390/foods13060879
Zhang Z, Ye W, Li C, Zhou H, Wang C, Liu P, Zhou B, Zhao H, Wang S, Yang J. Volatilomics-Based Discovery of Key Volatiles Affecting Flavor Quality in Tomato. Foods. 2024; 13(6):879. https://doi.org/10.3390/foods13060879
Chicago/Turabian StyleZhang, Zhonghui, Weizhen Ye, Chun Li, Haihong Zhou, Chao Wang, Penghui Liu, Binxin Zhou, Hanqing Zhao, Shouchuang Wang, and Jun Yang. 2024. "Volatilomics-Based Discovery of Key Volatiles Affecting Flavor Quality in Tomato" Foods 13, no. 6: 879. https://doi.org/10.3390/foods13060879
APA StyleZhang, Z., Ye, W., Li, C., Zhou, H., Wang, C., Liu, P., Zhou, B., Zhao, H., Wang, S., & Yang, J. (2024). Volatilomics-Based Discovery of Key Volatiles Affecting Flavor Quality in Tomato. Foods, 13(6), 879. https://doi.org/10.3390/foods13060879




