Effects of Extrusion Technology on Physicochemical Properties and Microstructure of Rice Starch Added with Soy Protein Isolate and Whey Protein Isolate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Extruded Samples
2.3. Preparation of RS, ERS, and SPI-WPI-ERS
2.4. Molecular Structure of RS, ERS, and SPI-WPI-ERS
2.4.1. Amylopectin Chain Length Distribution of RS, ERS, and SPI-WPI-ERS
2.4.2. Total and Amylose Content of RS, ERS, and SPI-WPI-ERS
2.4.3. Molecular Weight of RS, ERS, and SPI-WPI-ERS
2.4.4. XRD Measurements of RS, ERS, and SPI-WPI-ERS
2.4.5. FTIR Spectroscopy of RS, ERS, and SPI-WPI-ERS
2.4.6. Micromorphology of RS, ERS, and SPI-WPI-ERS
2.5. Physical Properties of RS, ERS, and SPI-WPI-ERS
2.5.1. Water Solubility Index (WSI) and Water Absorption Index (WAI) of RS, ERS, and SPI-WPI-ERS
2.5.2. RVA of RS, ERS, and SPI-WPI-ERS
2.5.3. Rheological Measurement of RS, ERS, and SPI-WPI-ERS
2.5.4. Thermal Properties of RS, ERS, and SPI-WPI-ERS
2.6. Statistical Analysis
3. Results and Discussion
3.1. The Amylopectin Chain Length Distribution of RS, ERS, and SPI-WPI-ERS
3.2. The Starch Content and Molecular Weight Distribution of RS, ERS, and SPI-WPI-ERS
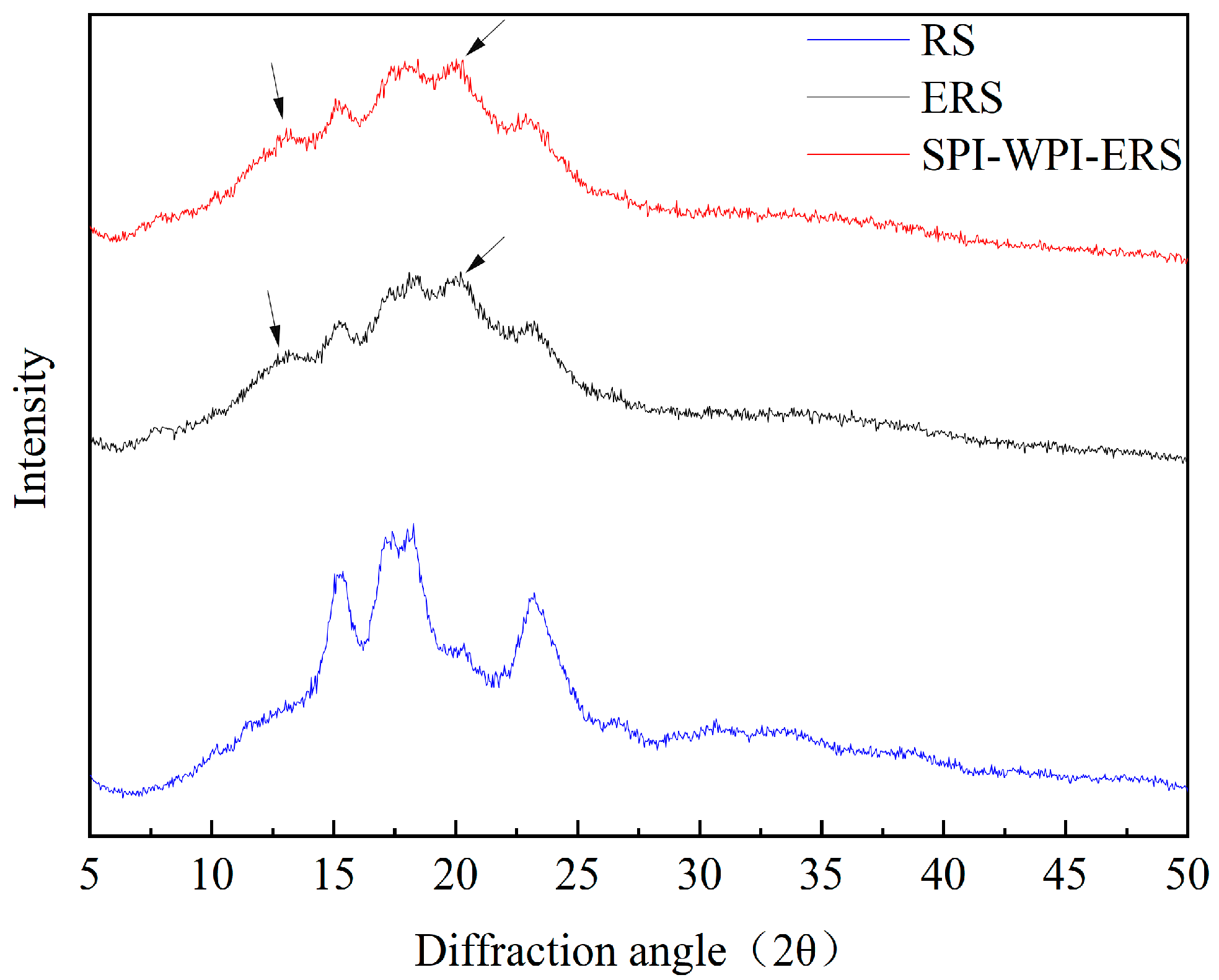
3.3. XRD Analysis of RS, ERS, and SPI-WPI-ERS
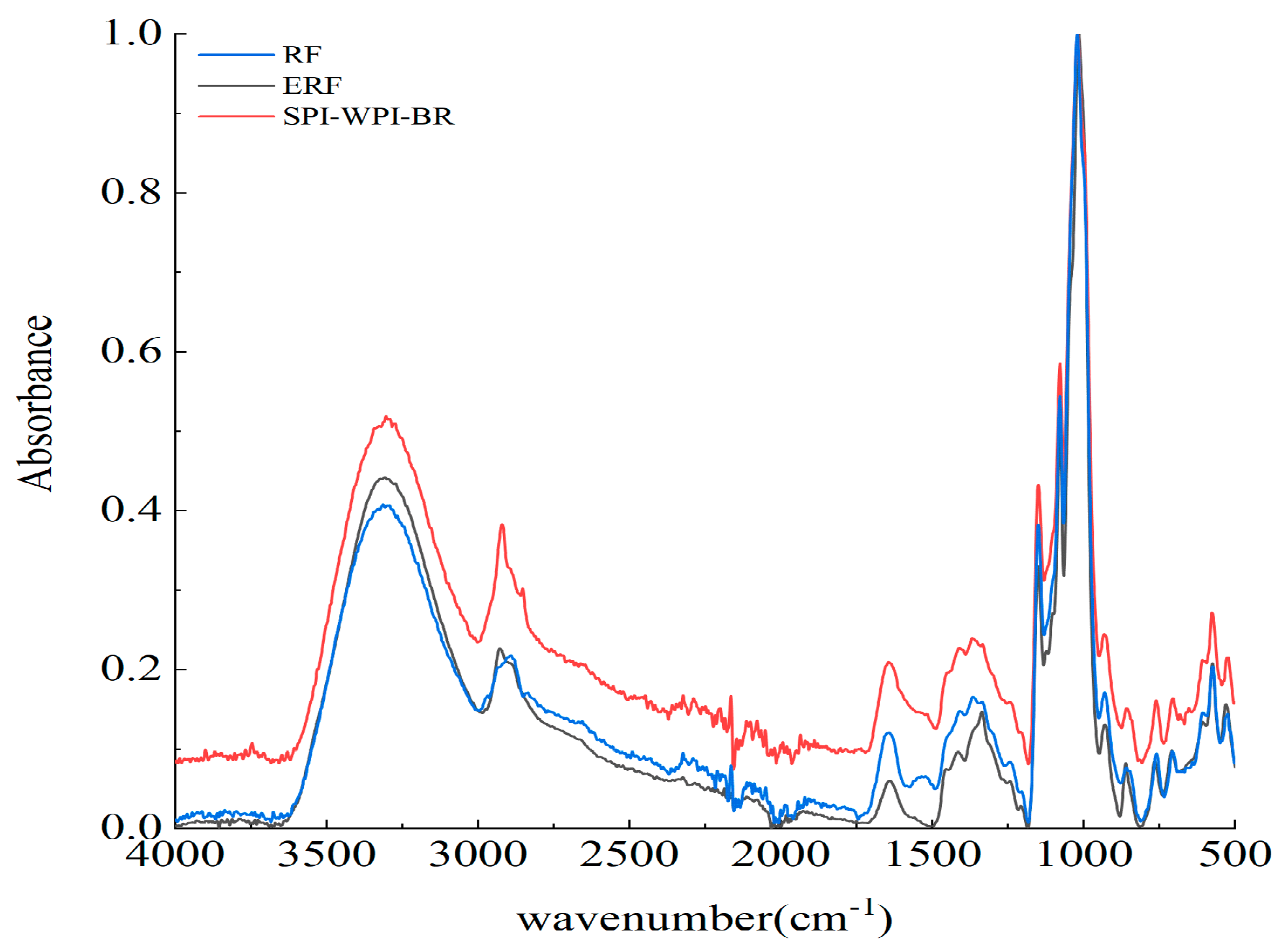
3.4. FTIR Spectra of RS, ERS, and SPI-WPI-ERS
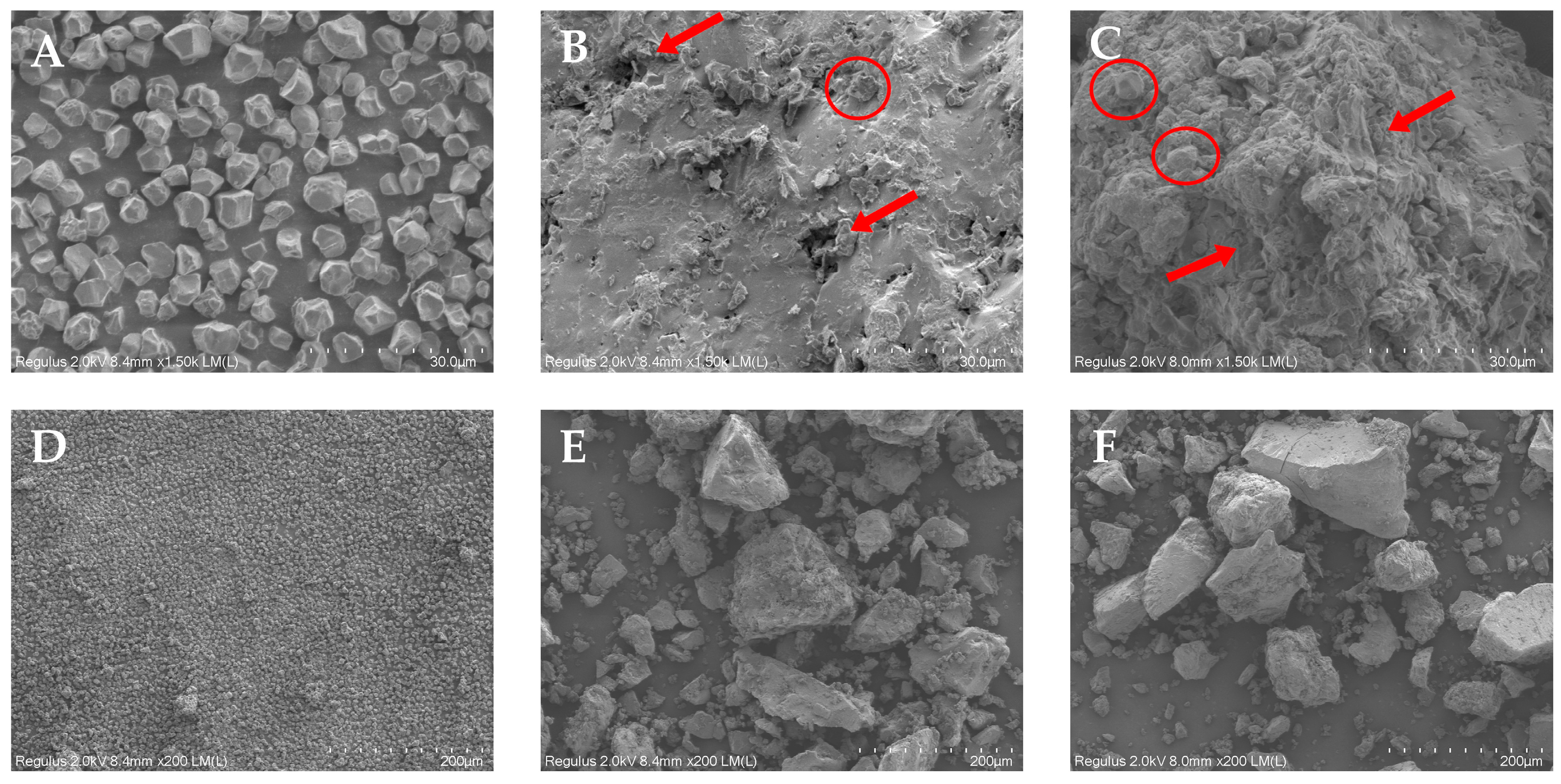
3.5. SEM Analysis of RS, ERS, and SPI-WPI-ERS
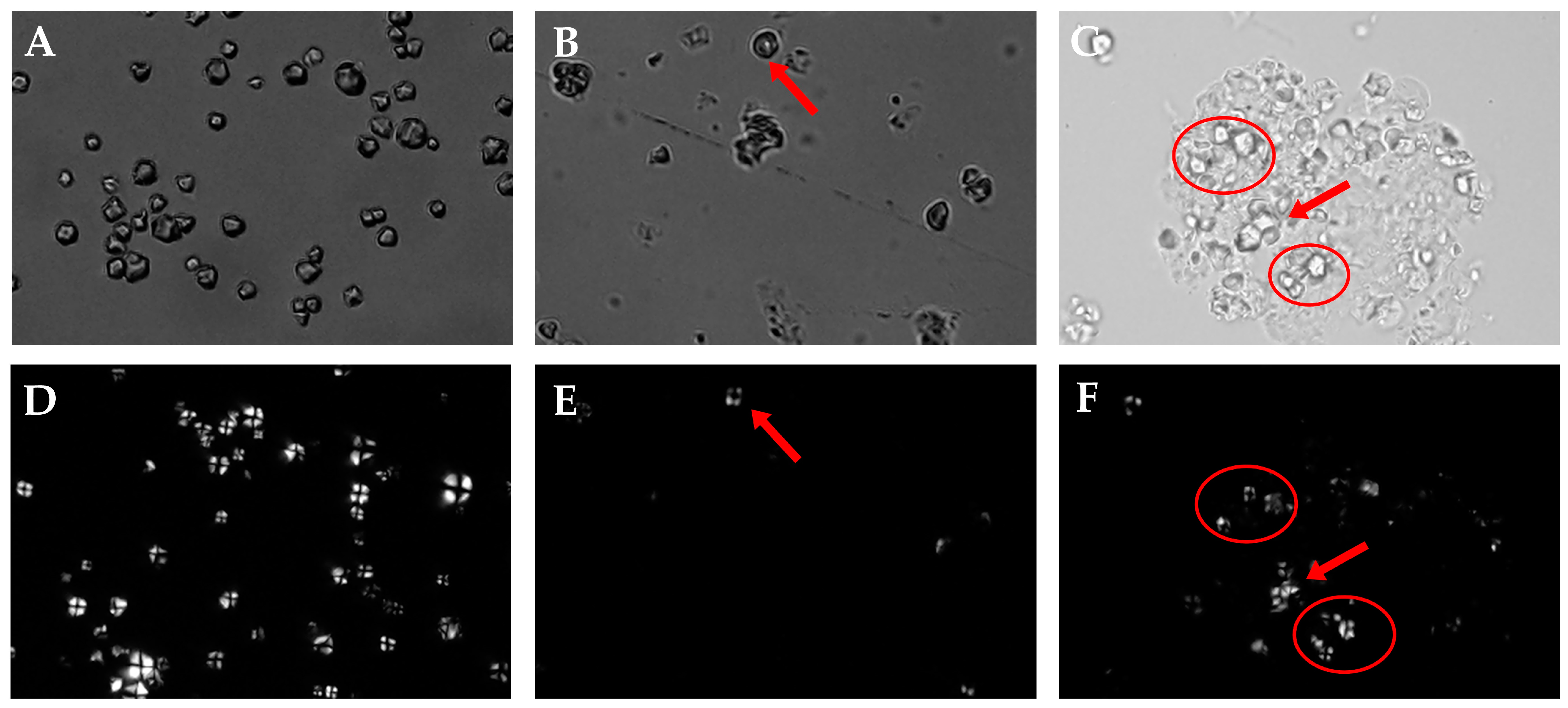
3.6. PLM Analysis of RS, ERS, and SPI-WPI-ERS
3.7. WAI and WSI Analysis of RS, ERS, and SPI-WPI-ERS
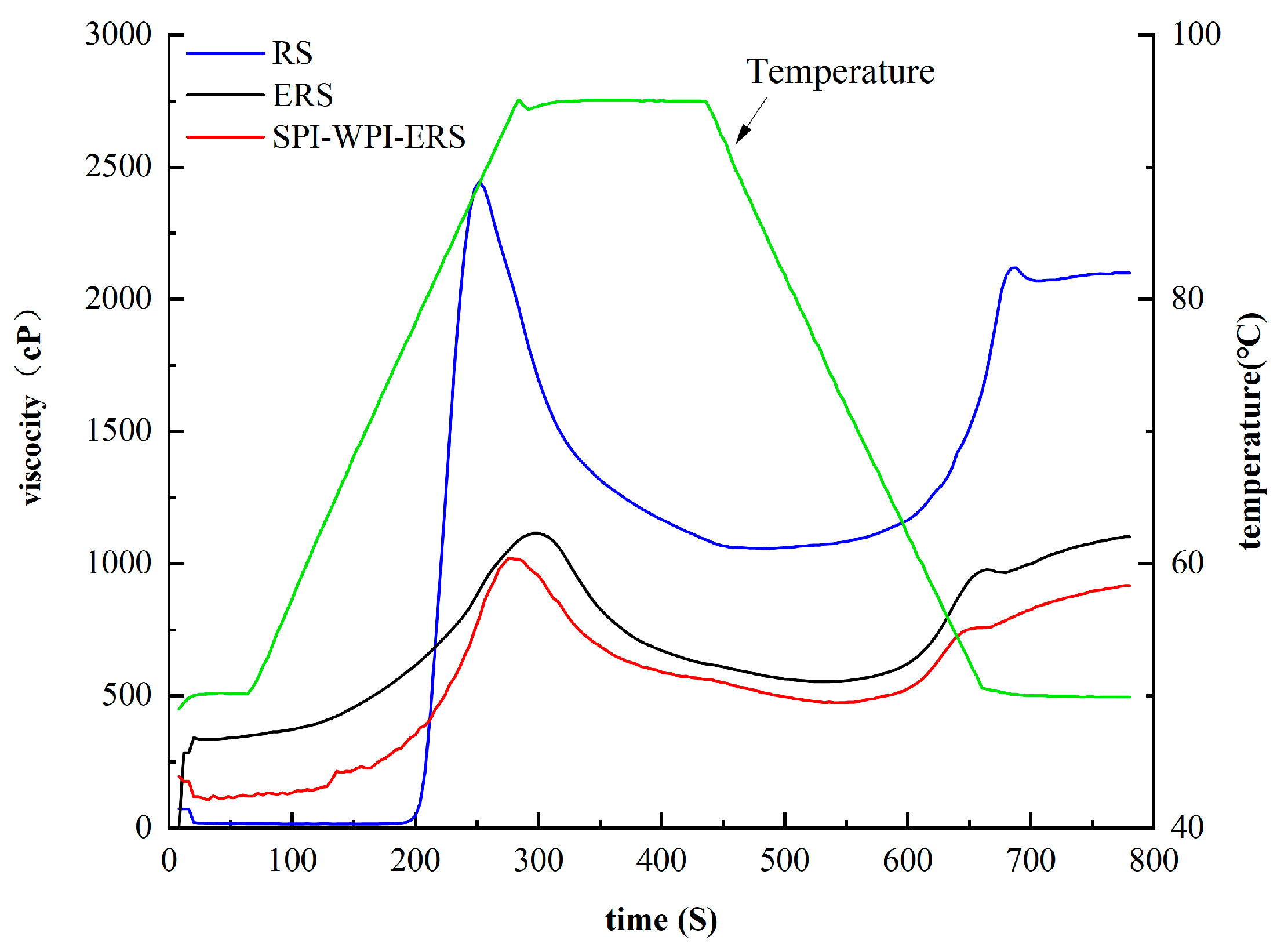
3.8. RVA Analysis of RS, ERS, and SPI-WPI-ERS
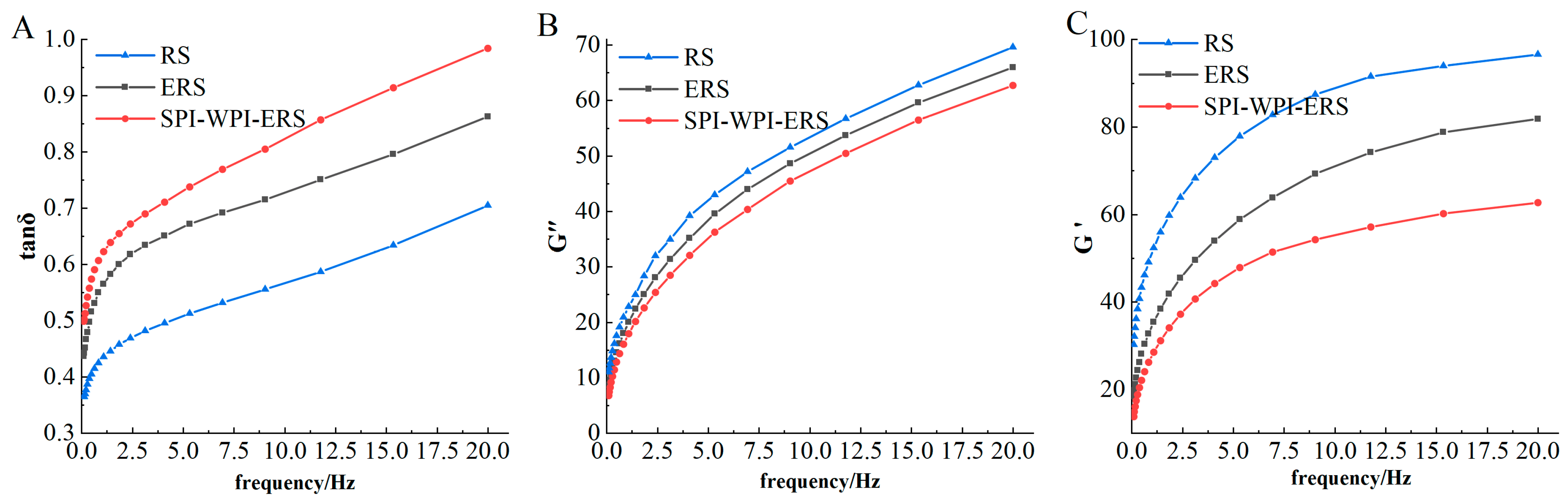
3.9. Rheological Properties of RS, ERS, and SPI-WPI-ERS
3.10. Thermal Properties of RS, ERS, and SPI-WPI-ERS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, S.; Aravind, S.M.; Charpe, P.; Ajlouni, S.; Ranadheera, C.S.; Chakkaravarthi, S. Traditional rice-based fermented roducts: Insight into their probiotic diversity and probable health benefits. Food Biosci. 2022, 50, 102082. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, Y.; Ko, S.; Yoon, M.R.; Lee, J.S.; Lee, S. Physicochemical characterization and in-vitro digestibility of extruded ce noodles with different amylose contents based on rheological approaches. J. Cereal Sci. 2016, 71, 258–263. [Google Scholar] [CrossRef]
- Dalbhagat, C.G.; Mahato, D.K.; Mishra, H.N. Effect of extrusion processing on physicochemical, functional and nutritional haracteristics of rice and rice-based products: A review. Trends Food Sci. Technol. 2019, 85, 226–240. [Google Scholar] [CrossRef]
- Huang, S.; Benchamas, G.; Huang, G. Whole processing and use of rice polishings. Innov. Food Sci. Emerg. Technol. 2020, 63, 02373. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Chemistry, structure, functionality and applications of rice starch. J. ereal Sci. 2016, 70, 291–300. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Wu, J.; Luo, S.; Chen, R.; Liu, C.; Gilbert, R.G. Modification of retrogradation property of rice starch by mproved extrusion cooking technology. Carbohydr. Polym. 2019, 213, 192–198. [Google Scholar] [CrossRef]
- Xu, E.; Wu, Z.; Chen, J.; Tian, J.; Cheng, H.; Li, D.; Jin, Z. Calcium—Lactate-induced enzymatic hydrolysis of extruded broken ce starch to improve Chinese rice wine fermentation and antioxidant capacity. LWT 2020, 118, 108803. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, L.; Li, D.; Adhikari, B. Effects of partial gelatinization on structure and thermal properties of corn starch after pray drying. Carbohydr. Polym. 2012, 88, 1319–1325. [Google Scholar] [CrossRef]
- Ai, J.; Witt, T.; Cowin, G.; Dhital, S.; Turner, M.S.; Stokes, J.R.; Gidley, M.J. Anti-staling of high-moisture starchy food: Effect of hydrocolloids, emulsifiers and enzymes on mechanics of steamed-rice cakes. Food Hydrocoll. 2018, 83, 454–464. [Google Scholar] [CrossRef]
- Lian, X.; Kang, H.; Sun, H.; Liu, L.; Li, L. Identification of the main retrogradation-related properties of rice starch. J. Agric. d Chem. 2015, 63, 1562–1572. [Google Scholar] [CrossRef]
- Zia-ud-din; Xiong, H.; Fei, P. Physical and chemical modification of starches—A review. Crit. Rev. Food Sci. Nutr. 2015, 1040, 6. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Luo, S.; Li, C.; Ye, J.; Liu, C.; Gilbert, R.G. Physicochemical and structural properties of pregelatinized starch pared by improved extrusion cooking technology. Carbohydr. Polym. 2017, 175, 265–272. [Google Scholar] [CrossRef]
- Gao, L.; Xu, Z.; Zhang, R.; Qin, Y.; Ji, N.; Wang, Y.; Sun, Q. Effects of erythritol on rheological properties of rice flour and ctural characteristics of extruded dried rice noodles with rapid rehydration behaviors. Food Hydrocoll. 2023, 144, 109007. [Google Scholar] [CrossRef]
- Yu, X.; Wang, L.; Zhang, J.; Duan, Y.; Xin, G.; Tong, L.; Wang, P. Effects of screw speed on the structure and physicochemical perties of extruded reconstituted rice composed of rice starch and glutelin. Food Struct. 2023, 36, 100313. [Google Scholar] [CrossRef]
- Day, L.; Swanson, B.G. Functionality of protein-fortified extrudates. Compr. Rev. Food Sci. Food Saf. 2013, 12, 546–564. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.A.; Derbyshire, E.; Tiwari, B.K.; Brennan, C.S. Ready-to-eat snack products: The role of extrusion technology in eloping consumer acceptable and nutritious snacks. Int. J. Food Sci. Technol. 2013, 48, 893–902. [Google Scholar] [CrossRef]
- Schreuders, F.K.; Dekkers, B.L.; Bodnár, I.; Erni, P.; Boom, R.M.; van der Goot, A.J. Comparing structuring potential of pea soy protein with gluten for meat analogue preparation. J. Food Eng. 2019, 261, 32–39. [Google Scholar] [CrossRef]
- Teba, C.d.S.; Silva, E.M.M.d.; Chávez, D.W.H.; Carvalho, C.W.P.d.; Ascheri, J.L.R. Effects of whey protein concentrate, d moisture and temperature on the physicochemical characteristics of a rice-based extruded flour. Food Chem. 2017, 228, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Onwulata, C.I.; Tunick, M.H.; Thomas-Gahring, A.E. Pasting and extrusion properties of mixed carbohydrate and whey tein isolate matrices. J. Food Process. Preserv. 2013, 38, 1577–1591. [Google Scholar] [CrossRef]
- Pastor-Cavada, E.; Drago, S.R.; Gonzalez, R.J.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Effects of the addition of wild umes (Lathyrus annuus and Lathyrus clymenum) on the physical and nutritional properties of extruded products based on ole corn and brown rice. Food Chem. 2011, 128, 961–967. [Google Scholar] [CrossRef]
- Yu, L.; Ramaswamy, H.S.; Boye, J. Protein rich extruded products prepared from soy protein isolate-corn flour blends. LWT-d Sci. Technol. 2013, 50, 279–289. [Google Scholar] [CrossRef]
- Berrios, J.D.J.; Cámara, M.; Torija, M.E.; Alonso, M. Effect of extrusion cooking and sodium bicarbonate addition on the bohydrate composition of black bean flours. J. Food Process. Preserv. 2010, 26, 113–128. [Google Scholar] [CrossRef]
- Alam, M.S.; Kaur, J.; Khaira, H.; Gupta, K. Extrusion and extruded products: Changes in quality attributes as affected by extrusion process parameters: A review. Crit. Rev. Food Sci. Nutr. 2015, 56, 445–473. [Google Scholar] [CrossRef] [PubMed]
- Mckeown, N.M.; Hruby, A.; Saltzman, E.; Choumenkovitch, S.F.; Jacques, P.F. Weighing in on Whole Grains: A Review of dence Linking Whole Grains to Body Weight; AACC International, Inc.: Saint Paul, MN, USA, 2012. [Google Scholar]
- Paul, S. Approved Methods of the American Association of Cereal Chemists; AACC: Saint Paul, MN, USA, 2020. [Google Scholar]
- Kong, X.; Zhu, Z.; Zhang, X.; Zhu, Y. Effects of cordyceps polysaccharides on pasting properties and in vitro starch digestity of wheat starch. Food Hydrocoll. 2020, 102, 105604. [Google Scholar] [CrossRef]
- Jeong, D.; Lee, J.H.; Chung, H.J. Effect of molecular structure on phase transition behavior of rice starch with different ylose contents. Carbohydr. Polym. 2021, 259, 117712. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.H.; Chung, H.J. Impact of diverse cultivars on molecular and crystalline structures of rice starch for food cessing. Carbohydr. Polym. 2017, 169, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hoover, R.; Hughes, T.; Chung, H.J.; Liu, Q. Composition, molecular structure, properties, and modification of pulse rches: A review. Food Res. Int. 2010, 43, 399–413. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Impact of different structural types of amylopectin on retrogradation. Food Hydrocoll. 2018, 80, 96. [Google Scholar] [CrossRef]
- Li, C.; Gong, B. Insights into chain-length distributions of amylopectin and amylose molecules on the gelatinization property ice starches. Int. J. Biol. Macromol. 2020, 155, 721–729. [Google Scholar] [CrossRef]
- Sofi, S.A.; Singh, J.; Mir, S.A.; Dar, B.N. In vitro starch digestibility, cooking quality, rheology and sensory properties of ten-free pregelatinized rice noodle enriched with germinated chickpea flour. LWT 2020, 133, 110090. [Google Scholar] [CrossRef]
- Bian, X.; Chen, J.R.; Yang, Y.; Yu, D.H.; Ma, Z.Q.; Ren, L.K.; Wu, N.; Chen, F.L.; Liu, X.F.; Wang, B. Effects of fermentation the structure and physical properties of glutinous proso millet starch. Food Hydrocoll. 2022, 123, 107144. [Google Scholar] [CrossRef]
- Gong, B.; Chengd, L.; Gilbert, R.G.; Li, C. Distribution of short to medium amylose chains are major controllers of in vitro estion of retrograded rice starch. Food Hydrocoll. 2019, 96, 634–643. [Google Scholar] [CrossRef]
- Wu, W.; Jiao, A.; Xu, E.; Chen, Y.; Jin, Z. Effects of extrusion technology combined with enzymatic hydrolysis on the strucl and physicochemical properties of porous corn starch. Food Bioprocess Technol. 2020, 13, 442–451. [Google Scholar] [CrossRef]
- Ichihara, T.; Fukuda, J.; Takaha, T.; Suzuki, S.; Yuguchi, Y.; Kitamura, S. Small-angle X-ray scattering measurements of gel duced from α-amylase-treated cassava starch granules. Food Hydrocoll. 2016, 55, 228–234. [Google Scholar] [CrossRef]
- Liang, R.; Liang, Q.; Li, Z.; Zhou, Q.; Li, L.; Sun, W. Assessment of the thermal hazards and oxidization mechanism of ured corn starch dust by TG–FTIR. J. Loss Prev. Process Ind. 2021, 69, 104380. [Google Scholar] [CrossRef]
- Su, C.; Saleh, A.S.; Zhang, B.; Feng, D.; Zhao, J.; Guo, Y.; Yan, W. Effects of germination followed by hot air and infrared ing on properties of naked barley flour and starch. Int. J. Biol. Macromol. 2020, 165, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Liu, C.; Luo, S.; Li, T.; Liu, Y.; Wu, D.; Zuo, Y. Retrogradation behaviour of high-amylose rice starch pared by improved extrusion cooking technology. Food Chem. 2014, 158, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Man, J.; Cai, C.; Yan, Q.; Hu, M.; Wei, C. Applications of infrared spectroscopy in the analysis of ordered structure of starch grain. Acta Agron. Sin. 2012, 38, 505–513. [Google Scholar] [CrossRef]
- Chu, L.; Yang, L.; Li, J.; Lin, L.; Zheng, G. Effect of smilax china l. starch on the gel properties and interactions of calcium ate-induced soy protein isolate gel. Int. J. Biol. Macromol. 2019, 135, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Phongthai, S.; D’Amico, S.; Schoenlechner, R.; Homthawornchoo, W.; Rawdkuen, S. Effects of protein enrichment on the perties of rice flour-based gluten-free pasta. LWT-Food Sci. Technol. 2017, 80, 378–385. [Google Scholar] [CrossRef]
- Li, W.; Bai, Y.; Mousaa, S.A.S.; Zhang, Q.; Shen, Q. Effect of high hydrostatic pressure on physicochemical and structural perties of rice starch. Food Bioprocess Technol. 2012, 5, 2233–2241. [Google Scholar] [CrossRef]
- Kou, F.; Wang, W.H.; Wang, L.D.; Li, W.J.; Cao, L.K. Effect of lactobacillus fermentation on molecular structure and pasting properof millet starch. Chin. J. Biol. 2017, 30, 695–701. [Google Scholar] [CrossRef]
- Deng, Y.; Jin, Y.; FLuo, Y.L.; Zhong, Y.; Yue, J.; Song, X.Y.; Zhao, Y.Y. Impact of continuous or cycle high hydrostatic pressure the ultrastructure and digestibility of rice starch granules. J. Cereal Sci. 2014, 60, 302–310. [Google Scholar] [CrossRef]
- Ye, F.; Xiao, L.; Liang, Y.; Zhou, Y.; Zhao, G. Spontaneous fermentation tunes the physicochemical properties of sweet potato ch by modifying the structure of starch molecules. Carbohydr. Polym. 2019, 213, 79–88. [Google Scholar] [CrossRef]
- Li, M.; Hasjim, J.; Xie, F.; Halley, P.J.; Gilbert, R.G. Shear degradation of molecular, crystalline, and granular structures of starch ing extrusion. Starch-Starke 2013, 66, 595–605. [Google Scholar] [CrossRef]
- Mesa, N.J.E.D.; Alavi, S.; Singh, N.; Shi, Y.C.; Dogan, H.; Sang, Y. Soy protein-fortified expanded extrudates: Baseline study g normal corn starch. J. Food Eng. 2008, 90, 262–270. [Google Scholar] [CrossRef]
- Allen, K.E.; Carpenter, C.E.; Walsh, M.K. Influence of protein level and starch type on an extrusion-expanded whey product. h State Univ. 2007, 42, 953–960. [Google Scholar] [CrossRef]
- Yan, X.; Wu, Z.Z.; Li, M.Y.; Yin, F.; Ren, K.X.; Tao, H. The combined effects of extrusion and heat-moisture treatment on the sicochemical properties and digestibility of corn starch. Int. J. Biol. Macromol. 2019, 134, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gutiérrez, J.A.; San Martín-Martínez, E.; Martínez-Bustos, F.; Cruz-Orea, A. Physicochemical properties of in-starch interaction obtained by extrusion process. Starch-Stärke 2004, 56, 190–198. [Google Scholar] [CrossRef]
- Valle, G.D.; Colonna, P.; Patria, A.; Vergnes, B. Influence of amylose content on the viscous behavior of low hydrated molten ches. J. Rheol. 1996, 40, 347–362. [Google Scholar] [CrossRef]
- Sun, Q.; Han, Z.; Wang, L.; Xiong, L. Physicochemical differences between sorghum starch and sorghum flour modified by t-moisture treatment. Food Chem. 2014, 145, 756–764. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Zhou, Y.; Wu, Y.; Ouyang, J. Influence of ultrasound and microwave treatments on the structural and thermal properties of normal maize starch and potato starch: A comparative study. Food Chem. 2022, 377, 131990. [Google Scholar] [CrossRef]
- Karlsson, M.E.; Eliasson, A.C. Gelatinization andretrogradation of potato (Solanum tuberosum) starch in situ as assessedby ferential scanning calorimetry (DSC). LWT-Food Sci. Technol. 2003, 36, 735–741. [Google Scholar] [CrossRef]
- Shanthilal, J.; Bhattacharya, S. Rheology of rice flour dough with gum arabic: Small and large-deformation studies, sensory essment and modeling. J. Food Sci. 2015, 80, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Koocheki, A.; Milani, E. Effect of extrusion cooking on chemical structure, morphology, crystallinity and thermal operties of sorghum flour extrudates. J. Cereal Sci. 2017, 75, 324–331. [Google Scholar] [CrossRef]
- Luo, Y.; Niu, L.; Li, D.; Xiao, J. Synergistic effects of plant protein hydrolysates and xanthan gum on the short-and long-term retrogradation of rice starch. Int. J. Biol. Macromol. 2019, 144, 967–977. [Google Scholar] [CrossRef]
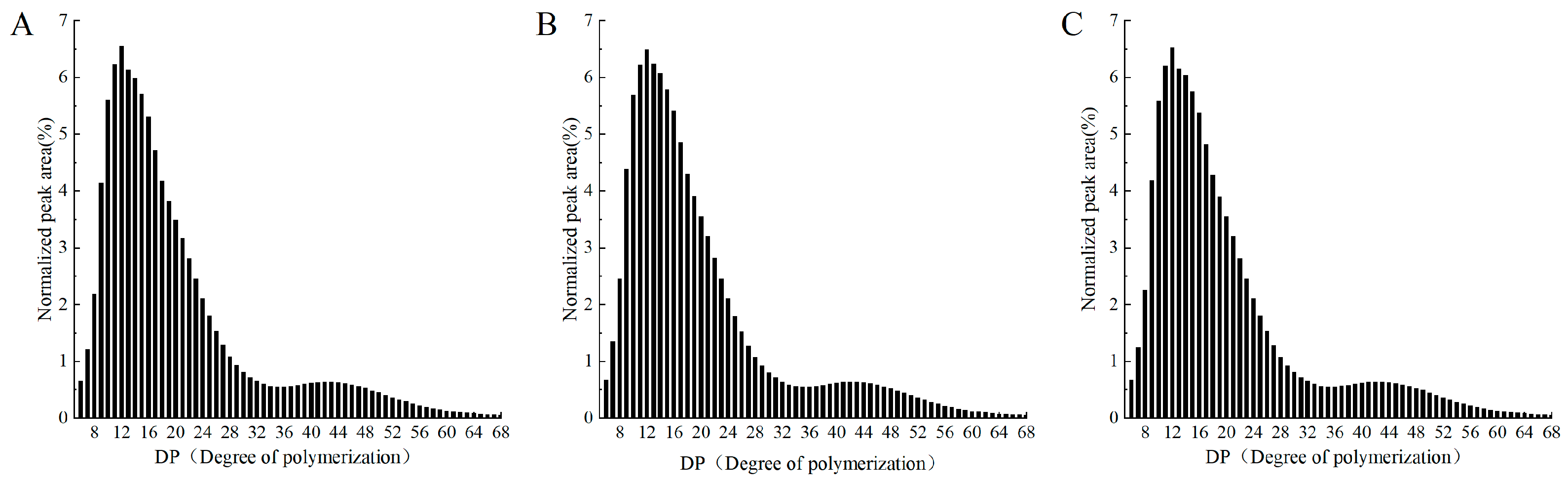

| Samples | A: 6 ≤ DP ≤ 12 | B1: 13 ≤ DP ≤ 24 | B2: 25 ≤ DP ≤ 36 | B3: 37 ≤ DP |
|---|---|---|---|---|
| RS | 26.63 ± 0.24 b | 49.97 ± 0.07 c | 11.18 ± 0.16 a | 11.54 ± 0.27 a |
| ERS | 27.32 ± 0.16 a | 50.82 ± 0.35 a | 11.09 ± 0.06 c | 11.45 ± 0.22 b |
| SPI-WPI-ERS | 26.73 ± 0.39 b | 50.56 ± 0.28 b | 11.15 ± 0.11 b | 11.53 ± 0.32 a |
| Samples | Total Starch (%) | Amylose Content (%) | Mn (kDa) | Mw (kDa) | Mw/Mn |
|---|---|---|---|---|---|
| RS | 77.40 ± 0.16 a | 17.10 ± 0.10 c | 26691.42 ± 26.16 a | 72246.39 ± 9.01 a | 2.71 ± 0.12 c |
| ERS | 74.00 ± 0.61 c | 21.29 ± 0.19 a | 20139.40 ± 14.64 b | 64082.54 ± 25.32 c | 3.18 ± 0.06 b |
| SPI-WPI-ERS | 75.78 ± 0.53 b | 18.12 ± 0.17 b | 20286.12 ± 10.58 b | 67605.68 ± 27.53 b | 3.33 ± 0.19 a |
| Samples | Crystal Pattern | Degree of Crystallinity (%) | IR Peak Ratio of 1047/1022 cm−1 | IR Peak Ratio of 1022/995 cm−1 |
|---|---|---|---|---|
| RS | A | 40.58 ± 0.24 a | 0.7375 ± 0.17 a | 1.24 ± 0.02 a |
| ERS | V | 30.00 ± 0.15 b | 0.7229 ± 0.15 b | 1.21 ± 0.01 b |
| SPI-WPI-ERS | V | 17.97 ± 0.11 c | 0.6873 ± 0.08 c | 1.20 ± 0.00 c |
| Samples | PV (cP) | TV (cP) | BD (cP) | FV (cP) | SB (cP) |
|---|---|---|---|---|---|
| RS | 2444 ± 31.36 a | 1056 ± 10.56 a | 1388 ± 12.43 a | 2099 ± 32.12 a | 1043 ± 13.09 a |
| ERS | 1115 ± 6.35 b | 553 ± 5.87 b | 562 ± 6.24 b | 1101 ± 10.33 b | 548 ± 6.27 b |
| SPI-WPI-ERS | 1021 ± 7.84 c | 474 ± 5.30 c | 547 ± 8.16 c | 917 ± 9.17 c | 443 ± 3.15 c |
| Samples | T0 (°C) | Tp (°C) | Tc (°C) | ∆H (J/g) | ∆T (°C) |
|---|---|---|---|---|---|
| RS | 38.82 ± 1.01 a | 96.66 ± 0.88 a | 157.23 ± 5.16 a | 276.25 ± 5.16 a | 118.41 ± 2.55 a |
| ERS | 37.15 ± 1.00 b | 91.67 ± 1.07 b | 143.24 ± 5.65 b | 207.55 ± 2.50 b | 106.09 ± 4.51 b |
| SPI-WPI-ERS | 35.19 ± 0.99 c | 90.05 ± 1.20 c | 140.48 ± 7.14 c | 183.08 ± 5.12 c | 105.29 ± 4.50 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhao, X.; Ma, C.; Wu, M.; Fan, Q.; Fu, Y.; Zhang, G.; Bian, X.; Zhang, N. Effects of Extrusion Technology on Physicochemical Properties and Microstructure of Rice Starch Added with Soy Protein Isolate and Whey Protein Isolate. Foods 2024, 13, 764. https://doi.org/10.3390/foods13050764
Liu X, Zhao X, Ma C, Wu M, Fan Q, Fu Y, Zhang G, Bian X, Zhang N. Effects of Extrusion Technology on Physicochemical Properties and Microstructure of Rice Starch Added with Soy Protein Isolate and Whey Protein Isolate. Foods. 2024; 13(5):764. https://doi.org/10.3390/foods13050764
Chicago/Turabian StyleLiu, Xiaofei, Xiangxiang Zhao, Chunmin Ma, Ming Wu, Qiqi Fan, Yu Fu, Guang Zhang, Xin Bian, and Na Zhang. 2024. "Effects of Extrusion Technology on Physicochemical Properties and Microstructure of Rice Starch Added with Soy Protein Isolate and Whey Protein Isolate" Foods 13, no. 5: 764. https://doi.org/10.3390/foods13050764
APA StyleLiu, X., Zhao, X., Ma, C., Wu, M., Fan, Q., Fu, Y., Zhang, G., Bian, X., & Zhang, N. (2024). Effects of Extrusion Technology on Physicochemical Properties and Microstructure of Rice Starch Added with Soy Protein Isolate and Whey Protein Isolate. Foods, 13(5), 764. https://doi.org/10.3390/foods13050764






