UPLC-ESI-MS/MS-Based Analysis of Various Edible Rosa Fruits Concerning Secondary Metabolites and Evaluation of Their Antioxidant Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Metabolites Extraction
2.4. Non-Targeted Metabolite Analysis Using UPLC-ESI-MS/MS
2.5. Multivariate Statistical Analysis
2.6. Determination of Total Polysaccharide (TPS) Content
2.7. Determination of Antioxidant Activity
2.7.1. Determining the Effects of Rosa Samples on H2O2-Damaged HaCaT Cells
2.7.2. Determination of Superoxide Dismutase (SOD), Glutathione Peroxidase (GSH-Px) Activity, and Malondialdehyde (MDA) Content
3. Results
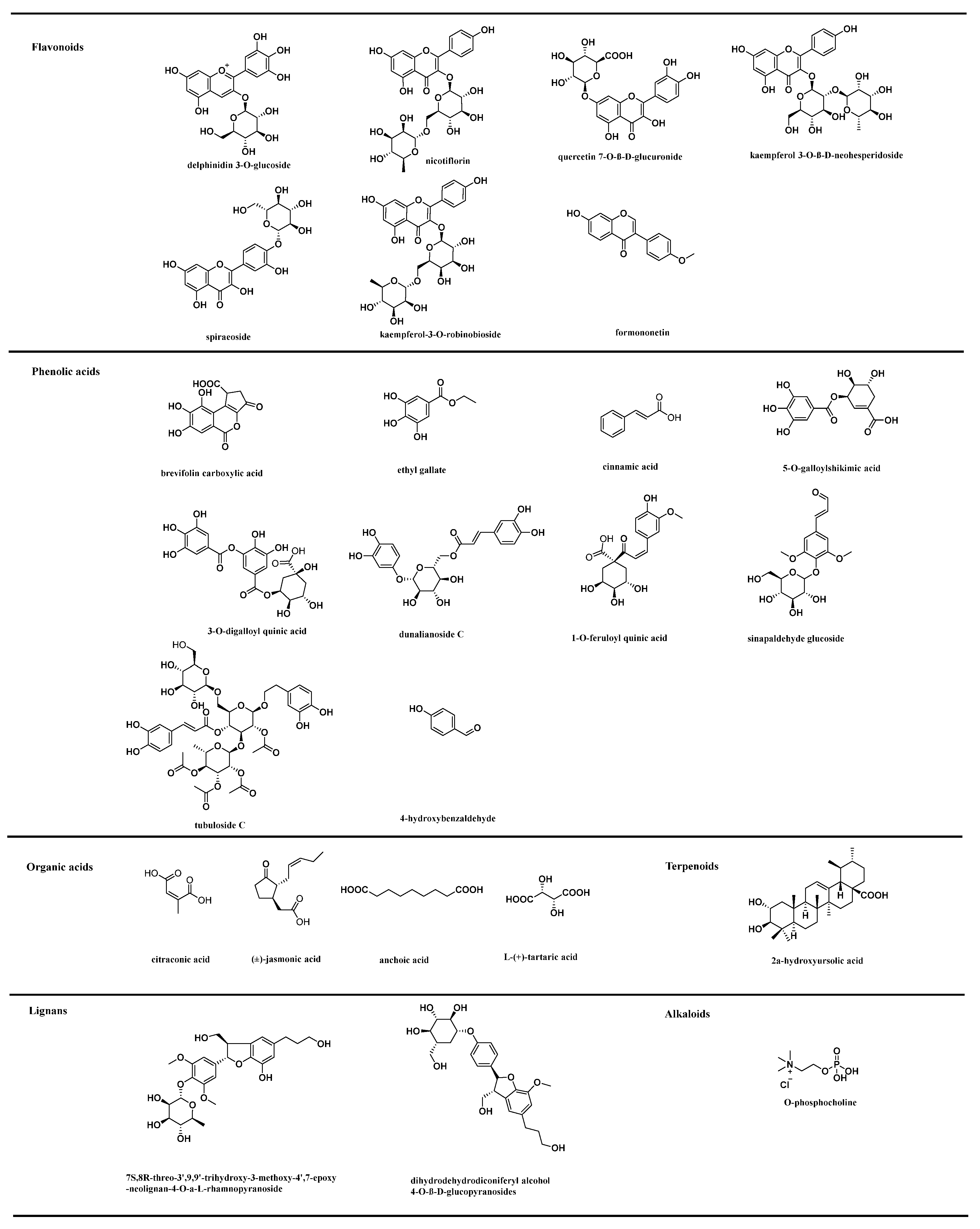
3.1. Metabolites Identification
3.2. Multivariate Analysis
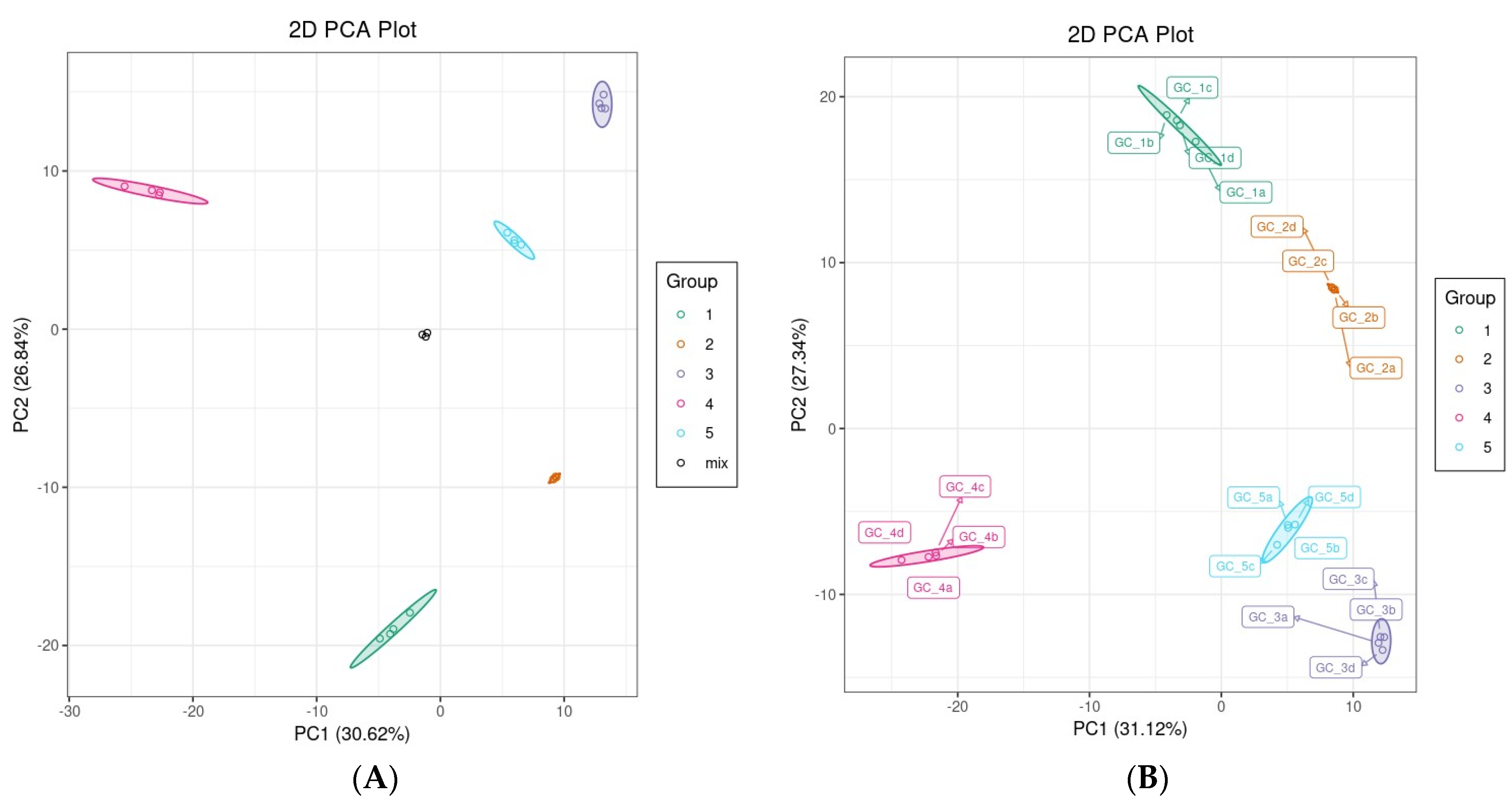
3.2.1. PCA and Hierarchical Clustering Analysis
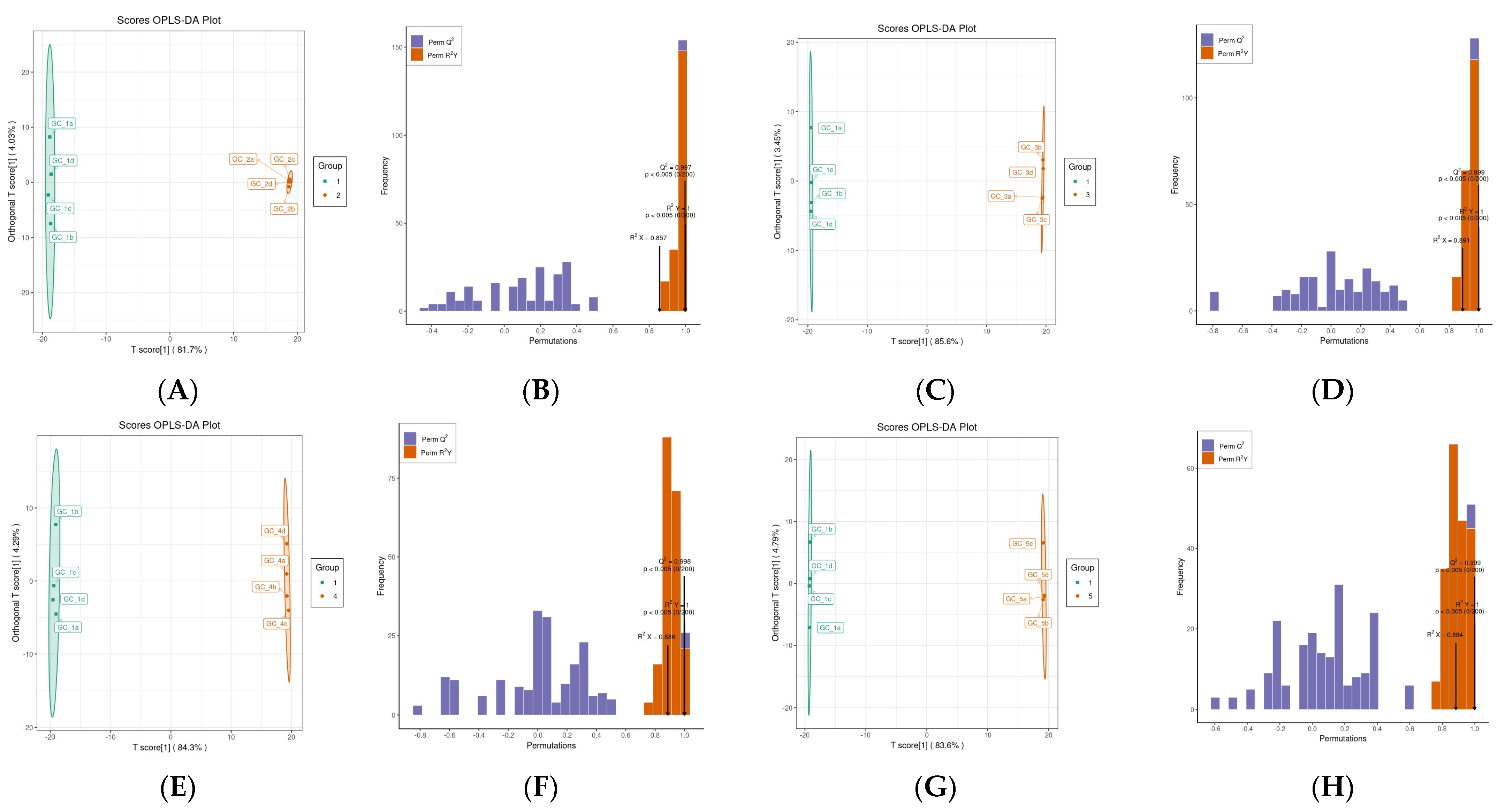
3.2.2. OPLS-DA
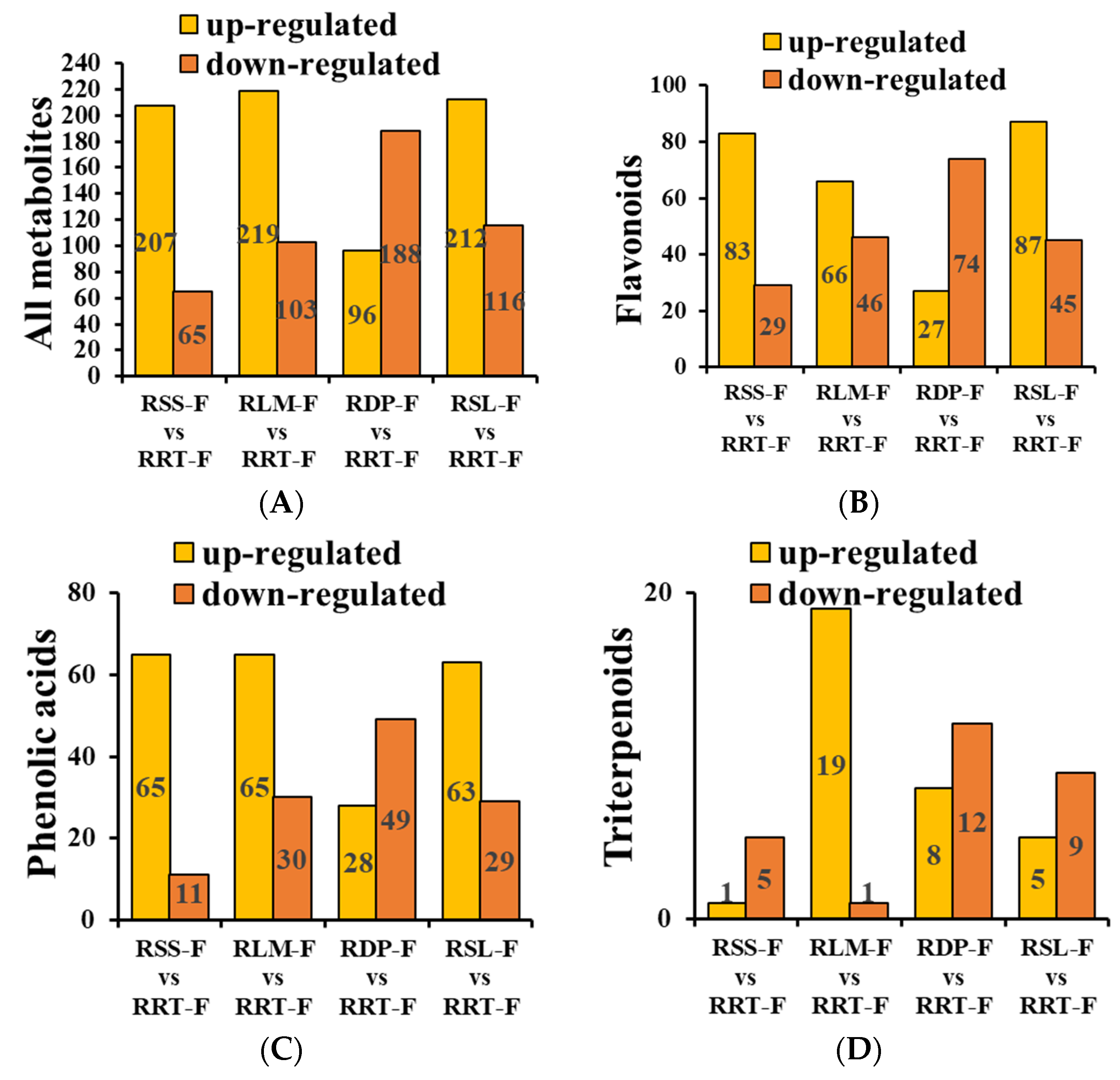
3.3. Differentially Expressed Metabolite Analysis of the Five Rosa Fruits
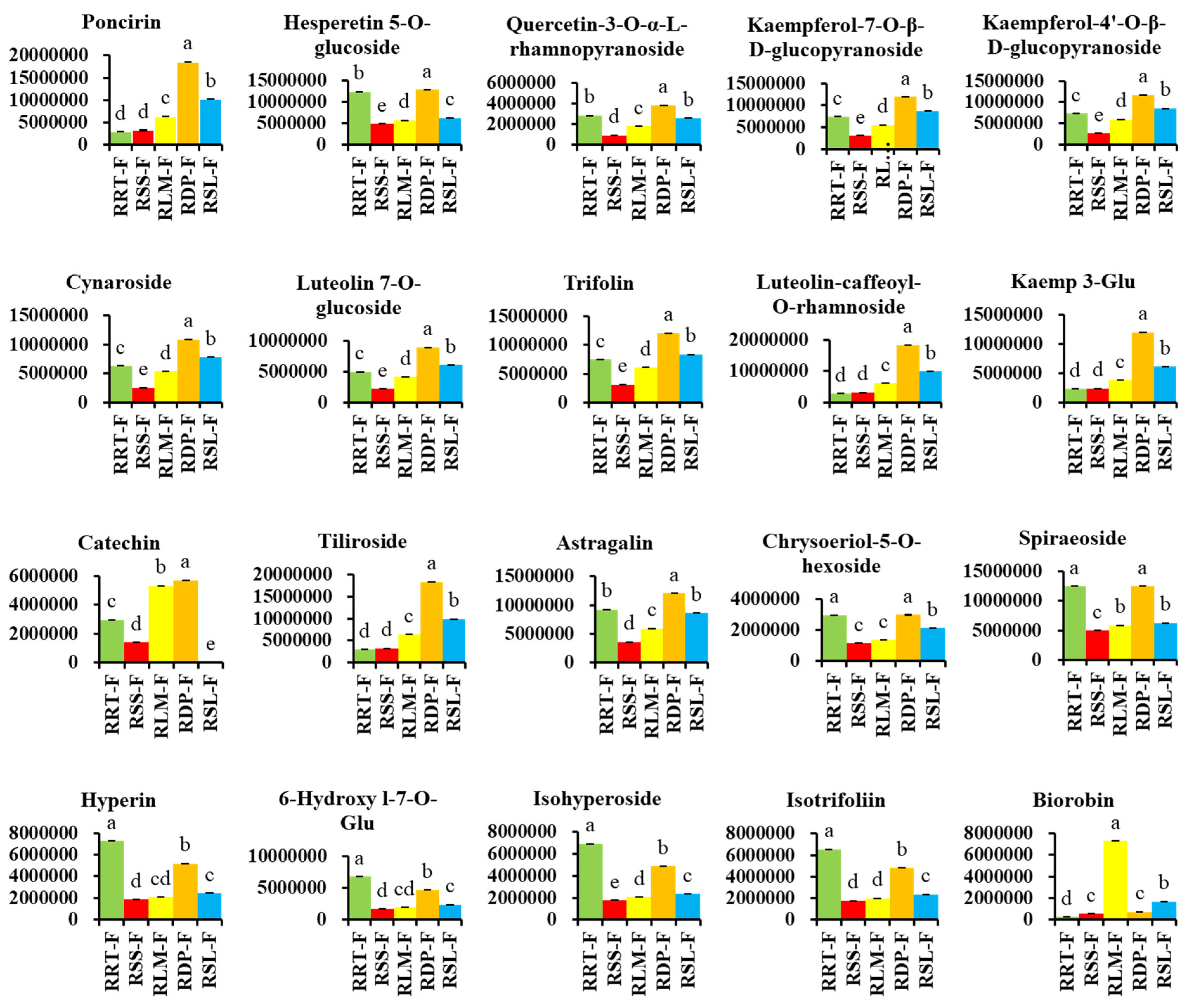
3.3.1. Differentially Expressed Flavonoids in Rosa Samples
3.3.2. Differentially Expressed Phenolic Acids in Rosa Samples
3.3.3. Differentially Expressed Terpenoids in Rosa Samples
3.3.4. Potential Markers in Five Samples
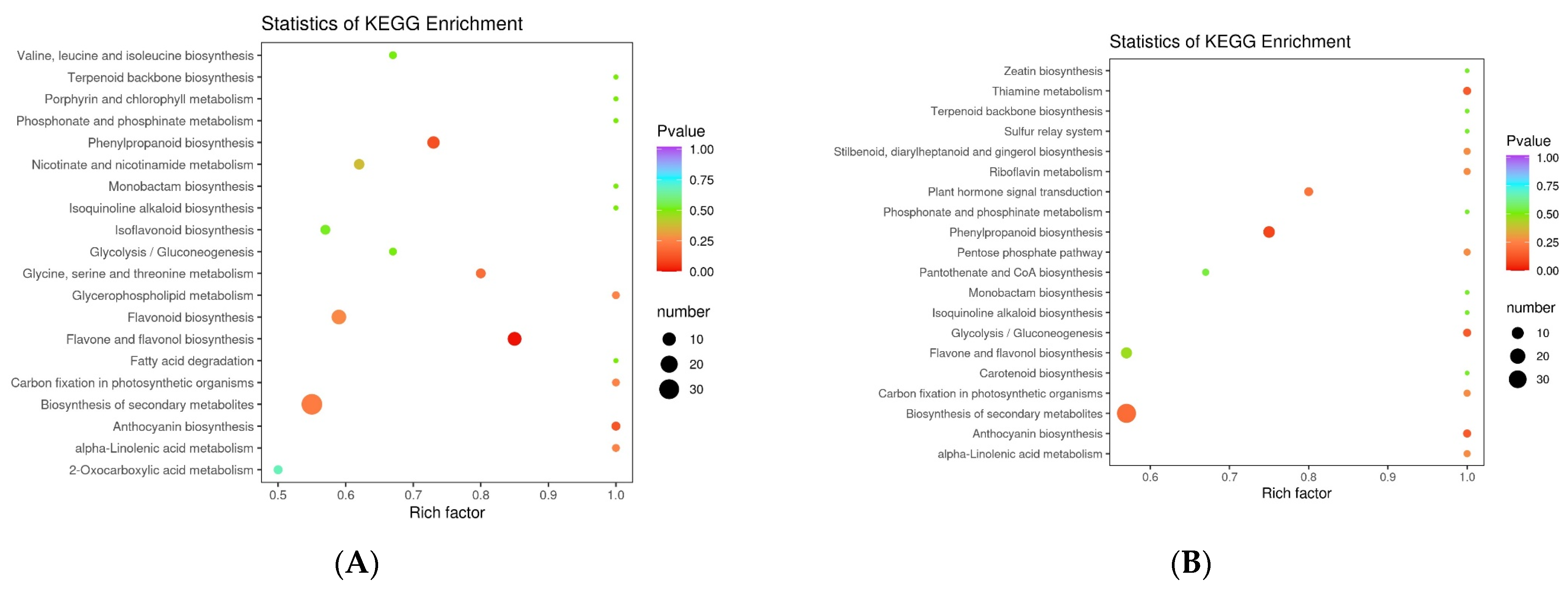
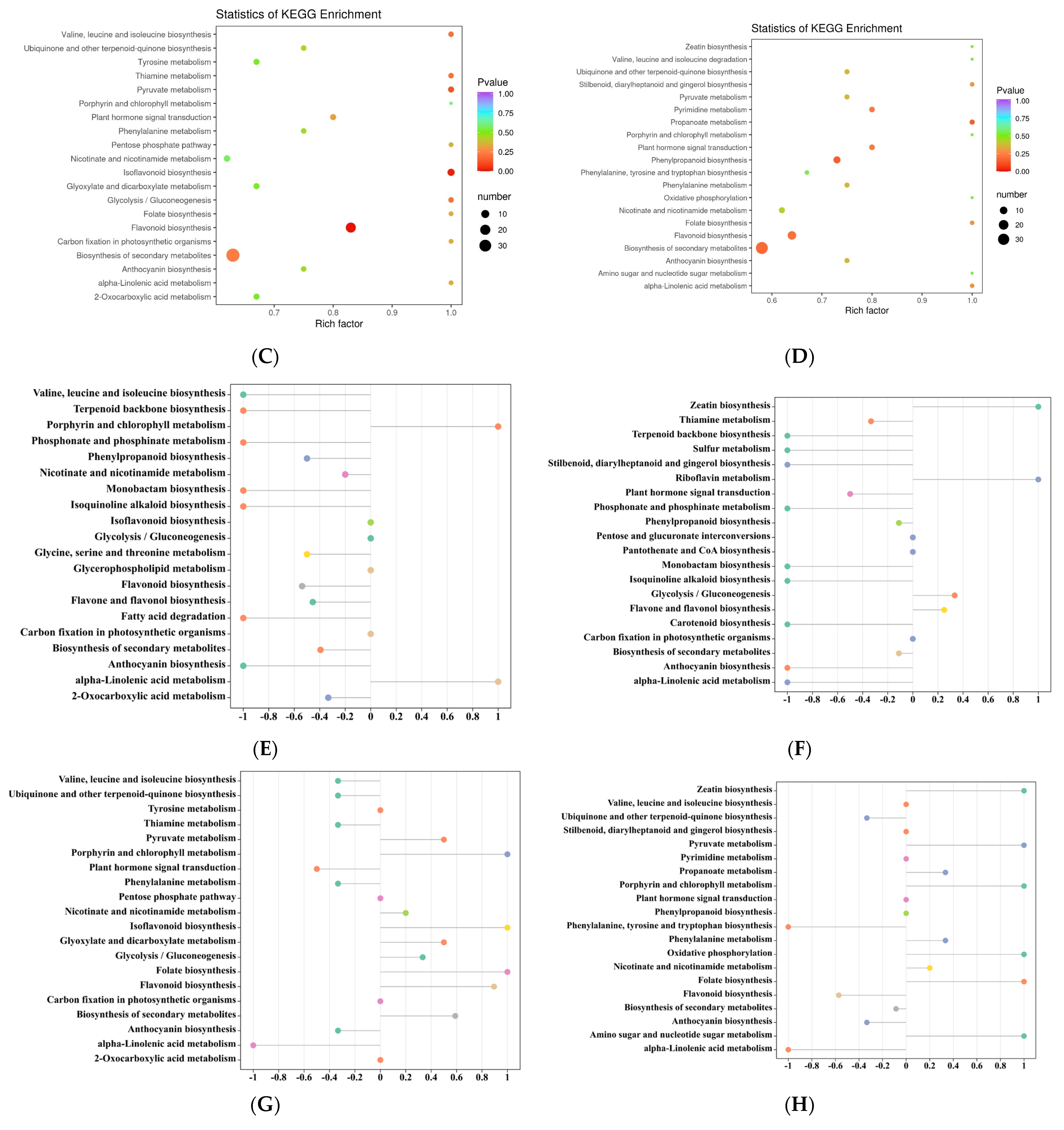
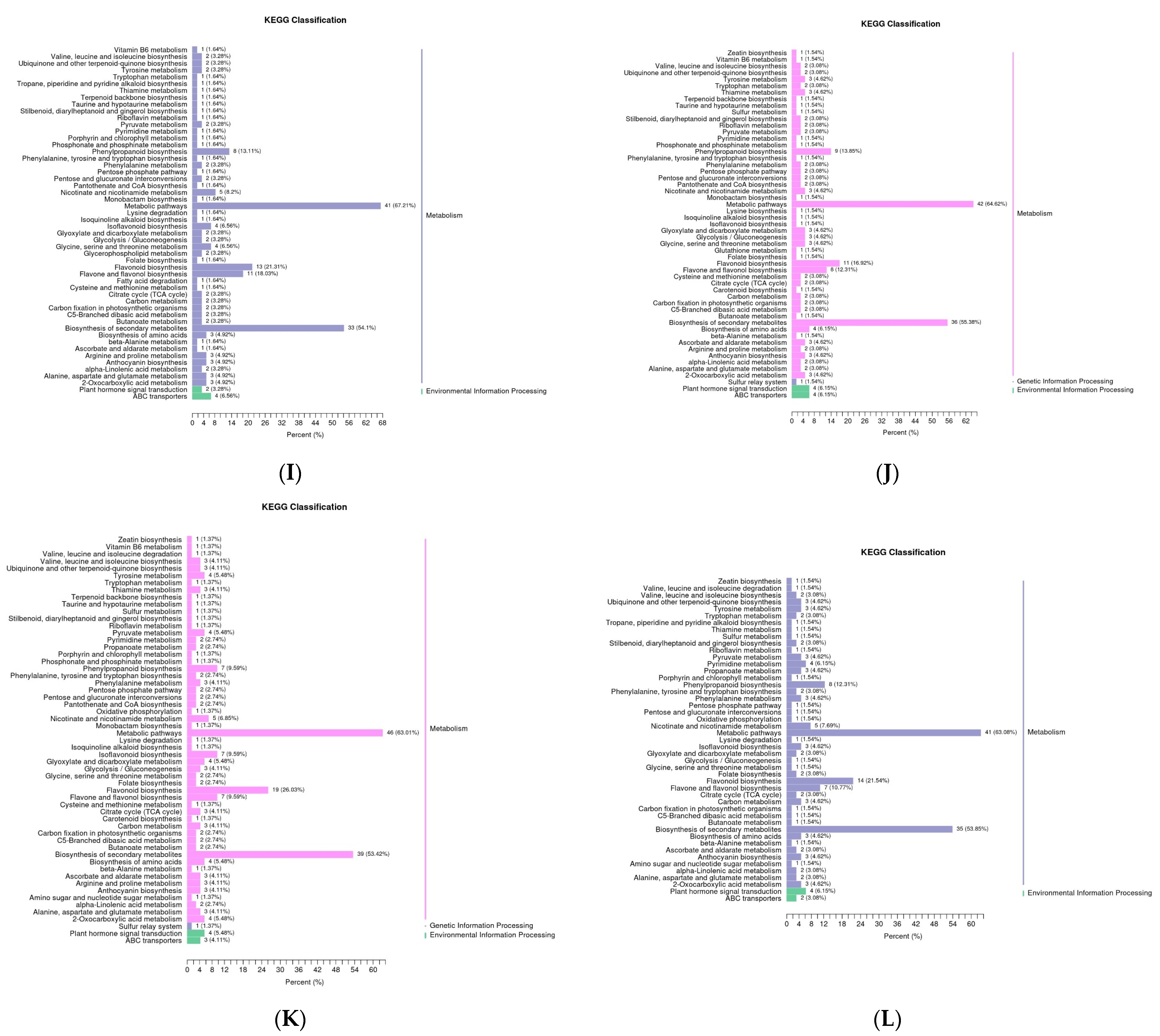
3.4. Analysis of Differential Metabolic Pathways in the Five Rosa Fruits
3.5. TPS Contents Detection
3.6. Antioxidant Activity
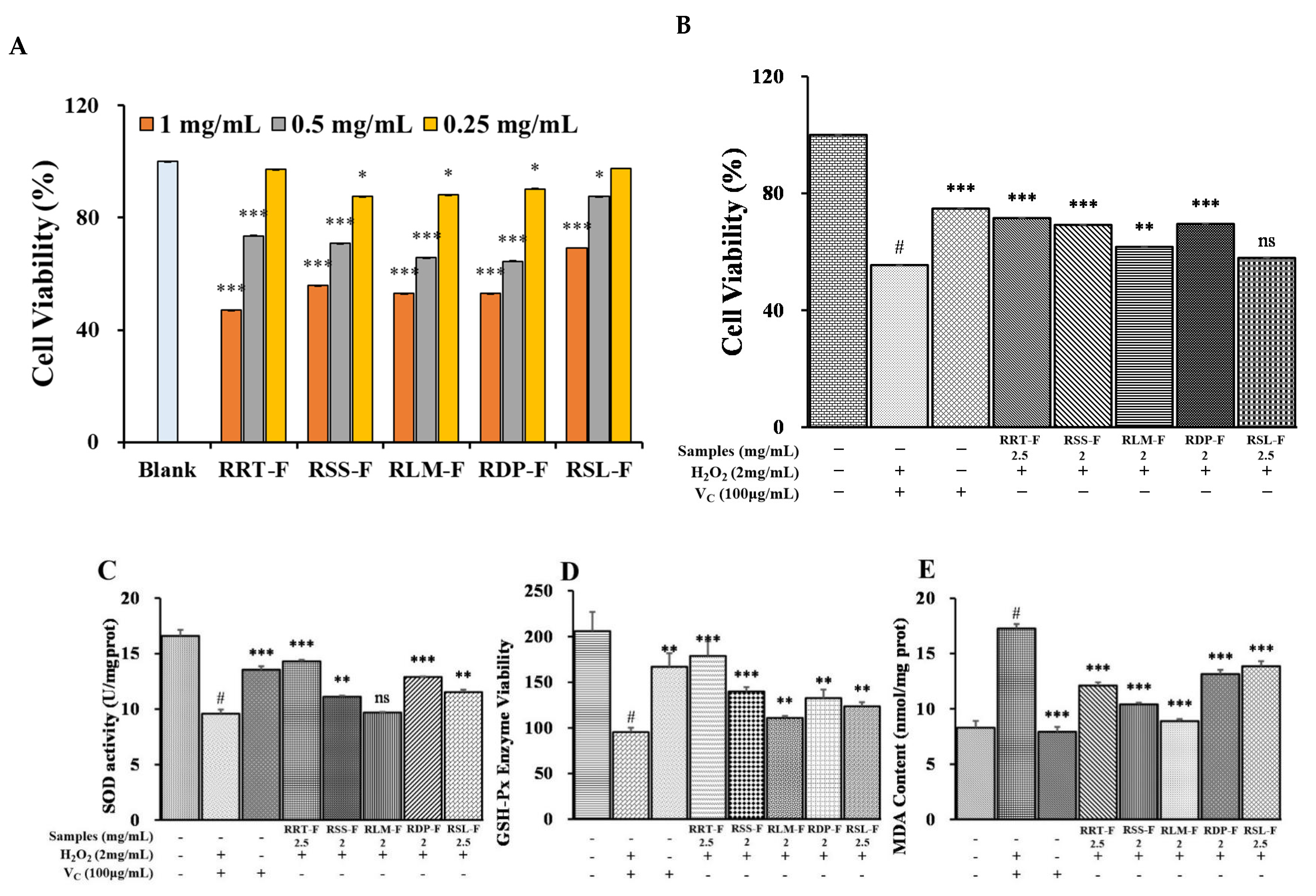
3.6.1. Cell Viability
3.6.2. Effects of the Five Rosa Samples on H2O2-Induced Cytotoxicity in HaCaT Cells
3.6.3. Effects of the Five Rosa Samples on SOD and GSH-Px Activities and MDA Content in H2O2-Induced HaCaT Cells
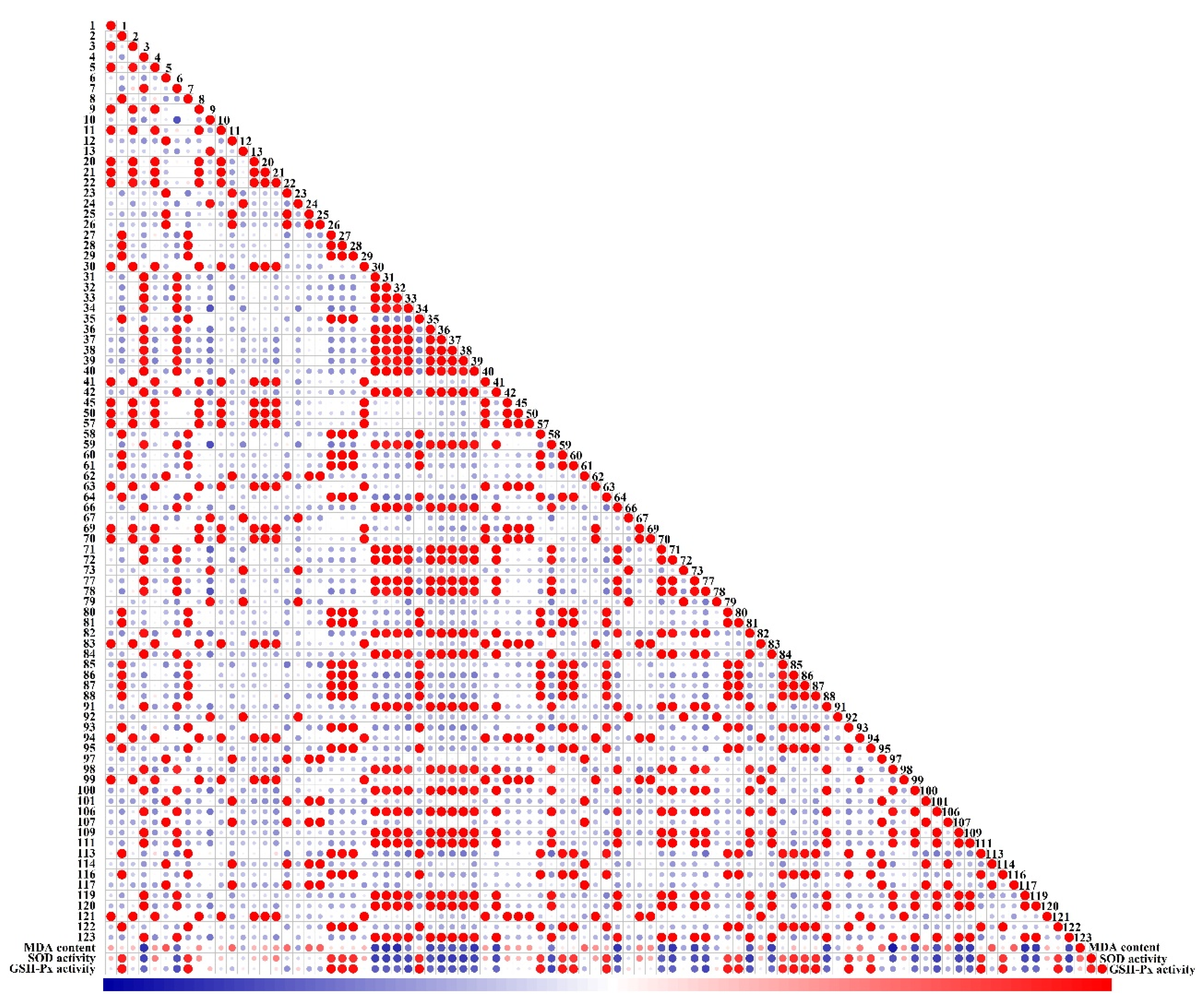
3.7. Pearson Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zheng, B.J.; Dong, L. Study on Plant Landscape of Beijing Urban Linear Parks Based on Bird Diversity Improvement. Landsc. Archit. 2019, 26, 53–57. [Google Scholar] [CrossRef]
- Ayati, Z.; Amiri, M.S.; Ramezani, M.; Delshad, E.; Sahebkar, A.; Emami, S.A. Phytochemistry, traditional uses and pharmacological profile of rose hip: A review. Curr. Pharm. Des. 2019, 24, 4101–4124. [Google Scholar] [CrossRef]
- Singh, K.; Sharma, Y.P.; Gairola, S. Distribution status and ethnomedicinal importance of genus Rosa L. (Rosaceae) in India. Ethnobot. Res. Appl. 2023, 25, 1–22. [Google Scholar] [CrossRef]
- Chen, G.J.; Kan, J.Q. Ultrasound-assisted extraction, characterization, and antioxidant activity in vitro and in vivo of polysaccharides from Chestnut rose (Rosa roxburghii tratt) fruit. J. Food Sci. Technol. 2018, 55, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Vidyarthi, S.K.; Bai, W.B.; Pan, Z.L. Nutritional constituents, health benefits and processing of Rosa Roxburghii: A review. J. Funct. Foods 2019, 60, 103456. [Google Scholar] [CrossRef]
- Liu, M.H.; Zhang, Q.; Zhang, Y.H.; Lu, X.Y.; Fu, W.M.; He, J.Y. Chemical analysis of dietary constituents in Rosa roxburghii and Rosa sterilis fruits. Molecules 2016, 21, 1204. [Google Scholar] [CrossRef]
- Deng, H.N.; Gao, X.F.; Li, X.Y.; Zhou, H.Y. Molecular evidence for hybridization origin of Rosa × sterilis (Rosaceae). J. Plant Resour. Environ. 2015, 24, 10–17. [Google Scholar]
- Zong, D.; Huang, J.C.; Duan, X.M.; Zhang, X.L.; Feng, J.Y.; Gan, P.H.; He, C.Z. Comparative analyses on chloroplast genome sequence of Rosa sterilis and its related species. J. Fujian Agric. For. Univ. 2024, 53, 39–47. [Google Scholar] [CrossRef]
- Dong, B.Y.; Zhu, D.Q.; Yao, Q.P.; Tang, H.M.; Ding, X.C. Hydrogen-rich water treatment maintains the quality of Rosa sterilis fruit by regulating antioxidant capacity and energy metabolism. LWT-Food Sci. Technol. 2022, 161, 113361. [Google Scholar] [CrossRef]
- He, J.; Zhang, H.; Ma, N.; Zhang, X.; Liu, M.; Fu, W. Comparative analysis of multiple ingredients in Rosa roxburghii and R. sterilis fruits and their antioxidant activities. J. Funct. Foods 2016, 27, 29–41. [Google Scholar] [CrossRef]
- Xu, L.; Yang, H.Z.; Li, C.Z.; Liu, S.Y.; Zhao, H.D.; Liao, X.J.; Zhao, L. Composition analysis of free and bound phenolics in chestnut rose (Rosa roxburghii Tratt.) fruit by UHPLC-IM-QTOF and UPLC-QQQ. LWT-Food Sci. Technol. 2023, 185, 115125. [Google Scholar] [CrossRef]
- Li, B.L.; Yuan, J.; Wu, J.W. A Review on the phytochemical and pharmacological properties of Rosa laevigata: A medicinal and edible plant. Chem. Pharm. Bull. 2021, 69, 421–431. [Google Scholar] [CrossRef]
- Gao, P.Y.; Li, L.Z.; Peng, Y.; Piao, S.J.; Zeng, N.; Lin, H.W.; Song, S.J. Triterpenes from fruits of Rosa laevigata. Biochem. Syst. Ecol. 2010, 38, 457–459. [Google Scholar] [CrossRef]
- Li, X.; Cao, W.; Shen, Y.; Li, N.; Dong, X.P.; Wang, K.J.; Cheng, Y.X. Antioxidant compounds from Rosa laevigata fruits. Food Chem. 2012, 130, 575–580. [Google Scholar] [CrossRef]
- Osada, A.; Horikawa, K.; Wakita, Y.; Nakamura, H.; Ukai, M.; Shimura, H.; Jitsuyama, Y.; Suzuki, T. Rosa davurica Pall., a useful Rosa species for functional rose hip production with high content of antioxidants and multiple antioxidant activities in hydrophilic extract. Sci. Hortic. 2022, 291, 110528. [Google Scholar] [CrossRef]
- Hwang, D.; Koh, P.O.; Kang, C.; Kim, E. Rosa davurica Pall. improves DNCB-induced atopic dermatitis in mice and regulated TNF-Alpa/IFN-gamma-induced skin inflammatory responses in HaCaT cells. Phytomedicine 2021, 91, 153708. [Google Scholar] [CrossRef]
- Tong, H.B.; Jiang, G.Q.; Guan, X.G.; Wu, H.; Song, K.X.; Cheng, K.; Sun, X. Characterization of a polysaccharide from Rosa davurica and inhibitory activity against neutrophil migration. Int. J. Biol. Macromol. 2016, 89, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Oh, S.; Nguyen, Q.; Kim, M.; Zheng, S.D.; Fang, M.Z.; Yi, T.H. Rosa davurica inhibited allergic mediators by regulating calcium and histamine signaling pathways. Plants 2023, 12, 1572. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.Z.; Lee, H.M.; Oh, S.; Zheng, S.D.; Bellere, A.D.; Kim, M.; Yu, D.; Yi, T.H. Rosa davurica inhibits skin photoaging via regulating MAPK/AP-1, NF-κB, and Nrf2/HO-1 signaling in UVB-irradiated HaCaTs. Photochem. Photobiol. Sci. 2022, 21, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Yeo, J.H.; Jiang, Y.; Heo, S.I.; Wang, M.H. The antidiabelic effects of an herbal formula composed of Alnus hirsuta, Rosa davurica, Acanthopanax senticosus and Panax schinseng in the streptozotocin-induced diabetic rats. Nutr. Res. Pract. 2013, 7, 103–108. [Google Scholar] [CrossRef]
- Huo, Y.Y.; Gao, Y.; Mi, J.; Wang, X.R.; Jiang, H.M.; Zhang, H.L. Isolation and simultaneous quantification of nine triterpenoids from Rosa davurica Pall. J. Chromatogr. Sci. 2017, 55, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Upman, K.; Sharma, A. Ethnobotany, phytochemistry, pharmacology, and toxicology of Rosa Sericea a medicinal plant. IOP Conf. Series Earth Environ. Sci. 2023, 1110, 012045. [Google Scholar] [CrossRef]
- Editorial Board of Chinese Materia Medica of National Administration of Traditional Chinese Medicine. Chinese Materia Medica; Shanghai Science and Technology Press: Shanghai, China, 1999. [Google Scholar]
- Xiao, C.N.; Wu, M.; Chen, Y.Y.; Zhang, Y.J.; Zhao, X.F.; Zheng, X.H. Revealing metabolomic variations in cortex moutan from different root parts using HPLC-MS method. Phytochem. Anal. 2015, 26, 86–93. [Google Scholar] [CrossRef]
- Xiao, Q.; Mu, X.L.; Liu, J.S.; Li, B.; Liu, H.T.; Zhang, B.G.; Xiao, P.G. Plant metabolomics: A new strategy and tool for quality evaluation of Chinese medicinal materials. Chin. Med. 2022, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.W.; Wang, S.X.; Jia, L.D.; Zhu, M.C.; Yang, J.; Zhou, B.J.; Yin, J.M.; Lu, K.; Wang, R.; Li, J.N.; et al. Identification and characterization of major constituents in different-colored rapeseed petals by UPLC-HESI-MS/MS. J. Agric. Food Chem. 2019, 67, 11053–11065. [Google Scholar] [CrossRef]
- Su, L.Y.; Zhang, T.; Wu, M.; Zhong, Y.; Cheng, Z.M. Transcriptome and metabolome reveal sugar and organic acid accumulation in Rosa roxburghii fruit. Plants 2023, 12, 3036. [Google Scholar] [CrossRef]
- Gu, W.; Wei, Y.H.; Fu, X.J.; Gu, R.H.; Chen, J.L.; Jian, J.Y.; Hao, X.J. HS-SPME/GCxGC-TOFMS-Based flavoromics and antimicrobial properties of the aroma components of Zanthoxylum motuoense. Foods 2023, 12, 2225. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Zeng, C.B.; Ye, G.Y.; Li, G.C.; Cao, H.; Wang, Z.H.; Ji, S.G. RID serve as a more appropriate measure than phenol sulfuric acid method for natural water-soluble polysaccharides quantification. Carbohydr. Polym. 2022, 278, 118928. [Google Scholar] [CrossRef]
- Ji, Z.; Mao, J.; Chen, S.; Mao, J. Antioxidant and anti-inflammatory activity of peptides from foxtail millet (Setaria italica) prolamins in HaCaT cells and RAW264.7 murine macrophages. Food Biosci. 2020, 36, 100636. [Google Scholar] [CrossRef]
- Zi, Y.S.; Zhang, B.; Jiang, B.; Yang, X.Y.; Liang, Z.L.; Liu, W.Y.; He, C.F.; Liu, L. Antioxidant action and protective and reparative effects of lentinan on oxidative damage in HaCaT cells. J. Cosmet. Dermatol. 2018, 17, 1108–1114. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, M.; Rao, T.; Liu, Z.; Wu, X.; An, H. Comparative analysis of fruit metabolome using widely targeted metabolomics reveals nutritional characteristics of different Rosa roxburghii genotypes. Foods 2022, 11, 850. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Bian, X.Q.; Yan, G.Y.; Sun, B.Q.; Miao, W.; Huang, M.Z.; Li, N.; Wu, J.L. Discovery of novel ascorbic acid derivatives and other metabolites in fruit of Rosa roxburghii Tratt through untargeted metabolomics and feature-based molecular networking. Food Chem. 2023, 405, 134807. [Google Scholar] [CrossRef]
- Sun, Y.L.; Zhou, M.C.; Luo, L.; Pan, H.T.; Zhang, Q.S.; Yu, C. Metabolic profiles, bioactive compounds and antioxidant activity of rosehips from Xinjiang, China. LWT-Food Sci. Technol. 2023, 174, 114451. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Mitsikaris, P.D.; Klaoudatos, D.; Papadopoulos, A.N.; Samanidou, V.F. A rapid HPLC-UV protocol coupled to chemometric analysis for the determination of the major phenolic constituents and tocopherol content in almonds and the discrimination of the geographical origin. Molecules 2021, 26, 5433. [Google Scholar] [CrossRef]
- Yan, N.; Du, Y.M.; Liu, X.M.; Chu, M.J.; Shi, J.; Zhang, H.B.; Liu, Y.H.; Zhang, Z.F. A comparative UHPLC-QqQ-MS-based metabolomics approach for evaluating Chinese and North American wild rice. Food Chem. 2019, 275, 618–627. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Huang, Y.; Xiao, J.C.; Liu, H.; Wang, Y.L.; Gong, Z.P.; Li, Y.T.; Wang, A.M.; Li, Y.J.; Zheng, L. Chemical profiling and quantification of multiple components in Jin-Gu-Lian capsule using a multivariate data processing approach based on UHPLC-Orbitrap Exploris 240 MS and UHPLC-MS/MS. J. Sep. Sci. 2022, 45, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Chapman, S.C.; Major, G.H.; Eggett, D.L.; Lunt, B.M.; Harrison, C.R.; Linford, M.R. Informatics analysis of capillary electropherograms of autologously doped and undoped blood. Anal. Methods 2019, 11, 1868–1878. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gaynor, R.B. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J. Clin. Investig. 2001, 107, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Wang, M.L.; Xing, S.; Zhang, C. Flavonoids of Rosa rugosa Thunb. inhibit tumor proliferation and metastasis in human hepatocellular carcinoma HepG2 cells. Food Sci. Hum. Wellness 2022, 11, 374–382. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, L.X.; Liu, B.; Xiao, J.B.; Cheng, K.W.; Chen, F.; Wang, M.F. Tricoumaroylspermidine from rose exhibits inhibitory activity against ethanol-induced apoptosis in HepG2 cells. Food Funct. 2021, 12, 5892–5902. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e370. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhou, K.; Ning, Y.; Zhao, G.H. Effect of the structure of gallic acid and its derivatives on their interaction with plant ferritin. Food Chem. 2016, 213, 260–267. [Google Scholar] [CrossRef]
- Ge, Y.H.; Li, X.; Huang, M.Z.; Huang, Z.X.; Wu, M.M.; Sun, B.Q.; Wang, L.S.; Wu, J.L.; Li, N. Aroma correlation assisted volatilome coupled network analysis strategy to unveil main aroma-active volatiles of Rosa roxburghii. Food Res. Int. 2023, 169, 112819. [Google Scholar] [CrossRef] [PubMed]
- Saaby, L.; Jäger, A.K.; Moesby, L.; Hansen, E.W.; Christensen, S.B. Isolation of immunomodulatory triterpene acids from a standardized rose hip powder (Rosa canina L.). Phytother. Res. 2010, 25, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.Q.; Yang, X.Z.; Miao, J.H.; Tang, C.P.; Ke, C.Q.; Zhang, J.B.; Ma, X.J.; Ye, Y. New triterpene glucosides from the roots of Rosa laevigata Michx. Molecules 2008, 13, 2229–2237. [Google Scholar] [CrossRef]
- Zhai, B.W.; Zhao, H.; Zhu, H.L.; Huang, H.; Zhang, M.Y.; Fu, Y.J. Triterpene acids from Rosa roxburghii Tratt fruits exert anti-hepatocellular carcinoma activity via ROS/JNK signaling pathway-mediated cell cycle arrest and mitochondrial apoptosis. Phytomedicine 2023, 119, 154960. [Google Scholar] [CrossRef]
- Liu, Y.J.; Liu, J.N.; Kong, Z.Y.; Huan, X.J.; Li, L.; Zhang, P.; Wang, Q.C.; Guo, Y.R.; Zhu, W.T.; Qin, P. Transcriptomics and metabolomics analyses of the mechanism of flavonoid synthesis in seeds of differently colored quinoa strains. Genomics 2022, 114, 138–148. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Y.H.; Lu, S.; Deng, X.Y.; Niu, X.L.; Guo, Z.; Qian, R.; Zhou, M.J.; Peng, X.B. Autophagy attenuates particulate matter 2.5-induced damage in HaCaT cells. Ann. Transl. Med. 2021, 9, 978. [Google Scholar] [CrossRef]
- Wang, Z.H.; Kang, K.A.; Zhang, R.; Piao, M.J.; Jo, S.H.; Kim, J.S.; Kang, S.S.; Lee, J.S.; Park, D.S.; Hyun, J.W. Myricetin suppresses oxidative stress-induced cell damage via both direct and indirect antioxidant action. Environ. Toxicol. Phar. 2010, 29, 12–18. [Google Scholar] [CrossRef]
- Butkevičiūtė, A.; Urbštaitė, R.; Liaudanskas, M.; Obelevičius, K.; Janulis, V. Phenolic content and antioxidant activity in fruit of the genus Rosa L. Antioxidants 2022, 11, 912. [Google Scholar] [CrossRef] [PubMed]
- Demir, N.; Yildiz, O.; Alpaslan, M.; Hayaloglu, A.A. Evaluation of volatiles, phenolic compounds and antioxidant activities of rose hip (Rosa L.) fruits in Turkey. LWT-Food Sci. Technol. 2014, 57, 126–133. [Google Scholar] [CrossRef]
- Koczka, N.; Stefanovits-Bányai, É.; Ombódi, A. Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fang, W.Y.; Wang, Z.; Chen, Y. Physicochemical, biological properties, and flavour profile of Rosa roxburghii Tratt, Pyracantha fortuneana, and Rosa laevigata Michx fruits: A comprehensive review. Food Chem. 2022, 366, 130509. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.P.; Wang, Q.; Lin, R.G.; He, P.; Lai, F.R.; Zhang, M.M.; Wu, H. Structural characterization and immunomodulatory activity of a novel acid polysaccharide isolated from the pulp of Rosa laevigata Michx fruit. Int. J. Biol. Macromol. 2020, 145, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, X.Q.; Rao, S.Q.; Li, Y.C.; Zang, Y.X.; Zhu, B.A. Identification and quantification of flavonoids in Okra (Abelmoschus esculentus L. Moench) and antiproliferative activity in vitro of four main components identified. Metabolites 2022, 12, 483. [Google Scholar] [CrossRef] [PubMed]
- Delazar, A.; Lasheni, S.; Fathi-Azad, F.; Nahar, L.; Rahman, M.M.; Asnaashari, S.; Mojarab, M.; Sarker, S.D. Free-radical scavenging flavonol 3-O-glycosides from the leaves of Ribes biebersteinii Berl. Rec. Nat. Prod. 2010, 4, 96–100. [Google Scholar]
- Li, X.; Yang, L.P.; Liu, S.Y.; Fei, D.Q.; Zhang, M.; Zhang, Y.X. Effect of quercetin-3-o-sambubioside isolated from Eucommia ulmoides male flowers on spontaneous activity and convulsion rate in mice. Planta Med. 2014, 80, 974–977. [Google Scholar] [CrossRef]
- Schumann, J.; Prockl, J.; Kiemer, A.K.; Vollmar, A.M.; Bang, R.; Tiegs, G. Silibinin protects mice from T cell-dependent liver injury. J. Hepatol. 2003, 39, 333–340. [Google Scholar] [CrossRef]
- Cheung, C.; Gibbons, N.; Johnson, D.W.; Nicol, D.L. Silibinin-A promising new treatment for cancer. Anti-Cancer Agent Med. Chem. 2010, 10, 186–195. [Google Scholar] [CrossRef]
- Deep, G.; Agarwal, R. Antimetastatic efficacy of silibinin: Molecular mechanisms and therapeutic potential against cancer. Cancer Metastasis Rev. 2010, 29, 447–463. [Google Scholar] [CrossRef]
- Yin, F.; Liu, J.H.; Ji, X.H.; Wang, Y.W.; Zidichouski, J.; Zhang, J.Z. Silibinin: A novel inhibitor of Aβ aggregation. Neurochem. Int. 2011, 58, 399–403. [Google Scholar] [CrossRef]
- Song, X.M.T.; Tan, L.; Wang, M.; Ren, C.X.; Guo, C.J.; Yang, B.; Ren, Y.L.; Cao, Z.X.; Li, Y.Z.; Pei, J. Myricetin: A review of the most recent research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef]
- Yan, M.; Zhu, Y.; Zhang, H.J.; Jiao, W.H.; Han, B.N.; Liu, Z.X.; Qiu, F.; Chen, W.S.; Lin, H.W. Anti-inflammatory secondary metabolites from the leaves of Rosa laevigata. Bioorgan. Med. Chem. 2013, 21, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.N.; Li, B.L.; Hu, J.J.; Xie, J.D.; Xiao, W.J.; Nie, L.H.; Wu, J.W. Rosanortriterpenes A–B, two new nortriterpenes from the fruits of Rosa laevigata var. leiocapus. Nat. Prod. Res. 2021, 35, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.W.; Zhou, H.; An, Y.A.; Shen, K.S.; Yu, L. Biological effects of corosolic acid as an anti-inflammatory, anti-metabolic syndrome and anti-neoplasic natural compound (Review). Oncol. Lett. 2021, 21, 84. [Google Scholar] [CrossRef]
- Wozniak, Ł.; Skapska, S.; Marszałek, K. Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules 2015, 20, 20614. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Sharma, A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Pironi, A.M.; Araujo, P.R.D.; Fernandes, M.A.; Salgado, H.R.N.; Chorilli, M. Characteristics, biological properties and analytical methods of ursolic acid: A review. Crit. Rev. Anal. Chem. 2018, 48, 86–93. [Google Scholar] [CrossRef] [PubMed]


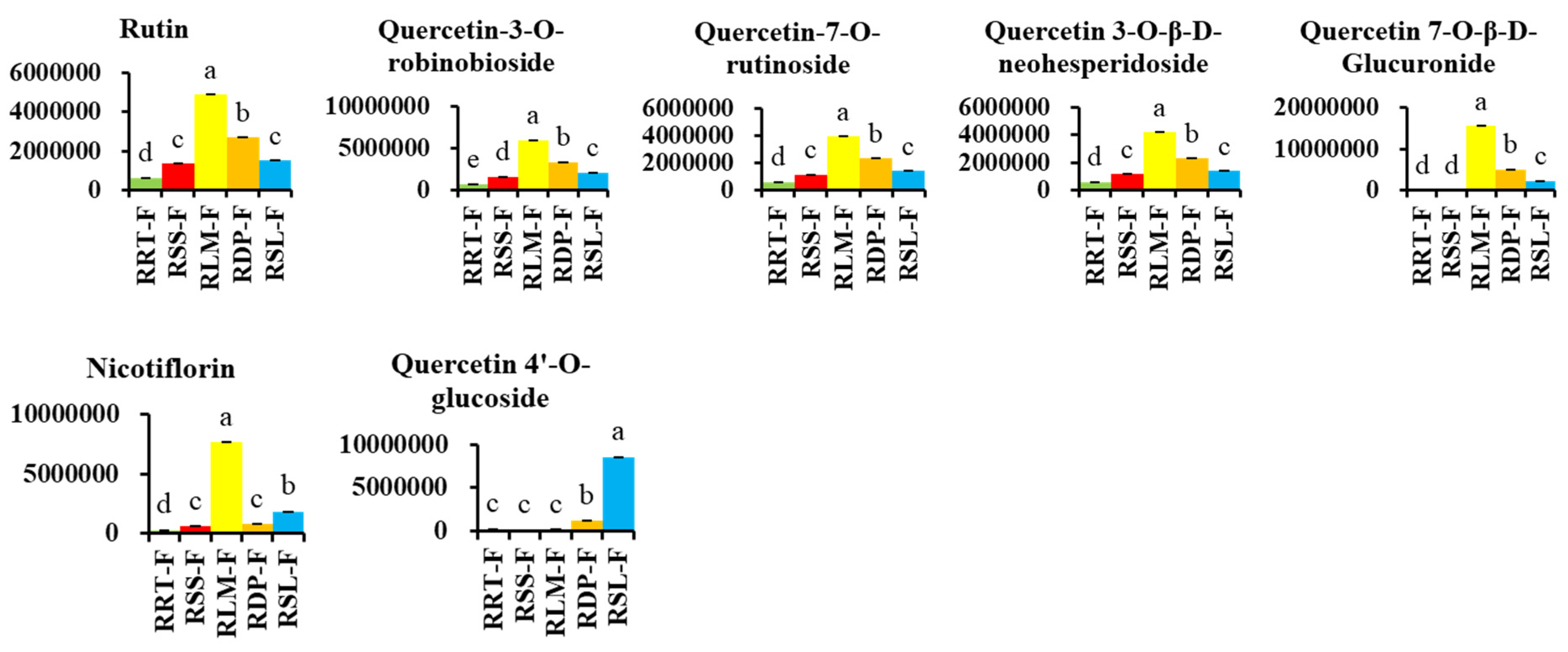
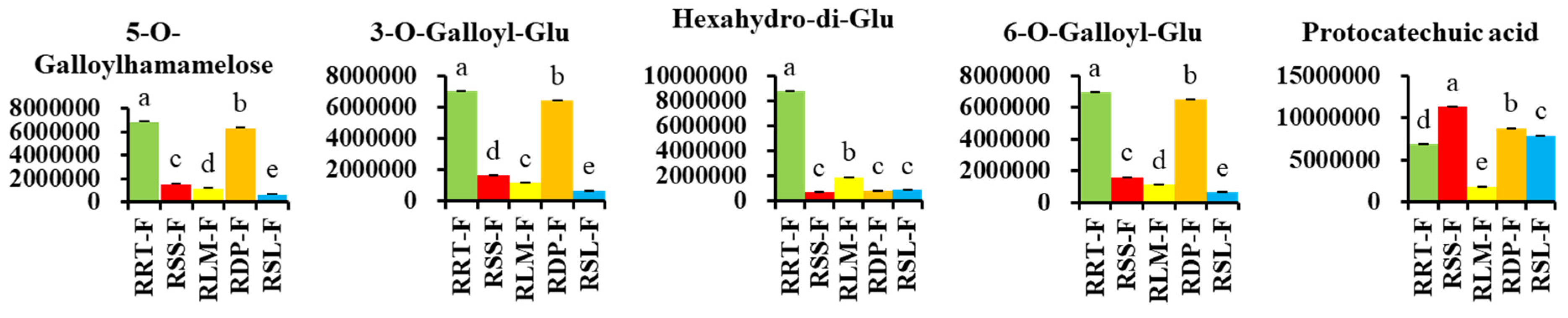
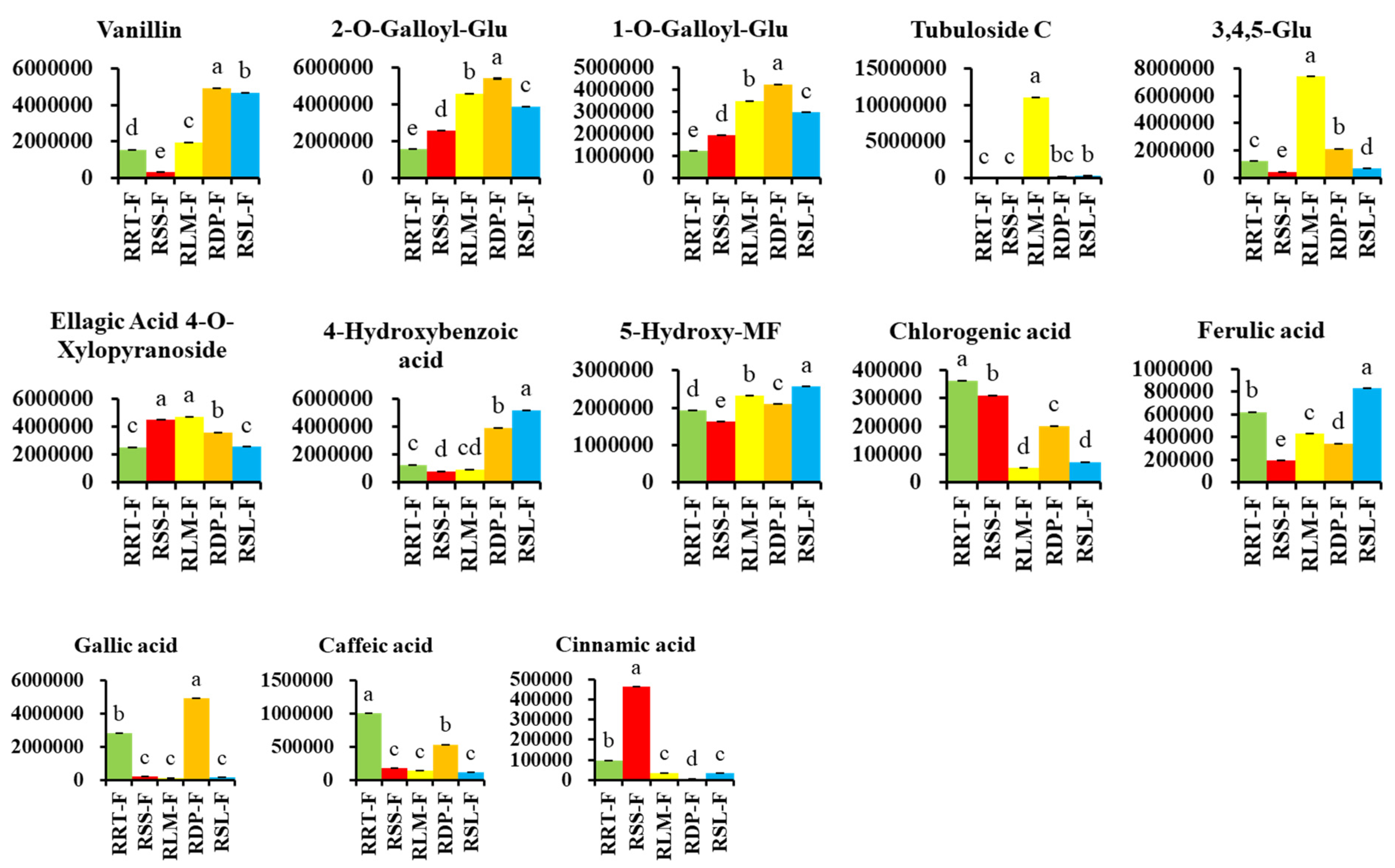
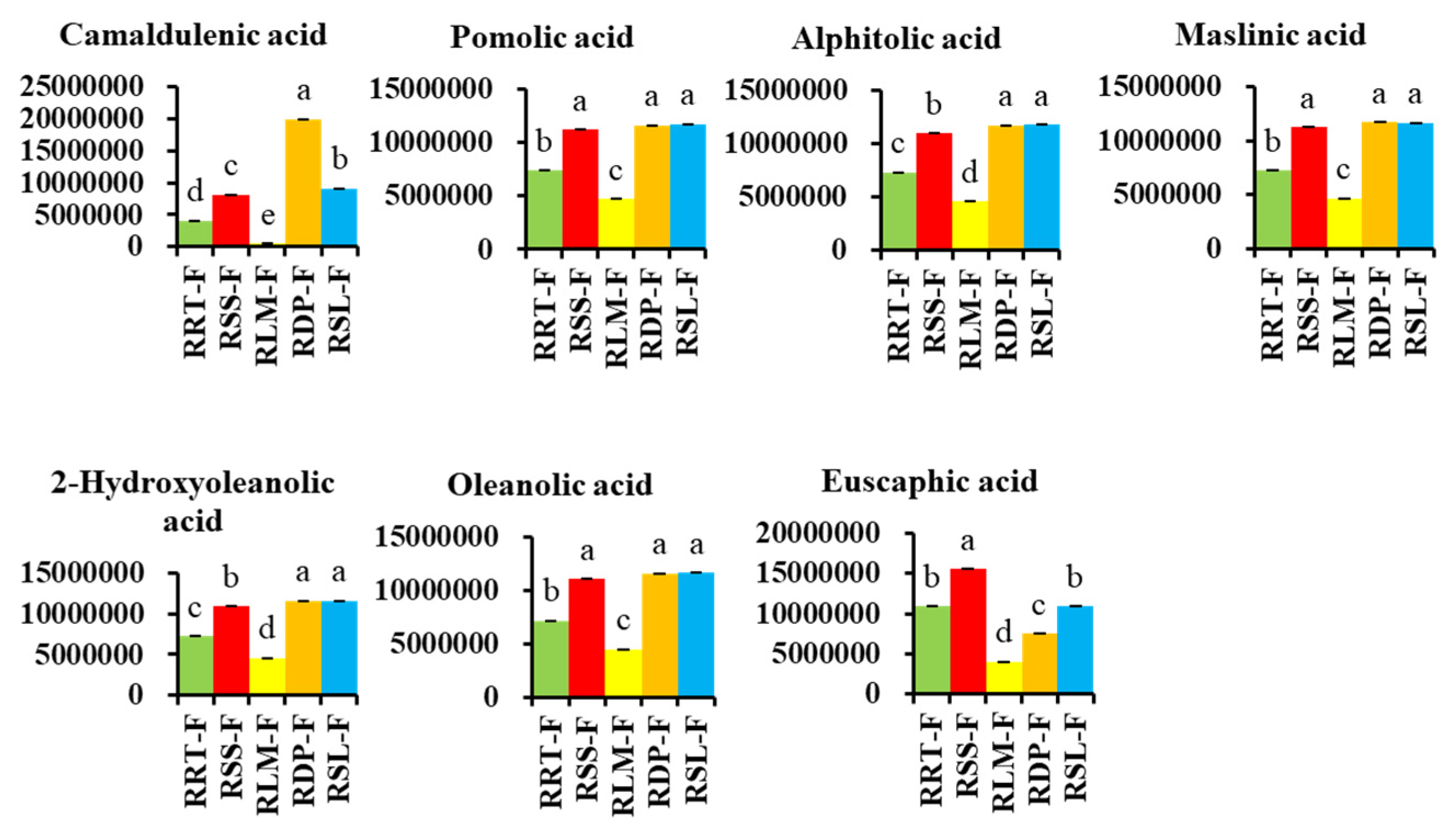
| Class | RRT-F | RSS-F | RLM-F | RDP-F | RSL-F |
|---|---|---|---|---|---|
| Flavonoids | 33.98% | 22.29% | 45.13% | 41.24% | 35.08% |
| Phenolic acids | 17.18% | 12.67% | 14.34% | 15.20% | 11.27% |
| Terpenoids | 12.22% | 24.81% | 7.70% | 16.21% | 19.07% |
| Organic acids | 12.29% | 9.77% | 8.56% | 9.59% | 12.18% |
| Lignins | 4.06% | 1.50% | 1.29% | 1.30% | 0.59% |
| Alkaloids | 3.45% | 8.02% | 3.40% | 3.17% | 3.77% |
| Vitamins | 0.82% | 0.87% | 0.98% | 0.68% | 0.79% |
| Coumarins | 0.25% | 0.23% | 0.44% | 0.09% | 0.53% |
| Others | 3.84% | 4.54% | 2.60% | 3.77% | 4.52% |
| Total | 88.08% | 84.71% | 84.44% | 91.25% | 87.78% |
| Samples | Contents (g/100 g ± SD, n = 3) |
|---|---|
| TPS | |
| RRT-F | 33.80 ± 0.07 c |
| RSS-F | 39.06 ± 0.04 bc |
| RLM-F | 64.48 ± 0.07 a |
| RDP-F | 49.11 ± 0.08 abc |
| RSL-F | 53.42 ± 0.10 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, M.; Chen, J.; Fu, M.; Li, H.; Bu, S.; Hao, X.; Gu, W. UPLC-ESI-MS/MS-Based Analysis of Various Edible Rosa Fruits Concerning Secondary Metabolites and Evaluation of Their Antioxidant Activities. Foods 2024, 13, 796. https://doi.org/10.3390/foods13050796
Ni M, Chen J, Fu M, Li H, Bu S, Hao X, Gu W. UPLC-ESI-MS/MS-Based Analysis of Various Edible Rosa Fruits Concerning Secondary Metabolites and Evaluation of Their Antioxidant Activities. Foods. 2024; 13(5):796. https://doi.org/10.3390/foods13050796
Chicago/Turabian StyleNi, Ming, Junlei Chen, Mao Fu, Huanyang Li, Shengqian Bu, Xiaojiang Hao, and Wei Gu. 2024. "UPLC-ESI-MS/MS-Based Analysis of Various Edible Rosa Fruits Concerning Secondary Metabolites and Evaluation of Their Antioxidant Activities" Foods 13, no. 5: 796. https://doi.org/10.3390/foods13050796
APA StyleNi, M., Chen, J., Fu, M., Li, H., Bu, S., Hao, X., & Gu, W. (2024). UPLC-ESI-MS/MS-Based Analysis of Various Edible Rosa Fruits Concerning Secondary Metabolites and Evaluation of Their Antioxidant Activities. Foods, 13(5), 796. https://doi.org/10.3390/foods13050796






