The Role of MaWRKY70 in Regulating Lipoxygenase Gene Transcription during Chilling Injury Development in Banana Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Assessment of CI Index and Cell Membrane Permeability
2.3. RNA Extraction and cDNA Synthesis
2.4. Subcellular Localization
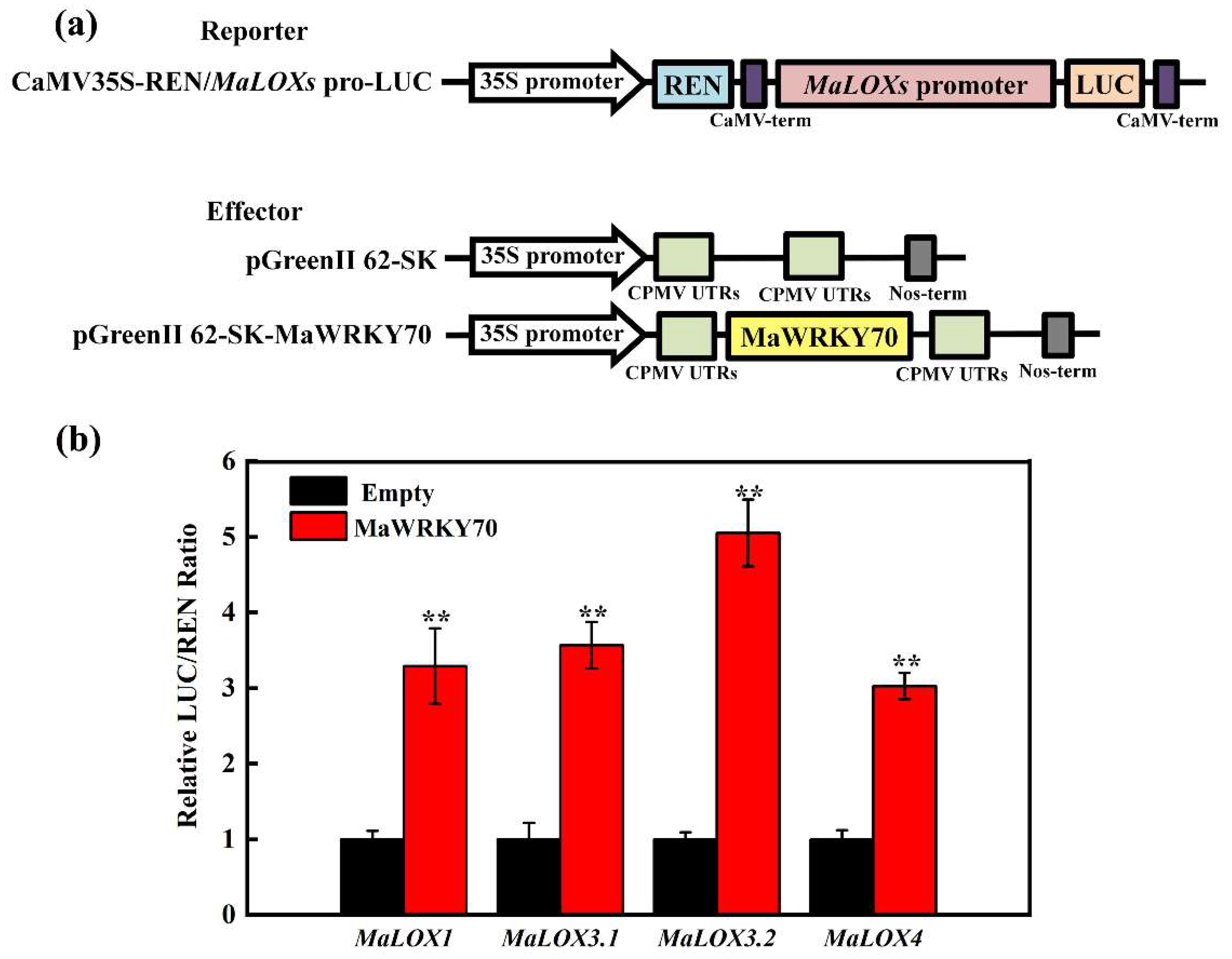
2.5. Dual-Luciferase Transient Expression Analysis
2.6. Y2H Assay
2.7. Electrophoretic Mobility Shift Assay (EMSA)
2.8. Statistical Analysis
3. Results
3.1. Physiological Changes during Banana Fruit CI Development
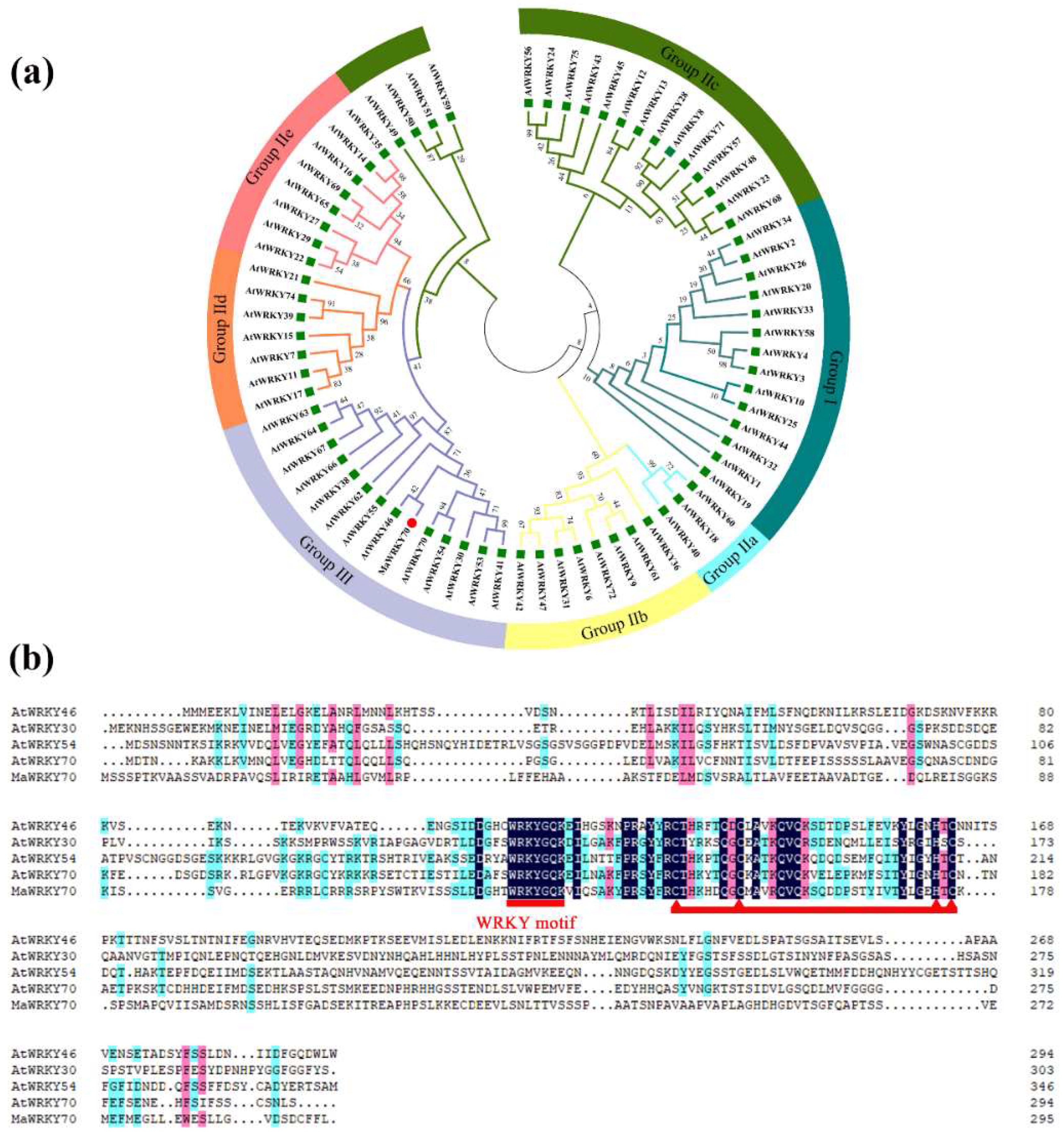
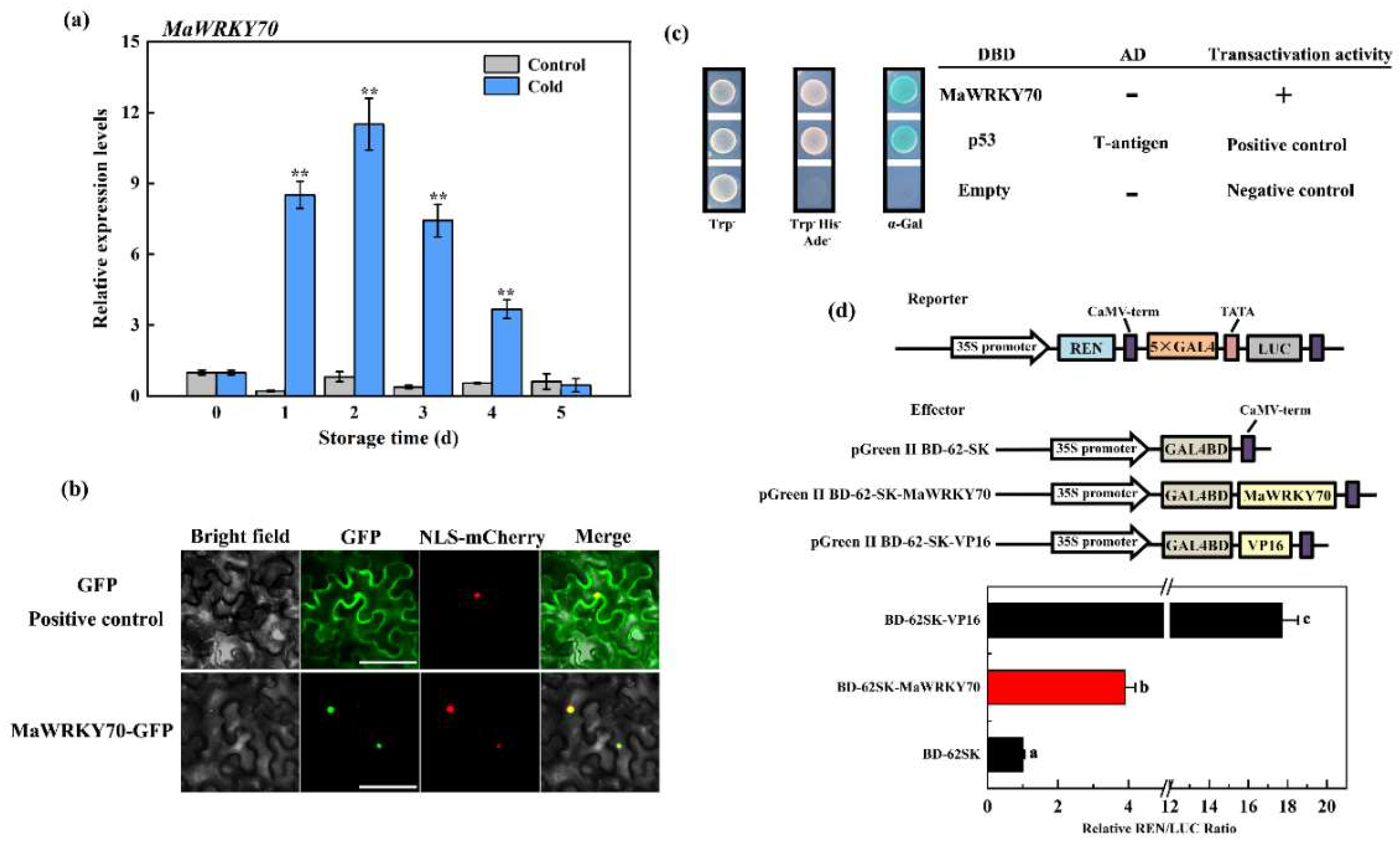
3.2. Isolation of MaWRKY70
3.3. Molecular Characterization of MaWRKY70
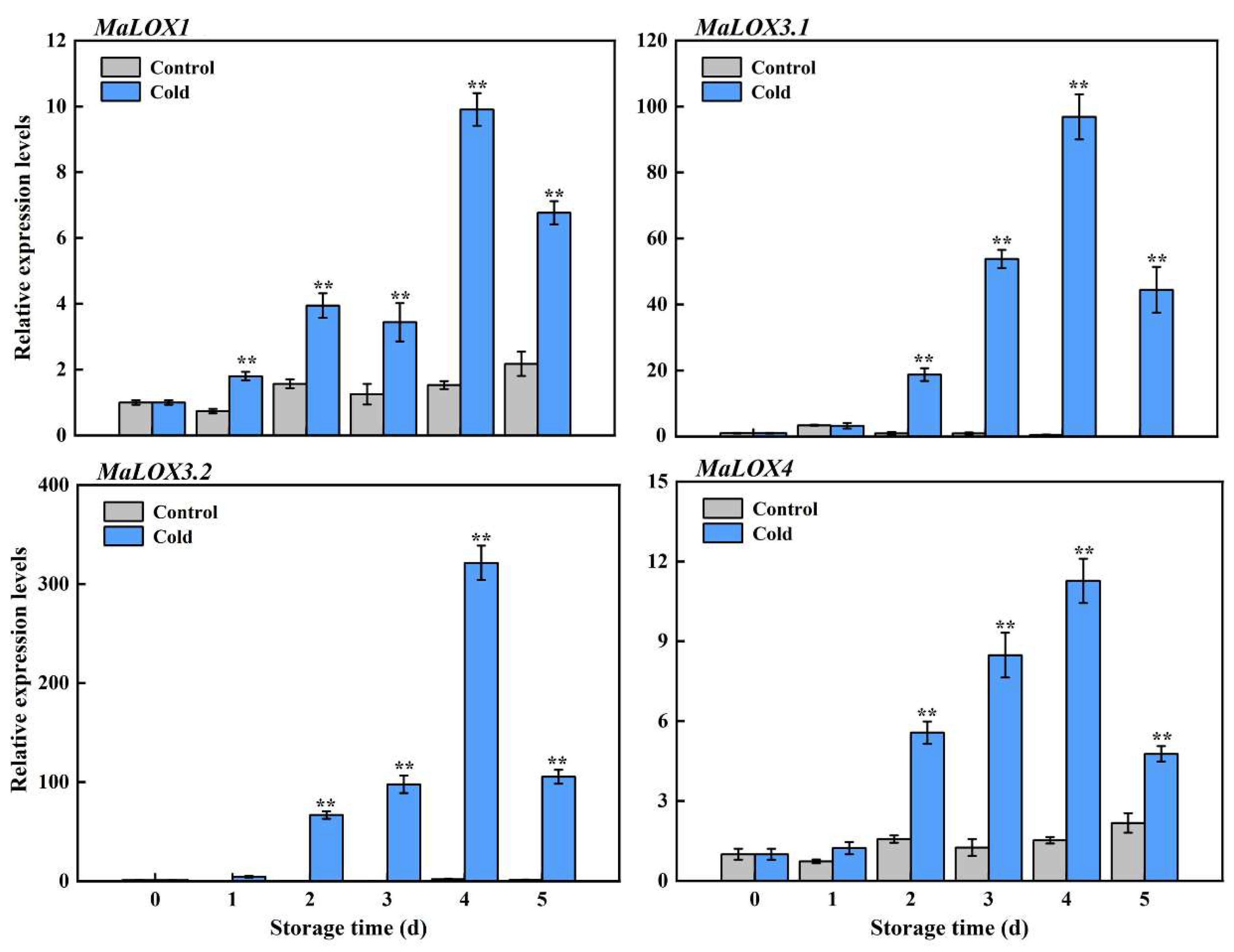
3.4. Expression Patterns of MaLOXs during Banana Fruit CI Development
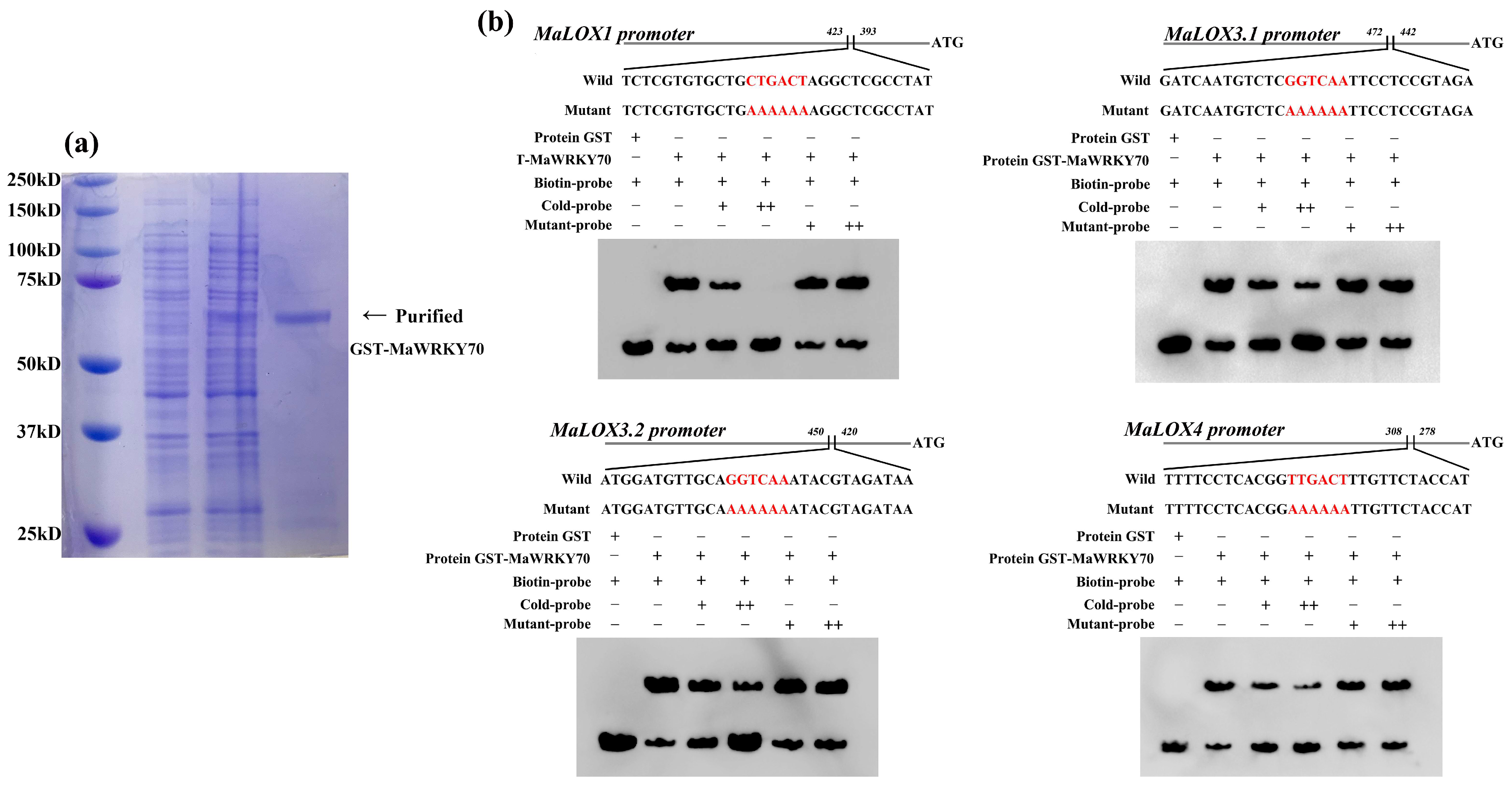
3.5. MaWRKY70 Activates Transcription of MaLOXs
4. Discussion
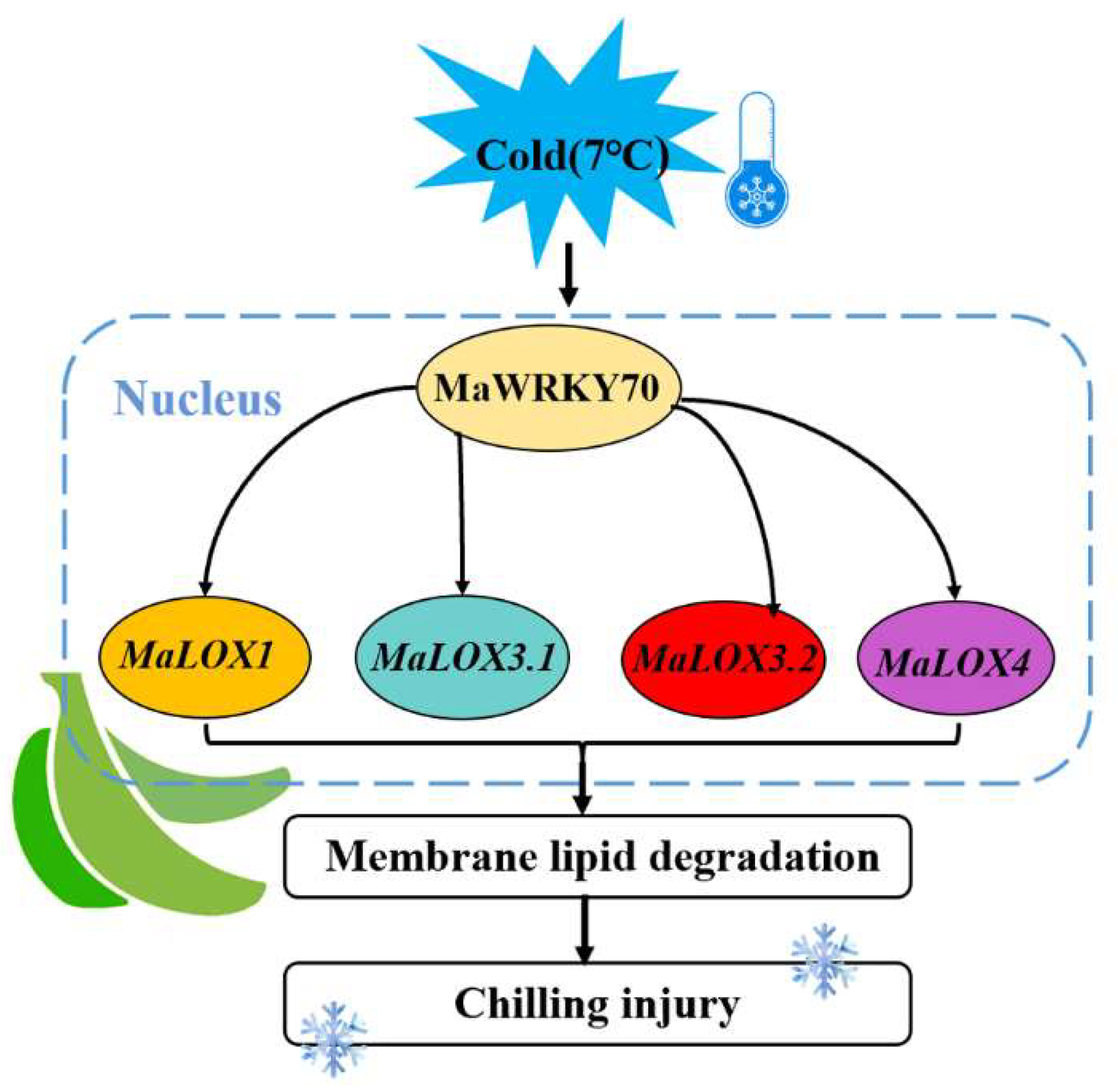
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qamar, S.; Shaikh, A. Therapeutic potentials and compositional changes of valuable compounds from banana—A review. Trends Food Sci. Technol. 2018, 79, 1–9. [Google Scholar] [CrossRef]
- Asif, M.H.; Lakhwani, D.; Pathak, S.; Gupta, P.; Bag, S.K.; Nath, P.; Trivedi, P.K. Transcriptome analysis of ripe and unripe fruit tissue of banana identifies major metabolic networks involved in fruit ripening process. BMC Plant Biol. 2014, 14, 316. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Bodjrenou, D.M.; Zhang, S.T.; Wang, B.; Pan, H.; Yeh, K.W.; Lai, Z.X.; Cheng, C.Z. The Endophytic Fungus Piriformospora indica Reprograms Banana to Cold Resistance. Int. J. Mol. Sci. 2021, 22, 4973. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lin, H.; Lin, H.T.; Lin, M.S.; Wang, H.; Wei, W.; Chen, J.Y.; Lu, W.J.; Shao, X.F.; Fan, Z.Q. The metabolism of membrane lipid participates in the occurrence of chilling injury in cold-stored banana fruit. Food Res. Int. 2023, 173, 113415. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Enders, T.A.; Myers, Z.A.; Magnusson, E.; Crisp, P.A.; Noshay, J.M.; Gomez-Cano, F.; Liang, Z.; Grotewold, E.; Greenham, K.; et al. Prediction of conserved and variable heat and cold stress response in maize using cis-regulatory information. Plant Cell 2022, 34, 514–534. [Google Scholar] [CrossRef] [PubMed]
- Madani, N.; Kimball, J.S.; Ballantyne, A.P.; Affleck, D.L.R.; van Bodegom, P.M.; Reich, P.B.; Kattge, J.; Sala, A.; Nazeri, M.; Jones, M.O.; et al. Future global productivity will be affected by plant trait response to climate. Sci. Rep. 2018, 8, 2870. [Google Scholar] [CrossRef] [PubMed]
- Eremina, M.; Rozhon, W.; Poppenberger, B. Hormonal control of cold stress responses in plants. Cell. Mol. Life Sci. 2015, 73, 797–810. [Google Scholar] [CrossRef]
- Zhang, X.D.; Liu, T.; Zhu, S.H.; Wang, D.; Sun, S.; Xin, L. Short-term hypobaric treatment alleviates chilling injury by regulating membrane fatty acids metabolism in peach fruit. J. Food Biochem. 2022, 46, e14113. [Google Scholar] [CrossRef]
- Song, C.B.; Wu, M.B.; Zhou, Y.; Gong, Z.H.; Yu, W.W.; Zhang, Y.; Yang, Z.F. NAC-mediated membrane lipid remodeling negatively regulates fruit cold tolerance. Hortic. Res. 2022, 9, uhac039. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Lin, D.; Yan, R.Y.; Xu, Y.H.; Xing, M.Y.; Liao, S.Y.; Wan, C.P.; Chen, C.Y.; Zhu, L.Q.; Kai, W.B.; et al. Amelioration of Chilling Injury by Fucoidan in Cold-Stored Cucumber via Membrane Lipid Metabolism Regulation. Foods 2023, 12, 301. [Google Scholar] [CrossRef]
- Liu, X.H.; Xiao, K.; Zhang, A.D.; Zhu, W.M.; Zhang, H.; Tan, F.; Huang, Q.R.; Wu, X.X.; Zha, D.S. Metabolomic Analysis, Combined with Enzymatic and Transcriptome Assays, to Reveal the Browning Resistance Mechanism of Fresh-Cut Eggplant. Foods 2022, 11, 1174. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.M.; Ge, W.Y.; Wei, B.D.; Zhou, Q.; Zhou, X.; Zhao, Y.B.; Ji, S.J. Melatonin ameliorates chilling injury in green bell peppers during storage by regulating membrane lipid metabolism and antioxidant capacity. Postharvest Biol. Technol. 2020, 170, 111315. [Google Scholar] [CrossRef]
- Yao, W.S.; Xu, T.T.; Farooq, S.U.; Jin, P.; Zheng, Y.H. Glycine betaine treatment alleviates chilling injury in zucchini fruit (Cucurbita pepo L.) by modulating antioxidant enzymes and membrane fatty acid metabolism. Postharvest Biol. Technol. 2018, 144, 20–28. [Google Scholar] [CrossRef]
- Meng, D.; Li, Y.Y.; Bai, Y.; Li, M.J.; Cheng, L.L. Genome-wide identification and characterization of WRKY transcriptional factor family in apple and analysis of their responses to waterlogging and drought stress. Plant Physiol. Biochem. 2016, 106, 71–83. [Google Scholar] [CrossRef]
- Wei, H.W.; Chen, S.Y.; Niyitanga, S.; Liu, T.; Qi, J.M.; Zhang, L.W. Genome-wide identification and expression analysis response to GA3 stresses of WRKY gene family in seed hemp (Cannabis sativa L.). Gene 2022, 822, 146290. [Google Scholar] [CrossRef]
- Zhang, M.X.; Zhao, R.R.; Huang, K.; Huang, S.Z.; Wang, H.T.; Wei, Z.Q.; Li, Z.; Bian, M.D.; Jiang, W.Z.; Wu, T.; et al. The OsWRKY63-OsWRKY76-OSDREB1B module regulates chilling tolerance in rice. Plant J. 2022, 112, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Vo, K.T.X.; Nguyen, C.D.; Jeong, D.H.; Lee, S.K.; Kumar, M.; Kim, S.R.; Park, S.H.; Kim, J.K.; Jeon, J.S. Functional analysis of a cold-responsive rice WRKY gene, OsWRKY71. Plant Biotechnol. Rep. 2016, 10, 13–23. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, T.T.; Sun, X.M.; Wang, Y.; Du, C.; Zhu, Z.F.; Gichuki, D.K.; Wang, Q.F.; Li, S.H.; Xin, H.P. Overexpression of VaWRKY12, a transcription factor from Vitis amurensis with increased nuclear localization under low temperature, enhances cold tolerance of plants. Plant Mol. Biol. 2019, 100, 95–110. [Google Scholar] [CrossRef]
- Fei, J.; Wang, Y.S.; Cheng, H.; Su, Y.B.; Zhong, Y.J.; Zheng, L. The Kandelia obovata transcription factor KoWRKY40 enhances cold tolerance in transgenic Arabidopsis. BMC Plant Biol. 2022, 22, 274. [Google Scholar] [CrossRef]
- Wang, Y.G.; Dong, B.; Wang, N.N.; Zheng, Z.F.; Yang, L.Y.; Zhong, S.W.; Fang, Q.; Xiao, Z.; Zhao, H.B. A WRKY Transcription Factor PmWRKY57 from Prunus mume Improves Cold Tolerance in Arabidopsis thaliana. Mol. Biotechnol. 2022, 65, 1359–1368. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.J.; Li, T.T.; Yun, Z.; Duan, X.W.; Jiang, Y.M. Fibroin treatment inhibits chilling injury of banana fruit via energy regulation. Sci. Hortic. 2019, 248, 8–13. [Google Scholar] [CrossRef]
- Chen, Y.T.; Liu, L.P.; Feng, Q.Q.; Liu, C.; Bao, Y.J.; Zhang, N.; Sun, R.H.; Yin, Z.N.; Zhong, C.F.; Wang, Y.H.; et al. FvWRKY50 is an important gene that regulates both vegetative growth and reproductive growth in strawberry. Hortic Res. 2023, 10, uhad115. [Google Scholar] [CrossRef] [PubMed]
- Song, C.B.; Yang, Y.Y.; Yang, T.W.; Ba, L.J.; Zhang, H.; Han, Y.C.; Xiao, Y.Y.; Shan, W.; Kuang, J.F.; Chen, J.Y.; et al. MaMYB4 recruits histone deacetylase MaHDA2 and modulates the expression of ω-3 fatty acid desaturase genes during cold stress response in banana fruit. Plant Cell Physiol. 2019, 60, 2410–2422. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, F.; Thuenemann, E.C.; Lomonossoff, G.P. pEAQ: Versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol. J. 2009, 7, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Sun, P.P.; Kong, W.N.; Xie, Z.Z.; Li, C.L.; Liu, J.H. SnRK2.4-mediated phosphorylation of ABF2 regulates ARGININE DECARBOXYLASE expression and putrescine accumulation under drought stress. New Phytol. 2023, 238, 216–236. [Google Scholar] [CrossRef]
- Han, Y.C.; Fu, C.C. Cold-inducible MaC2H2s are associated with cold stress response of banana fruit via regulating MaICE1. Plant Cell Rep. 2019, 38, 673–680. [Google Scholar] [CrossRef]
- Li, Z.W.; Zhou, Y.J.; Liang, H.Z.; Li, Q.; Jiang, Y.M.; Duan, X.W.; Jiang, G.X. MaMYB13 is involved in response to chilling stress via activating expression of VLCFAs and phenylpropanoids biosynthesis-related genes in postharvest banana fruit. Food Chem. 2023, 405, 134957. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Luo, J.; Li, Q.M.; Li, T.X.; Wang, R.; Chen, W.X.; Li, X.P. Low temperature storage reduces aroma-related volatiles production during shelf-life of banana fruit mainly by regulating key genes involved in volatile biosynthetic pathways. Postharvest Biol. Technol. 2018, 146, 68–78. [Google Scholar] [CrossRef]
- Nukuntornprakit, O.A.; Chanjirakul, K.; van Doorn, W.G.; Siriphanich, J. Chilling injury in pineapple fruit: Fatty acid composition and antioxidant metabolism. Postharvest Biol. Technol. 2015, 99, 20–26. [Google Scholar] [CrossRef]
- Purwanto, Y.A.; Okvitasari, H.; Mardjan, S.S.; Ahmad, U.; Makino, Y.; Oshita, S.; Kawagoe, Y. Chilling injury in green mature ‘Gedong Gincu’ mango fruits based on the changes in ion leakage. Acta Hortic. 2013, 1011, 219–226. [Google Scholar] [CrossRef]
- Sun, H.J.; Zhou, X.; Zhou, Q.; Zhao, Y.B.; Kong, X.M.; Luo, M.L.; Ji, S.J. Disorder of membrane metabolism induced membrane instability plays important role in pericarp browning of refrigerated ‘Nanguo’ pears. Food Chem. 2020, 320, 126684. [Google Scholar] [CrossRef]
- Huang, S.; Bi, Y.; Li, H.; Liu, C.H.; Wang, X.; Wang, X.Y.; Lei, Y.X.; Zhang, Q.; Wang, J. Reduction of Membrane Lipid Metabolism in Postharvest Hami Melon Fruits by n-Butanol to Mitigate Chilling Injury and the Cloning of Phospholipase D-β Gene. Foods 2023, 12, 1904. [Google Scholar] [CrossRef]
- Wang, L.; Bokhary, S.U.F.; Xie, B.; Hu, S.Q.; Jin, P.; Zheng, Y.H. Biochemical and molecular effects of glycine betaine treatment on membrane fatty acid metabolism in cold stored peaches. Postharvest Biol. Technol. 2019, 154, 58–69. [Google Scholar] [CrossRef]
- Kong, X.M.; Zhou, Q.; Zhou, X.; Wei, B.D.; Ji, S.J. Transcription factor CaNAC1 regulates low-temperature-induced phospholipid degradation in green bell pepper. J. Exp. Bot. 2020, 71, 1078–1091. [Google Scholar] [CrossRef] [PubMed]
- Kidokoro, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. 2022, 27, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, J.; Zhou, X.H.; Liu, S.Y.; Zhuang, Y. Identification of WRKY gene family and characterization of cold stress-responsive WRKY genes in eggplant. Plant Biol. 2020, 8, e8777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, H.J.; Yang, X.Y.; Li, Q.; Ling, J.; Wang, H.; Gu, X.F.; Huang, S.W.; Jiang, W.J. CsWRKY46, a WRKY transcription factor from cucumber, confers cold resistance in transgenic-plant by regulating a set of cold-stress responsive genes in an ABA-dependent manner. Plant Physiol. Biochem. 2016, 108, 478–487. [Google Scholar] [CrossRef]
- Liu, W.; Liang, X.Q.; Cai, W.J.; Wang, H.; Liu, X.; Cheng, L.F.; Song, P.H.; Luo, G.J.; Han, D.G. Isolation and Functional Analysis of VvWRKY28, a Vitis vinifera WRKY Transcription Factor Gene, with Functions in Tolerance to Cold and Salt Stress in Transgenic Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 13418. [Google Scholar] [CrossRef]
- Sheikh, A.H.; Eschen-Lippold, L.; Pecher, P.; Hoehenwarter, W.; Sinha, A.K.; Scheel, D.; Lee, J. Regulation of WRKY46 Transcription Factor Function by Mitogen-Activated Protein Kinases in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 61. [Google Scholar] [CrossRef]
- Li, S.; Nayar, S.; Jia, H.; Kapoor, S.; Wu, J.; Yukawa, Y. The Arabidopsis hypoxia inducible AtR8 long non-coding RNA also contributes to plant defense and root elongation coordinating with WRKY genes under low levels of salicylic acid. Non-Coding RNA 2020, 6, 8. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Choi, D.S.; Kim, M.S.; Deslandes, L.; Jayaraman, J.; Sohn, K.H. Molecular basis for the interference of the Arabidopsis WRKY54-mediated immune response by two sequence-unrelated bacterial effectors. Plant J. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Zhu, W.J.; Li, H.; Dong, P.F.; Ni, X.T.; Fan, M.L.; Yang, Y.J.; Xu, S.Y.; Xu, Y.B.; Qian, Y.W.; Chen, Z.; et al. Low temperature-induced regulatory network rewiring via WRKY regulators during banana peel browning. Plant Physiol. 2023, 193, 855–873. [Google Scholar] [CrossRef]
- Luo, D.L.; Ba, L.J.; Shan, W.; Kuang, J.F.; Lu, W.J.; Chen, J.Y. Involvement of WRKY Transcription Factors in Abscisic-Acid-Induced Cold Tolerance of Banana Fruit. J. Agric. Food Chem. 2017, 65, 3627–3635. [Google Scholar] [CrossRef]
- Niu, C.F.; Wei, W.; Zhou, Q.Y.; Tian, A.G.; Hao, Y.J.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, Z.B.; Zhang, J.S.; et al. Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Environ. 2012, 35, 1156–1170. [Google Scholar] [CrossRef]
- Xu, P.Y.; Xu, L.; Xu, H.F.; He, X.W.; He, P.; Chang, Y.S.; Wang, S.; Zheng, W.Y.; Wang, C.Z.; Chen, X.; et al. MdWRKY40 is directly promotes anthocyanin accumulation and blocks MdMYB15L, the repressor of MdCBF2, which improves cold tolerance in apple. J. Integr. Agric. 2023, 22, 1704–1719. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Bai, L.; Wei, W.; Su, W.; Wu, Y.; Wu, R.; Wang, H.; Chen, J.; Lin, H.; Fan, Z. The Role of MaWRKY70 in Regulating Lipoxygenase Gene Transcription during Chilling Injury Development in Banana Fruit. Foods 2024, 13, 854. https://doi.org/10.3390/foods13060854
Lin H, Bai L, Wei W, Su W, Wu Y, Wu R, Wang H, Chen J, Lin H, Fan Z. The Role of MaWRKY70 in Regulating Lipoxygenase Gene Transcription during Chilling Injury Development in Banana Fruit. Foods. 2024; 13(6):854. https://doi.org/10.3390/foods13060854
Chicago/Turabian StyleLin, Han, Lijuan Bai, Wei Wei, Wenbing Su, Yanting Wu, Rong Wu, Hui Wang, Jianye Chen, Hetong Lin, and Zhongqi Fan. 2024. "The Role of MaWRKY70 in Regulating Lipoxygenase Gene Transcription during Chilling Injury Development in Banana Fruit" Foods 13, no. 6: 854. https://doi.org/10.3390/foods13060854
APA StyleLin, H., Bai, L., Wei, W., Su, W., Wu, Y., Wu, R., Wang, H., Chen, J., Lin, H., & Fan, Z. (2024). The Role of MaWRKY70 in Regulating Lipoxygenase Gene Transcription during Chilling Injury Development in Banana Fruit. Foods, 13(6), 854. https://doi.org/10.3390/foods13060854







