Deep Eutectic Solvents as New Extraction Media for Flavonoids in Mung Bean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Preparation of Deep Eutectic Solvents
2.4. Ultrasound-Assisted Extraction (UAE) of Total Amounts of Flavonoids from Mung Bean by Deep Eutectic Solvents and EtOH
2.5. Single-Factor Experiment
2.6. Response Surface Methodology (RSM)
2.7. High Performance Liquid Chromatography Analysis
2.8. Assessment of Antioxidant Capacity
2.9. Scanning Electron Microscope (SEM)
2.10. Fourier Transform Infrared Spectrometer
2.11. Statistical Analyses
3. Results and Discussion
3.1. Selection of Deep Eutectic Solvents
3.2. Effect of Hydrogen Bond Acceptors-Hydrogen Bond Donors Molar Ratios on the Extraction Yield of Total Amounts of Flavonoids
3.3. Effect of Water Content in Deep Eutectic Solvent on the Extraction Yield of Total Amounts of Flavonoids
3.4. Effect of Liquid–Solid Ratio on the Extraction Yield of Total Amounts of Flavonoids
3.5. Effect of Ultrasonic Power on the Extraction Yield of Total Amounts of Flavonoids
3.6. Effect of Extraction Temperature on the Extraction Yield of Total Amounts of Flavonoids
3.7. Effect of Extraction Time on the Extraction Yield of Total Amounts of Flavonoids
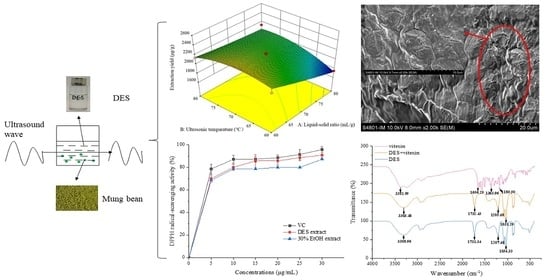
3.8. Model Fitting and Response Surface Methodology
3.9. Model Validation
3.10. Antioxidant Capacities of the Flavonoids
3.10.1. 2,2-Diphenyl-1-picrylhydrazyl Radical-Scavenging Activity
3.10.2. 2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) Radical-Scavenging Activity
3.11. Scanning Electron Microscope
3.12. Fourier Transform Infrared Spectrometer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Hung, P.; Hoang Yen, N.T.; Lan Phi, N.T.; Ha Tien, N.P.; Thu Trung, N.T. Nutritional Composition, Enzyme Activities and Bioactive Compounds of Mung Bean (Vigna radiata L.) Germinated under Dark and Light Conditions. LWT 2020, 133, 110100. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A Critical Review on Phytochemical Profile and Health Promoting Effects of Mung Bean (Vigna radiata). Food Sci. Hum. Well. 2018, 7, 11–33. [Google Scholar] [CrossRef]
- Cao, D.; Li, H.; Yi, J.; Zhang, J.; Che, H.; Cao, J.; Yang, L.; Zhu, C.; Jiang, W. Antioxidant Properties of the Mung Bean Flavonoids on Alleviating Heat Stress. PLoS ONE 2011, 6, e21071. [Google Scholar] [CrossRef] [PubMed]
- Supasatyankul, B.; Saisriyoot, M.; Klinkesorn, U.; Rattanaporn, K.; Sae-Tan, S. Extraction of Phenolic and Flavonoid Compounds from Mung Bean (Vigna radiata L.) Seed Coat by Pressurized Liquid Extraction. Molecules 2022, 27, 2085. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Cai, W.; Wu, T.; Xu, B. Phytochemical Distribution in Hull and Cotyledon of Adzuki Bean (Vigna angularis L.) and Mung Bean (Vigna radiate L.), and Their Contribution to Antioxidant, Anti-Inflammatory and Anti-Diabetic Activities. Food Chem. 2016, 201, 350–360. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Min, J.W.; Kong, W.L.; He, X.H.; Li, J.X.; Peng, B.W. A Review on the Pharmacological Effects of Vitexin and Isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Cai, W.; Xu, B. Kinetic Changes of Nutrients and Antioxidant Capacities of Germinated Soybean (Glycine max L.) and Mung Bean (Vigna radiata L.) with Germination Time. Food Chem. 2014, 143, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, X.-Z.; Ren, G.X. Application of Near-Infrared Reflectance Spectroscopy to the Evaluation of D-Chiro-Inositol, Vitexin, and Isovitexin Contents in Mung Bean. Agric. Sci. China 2011, 10, 1986–1991. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Optimization of Ultrasound-Assisted Extraction of Antioxidants from the Mung Bean Coat. Molecules 2017, 22, 638. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and Thermal Behavior of Natural Deep Eutectic Solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- Maugeri, Z.; Domínguez de María, P. Novel Choline-Chloride-Based Deep-Eutectic-Solvents with Renewable Hydrogen Bond Donors: Levulinic Acid and Sugar-Based Polyols. RSC Adv. 2012, 2, 421–425. [Google Scholar] [CrossRef]
- Boateng, I.D. Evaluating the Status Quo of Deep Eutectic Solvent in Food Chemistry. Potentials and Limitations. Food Chem. 2023, 406, 135079. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.; Prieto, M.A.; Barros, L.; Coutinho, J.A.P.; Ferreira, I.C.F.R.; Ferreira, O. Enhanced Extraction of Phenolic Compounds Using Choline Chloride Based Deep Eutectic Solvents from Juglans regia L. Ind. Crops Prod. 2018, 115, 261–271. [Google Scholar] [CrossRef]
- Pan, X.; Niu, G.; Liu, H. Microwave-Assisted Extraction of Tanshinones from Salvia miltiorrhiza bunge with Analysis by High-Performance Liquid Chromatography. J. Chromatogr. A 2001, 922, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Dou, L.-L.; Guo, L.; Li, P.; Liu, E.-H. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Islamčević Razboršek, M.; Ivanović, M.; Krajnc, P.; Kolar, M. Choline Chloride Based Natural Deep Eutectic Solvents as Extraction Media for Extracting Phenolic Compounds from Chokeberry (Aronia melanocarpa). Molecules 2020, 25, 1619. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Marques, C.; Igarashi-Mafra, L.; Coutinho, J.A.P.; Mafra, M.R. Extraction of Phenolic Compounds from Rosemary Using Choline Chloride—Based Deep Eutectic Solvents. Sep. Purif. Technol. 2021, 258, 117975. [Google Scholar] [CrossRef]
- Almusallam, I.A.; Mohamed Ahmed, I.A.; Babiker, E.E.; Al Juhaimi, F.Y.; Fadimu, G.J.; Osman, M.A.; Al Maiman, S.A.; Ghafoor, K.; Alqah, H.A.S. Optimization of Ultrasound-Assisted Extraction of Bioactive Properties from Date Palm (Phoenix dactylifera L.) Spikelets Using Response Surface Methodology. LWT 2021, 140, 110816. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Xu, T.; Fan, L.; Zhou, X.; Wu, S. Ionic Deep Eutectic Solvents for the Extraction and Separation of Natural Products. J. Chromatogr. A 2019, 1598, 1–19. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, H.; Cui, L.; Hussain, H.; Nadolnik, L.; Zhang, Z.; Zhao, Y.; Qin, X.; Li, J.; Park, J.H.; et al. Ultrasonic-Assisted Extraction of Flavonoids from Peanut Leave and Stem Using Deep Eutectic Solvents and Its Molecular Mechanism. Food Chem. 2024, 434, 137497. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Serna, C.L.; Ochoa-Martínez, C.I.; Vélez-Pasos, C. Microwave-Assisted Extraction of Phenolic Compounds from Pineapple Peel Using Deep Eutectic Solvents. Horticulturae 2022, 8, 791. [Google Scholar] [CrossRef]
- Shang, X.; Dou, Y.; Zhang, Y.; Tan, J.N.; Liu, X.; Zhang, Z. Tailor-Made Natural Deep Eutectic Solvents for Green Extraction of Isoflavones from Chickpea (Cicer arietinum L.) Sprouts. Ind. Crops Prod. 2019, 140, 111724. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, G.; Wu, G.; Bo, Y.; Yang, D.; Guo, J.; Ma, Y.; An, M. An Ultrasound-Assisted Extraction Using an Alcohol-Based Hydrophilic Natural Deep Eutectic Solvent for the Determination of Five Flavonoids from Platycladi cacumen. Microchem. J. 2024, 110076. [Google Scholar] [CrossRef]
- Lu, C.; Cao, J.; Wang, N.; Su, E. Significantly Improving the Solubility of Non-Steroidal Anti-Inflammatory Drugs in Deep Eutectic Solvents for Potential Non-Aqueous Liquid Administration. Med. Chem. Commun. 2016, 7, 955–959. [Google Scholar] [CrossRef]
- Wei, Z.; Qi, X.; Li, T.; Luo, M.; Wang, W.; Zu, Y.; Fu, Y. Application of Natural Deep Eutectic Solvents for Extraction and Determination of Phenolics in Cajanus cajan Leaves by Ultra Performance Liquid Chromatography. Sep. Purif. Technol. 2015, 149, 237–244. [Google Scholar] [CrossRef]
- Tian, H.; Wang, J.; Li, Y.; Bi, W.; Chen, D.D.Y. Recovery of Natural Products from Deep Eutectic Solvents by Mimicking Denaturation. ACS Sustain. Chem. Eng. 2019, 7, 9976–9983. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The Effects of Ultrasound Assisted Extraction on Yield, Antioxidant, Anticancer and Antimicrobial Activity of Polyphenol Extracts: A Review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Oroian, M.; Ursachi, F.; Dranca, F. Ultrasound-Assisted Extraction of Polyphenols from Crude Pollen. Antioxidants 2020, 9, 322. [Google Scholar] [CrossRef]
- Nie, F.; Feng, C.; Ahmad, N.; Tian, M.; Liu, Q.; Wang, W.; Lin, Z.; Li, C.; Zhao, C. A New Green Alternative Solvent for Extracting Echinacoside and Acteoside from Cistanche deserticola Based on Ternary Natural Deep Eutectic Solvent. J. Ind. Eng. Chem. 2023, 118, 499–510. [Google Scholar] [CrossRef]
- He, Q.; Lei, Q.; Huang, S.; Zhou, Y.; Liu, Y.; Zhou, S.; Peng, D.; Deng, X.; Xue, J.; Li, X.; et al. Effective Extraction of Bioactive Alkaloids from the Roots of Stephania tetrandra by Deep Eutectic Solvents-Based Ultrasound-Assisted Extraction. J. Chromatogr. A 2023, 1689, 463746. [Google Scholar] [CrossRef] [PubMed]
- Ealias, A.M.; Saravanakurnar, M.P. Facile Synthesis and Characterisation of AlNs Using Protein Rich Solution Extracted from Sewage sludge and Its Application for Ultrasonic Assisted Dye Adsorption: Isotherms, Kinetics, Mechanism and RSM Design. J. Environ. Manag. 2018, 206, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yue, S.-J.; Gao, H.; Zhang, Q.; Xu, D.-Q.; Zhou, J.; Li, J.J.; Tang, Y.P. Natural Deep Eutectic Solvent-Ultrasound Assisted Extraction: A Green Approach for Ellagic Acid Extraction from Geum japonicum. Front. Nutr. 2023, 9, 1079767. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Liu, Q.; Jing, W.; Tian, H.; Yan, H.; Bi, W.; Jiang, Y.; Chen, D.D.Y. Insight into the Deep Eutectic Solvent Extraction Mechanism of Flavonoids from Natural Plant. ACS Sustain. Chem. Eng. 2020, 8, 19169–19177. [Google Scholar] [CrossRef]
- Feng, Z.; Yang, D.; Guo, J.; Bo, Y.; Zhao, L.; An, M. Optimization of Natural Deep Eutectic Solvents Extraction of Flavonoids from Xanthoceras sorbifolia Bunge by Response Surface Methodology. Sustain. Chem. Pharm. 2023, 31, 100904. [Google Scholar] [CrossRef]
- Xu, M.; Ran, L.; Chen, N.; Fan, X.; Ren, D.; Yi, L. Polarity-Dependent Extraction of Flavonoids from Citrus Peel Waste Using a Tailor-Made Deep Eutectic Solvent. Food Chem. 2019, 297, 124970. [Google Scholar] [CrossRef] [PubMed]
- Elik, A.; Altunay, N. Optimization of Vortex-Assisted Switchable Hydrophilicity Solvent Liquid Phase Microextraction for the Selective Extraction of Vanillin in Different Matrices Prior to Spectrophotometric Analysis. Food Chem. 2023, 399, 133929. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, Z.; Li, S.; Wang, L.; Zhang, J. Eco-Friendly and High-Efficient Extraction of Natural Antioxidants from Polygonum aviculare Leaves Using Tailor-Made Deep Eutectic Solvents as Extractants. Sep. Purif. Technol. 2021, 262, 118339. [Google Scholar] [CrossRef]
- Zhang, G.; He, L.; Hu, M. Optimized Ultrasonic-Assisted Extraction of Flavonoids from Prunella vulgaris L. and Evaluation of Antioxidant Activities in vitro. Innov. Food Sci. Emerg. 2011, 12, 18–25. [Google Scholar] [CrossRef]
- Yao, Y.; Cheng, X.; Wang, L.; Wang, S.; Ren, G. Biological Potential of Sixteen Legumes in China. Int. J. Mol. Sci. 2011, 12, 7048–7058. [Google Scholar] [CrossRef]
- Nagai, T.; Inoue, R.; Inoue, H.; Suzuki, N. Preparation and Antioxidant Properties of Water Extract of Propolis. Food Chem. 2003, 80, 29–33. [Google Scholar] [CrossRef]
- Chen, K.; Yang, X.; Huang, Z.; Jia, S.; Zhang, Y.; Shi, J.; Hong, H.; Feng, L.; Luo, Y. Modification of Gelatin Hydrolysates from Grass Carp (Ctenopharyngodon idellus) Scales by Maillard Reaction: Antioxidant Activity and Volatile Compounds. Food Chem. 2019, 295, 569–578. [Google Scholar] [CrossRef]






| DES Groups | Component 1 | Component 2 | Molar Ratio |
|---|---|---|---|
| DES-1 | choline chloride | urea | 1:2 |
| DES-2 | choline chloride | 1,2-propanediol | 1:2 |
| DES-3 | choline chloride | ethylene glycol | 1:2 |
| DES-4 | choline chloride | citric acid | 1:2 |
| DES-5 | choline chloride | malic acid | 1:2 |
| DES-6 | ethylene glycol | malonate | 1:2 |
| DES-7 | 1,2-propanediol | glycolic acid | 1:2 |
| DES-8 | ethylene glycol | glycolic acid | 1:2 |
| Factors | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Liquid–solid ratio (X1) (mL/g) | 60 | 70 | 80 |
| Ultrasonic temperature (X2) (°C) | 60 | 70 | 80 |
| Water content in DES (X3) (%) | 30 | 40 | 50 |
| Std. | X1 | X2 | X3 | Extraction Yield (μg/g) |
|---|---|---|---|---|
| 1 | 0 | 1 | −1 | 2018.97 |
| 2 | 1 | 0 | 1 | 1951.25 |
| 3 | 0 | −1 | 1 | 2029.34 |
| 4 | 0 | 0 | 0 | 2175.33 |
| 5 | 0 | 0 | 0 | 2139.67 |
| 6 | −1 | 0 | −1 | 2463.26 |
| 7 | 0 | −1 | −1 | 2034.37 |
| 8 | 0 | 0 | 0 | 2045.17 |
| 9 | −1 | −1 | 0 | 2152.30 |
| 10 | 1 | 0 | −1 | 2006.24 |
| 11 | −1 | 0 | 1 | 2319.49 |
| 12 | 1 | −1 | 0 | 1748.71 |
| 13 | 0 | 1 | 1 | 2132.12 |
| 14 | 1 | 1 | 0 | 2136.82 |
| 15 | −1 | 1 | 0 | 2137.94 |
| 16 | 0 | 0 | 0 | 2189.57 |
| 17 | 0 | 0 | 0 | 2172.41 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 321,245.700 | 9 | 35,693.960 | 4.324 | 0.033 * |
| A-Liquid–solid ratio | 189,102.300 | 1 | 189,102.300 | 22.910 | 0.002 ** |
| B-Ultrasonic temperature | 26,581.520 | 1 | 26,581.520 | 3.220 | 0.116 |
| C-Water content | 1026.892 | 1 | 1026.892 | 0.124 | 0.735 |
| AB | 40,495.180 | 1 | 40,495.180 | 4.906 | 0.062 |
| AC | 1970.830 | 1 | 1970.830 | 0.239 | 0.640 |
| BC | 3491.468 | 1 | 3491.468 | 0.423 | 0.536 |
| A2 | 1003.442 | 1 | 1003.442 | 0.122 | 0.738 |
| B2 | 56,581.020 | 1 | 56,581.02 | 6.855 | 0.035 |
| C2 | 2671.865 | 1 | 2671.865 | 0.324 | 0.587 |
| Residual | 57,778.800 | 7 | 8254.114 | ||
| Lack of fit | 44,128.860 | 3 | 14,709.620 | 4.310 | 0.096 |
| Pure error | 13,649.940 | 4 | 3412.484 | ||
| Total | 379,024.500 | 16 | |||
| R-Squared = 0.948 | Std.Dev. = 90.852 | ||||
| Adjusted R-Squared = 0.852 | Mean = 2109 | ||||
| Predicted R-Squared = 0.919 | C.V. % = 4.310 | ||||
| Adequacy precision = 9.190 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Xie, L.; Peng, Y.; Li, M.; Li, J.; Ni, Y.; Wen, X. Deep Eutectic Solvents as New Extraction Media for Flavonoids in Mung Bean. Foods 2024, 13, 777. https://doi.org/10.3390/foods13050777
Gao J, Xie L, Peng Y, Li M, Li J, Ni Y, Wen X. Deep Eutectic Solvents as New Extraction Media for Flavonoids in Mung Bean. Foods. 2024; 13(5):777. https://doi.org/10.3390/foods13050777
Chicago/Turabian StyleGao, Jingyu, Longli Xie, Yu Peng, Mo Li, Jingming Li, Yuanying Ni, and Xin Wen. 2024. "Deep Eutectic Solvents as New Extraction Media for Flavonoids in Mung Bean" Foods 13, no. 5: 777. https://doi.org/10.3390/foods13050777
APA StyleGao, J., Xie, L., Peng, Y., Li, M., Li, J., Ni, Y., & Wen, X. (2024). Deep Eutectic Solvents as New Extraction Media for Flavonoids in Mung Bean. Foods, 13(5), 777. https://doi.org/10.3390/foods13050777