Occurrence of Helicobacter pullorum in Retail Chicken Meat: A One-Health Approach to Consumer Health Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Bacteriological Isolation
2.3. Biochemical Identification
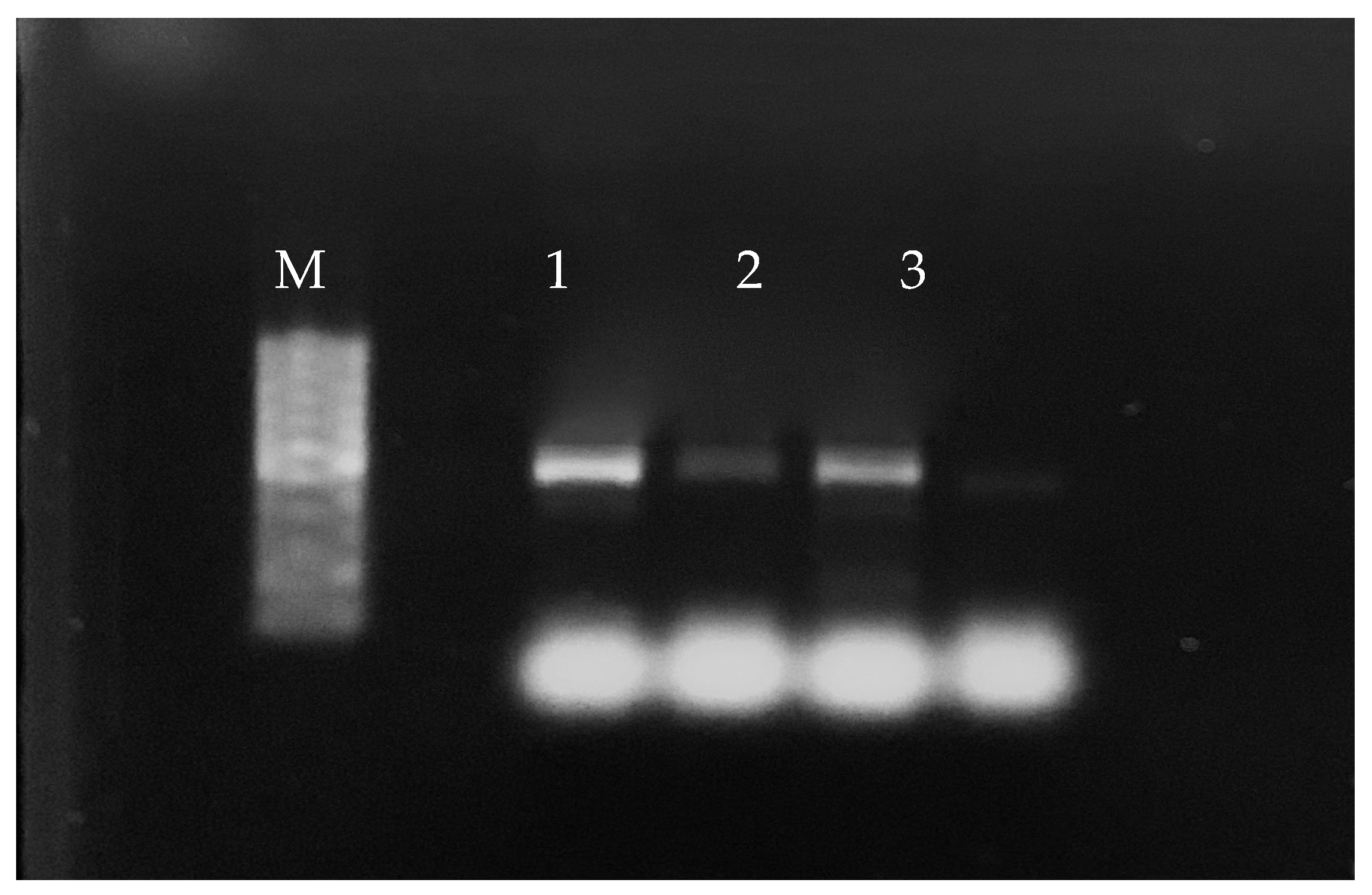
2.4. PCR Analysis
2.5. DNA Sequencing
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhlaghi, H.; Jebelli, A.J.; Chashmi, S.H.E. Helicobacter pullorum and Helicobacter canadensis: Etiology, pathogenicity, epidemiology, identification, and antibiotic resistance implicating food and public health. Int. J. Food Microbiol. 2024, 413, 110573. [Google Scholar] [CrossRef]
- Javed, K.; Gul, F.; Abbasi, R.; Zaidi, R.A.; Noreen, Z.; Bokhari, H.; Javed, S. Prevalence and Role of Type Six Secretion System in Pathogenesis of Emerging Zoonotic Pathogen Helicobacter pullorum from Retail Poultry. Avian Pathol. 2019, 48, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Wai, S.S.; Abdul-Aziz, S.; Bitrus, A.A.; Zunita, Z.; Abu, J. Helicobacter pullorum in broiler chickens and the farm environment: A one health approach. Int. J. One Health 2019, 5, 20–25. [Google Scholar] [CrossRef]
- Akhlaghi, H.; Jebelli, A.J.; Chashmi, S.H.E. Public health significance of Helicobacter pullorum, a putative food-associated emerging zoonotic pathogen in Iran. Comp. Immunol. Microbiol. Infect. Dis. 2022, 87, 101849. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.; Linton, D.; Burnens, A.P.; Dewhirst, F.E.; On, S.L.W.; Porter, A.; Owen, R.J.; Costas, M. Helicobacter pullorum sp. nov. genotype and phenotype of new species isolated from poultry and from human patients with gastroenteritis. Microbiology 1994, 140, 3441–3449. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Shahata, M.; Refaie, E.; Ibrahim, R. Pathogenicity testing and antimicrobial susceptibility of Helicobacter pullorum isolates from chicken origin. Int. J. Vet. Sci. Med. 2014, 2, 72–77. [Google Scholar] [CrossRef]
- Hameed, K.G.A.; Sender, G. Prevalence of Helicobacter pullorum in Egyptian hen’s eggs and in vitro susceptibility to different antimicrobial agents. Anim. Sci. Pap. Rep. 2011, 29, 257–264. [Google Scholar]
- Burnens, A.P.; Stanley, J.; Nicolet, J. Possible association of Helicobacter pullorum with lesions of vibrionic hepatitis in poultry. In Campylobacters, Helicobacters and Related Organisms; Newell, D.G., Ketley, J.M., Feldman, R.A., Eds.; Plenum Press: New York, NY, USA, 1996; pp. 291–293. [Google Scholar]
- Zanoni, R.G.; Rossi, M.; Giacomucci, D.; Sanguinetti, V.; Manfreda, G. Occurrence and antibiotic susceptibility of Helicobacter pullorum from broiler chickens and commercial laying hens in Italy. Int. J. Food Microbiol. 2007, 116, 168–173. [Google Scholar] [CrossRef]
- Behroo, S.; Javadi, A.; Mahdi, G.R. Helicobacter pullorum prevalence in patients with gastroenteritis in humans and chicken in the province of Ardabil in 2014. Indian J. Fundam. Appl. Life Sci. 2015, 5, 87–94. [Google Scholar]
- Ceelen, L.; Decostere, A.; Verschraegen, G.; Ducatelle, R.; Haesebrouck, F. Prevalence of Helicobacter pullorum among patients with gastrointestinal disease and clinically healthy persons. J. Clin. Microbiol. 2005, 43, 2984–2986. [Google Scholar] [CrossRef]
- Steinbrueckner, B.; Haerter, G.; Pelz, K.; Weiner, S.; Rump, J.; Deissler, W. Isolation of Helicobacter pullorum from patients with enteritis. Scand. J. Infect. Dis. 1997, 29, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Varon, C.; Duriez, A.; Lehours, P.; Menard, A.; Laye, S.; Zerbib, F. Study of Helicobacter pullorum proinflammatory properties on human epithelial cells in vitro. Gut 2009, 58, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Parente, M.R.; Monteiro, J.T.; Martins, G.G.; Saraiva, L.M. Helicobacter pullorum induces nitric oxide release in murine macrophages that promotes phagocytosis and killing. Microbiology 2016, 162, 503–512. [Google Scholar] [CrossRef]
- Zhou, G.; Liang, H.; Gu, Y.; Ju, C.; He, L.; Guo, P. Comparative genomics of Helicobacter pullorum from different countries. Gut Pathol. 2020, 12, 56. [Google Scholar] [CrossRef]
- Cacioppo, L.D.; Turk, M.L.; Shen, Z.; Ge, Z.; Parry, N.; Whary, M.T. Natural and experimental Helicobacter pullorum infection in Brown Norway rats printed in Great Britain. J. Med. Microbiol. 2012, 61, 1319–1323. [Google Scholar] [CrossRef][Green Version]
- Borges, V.; Santos, A.; Correia, C.B.; Saraiva, M.; Menard, A.; Vieira, L.; Sampaio, D.A.; Pinheiro, M.; Gomes, J.P.; Oleastro, M. Helicobacter pullorum isolated from fresh chicken meat: Antibiotic resistance and genomic traits of an emerging foodborne pathogen. Appl. Environ. Microbiol. 2015, 81, 8155–8163. [Google Scholar] [CrossRef]
- Ahangaran, M.G.; Haddadi, I.; Karimi, Y.; Omrani, E. Molecular evidence of Helicobacter pullorum, as a foodborne pathogen in broiler carcasses in Iran. Eur. Poult. Sci. 2015, 79, 82–85. [Google Scholar] [CrossRef]
- Akhlaghi, H.; Chashmi, S.H.E.; Jebelli, A.J. Prevalence and antibiotic resistance of Helicobacter pullorum isolates in poultry from Semnan province, Iran. Int. J. Enteric Pathog. 2020, 8, 101–106. [Google Scholar] [CrossRef]
- Jamshidi, A.; Bassami, R.M.; Salami, H.; Mohammadi, S. Isolation and identification of Helicobacter pullorum from caecal content of broiler chickens in Mashhad, Iran. Iranian J. Vet. Res. 2014, 15, 179–182. [Google Scholar]
- Mohamed, M.; Ragab, I.; Shahata, M.; EI Refaie, M. Helicobacter pullorum among poultry in assiut-egypt: Genetic characterization, virulence and MIC. Int. J. Poult. Sci. 2010, 9, 521–526. [Google Scholar] [CrossRef][Green Version]
- Akhlaghi, H.; Chashmi, S.H.E.; Jebelli, A.J. Development of a novel and specialized cultivation method for isolating Helicobacter pullorum from chicken meat, Iran. J. Vet. Res. 2021, 22, 76–80. [Google Scholar] [CrossRef]
- Akhlaghi, H.; Chashmi, S.H.E.; Jebelli, A.J. Isolation and antibiotic resistance of Helicobacter pullorum from chicken wings. J. Nutr. Fasting Health 2021, 9, 306–311. [Google Scholar] [CrossRef]
- Gonzalez, A.; Piqueres, P.; Moreno, Y.; Canigral, I.; Owen, R.J.; Hernandez, J. A novel real-time PCR assay for the detection of Helicobacter pullorum-like organisms in chicken products. Int. Microbiol. 2008, 11, 203–208. [Google Scholar] [CrossRef]
- Jebelli, A.J.; Emadi Chashmi, S.H.; Staji, H.; Akhlaghi, H.A. Comparison of culture and PCR method to determine the prevalence and antibiotic resistance of Helicobacter pullorum isolated from chicken thigh samples in Semnan, Iran. J. Hum. Environ. Health Prom. 2020, 6, 167–172. [Google Scholar]
- Quaglia, N.C.; Dambrosio, A. Helicobacter pylori: A foodborne pathogen? World J. Gastroenterol. 2018, 24, 3472–3487. [Google Scholar] [CrossRef]
- Steele, T.W.; McDermott, S.N. The use of membrane filters applied directly to the surface of agar plates for the isolation of Campylobacter jejuni from feces. Pathology 1984, 16, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Morshed, M.G.; Karita, M.; Konishi, H.; Okita, K.; Nakazawa, T. Growth Concentration Helicobacter Medium Containing Cyclodextrin and Low of Horse Serum for Cultivation of Helicobacter pylori. Microbiol. Immunol. 1995, 39, 897–900. [Google Scholar] [CrossRef] [PubMed][Green Version]
- On, S.L.W.; Holmes, B.; Sackin, M.J. A probability matrix for the identification of Campylobacters, Helicobacters and allied taxa. J. Appl. Bacteriol. 1996, 81, 425–432. [Google Scholar] [CrossRef] [PubMed]
- El-Shibiny, A.; Connerton, P.; Connerton, I. Survival at refrigeration and freezing temperatures of Campylobacter coli and Campylobacter jejuni on chicken skin applied as axenic and mixed inoculums. Int. J. Food Microbiol. 2009, 131, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Public Health England Identification of Helicobacter Species. UK Standards for Microbiology Investigations. 2015. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/443464/ID_26i3.pdf (accessed on 9 March 2024).
- Atabay, H.I.; Corry, J.E.; On, S.L.W. Identification of unusual Campylobacter like isolates from poultry products as Helicobacter pullorum. J. Appl. Microbiol. 1998, 84, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, 7666. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) Panel on Biological Hazards (BIOHAZ). Scientific Opinion on Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011, 9, 2105. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A. Helicobacter pullorum: A potential hurdle emerging pathogen for public health. J. Infec. Develop. Coutr. 2020, 14, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Butzler, J.P. Campylobacter, from obscurity to celebrity. Clin. Microbiol. Infect. Dis. 2004, 10, 868–876. [Google Scholar] [CrossRef]
| Samples | n. of Analyzed Samples | Positive Samples n. (%) | Broth Culture Positive to PCR n. (%) | Colonies Biochemically Identified as H. pullorum n. (%) |
|---|---|---|---|---|
| chicken thighs | 120 | 51 (42.5%) | 66 (55%) | 216 (84.7%) * |
| chicken breasts | 120 | 33 (27.5%) | 42 (35%) | 135 (81.8%) * |
| 240 | 84 (35%) | 108 (45%) | 350 (83.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quaglia, N.C.; Capuozzo, F.; Ioanna, F.; De Rosa, M.; Dambrosio, A. Occurrence of Helicobacter pullorum in Retail Chicken Meat: A One-Health Approach to Consumer Health Protection. Foods 2024, 13, 845. https://doi.org/10.3390/foods13060845
Quaglia NC, Capuozzo F, Ioanna F, De Rosa M, Dambrosio A. Occurrence of Helicobacter pullorum in Retail Chicken Meat: A One-Health Approach to Consumer Health Protection. Foods. 2024; 13(6):845. https://doi.org/10.3390/foods13060845
Chicago/Turabian StyleQuaglia, Nicoletta C., Flavia Capuozzo, Federica Ioanna, Michele De Rosa, and Angela Dambrosio. 2024. "Occurrence of Helicobacter pullorum in Retail Chicken Meat: A One-Health Approach to Consumer Health Protection" Foods 13, no. 6: 845. https://doi.org/10.3390/foods13060845
APA StyleQuaglia, N. C., Capuozzo, F., Ioanna, F., De Rosa, M., & Dambrosio, A. (2024). Occurrence of Helicobacter pullorum in Retail Chicken Meat: A One-Health Approach to Consumer Health Protection. Foods, 13(6), 845. https://doi.org/10.3390/foods13060845








